Abstract
Although gagging is a frequent problem that, when severe, can jeopardize the dental procedure, no single protocol is used to alleviate this phenomenon. Selective 5-HT3 antagonists, such as granisetron, may attenuate gagging. In this study, granisetron and placebo were administered intravenously, in a crossover, double-blind manner, to 25 healthy volunteers in 2 different sessions. Gagging levels were recorded before and after administration, as were BP, pulse, and O2 saturation. Recorded results were analyzed with the use of tests for nonparametric values (P = .05). A significant increase in the depth of swab insertion was noted after administration of both placebo and drug. The increase in drug effectiveness correlated with decreased body weight. The true efficacy of granisetron in gagger patients with this treatment protocol has yet to be fully established, although it has been theorized that an increased dosage of granisetron may have a better effect.
Keywords: Granisetron, gag reflex, 5-HT3 inhibitor
Introduction
The gag reflex is a primitive physiological defense mechanism, tailored to prevent ingestion of harmful or offensive substances and foreign bodies into the gastrointestinal tract. Primarily, the reflex involves constriction of the oropharyngeal muscles, followed immediately by spastic and periodic reversed movements, which are combined with abdominal, thoracic, and diaphragmatic activity. Common effects of this reflex include hypersalivation, tears, diaphoresis, tachycardia, and, in extreme instances, vomiting, panic behavior, and loss of consciousness.1
Gagging can present a significant obstacle to the delivery of routine dental treatment. Dental instruments, materials, and even odors can serve as a stimulus to the reflex pathway, starting from the autonomic center in the medulla oblongata. The reflex is modified further by the emetic nucleus located at the area postrema of the fourth ventricle, and by the enteric nucleus of the vagus nerve (CN X). The chemoreceptor trigger zone (CTZ) of the emetic nucleus contains a large quantity of 5-HT3 receptors. These receptors, which are also found in the stomach and the proximal small intestine, regulate emesis in situations such as infection, drug therapy, and, primarily, antineoplastic treatment. A suggested mechanism is that serotonin, secreted from the vagus nerve endings, binds to these receptors and induces emesis.2
The medullary center, despite its being an autonomic one, is partially controlled by the cerebral cortex, thus making it possible to initiate gagging just by imagining a disagreeable experience, or, conversely, by controlling the reflex to some extent by distractive action.1,3
Different levels of severity in gagging have been noted. In most cases, successful dental treatment can be given with minor necessary adjustments. Yet, some patients react with such an acute reflex that any treatment attempt might result in lowered quality or may not succeed at all. The level of gagging is proportionate to the quantity of neural endings in the stimulated area, and gagging can be affected by local or transient factors such as a common cold, gastrointestinal disease, or mental stress.1
No definitive technique can completely attenuate the gag reflex. Among the most prevalent methods are behavioral approaches,4 distraction or relaxation, and drug therapy, provided primarily through topical anesthesia of the oral mucosa. Research into the use of nitrous oxide (N2O) sedation has led to conflicting results, with most studies pointing to a significant decrease in gagging5; others suggest that N2O itself may cause nausea and vomiting, especially when combined with local anesthetics.6 Other suggested methods of controlling dental gagging include administration of propofol IV,7 acupuncture,8,9 hypnosis,10 and combinations of these.11,12
Selective 5-HT3 antagonists were synthesized for palliative treatment of patients during antineoplastic treatment.13 The drug of choice in such cases is ondansetron (Zofran), which has been used since the beginning of the 1990s.14 This drug and other 5-HT3 antagonists (palonosetron, dolasetron, granisetron, and tropisetron) are given enterally or parenterally and influence the peripheral and central nervous system in prevention of emesis. Their primary use is in preventing nausea and emesis during antineoplastic treatment or post surgery, yet some are used to minimize nausea at pregnancy. Their therapeutic activity is incomplete, as it depends on genetic polymorphism of 5-HT3 receptors.15
The objective of this study was to examine the prophylactic effect of granisetron, a commonly used antiemetic drug, during palatal and oropharyngeal stimulation mimicking the dental situation, as a first step in establishing a protocol for extensive gaggers.
Methods
Subjects and Study Design
This study was designed as a double-blind crossover placebo-controlled trial and was conducted at the Center of Sedation and Anesthesia in Dentistry in the Oral Medicine Department of the Hebrew University-Hadassah School of Dental Medicine in Jerusalem, Israel.
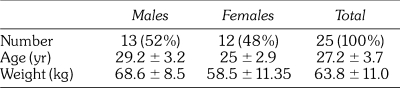
Volunteers were requested to fill out a medical history form, and those with health problems (The American Society of Anesthesiologists Physical Status Classification System, as it appears in the 2008 ASA Relative Value Guide [ASA] ASA >2), as well as smokers, or those using systemic medications or recreational drugs were excluded. Twenty-five volunteers were included in the study, most of them students at the medical campus of the Hebrew University. The volunteers were in good general health (ASA 1-2) with no history of extensive gagging that would interfere with dental treatment. Tested group data are presented in the Table.
Subject Data
Informed signed consent was required before participation in the study. The study was performed in accordance with the Declaration of Helsinki, and the protocol was approved by the local ethics committee of the Hadassah Medical Organization.
In the study, a standard dose of 3 mg granisetron (Granisetron-Teva, Teva Pharmaceutical Industries, Jerusalem, Israel) was administered to all volunteers in a manner similar to that used in routine clinical situations. Each subject received the drug and the placebo at 2 different appointments, separated by at least 1 week. A 10 mL syringe containing the placebo (10 mL of saline) or the drug (3 mg/3 mL of granisetron diluted in 7 mL of saline) was prepared for each volunteer in each session in computer-generated random order by an investigator not involved in the data collection. Each syringe was marked with a serial number, and its contents were unknown to both examiners and subjects. Decoding of syringe contents was done upon completion of the study.
Procedures
Each meeting was designed to last 30 minutes. Blood pressure, pulse, and O2 saturation values were recorded (S/5 Anesthesia Monitor, Datex-Ohmeda, GE Healthcare, Oy, Finland) 4 times during the appointment: (1) upon arrival, (2) after IV line insertion, (3) 5 minutes after injection of the drug or placebo, and (4) after completion of the check.
Following primary data intake (time point 1), an intravenous line was started using standard 24G venflon (BD Venflon, Becton Dickinson Infusion Therapy, Helsingborg, Sweden), which was connected to an IV bag containing 150 mL of normal saline solution dripping at a rate of 100 mL/hr. BP, pulse, and saturation values then were taken again (time point 2), and gagging was checked with a standard 130 mm bacteriology transport swab (Fastidious Anaerobe Transport Swab, 108C.US, COPAN Innovation, Brescia, Italy). The swab was placed gently on the anterior hard palate and was moved posteriorly until gagging was initiated. At this point, the depth of insertion was measured in millimeters, with the edge of the upper central incisors serving as the reference point.
Either the drug or the placebo was injected through the IV line via the pre-prepared 10 mL syringe. A slow, manual injection with a simple disposable plastic syringe (Medi-Plus, Shandong Zibo Shanchuan Medical Instrument Co. Ltd., Shandong, China) lasted 90 seconds, and after a 5 minute pause, BP, pulse oximetry, and swab insertion depth measurements were retaken (time point 3). BP, pulse, and saturation values were recorded again before the volunteer was discharged (time point 4).
Statistical Analysis
Blood pressure, pulse, and O2 saturation were measured and recorded 4 times during each session. The length of swab insertion was measured 3 times before and 3 times after drug or placebo injection (time points 2 and 3) and each time the mean result was calculated. The difference between insertions before and after injection of drug/placebo was calculated.
Because of the experimental design, the nonparametric Wilcoxon test was used to compare paired median values. The P value in our trial was equal to .05.
Results
Results were obtained after recorded data were correlated with the type of treatment (drug or placebo) given to each volunteer.
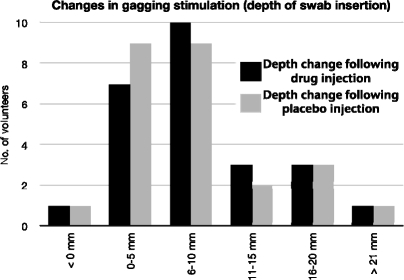
A significant difference between depth of insertion before and after injection was noted, with both placebo and drug showing an increase in depth of insertion (Figure 1). The mean increase after drug injection was 8.57 ± 5.41 mm, and after placebo injection, 7.81 ± 6.30 mm (P = .388, using the Monte-Carlo simulation). Thus, no statistically significant difference between depths reached after injection of drug or placebo was observed.
Figure 1.
The change in swab insertion depth was measured following administration of drug (black) and placebo (grey). The figure shows that both drug and placebo caused a significant increase in the volunteer's handling of the swab insertion.
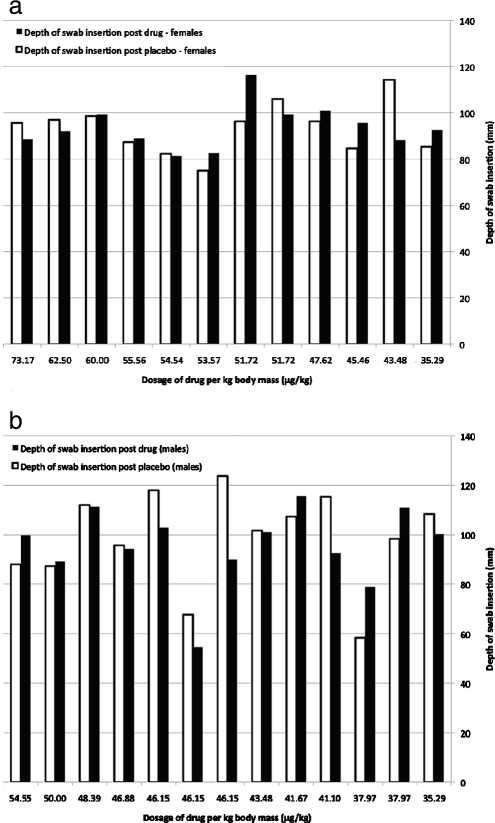
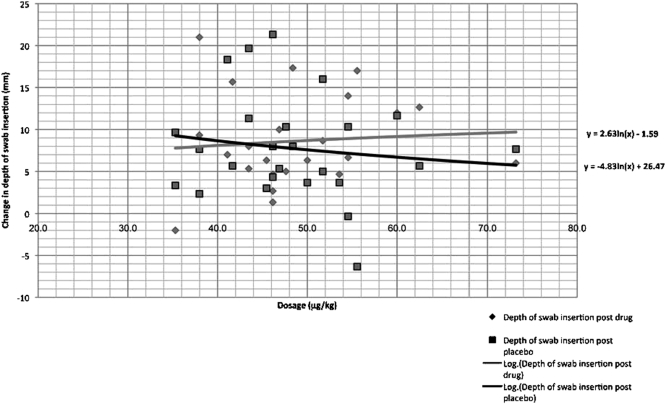
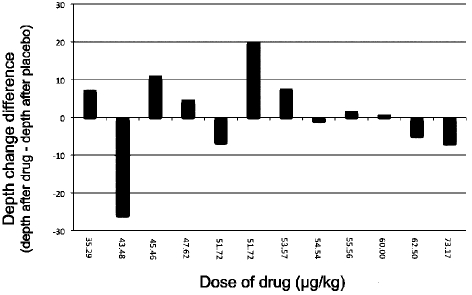
When the dose per kilogram was calculated in each volunteer, and in spite of the similarity of body masses of some volunteers, variability in responses to drug and to placebo was evident (Figure 2). However, a positive trend of increased drug effect with decreased body weight was seen (Figure 3). The effect of the drug was statistically greater among females, whose average weight was lower (P = .031) (Figure 4).
Figure 2.
Depths of swab insertion in male (a) and female (b) volunteers following drug (black) and placebo (white) administration. The females, who received higher dosages because of lower body mass, showed a more significant increase in drug action contrary to placebo.
Figure 3.
A logarithmic reduction of changes in depth following administration of drug and placebo shows a positive trend in the increase of drug efficacy correlated with the increase in dosage per kilogram. The placebo does not follow the same trend. The increase in drug effectiveness is best seen in doses greater than ∼45 µg/kg.
Figure 4.
Subtraction of the placebo effect from the drug effect shows that in females, whose average weight was lower (and thus the dosage per kilogram was higher), a significant increase was seen in drug efficacy proportional to the increase in dosage per kg (P < .031).
No statistically significant change in volunteer heart rate, blood pressure, and O2 saturation was noted after injection of drug or placebo.
Discussion
Granisetron (Kytril) is a selective, high-affinity antagonist of the 5-HT3 receptor, with very low affinity to other amine receptors. It is metabolized by the liver by CYP-4503A, together with other drugs (e.g., cyclophosphamide, diazepam), yet no drug interactions have been reported.16 Granisetron is not cardiotoxic in dosages lower than 300 µg/kg.16,17 The recommended dose of granisetron is 40 µg/kg. In more than 60% of patients, this dose prevents emesis for 24 hours post chemotherapy or radiation therapy.17 Side effects have been noted in a small percentage of patients; the most common is headache, which occurs in 15% of cases. The recommended dose of granisetron has proved safe and effective in children.18 In this study, no adverse effects were reported by volunteers.
A significant change in the reaction of volunteers to swab insertion occurred after injection of either drug or placebo. Some volunteers with similar body mass responded differently to drug and placebo, enabling deeper insertion of the swab on the second appointment, independent of the injection contents. This phenomenon is probably due to order effect. In most volunteers (78%), depth of insertion significantly increased by 5 mm or more after injection. It is well documented that the gag reflex is affected by psychological factors; thus it is possible that the gag reflex decreased on the second measurement as subjects became more relaxed and better acquainted with the procedure.
Although some placebo effect was noted, it is important to emphasize that a positive trend in drug action was seen (Figure 3). Although not statistically significant, the drug effect increased proportionately to the increase in drug dosage per kilogram of body weight. In the study, a standard dose of 3 mg of granisetron was administered to all volunteers. It was found that the drug was more effective in volunteers with lower body weight (who, because of this, received a higher drug dosage per kilogram). Body weight had no such similar effect on those receiving the placebo injection.
Various pilot studies have been conducted throughout the world to evaluate the efficacy of granisetron and its side effects following its original protocol as a palliative, antinausea-antiemetic treatment, given during chemotherapy or post surgery. In a recent double-blind study, small doses (10 µg/kg-40 µg/kg) of granisetron were given to children and adolescents aged 1 to 23 years, during carboplatin therapy. The 2 dosages were found to be equally effective in preventing emesis, without causing side effects.18 In studies conducted in the mid 1990s by the Granisetron Study Group, who studied the antiemetic effects of granisetron during cisplatin chemotherapy courses, doses of 10 µg/kg and 40 µg/kg were found to have similar efficacy.19 When dosages of 40 µg/kg and 160 µg/kg were compared, no significant difference between their efficacies was found.20 Nonetheless, Carlisle and Stevenson stated that reducing the dosage of granisetron by 50% increases the risk of nausea or emesis.21
In a recent study in which the efficacy of prophylactic granisetron in preventing intraoperative nausea and vomiting during cesarean section was checked, doses of 1 mg granisetron proved ineffective in comparison with placebo.22 Another study suggested that granisetron is more effective than inhalation isopropyl alcohol in preventing postoperative nausea and vomiting (PONV) following laparoscopic procedures, yet no significant difference was found between granisetron prophylaxis and no prophylaxis at all.23 Other studies have shown granisetron to be more effective than other drugs in preventing PONV post laparoscopy,24 especially when combined with steroid agents.25,26 Subjects received doses ranging from 35.3 µg/kg to 77.5 µg/kg, and only when given in doses greater than 45 µg/kg was the drug more effective than the placebo, with its efficacy increasing proportionately with dosage. It is possible, therefore, that although the recommended dosage of 40 µg/kg is effective in the prevention of chemotherapy-induced delayed nausea and vomiting, it might not be enough to prevent dental gagging induced by direct triggering of the oropharyngeal region during dental procedures.
All of the volunteers in this study were young and generally healthy and had no known gagging problems during past dental treatments. Thus, they may or may not represent those with severe gagging, and it is difficult to determine the potential effect of granisetron on gagger patients, on whom the second phase of the study will be conducted.
None of the subjects in this study reported side effects of any kind after a 1-time injection of granisetron; thus the drug can be considered safe to use as part of a dental treatment protocol. Indications suggest that granisetron may have an effect on the prevention of gagging during dental treatment when given in the appropriate dosage. However, the true efficacy of granisetron in this treatment protocol has yet to be established fully.
Acknowledgments
This study was performed as part of the DMD degree requirements at the Hebrew University and Hadassah School of Dental Medicine. Special thanks to Dr. Yaron Haviv and Dr. Igal Granot for their kind assistance during the trials, and to Ms. Rita Gottloiber for performing the statistical analysis.
Footnotes
Address correspondence to Dr Eliezer Kaufman, Department of Oral Medicine, The Hebrew University-Hadassah School of Dental Medicine, POB 12272, Jerusalem, 91120, Israel, e-mail: ekaufman@cc.huji.ac.il
References
- Bassi G.S, Humphris G.M, Longman L.P. The etiology and management of gagging: a review of the literature. J Prosthet Dent. 2004;91:459–467. doi: 10.1016/S0022391304000939. [DOI] [PubMed] [Google Scholar]
- Pastricha P.J. Treatment of disorders of bowel motility and water flux. In: Brunton L, Lazo J, Parker K, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2006. pp. 1001–1004. [Google Scholar]
- Yagiela J.A. Making patients safe and comfortable for a lifetime of dentistry: frontiers in office-based sedation. J Dent Educ. 2005;65:1348–1356. [PubMed] [Google Scholar]
- Neumann J.K, McCarty G.A. Behavioral approaches to reduce hypersensitive gag response. J Prosthet Dent. 2001;85:305. doi: 10.1067/mpr.2001.114273. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Sommers E, Soletero D, Weinstein P. An experimental study of the control of the gag reflex with nitrous oxide. Anesth Prog. 1988;35:155–157. [PMC free article] [PubMed] [Google Scholar]
- Collado V, Nicolas E, Faulks D, Hennequin M. A review of the safety of 50% nitrous oxide/oxygen in conscious sedation. Expert Opin Drug Saf. 2007;6:559–571. doi: 10.1517/14740338.6.5.559. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Ayuse T, Ishizaka S, Ishitobi S, Nogami T, Oi K. Management of exaggerated gag reflex using intravenous sedation in prosthodontic treatment. Tohoku J Exp Med. 2007;212:373–378. doi: 10.1620/tjem.212.373. [DOI] [PubMed] [Google Scholar]
- Thayer C.C. The use of acupuncture in dentistry. Dent Update. 2007;34:244–246. 249–250. doi: 10.12968/denu.2007.34.4.244. [DOI] [PubMed] [Google Scholar]
- Rosted P, Bundgaard M, Fiske J, Pedersen A.M. The use of acupuncture in controlling the gag reflex in patients requiring an upper alginate impression: an audit. Br Dent J. 2006;201:721–725. doi: 10.1038/sj.bdj.4814305. [DOI] [PubMed] [Google Scholar]
- Roberts K. Hypnosis in dentistry. Dent Update. 2006;33:312–314. doi: 10.12968/denu.2006.33.5.312. [DOI] [PubMed] [Google Scholar]
- Eitner S, Wichmann M, Holst S. A long-term therapeutic treatment for patients with a severe gag reflex. Int J Clin Exp Hypn. 2005;53:74–86. doi: 10.1080/00207140490914252. [DOI] [PubMed] [Google Scholar]
- Eitner S, Wichmann M, Holst S. “Hypnopuncture”—a dental-emergency treatment concept for patients with a distinctive gag reflex. Int J Clin Exp Hypn. 2005;53:60–73. doi: 10.1080/00207140490914243. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Tremblay P.B, Sezer O, Possinger K, Roots I, Brockmoeller J. Investigation of the association between 5-HT3A receptor gene polymorphisms and efficiency of antiemetic treatment with 5-HT3 receptor antagonists. Pharmacogenetics. 2004;14:271–278. doi: 10.1097/00008571-200405000-00001. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Mayer S.E. 5-Hydroxytryptamine (serotonin): receptor agonists and antagonists. In: Brunton L, Lazo J, Parker K, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2004. pp. 297–315. [Google Scholar]
- Kaiser R, Sezer O, Papies A, et al. Patient tailored antiemetic treatment with 5-hydroxytriptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol. 2002;20:2805–2811. doi: 10.1200/JCO.2002.09.064. [DOI] [PubMed] [Google Scholar]
- Keefe D.L. The cardiotoxic potential of the 5-HT3 receptor antagonist antiemetics: is there cause for concern. Oncologist. 2002;7:65–72. doi: 10.1634/theoncologist.7-1-65. [DOI] [PubMed] [Google Scholar]
- Aapro M. Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist. 2004;9:673–686. doi: 10.1634/theoncologist.9-6-673. [DOI] [PubMed] [Google Scholar]
- Berrak S.G, Ozdemir N, Bakirci N, et al. A double-blind, crossover, randomized dose-comparison trial of granisetron for the prevention of acute and delayed nausea and emesis in children receiving moderately emetogenic carboplatin-based chemotherapy. Support Care Cancer. 2007;15:1163–1168. doi: 10.1007/s00520-007-0242-y. [DOI] [PubMed] [Google Scholar]
- Soukop M. A dose-finding study of granisetron, a novel antiemetic, in patients receiving high-dose cisplatin. Granisetron Study Group. Support Care Cancer. 1994;2:177–183. doi: 10.1007/BF00417477. [DOI] [PubMed] [Google Scholar]
- Carlisle J.B, Stevenson C.A. Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2006;3:CD004125. doi: 10.1002/14651858.CD004125.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balki M, Casodekar S, Dhumne S, Carvalho J. The prophylactic granisetron does not prevent postdelivery nausea and vomiting during elective cesarean delivery under spinal anesthesia. Anesth Analg. 2007;104:679–683. doi: 10.1213/01.ane.0000253036.06307.5c. [DOI] [PubMed] [Google Scholar]
- Teran L, Hawkins J.K. The effectiveness of inhalation isopropyl alcohol vs. granisetron for the prevention of postoperative nausea and vomiting. AANA J. 2007;75:417–422. [PubMed] [Google Scholar]
- Oksuz H, Zencirci B, Ezberci M. Comparison of the effectiveness of metoclopramide, ondansetron, and granisetron on the prevention of nausea and vomiting after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2007;17:803–808. doi: 10.1089/lap.2006.0243. [DOI] [PubMed] [Google Scholar]
- Moussa A.A, Oregan P.J. Prevention of postoperative nausea and vomiting in patients undergoing laparoscopic bariatric surgery—granisetron alone vs granisetron combined with dexamethasone/droperidol. Middle East J Anesthesiol. 2007;19:357–367. [PubMed] [Google Scholar]
- Gombar S, Kaur J, Kumar Gombar K, Dass A, Singh A. Superior anti-emetic efficacy of granisetron-dexamethasone combination in children undergoing middle ear surgery. Acta Anaesthesiol Scand. 2007;51:621–624. doi: 10.1111/j.1399-6576.2007.01296.x. [DOI] [PubMed] [Google Scholar]