Abstract
The developmental requirements of ovarian follicles are dependent on the maturation stage of the follicle; in particular, elegant studies with genetic models have indicated that FSH is required for antral, but not preantral, follicle growth and maturation. To elucidate further the role of FSH and other regulatory molecules in preantral follicle development, in vitro culture systems are needed. We employed a biomaterials-based approach to follicle culture, in which follicles were encapsulated within matrices that were tailored to the specific developmental needs of the follicle. This three-dimensional system was used to examine the impact of increasing doses of FSH on follicle development for two-layered secondary (100–130 µm; two layers of granulosa cells surrounding the oocyte) and multilayered secondary (150–180 µm, several layers of granulosa cells surrounding the oocyte) follicles isolated from mice. Two-layered secondary follicles were FSH responsive when cultured in alginate-collagen I matrices, exhibiting FSH dose-dependent increases in follicle growth, lactate production, and steroid secretion. Multilayered secondary follicles were FSH dependent, with follicle survival, growth, steroid secretion, metabolism, and oocyte maturation all regulated by FSH. However, doses greater than 25 mIU/ml of FSH negatively impacted multilayered secondary follicle development (reduced follicle survival). The present results indicate that the hormonal and environmental needs of the follicular complex change during the maturation process. The culture system can be adapted to each stage of development, which will be especially critical for translation to human follicles that have a longer developmental period.
Keywords: follicle, follicle-stimulating hormone, follicular development, oocyte development, ovary
INTRODUCTION
Ovarian follicle maturation is a complex developmental process involving the interaction of local regulatory factors and endocrine signals. Follicle-stimulating hormone plays a critical role in this process, regulating estradiol secretion [1], development of antral follicles [2–4], and selection of the dominant follicle [5]. Additionally, FSH is widely employed in assisted reproduction technologies to recruit supernumerary follicles for oocyte collection and for in vitro maturation of immature oocytes [6]. In vivo treatment of mice with FSH resulted in retraction of transzonal projections and improved oocyte meiotic competence [7]; however, altered hormone levels impacted gamete quality [8]. Many fundamental questions remain regarding the role of FSH in follicle and oocyte development, including the precise role of FSH in early follicle development and the mechanism of action at successive stages of development. Although follicles are able to progress to the preantral stage in the absence of FSHβ [2] or the FSH receptor [4], FSH levels are elevated during the first 10 days of life in female mice [9], which corresponds to a period of rapid follicle growth and development.
In vitro systems have been developed to better understand the complex mechanisms that regulate follicle maturation. These systems have been developed for a variety of species, including bovine [10], rat [11], and nonhuman primates [12], with the majority of these efforts centered on the development of systems for mouse follicle culture. Follicle-stimulating hormone is a central component in such systems, but interpretations conflict regarding the appropriate dosage and timing of FSH presentation. It is difficult to compare these different culture systems directly because of differences in isolation and culture conditions. However, the dependence of follicle development on FSH may depend on the stage of the follicle in the culture system. Although most studies have been restricted to examining a particular stage and have not compared different stages, FSH appears to be critical for continued development of late preantral follicles [13] or early antral follicles [14]. The exact role of FSH in earlier follicle development is less clear: Two-layered secondary follicles (two layers of granulosa cells surrounding the oocyte) isolated from immature mice did not respond to FSH alone, whereas two-layered secondary follicles isolated from adult mice grew larger in response to FSH [15, 16]. Additional studies demonstrated that 8-bromo-cAMP or forskolin, but not FSH, could stimulate two-layered secondary follicles isolated from immature mice to grow in serum-free culture [17]. However, in serum-supplemented cultures of two-layered secondary follicles isolated from immature mice, FSH was critical for follicle survival, growth, and antrum formation [18]. In addition to the possible effect of FSH on different stages of follicles, the dose of FSH may impact follicle maturation. For example, in early studies of in vitro-cultured, two-layered secondary follicles, a dose of 100 mIU/ml of FSH was used to promote follicle survival and oocyte maturation [18], but a dose of 10 mIU/ml of FSH was later reported to be the minimal dose required for oocytes in these cultured follicles to obtain meiotic competence [13]. In a study of multilayered secondary follicles (follicles with several layers of granulosa cells surrounding the oocyte), a dose of 100 mIU/ml of FSH produced the maximum rate of growth, but estradiol secretion was significantly higher with increased doses of FSH [19].
One potential limitation of these systems is the disruption of follicle architecture that can occur when follicles are cultured on a two-dimensional substrate [20]. The change in follicle morphology may alter the paracrine signaling that is critical to follicle maturation, because the altered cell-cell orientation could result in diffusion of paracrine signals away from the target cells. Additionally, in vivo, the inner layers of granulosa cells are not directly exposed to endocrine signals because of the exclusion of the vascular system by the basal lamina [21], whereas in the disrupted architecture of two-dimensional systems, few granulosa cell layers are between the oocyte and the media. As an alternative, we have developed a novel, three-dimensional culture system in which individual, immature mouse granulosa-oocyte complexes [22] or intact follicles are encapsulated within alginate beads for culture. In this system, the alginate matrix provides a mechanical support for the follicle as it increases in size, allowing examination of the role of various factors in follicle maturation while maintaining an in vivo-like morphology. Additionally, encapsulating the follicle within a three-dimensional matrix allows for studies of how interactions of the outer layers of somatic cells and insoluble factors, such as the extracellular matrix, direct follicle maturation.
We hypothesized that the level of FSH in a culture system must be coordinated with the developmental stage of the follicle for appropriate granulosa cell proliferation and differentiation and for production of healthy oocytes. In this study, we undertook a systematic study of FSH in our alginate-based hydrogel culture system. Alginate, a linear polysaccharide derived from algae and composed of repeating units of β-d-mannuronic acid and α-l-guluronic acid [23], gels by ionic cross-linking of the guluronic residues [24]. This mild gelation process maintains cell viability [25]. Additionally, granulosa cells do not interact with alginate [26], allowing intact follicles to be retrieved from the matrix for in vitro maturation of the oocyte. Two-layered as well as multilayered secondary follicles were cultured in alginate-based matrices with increasing doses of recombinant human FSH to determine the effect of FSH dose on follicle survival, growth, metabolism, steroid production, and oocyte development.
MATERIALS AND METHODS
Animals and Materials
The C57BL/6 female mice and CBA male mice were purchased (Harlan, Indianapolis, IN) and maintained as a breeder colony at Northwestern University (Evanston, IL). Animals were housed in a temperature- and light-controlled environment on a 12L:12D photoperiod and were provided with food and water ad libitum. Chow provided was Harlan Teklad Global irradiated 2919, which does not contain soybean or alfalfa meal and, therefore, contains minimal phytoestrogens. Animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the established Institutional Animal Care and Use committee protocol at Northwestern University.
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO), and media formulations were purchased from Invitrogen (Carlsbad, CA). Sodium alginate (55–65% guluronic acid) was provided by FMC BioPolymers (Philadelphia, PA).
Follicle Isolation, Encapsulation, and Culture
Two-layered secondary follicles (100–130 µm; oocyte, 53–63 µm) and multilayered secondary follicles (150–180 µm; oocyte, 61–74 µm) were mechanically isolated using insulin-gauge needles in L-15 media from Day-12 and Day-16 C57BL/6 × CBA F1 mice, respectively. Two-layered secondary follicles are type 4 or 5a, and multilayered secondary follicles are type 5b, according to the classification of Pedersen and Peters [27]. Efforts were made to maintain the follicles at 37°C and pH 7 throughout the isolation and encapsulation. Two-layered secondary follicles were encapsulated into sterile alginate-collagen I matrices composed of 1.5% (w/v) alginate and 0.2 mg/ml of collagen I (BD Biosciences, Bedford, MA), and multilayered secondary follicles were encapsulated into sterile alginate matrices composed of 1.5% (w/v) alginate. These matrix formulations promoted the maximum follicle growth (unpublished observations). Droplets of alginate or alginate-collagen I solution (~2–3 µl) were suspended on a polypropylene mesh (0.1-mm opening; McMaster-Carr, Atlanta, GA). A single follicle was pipetted into each droplet in a minimal amount of media. After all droplets had been filled, the mesh was immersed in sterile, 50 mM CaCl2 for 2 min to cross-link the alginate and then rinsed in L-15 media. Beads were plated (one follicle/well) in 96-well plates in 100 µl of culture media composed of α-minimum essential medium (MEM), 3 mg/ml of BSA, 5 µg/ml of insulin, 5 µg/ml of transferrin, and 5 ng/ml of selenium [22] without androgen supplementation. Media were supplemented with FSH to final concentrations from 0 to 50 mIU/ml with recombinant human FSH (obtained through the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases [Bethesda, MD], and Dr. A.F. Parlow [Harbor-UCLA Medical Center, Torrance, CA]). Follicles were cultured at 37°C in 5% CO2 for 8 days. Every 2 days, half the media volume was exchanged, and follicles were examined for survival and size measurements using an inverted Leica DM IRB microscope with transmitted light and phase objectives (Leica, Bannockburn, IL). Follicles were designated as dead if the oocyte was no longer contained within the granulosa cells or if the granulosa cells had become dark and fragmented. Two diameters were measured for each follicle, and collected media were frozen at –80°C until assayed.
Progesterone, Estradiol, and l-Lactate Measurements
17β-Estradiol and progesterone levels were determined by immunoassay (Assay Designs, Ann Arbor, MI). Data generated by ELISA were fit using a four-point logistic equation. Intra- and interassay coefficients of variation were determined to be 3.1% and 8.2%, respectively, for 17β-estradiol and 4.4% and 9.1%, respectively, for progesterone. The sensitivity limit for 17β-estradiol was 30 pg/ml, and the sensitivity limit for progesterone was 62.5 pg/ml. Collected media also were analyzed on a YSI 2700 Select Biochemistry Analyzer (YSI Incorporated, Yellow Springs, OH) for l-lactate and glucose levels.
Oocyte Maturation
At the conclusion of the culture, follicles were removed from the alginate beads by degrading the gel with 10 U/ml of alginate lyase for 30 min at 37°C with 5% CO2. Released follicles were then transferred to maturation media composed of α-MEM, 1.5 IU/ml of hCG, and 5 ng/ml of epidermal growth factor [28]. After an incubation of 14–16 h at 37°C with 5% CO2, oocytes were classified morphologically based on the presence or absence of a germinal vesicle and polar body. Oocytes were classified as degenerated if the cytoplasm was fragmented or shrunken from the zona pellucida. Oocytes were then fixed and processed for immuno-fluorescence as described previously [8]. Oocytes were stained with a 1:400 dilution of monoclonal anti-α-tubulin (Sigma), detected with a 1:500 dilution of AlexaFluor 488 Goat Anti-Mouse (Molecular Probes, Eugene, OR), and mounted in VectaShield with 4′,6′-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) to examine the meiotic spindles. For control in vitro-maturation oocytes for two-layered secondary follicle cultures, Day-18 mice were primed with 5 IU of eCG, and then denuded oocytes were collected from large follicles on Day 20. For control in vitro-maturation oocytes for multilayered secondary follicle cultures, Day-22 mice were primed with 5 IU of eCG, and then cumulus-oocyte complexes were collected on Day 24. Control in vivo-matured oocytes for multilayered secondary follicle cultures were obtained from ovulated cumulus-oocyte complexes from Day-24 mice primed with 5 IU of eCG for 48 h and 5 IU of hCG for 14 h before collection.
Histology
Follicles cultured in alginate beads were fixed with 4% paraformal-dehyde for 1 h at the completion of the culture period, dehydrated through an ethanol series, and then embedded in LR White (Electron Microscopy Sciences, Hatfield, PA). The embedded beads were then sectioned (thickness, 1 µm; Cell Imaging Facility, Northwestern University, Chicago, IL) and stained with hematoxylin for 5 min to examine granulosa cell morphology.
[3H]Thymidine Incorporation
Follicles were cultured as described above for the first 2 days of culture. Media were then exchanged and replaced by media supplemented with 0.2 µCi [methyl-3H]thymidine per follicle (Amersham Biosciences, Piscataway, NJ). After 24 h, five beads were collected for each replicate, washed twice with 1 × PBS, and then dissolved in 10 mM EDTA. Next, [3H]thymidine incorporation was assayed as described previously [29, 30]. Nonspecific incorporation was determined using empty alginate gels.
Statistical Analysis
For two-layered secondary follicle cultures, two or three independent cultures of 30–50 follicles each were performed for each FSH dose. For multilayered secondary follicles, two to four independent cultures of 10–30 follicles each were performed for each FSH dose. Follicle size, steroid, and lactate data were analyzed using a two-way ANOVA with repeated measures or a one-way ANOVA followed by the Tukey honestly significant difference test for isolated time points with a Bonferroni correction for multiple comparisons. Categorical data were analyzed by chi-square analysis. A P value of less than 0.05 was considered to be statistically significant. All statistical calculations were done with the JMP 4.0.4 software package (SAS Institute, Cary, NC).
RESULTS
FSH Regulation of Two-Layered Secondary Follicles
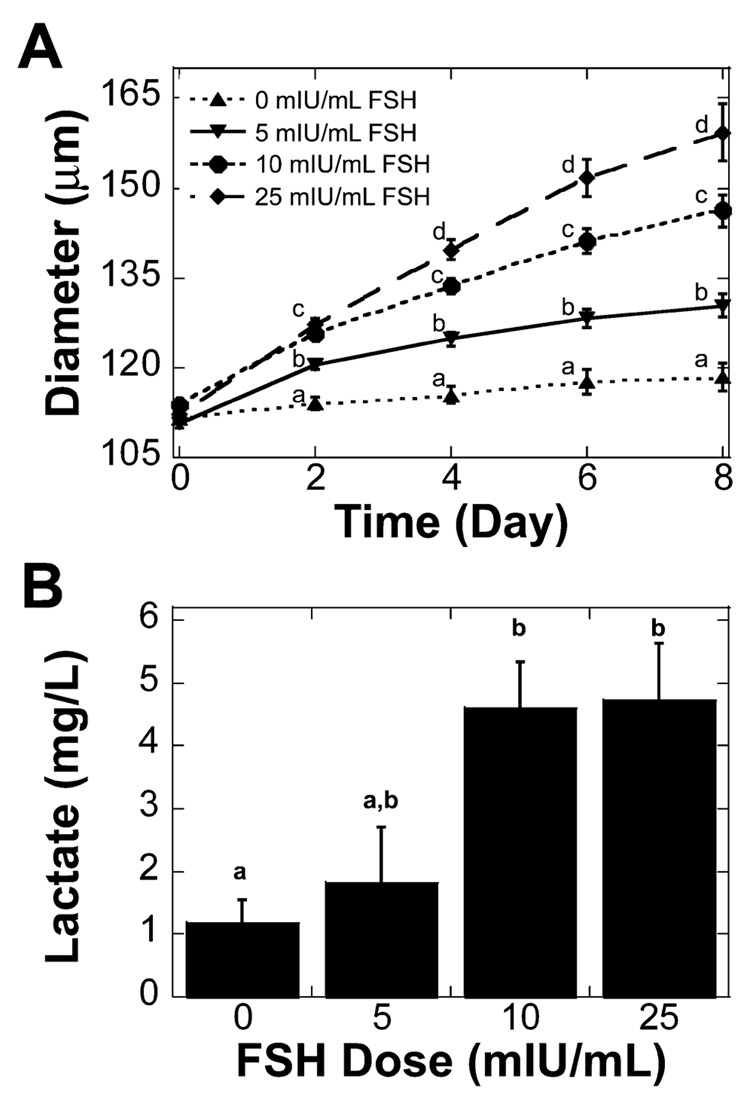
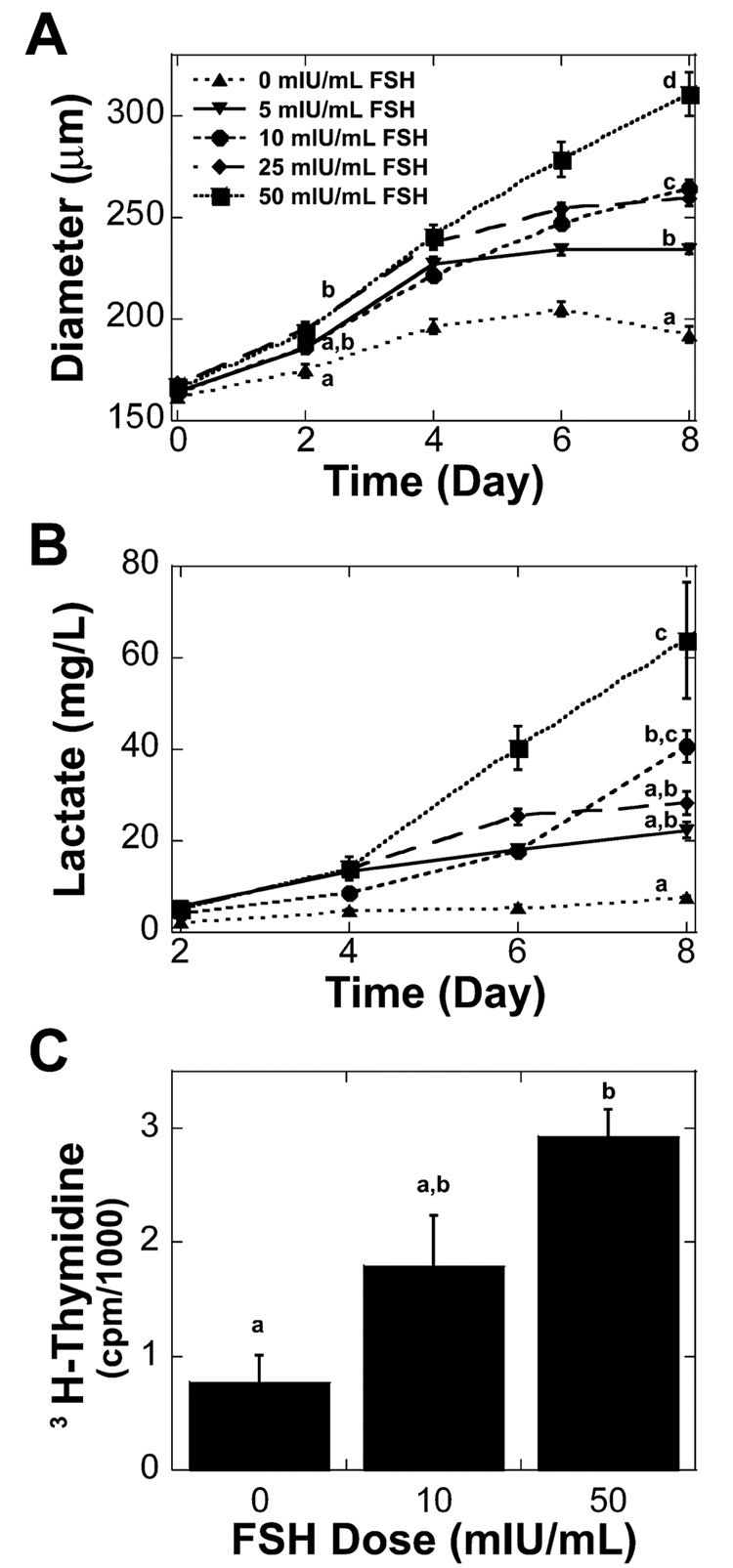
Two-layered secondary follicles (type 4 or 5a, 100–130 µm) were cultured in alginate-collagen I gels with 0, 5, 10, or 25 mIU/ml of recombinant human FSH for 8 days. Alginate-collagen I matrices promoted growth of two-layered secondary follicles in the absence of FSH. Survival of two-layered secondary follicles was not significantly affected by FSH dose, but two-layered secondary follicles grew significantly larger with FSH treatment (Table 1). Follicles were designated as dead if the oocyte was no longer contained within the granulosa cells or if the granulosa cells had become dark and fragmented. The effect of FSH on two-layered secondary follicle growth was apparent by the second day of culture, with follicles cultured in 10 and 25 mIU/ml of FSH being significantly larger than those cultured in 0 or 5 mIU/ml of FSH (Fig. 1A). Increased dosages of FSH also resulted in a significant increase in the accumulation of lactate in the media at the end of culture (Fig. 1B). Lactate production did not correspond linearly with follicle size, indicating that granulosa cells of two-layered secondary follicles cultured with higher doses of FSH had an increased metabolism.
TABLE 1.
Follicle survival and size increase for two-layered secondary follicles cultured in alginate-collagen I matrices and multilayered secondary follicles cultured in alginate matrices.
| FSH dose (mIU/mL) |
Two-layered secondary folliclesa | Multilayered secondary follicles | ||||
|---|---|---|---|---|---|---|
| nb | Survival (%) | Size increase (%)c | nb | Survival (%) | Size increase (%)c | |
| 0 | 102 | 77.4d | 6.0 ± 1.6d | 55 | 41.8d | 19.0 ± 1.8d |
| 5 | 99 | 75.8d | 18.1± 1.8e | 50 | 72.0e | 42.7 ± 1.0e |
| 10 | 96 | 75.0d | 28.5 ± 2.2f | 107 | 69.2e | 62.2 ± 2.9f |
| 25 | 98 | 66.3d | 41.4 ± 3.7g | 62 | 56.5d | 54.3 ± 2.2e,f |
| 50 | NE | NE | NE | 63 | 30.2d | 86.6 ± 6.6g |
NE = not examined.
n = number of follicles examined.
Values are the averages ± SEM.
Values without common superscripts differ significantly between treatments, P < 0.05.
FIG. 1. Two-layered secondary follicle growth and metabolism in alginate-collagen I gels.
A) Two-layered secondary follicles cultured with increased levels of FSH grew significantly larger. B) The increase in FSH dosage resulted in a significant increase in lactate accumulation on Day 8 of culture. Data represented as average ± SEM, the number examined for each condition is given in Table 1. Points or bars without common superscripts differ significantly between treatments for isolated time points (P < 0.05).
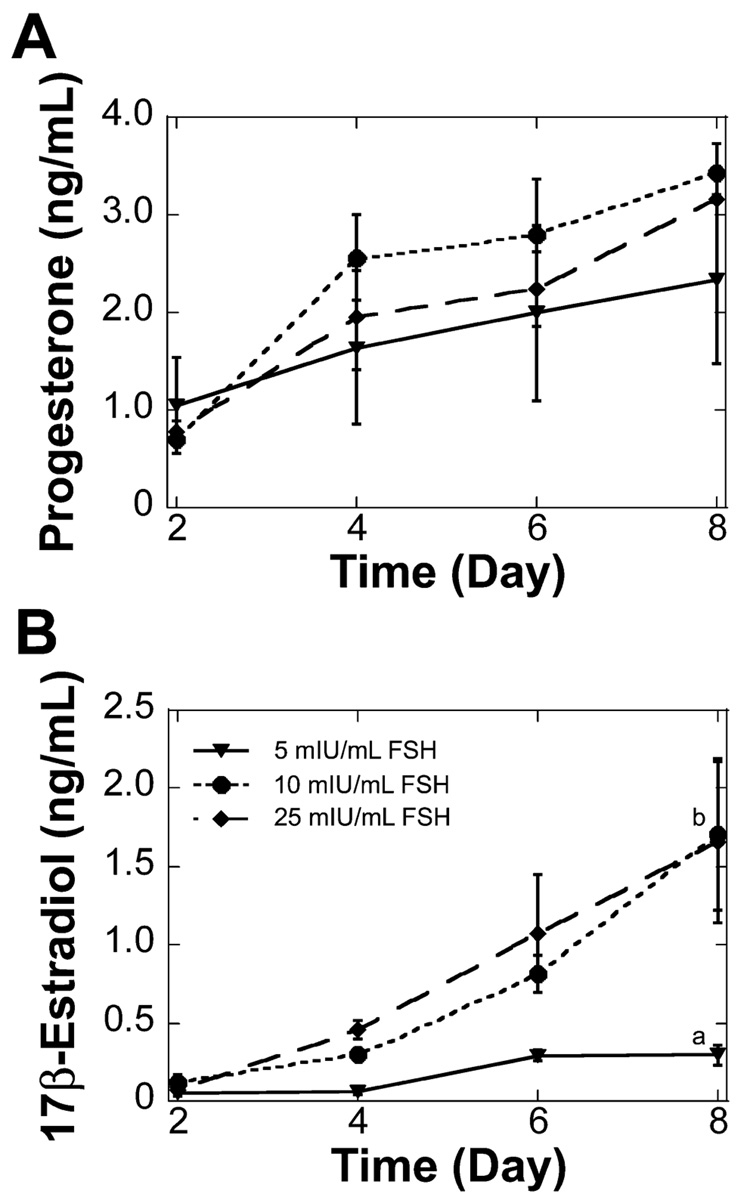
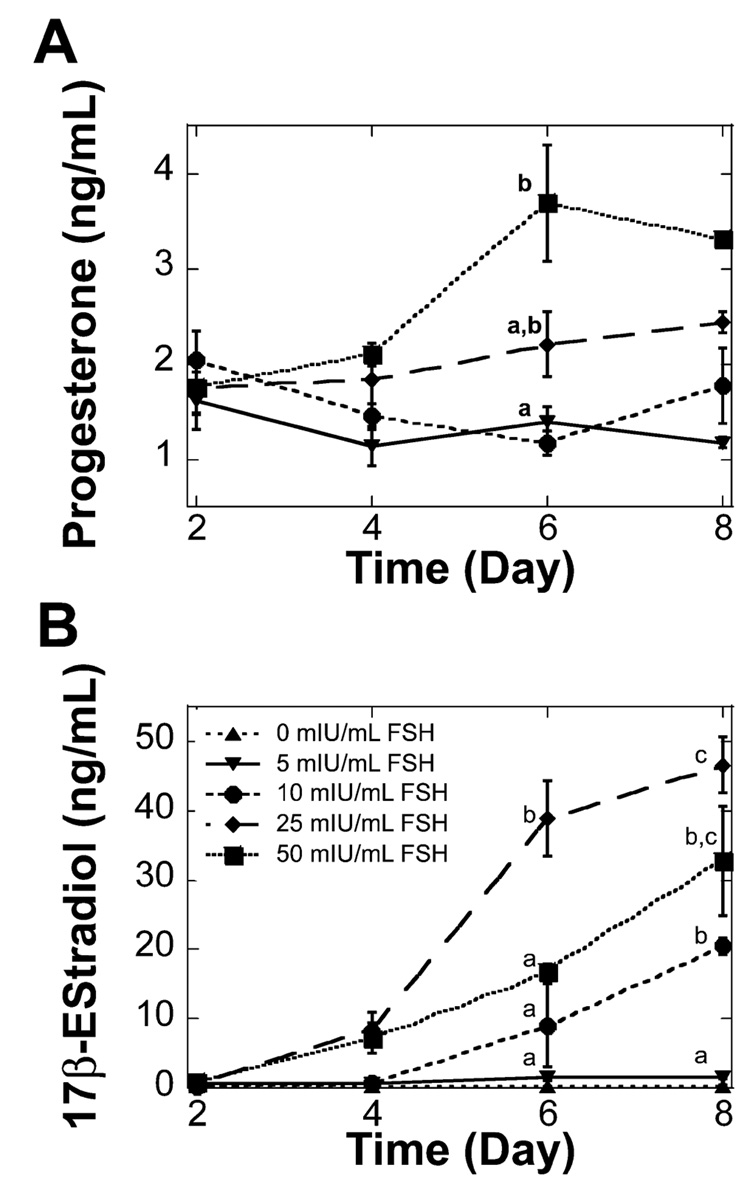
Progesterone and estradiol were not detected at any time for two-layered secondary follicles cultured without FSH. Progesterone levels increased significantly between Day 2 and Day 8 of the culture for follicles cultured with 10 or 25 mIU/ml of FSH, but no significant difference was found between FSH doses at the individual time points (Fig. 2A). Estradiol levels also were significantly higher at the end of the culture period, even though culture media were not supplemented with exogenous androgen. Additionally, culture with either 10 or 25 mIU/ml of FSH resulted in significantly higher estradiol levels on Day 8 of culture compared to those of culture with 5 mIU/ml of FSH (Fig. 2B). The dose of FSH did not significantly affect the percentage of oocytes that were competent to resume meiosis at the conclusion of culture (Table 2). The majority of the oocytes examined were arrested at prophase I, with an intact germinal vesicle (Fig. 3A). Oocytes that had resumed meiosis were arrested in metaphase I (Fig. 3B).
FIG. 2. Two-layered secondary follicle steroid secretion in alginate-collagen I gels.
A) Two-layered secondary follicles cultured with 10 or 25 mIU/ml of FSH secreted significantly more progesterone at the end of culture compared to Day 2 (P < 0.05); progesterone levels were not significantly different in response to increased doses of FSH. B) Two-layered secondary follicles cultured with 5, 10, or 25 mIU/ml of FSH secreted significantly more 17β-estradiol at the end of culture compared to Day 2 (P < 0.05). Increased levels of FSH also resulted in a significant increase in 17β-estradiol at the end of the culture. Data are presented as the average ± SEM (n = 3). Points without common superscripts differ significantly between treatments for isolated time points (P < 0.05). Follicles cultured without FSH did not secrete detectable levels of 17β-estradiol or progesterone.
TABLE 2.
Oocyte meiotic competence for two-layered secondary follicles cultured in alginate-collagen I matrices and multilayered secondary follicles cultured in alginate matrices.
| FSH dose (mIU/mL)a |
Two-layered secondary folliclesb | Multilayered secondary folliclesb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| nc | DG (%) | GV (%) | GVBD (%) | nc | DG (%) | GV (%) | GVBD (%) | PB (%) | |
| 0 | 21 | 23.8d | 61.9 | 14.3d | 13 | 84.6d | 0.0 | 15.4 | 0.0 |
| 5 | 28 | 17.9d | 60.7 | 21.4d | 32 | 12.5e | 0.0 | 9.4 | 78.1d |
| 10 | 44 | 36.4d | 36.4 | 27.3d | 40 | 15.0e | 7.5 | 37.5 | 40.0e |
| 25 | 39 | 33.3d | 43.6 | 23.1d | 30 | 10.0e | 0.0 | 26.7 | 63.3d,e |
| 50 | NE | NE | NE | NE | 14 | 14.3e | 0.0 | 42.9 | 42.9d,e |
| IVM | 72 | 0.0 | 0.0 | 100.0 | 129 | 0.0 | 0.0 | 6.2 | 93.8 |
| IVO | NE | NE | NE | NE | 207 | 2.9 | 0.0 | 39.1 | 58.0 |
IVM, in vitro matured control; IVO, in vivo ovulated control.
DG, degenerated; GV, germinal vesicle stage; GVBD, germinal vesicle breakdown; PB, polar body; NE, not examined.
n = number of oocytes examined.
Values without common superscripts differ significantly between treatments, P < 0.05.
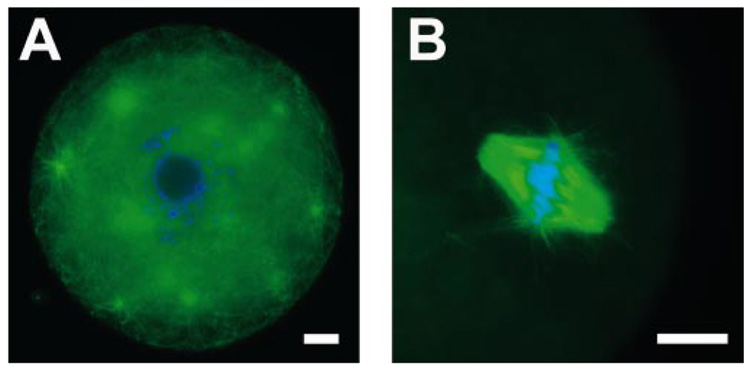
FIG. 3. Oocytes isolated from two-layered secondary follicles cultured in alginate-collagen I gels.
A) Oocyte arrested in prophase I from two-layered secondary follicle cultured with 0 mIU/ml of FSH. B) Spindle from a metaphase I-arrested oocyte from two-layered secondary follicle cultured with 25 mIU/ml of FSH. Oocytes were stained with anti-α-tubulin (green) and 4′,6′-diamidino-2-phenylindole (blue). Bar = 10 µm.
FSH Regulation of Multilayered Secondary Follicles
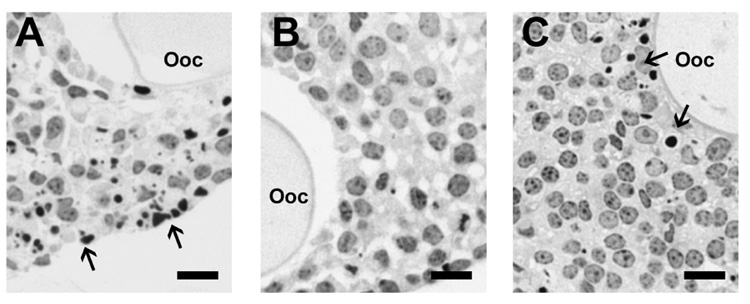
Multilayered secondary follicles (Type 5b, 150–180 µm) were cultured in alginate hydrogels for 8 days with 0, 5, 10, 25, or 50 mIU/ml of FSH. Multilayered secondary follicle survival was significantly affected by FSH dose, with a maximum survival of 72.0% and 69.2% at 5 and 10 mIU/ ml of FSH, respectively (Table 1). Sections of follicles cultured with 0, 10, or 50 mIU/ml of FSH were examined to characterize better the health of the granulosa cells. Culture without FSH resulted in a large number of pyknotic nuclei throughout the follicle (Fig. 4A). This morphology was not observed in sections of follicles cultured with 10 mIU/ml of FSH (Fig. 4B). With the further increase in dose to 50 mIU/ml of FSH, a large number of pyknotic nuclei were again observed (Fig. 4C). Unlike the follicles cultured without FSH, however, pyknotic cells from FSH-treated cultures were found primarily around the oocyte rather than the periphery of the follicle.
FIG. 4. Cross-sections of follicles on Day 8 of culture stained with hematoxylin.
Follicles cultured with 0 mIU/ml of FSH (A) or 50 mIU/ml of FSH (C) showed a large number of pyknotic nuclei; this was not observed in follicles cultured with 10 mIU/ml of FSH (B). Arrows indicate pyknotic nuclei. Ooc, Oocyte. Bar = 10 µm.
Multilayered secondary follicle growth was dependent on FSH dose (Table 1). The difference in follicle size was first detected on Day 2 of the culture, with follicles cultured using 25 and 50 mIU/ml of FSH being significantly larger than those cultured without FSH (Fig. 5A). At the completion of the culture period, follicle size showed a dose-dependent response. In addition, multilayered secondary follicles had an increased production of lactate with increased doses of FSH (Fig. 5B). A corresponding decrease in glucose was observed in the conditioned media (data not shown). Somatic cell proliferation also was assessed using a [3H]thymidine incorporation assay for culture with 0, 10, and 50 mIU/ml of FSH. For the culture period from Day 2 to Day 3, follicles in all media conditions incorporated [3H]thymidine, indicating that DNA replication and, therefore, cellular proliferation had occurred. Follicles cultured with 50 mIU/ml of FSH incorporated significantly more [3H]thymidine compared to follicles cultured without FSH (Fig. 5C), which was in agreement with the observed increase in follicle size on Day 2 for follicles treated with 50 mIU/ml of FSH (Fig. 5A).
FIG. 5. Multilayered secondary follicle growth and metabolism in alginate gels.
A) Multilayered secondary follicles cultured with increased levels of FSH grew significantly larger. Data represented as average ± SEM, the number examined for each condition is given in Table 1. B) The increase in FSH dosage resulted in a significant increase in lactate accumulation on day 8 of culture. Data are presented as the average ± SEM (n = 3). C) Multilayered secondary follicles cultured with 50 mIU/ml of FSH incorporated more [3H]thymidine from the second to third day of culture. Data are presented as the average ± SEM (n = 5). Points or bars without common superscripts differ significantly between treatments for isolated time points (P < 0.05).
Progesterone and estradiol secretion by multilayered secondary follicles was regulated by FSH in a dose-dependent manner. Progesterone was not detected from follicles cultured without FSH (data not shown) but was significantly increased on Day 6 from cultures with 50 mIU/ml of FSH relative to cultures with 5 or 10 mIU/ml of FSH (Fig. 6A). However, this difference was no longer significant on Day 8 of culture. Estradiol levels also were dependent on FSH dose, with levels being significantly higher at the conclusion of the culture for all FSH doses but not for follicles cultured without FSH. Follicle-stimulating hormone induced a dose-dependent increase in estradiol secretion for multilayered secondary follicles cultured with 5, 10, and 25 mIU/ml of FSH (Fig. 6B). Further increases in the FSH dose to 50 mIU/ml resulted in a small, nonsignificant decrease in estradiol compared to that with 25 mIU/ml.
FIG. 6. Multilayered secondary follicle steroid secretion in alginate gels.
A) Progesterone was increased significantly at the highest dose of FSH examined. Follicles cultured without FSH did not secrete detectable levels of progesterone. B) 17β-Estradiol increased significantly with time for multilayered secondary follicles cultured with FSH supplemented media. Increased levels of FSH also resulted in significant differences in 17β-estradiol levels. Data are presented as the average ± SEM (n = 3). Points without common superscripts differ significantly between treatments for isolated time points (P < 0.05).
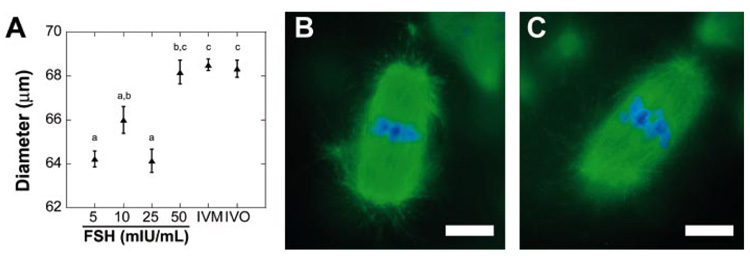
Oocyte meiotic competence was affected by FSH dose as well, with 84.6% of oocytes from cultures without FSH appearing to be degenerated, which was significantly higher than any FSH-treated culture (Table 2). Follicles cultured with 5 mIU/ml of FSH had the highest rate of progression to metaphase II, as evidenced by a polar body (Table 2). Oocytes cultured with 50 mIU/ml of FSH were the largest in size, however, and were not significantly different than in vitro- and in vivo-matured controls (P > 0.05) (Fig. 7A). Oocyte metaphase II spindles were a characteristic barrel shape, with chromatin aligned at the spindle equator (Fig. 7, B and C). No significant difference was found in the percentage of aligned spindles among the FSH treatments (data not shown).
FIG. 7. In vitro-matured oocytes isolated from multilayered secondary follicles cultured in alginate gels.
A) Oocytes from follicles cultured in 50 mIU/ml of FSH were not significantly different in size compared to in vitro or in vivo controls. Data represented as average ± SEM, the number examined for each condition is given in Table 2. Points without common superscripts differ significantly between treatments (P < 0.05). B and C) Representative metaphase II spindles from follicles cultured with 5 mIU/ml of FSH (B) or 50 mIU/ml of FSH (C). Oocytes were stained with anti-α-tubulin (green) and 4′,6′-diamidino-2-phenylindole (blue). Bar = 5 µm.
DISCUSSION
We examined the stage-dependent regulation of follicle development by FSH and the impact of FSH dose on follicle and oocyte maturation using a three-dimensional culture system for murine ovarian follicles. The alginate culture system maintains an in vivo-like morphology, with a centrally located oocyte and surrounding layers of granulosa and theca cells, and it supports cell-cell connections [22], which may provide a more physiological environment than two-dimensional culture systems. Small molecules with molecular weights of less than 20 kDa diffuse through alginate gels with the same diffusivity as in water [31], whereas larger molecules diffuse through the pores, which have diameters ranging from 5 to 200 nm, in a molecular weight-dependent manner [24]. The high porosity of the alginate gels allowed FSH in the culture media to diffuse into the matrix within 15 min and to reach a steady level within 4 h (data not shown). The alginate matrix helps to maintain paracrine signaling and can be modified to mimic the ovarian stroma [26]; therefore, incorporating endocrine signals, such as FSH, will further mimic the ovarian environment.
Two-layered as well as multilayered secondary follicles were encapsulated in alginate-based hydrogels and cultured in media supplemented with various doses of FSH for 8 days. The effects of FSH on follicle survival, growth, steroid production, and oocyte meiotic competence were examined. In this system, two-layered secondary follicles were FSH responsive, with increased follicle growth and steroid secretion; however, FSH did not affect survival and oocyte development. In contrast, multilayered secondary follicles were FSH dependent, with increased survival, follicle growth, steroid secretion, and improved oocyte development. However, these results were not strictly dose dependent, indicating a critical balance of FSH to ensure appropriate follicle development.
FSH in Two-Layered Secondary Follicle Development
Studies regarding the role of FSH in early follicle development, both in vivo and in vitro, have been inconclusive. The earliest stages of follicle development appear to be independent of FSH regulation, because ovaries from mice with null mutations for either FSHβ [2] or FSH receptor [4] contained follicles that progressed beyond the primordial stage but were blocked before antrum formation. However, full-length transcripts for FSH receptor were seen as early as Day 5 in immature mice [32], and high circulating levels of FSH have been measured in juvenile mice [9]. A study of intact newborn mouse ovaries cultured with and without FSH indicated that primary and two-layered secondary follicles had increased granulosa cell proliferation and follicle development with FSH [33].
In the three-dimensional system, two-layered secondary follicles cultured in alginate-collagen I gels were FSH responsive, with increased follicle growth and lactate production (Fig. 1) and increased estradiol secretion relative to follicles cultured without FSH (Fig. 2). An increase in lactate production has been shown previously to coincide with rapid growth and the onset of estradiol secretion in cultured, intact follicles [34], indicating that the two-layered secondary follicles cultured in alginate-collagen I gels differentiated in response to the increased doses of FSH. This result was in contrast to those of previous studies involving two-layered secondary follicles isolated from immature mice and cultured in serum-free conditions, which did not respond to FSH without additional supplementation with activin [15] or treatment with diethylstilbestrol [35]. Follicle-stimulating hormone has been shown to promote growth and differentiation in serum-supplemented culture of two-layered secondary follicles on two-dimensional substrates in a manner similar to the results of the present serum-free studies in alginate-collagen I gels (Fig. 1 and Fig. 2). Without FSH, estradiol and progesterone were not detected [36], and little granulosa cell proliferation occurred [18]. However, FSH was not necessary for survival of two-layered secondary follicles in the alginate-collagen I matrices (Table 1), and limited growth occurred without FSH (Fig. 1), indicating that this stage of follicle was not FSH dependent in this system. In contrast, follicles of the same size class cultured on two-dimensional substrates with serum required FSH for survival and development [18]. Thus, the three-dimensional alginate culture system provided a more in vivo-like dynamic for follicle progression.
The oocytes from two-layered secondary follicles in the alginate cultures were immature in comparison to age-matched, in vitro-matured controls (Table 2). The apparent slower development of the oocytes cultured in vitro has been reported previously for two-dimensional culture systems [37]. Because FSH is not a constant presence in the ovary [38], an intermittent-dosing schedule may be more physiological and improve oocyte development. Indeed, for culture of immature granulosa-oocyte complexes, constant exposure to FSH may contribute to reduced oocyte quality [39]. However, the lower rate of meiotic competence for oocytes from two-layered secondary follicle cultures relative to that of in vitro controls also may have been a result of different classes of follicles being compared. For example, oocytes used for the in vitro controls were isolated from eCG-primed ovaries, which include follicles of larger size than cultured two-layered secondary follicles. Immunostaining for tubulin and chromatin of oocytes from two-layered secondary follicles cultured in alginate-collagen I gels without FSH indicated that most of the oocytes were in the early stages of meiosis (Fig. 3), with some oocytes arrested at metaphase I. Germinal vesicle-stage oocytes displayed an array of cytoplasmic microtubules and several tubulin foci, which are characteristic of oocytes isolated from multilayered preantral follicles [40] and consistent with the observed increase in follicle size (Fig. 1).
FSH in Multilayered Secondary Follicle Development
Multilayered secondary follicles cultured in alginate matrices were FSH dependent, which is consistent with previous in vivo studies [2]. Follicle-stimulating hormone was determined to be a major survival factor for early antral rat follicles in vitro [41]. The multilayered secondary follicles cultured in the present study had improved survival at low, but not at the highest, doses of FSH (Table 1). Examination of morphologically normal follicles indicated that granulosa cell viability was also regulated by FSH dose, with follicles cultured without FSH containing many pyknotic nuclei (Fig. 4A), which is an early sign of atresia [42]. However, increased doses of FSH resulted in an eventual decline in multilayered secondary follicle survival, which was accompanied by the appearance of granulosa cells with pyknotic nuclei surrounding the oocyte (Table 1 and Fig. 4C). The observed pyknotic nuclei may have indicated that the inner layers of granulosa cells were damaged by an accumulation of metabolic by-products, such as lactate, which accompanied the rapid proliferation and increase in cell number seen with 50 mIU/ml of FSH (Fig. 5, B and C). Follicle metabolism for the multilayered secondary to preovulatory stages has been shown previously to occur primarily through the glycolytic pathway [43]. The increased levels of lactate were not solely accounted for by the increased follicle size with increased FSH doses in our system (data not shown) or in two-dimensional cultures [34], indicating that FSH increased metabolic activity of the granulosa cells. Neither class of follicle regularly formed antra in the alginate culture system, which likely would improve nutrient and waste transport [44]; however, the precise role of the antrum is unclear in both in vivo and in vitro follicle development. For instance, some mammalian species do not develop an antrum (e.g., tenrecs [45]). Modifications to the soluble factors in the culture media or to the mechanical properties of the matrix may allow for the regulation of antrum formation, making the alginate culture system an ideal model to examine the role of antrum development in follicle and oocyte maturation.
Multilayered secondary follicle growth in the alginate matrix was a result of granulosa cell proliferation, the rate of which depended on the FSH dose (Fig. 5C). Multilayered secondary follicles grew without FSH. Follicle growth slowed after Day 2, however, and follicles actually decreased slightly in size from Day 6 to Day 8 (Fig. 5A). A similar trend was seen for early antral follicles cultured without FSH on a two-dimensional substrate [14, 19]. Follicles grew significantly larger with increased doses of FSH, indicating that the three-dimensional support of the alginate matrix did not restrict follicle growth. The increased rate of granulosa cell proliferation may have uncoupled granulosa cell-granulosa cell interactions or granulosa cell-oocyte communication, affecting follicle survival. Alternatively, the continual exposure to FSH may have led to FSH-receptor desensitization [46], resulting in lowered follicle survival and flattened follicle growth curves, as observed for multilayered secondary follicles cultured with 5 or 25 mIU/ml of FSH.
Granulosa cells from mature follicles secrete large amounts of steroids, particularly in response to gonadotropin signaling as the dominant follicle matures [47]. In the alginate culture system, granulosa cells secreted progesterone in response to increased FSH, but when cultured in the absence of FSH, progesterone was not detected (Fig. 6A). A significant increase was found on Day 6 for cultures treated with 50 mIU/ml of FSH, indicating a possible premature luteinization of the granulosa cells. The production of estradiol by the cultured multilayered secondary follicles indicated a functioning theca layer, because the cultures were not supplemented with androgen. Additionally, FSH regulated estradiol secretion, as expected from the two cell-two gonadotropin model [48] and cultures of multilayered secondary follicles on two-dimensional substrates [19]. Unlike follicle growth and lactate production, this was not a strictly dose-dependent response, because the maximum amount of estradiol was achieved with 25 mIU/ml, not 50 mIU/ml, of FSH (Fig. 6B). This result may correspond to follicle growth, because 25 mIU/ml of FSH did not significantly increase the follicle growth relative to that with 10 or 50 mIU/ml of FSH. Therefore, granulosa cells at this dose of FSH may be proliferating less, resulting in increased differentiation [49].
Multilayered secondary follicles also produced oocytes that were competent to resume meiosis and progress to metaphase II, an important functional end point of the culture system. Oocytes from follicles cultured without FSH were not healthy, appearing dark and with a fragmented cytoplasm. The poor morphology of oocytes from cultures without FSH was not unexpected based on the reduced follicle survival (Table 1) and extensive granulosa cell apoptosis (Fig. 4A) seen in these follicles in the present study as well as previous reports of poor oocyte quality from follicles cultured without FSH [36]. Culture with even the lowest dose of FSH significantly improved oocyte health compared to that with no FSH. In vivo treatment with FSH induced withdrawal of transzonal projections, which corresponded to changes in oocyte transcriptional activity and increased rates of oocyte meiotic competence [7].
The improved morphology of granulosa cells that was observed with the addition of 10 mIU/ml of FSH (Fig. 4B) also may have resulted in improved support of the oocyte, enhancing its development [50]. Follicle-stimulating hormone has been shown previously to increase expression of connexin 43, a gap junction protein, in cultured granulosa cells [51]. Connexin 43 was found between granulosa cells in increased amounts as the follicle matures and in decreased amounts in atretic follicles [52]. Oocytes from ovaries of mice deficient in connexin 43 were meiotically incompetent [53], indicating that regulating communication between granulosa cells can impact oocyte quality. The doses of FSH examined in the present study were not found to impact the percentage of oocytes with aligned chromatin in meiotic spindles (data not shown). It recently was shown that increased dosages of FSH can negatively impact meiotic spindle formation [54]; however, the dosages examined in the present study were similar to those that did not impact spindle formation.
Several studies have indicated that FSH regulates oocyte growth through the kit ligand (KL1 and KL2) system. In vitro cultures of granulosa-oocyte complexes treated with low doses of FSH had upregulated expression of KL2 and increased oocyte growth compared to cultures with high doses of FSH, which had an increased ratio of KL1 to KL2 and no oocyte growth [55]. The promotion of oocyte growth by low doses of FSH was inhibited by treatment either with a KL inhibitor or with exogenous KL1. Additionally, mice with a null mutation for growth differentiation factor-9 (Gdf9) had increased KL expression [56] and precocious oocyte growth, with oocytes that grew larger than those of heterozygous littermates [57]. Our results indicated that increased doses of FSH resulted in larger oocytes (Fig. 7). It will be interesting to examine how FSH regulates KL and growth differentiation factor-9 in a three-dimensional, intact-follicle, in vitro culture system.
The three-dimensional nature of this culture system allows for examination of many fundamental questions regarding follicle development. We have shown previously that the culture maintains cell-cell connections [22]. In the present study, we incorporated endocrine signals by supplementing the culture with varying doses of FSH, and we observed that two-layered secondary follicles were FSH responsive and that multilayered secondary follicles were FSH dependent, which is consistent with in vivo observations [58]. The culture system described is flexible; signals can be examined either in isolation or in combination. Follicles of different developmental stages were used, and our results indicate that the culture system will need to be adapted to the developmental needs of the follicle. Culture systems such as this three-dimensional one that can be tailored to the developmental stage of the follicle will be especially critical for translation to human follicles, which require several months to produce mature oocytes.
ACKNOWLEDGMENTS
We would like to thank Courtney Berkholtz for providing the sectioning protocol and Dr. Alfred Rademaker for helpful discussions regarding data analysis. We would also like to thank Dr. William Miller and Dr. Annelise Barron for use of equipment. T.K.W. and L.D.S. are members of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Footnotes
Supported by NIH U54 HD41857 and a NDSEG research fellowship for P.K.K. T.K.W. and L.D.S. contributed equally to this work.
REFERENCES
- 1.Dorrington JH, Moon YS, Armstrong DT. Estradiol-17β biosynthesis in cultured granulosa cells from hypophysectomized immature rats: stimulation by follicle-stimulating hormone. Endocrinology. 1975;97:1328–1331. doi: 10.1210/endo-97-5-1328. [DOI] [PubMed] [Google Scholar]
- 2.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle-stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 3.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 5.Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18:71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- 6.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 7.Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev. 2004;69:347–355. doi: 10.1002/mrd.20128. [DOI] [PubMed] [Google Scholar]
- 8.Hodges CA, Ilagan A, Jennings D, Keri R, Nilson J, Hunt PA. Experimental evidence that changes in oocyte growth influence meiotic chromosome segregation. Hum Reprod. 2002;17:1171–1180. doi: 10.1093/humrep/17.5.1171. [DOI] [PubMed] [Google Scholar]
- 9.Halpin DM, Jones A, Fink G, Charlton HM. Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic-pituitary ovarian axis. J Reprod Fertil. 1986;77:287–296. doi: 10.1530/jrf.0.0770287. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000;62:1322–1328. doi: 10.1095/biolreprod62.5.1322. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Rippstein PU, Tsang BK. Role and gonadotrophic regulation of X-linked inhibitor of apoptosis protein expression during rat ovarian follicular development in vitro. Biol Reprod. 2003;68:610–619. doi: 10.1095/biolreprod.102.007807. [DOI] [PubMed] [Google Scholar]
- 12.Wandji SA, Srsen V, Nathanielsz PW, Eppig JJ, Fortune JE. Initiation of growth of baboon primordial follicles in vitro. Hum Reprod. 1997;12:1993–2001. doi: 10.1093/humrep/12.9.1993. [DOI] [PubMed] [Google Scholar]
- 13.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi: 10.1093/humrep/deh074. [DOI] [PubMed] [Google Scholar]
- 14.Spears N, Murray AA, Allison V, Boland NI, Gosden RG. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil. 1998;113:19–26. doi: 10.1530/jrf.0.1130019. [DOI] [PubMed] [Google Scholar]
- 15.Yokota H, Yamada K, Liu X, Kobayashi J, Abe Y, Mizunuma H, Ibuki Y. Paradoxical action of activin A on folliculogenesis in immature and adult mice. Endocrinology. 1997;138:4572–4576. doi: 10.1210/endo.138.11.5526. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Andoh K, Yokota H, Kobayashi J, Abe Y, Yamada K, Mizunuma H, Ibuki Y. Effects of growth hormone, activin, and follistatin on the development of preantral follicle from immature female mice. Endocrinology. 1998;139:2342–2347. doi: 10.1210/endo.139.5.5987. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi N, Andoh K, Abe Y, Yamada K, Mizunuma H, Ibuki Y. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Reprod. 2001;65:66–71. doi: 10.1095/biolreprod65.1.66. [DOI] [PubMed] [Google Scholar]
- 18.Cortvrindt R, Smitz J, Van Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod. 1997;12:759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- 19.Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349–362. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 20.Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123:185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- 21.Irving-Rodgers HF, Rodgers RJ. Ultrastructure of the basal lamina of bovine ovarian follicles and its relationship to the membrana granulosa. J Reprod Fertil. 2000;118:221–228. [PubMed] [Google Scholar]
- 22.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 23.Amsden B, Turner N. Diffusion characteristics of calcium alginate gels. Biotechnol Bioeng. 1999;65:605–610. doi: 10.1002/(sici)1097-0290(19991205)65:5<605::aid-bit14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 24.Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 25.Machluf M, Orsola A, Boorjian S, Kershen R, Atala A. Microencapsulation of Leydig cells: a system for testosterone supplementation. Endocrinology. 2003;144:4975–4979. doi: 10.1210/en.2003-0411. [DOI] [PubMed] [Google Scholar]
- 26.Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003;205:1–10. doi: 10.1016/s0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 28.Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization, and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–2666. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- 29.Roy SK, Greenwald GS. Quantitative analysis of in-vitro incorporation of [3H]thymidine into hamster follicles during the estrous cycle. J Reprod Fertil. 1986;77:143–152. doi: 10.1530/jrf.0.0770143. [DOI] [PubMed] [Google Scholar]
- 30.Wang XN, Roy SK, Greenwald GS. In vitro DNA synthesis by isolated preantral to preovulatory follicles from the cyclic mouse. Biol Reprod. 1991;44:857–863. doi: 10.1095/biolreprod44.5.857. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Matsumura M, Veliky IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;26:53–58. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- 32.O’Shaughnessy PJ, Dudley K, Rajapaksha WR. Expression of follicle stimulating hormone-receptor mRNA during gonadal development. Mol Cell Endocrinol. 1996;125:169–175. doi: 10.1016/s0303-7207(96)03957-3. [DOI] [PubMed] [Google Scholar]
- 33.Ryle M. The growth in vitro of mouse ovarian follicles of different sizes in response to purified gonadotrophins. J Reprod Fertil. 1972;30:395–405. doi: 10.1530/jrf.0.0300395. [DOI] [PubMed] [Google Scholar]
- 34.Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, Mizunuma H, Ibuki Y. A comparative study on transforming growth factor-beta and activin A for preantral follicles from adult, immature, and diethylstilbestrol-primed immature mice. Endocrinology. 1999;140:2480–2485. doi: 10.1210/endo.140.6.6827. [DOI] [PubMed] [Google Scholar]
- 36.Cortvrindt R, Hu Y, Smitz J. Recombinant luteinizing hormone as a survival and differentiation factor increases oocyte maturation in recombinant follicle stimulating hormone-supplemented mouse preantral follicle culture. Hum Reprod. 1998;13:1292–1302. doi: 10.1093/humrep/13.5.1292. [DOI] [PubMed] [Google Scholar]
- 37.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 38.Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- 39.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–1686. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 40.Mattson BA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev. 1990;25:374–383. doi: 10.1002/mrd.1080250411. [DOI] [PubMed] [Google Scholar]
- 41.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137:1447–1456. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- 42.Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–724. doi: 10.1210/edrv-15-6-707. [DOI] [PubMed] [Google Scholar]
- 43.Boland NI, Humpherson PG, Leese HJ, Gosden RG. Characterization of follicular energy metabolism. Hum Reprod. 1994;9:604–609. doi: 10.1093/oxfordjournals.humrep.a138557. [DOI] [PubMed] [Google Scholar]
- 44.Rodgers RJ, Irving-Rodgers HF, van Wezel IL, Krupa M, Lavranos TC. Dynamics of the membrana granulosa during expansion of the ovarian follicular antrum. Mol Cell Endocrinol. 2001;171:41–48. doi: 10.1016/s0303-7207(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 45.Bedford JM, Mock OB, Goodman SM. Novelties of conception in insectivorous mammals (Lipotyphla), particularly shrews. Biol Rev Camb Philos Soc. 2004;79:891–909. doi: 10.1017/s1464793104006529. [DOI] [PubMed] [Google Scholar]
- 46.Jonassen JA, Richards JS. Granulosa cell desensitization: effects of gonadotropin on antral and preantral follicles. Endocrinology. 1980;106:1786–1794. doi: 10.1210/endo-106-6-1786. [DOI] [PubMed] [Google Scholar]
- 47.Hsueh AJ, Adashi EY, Jones PB, Welsh TH., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;5:76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- 48.Gore-Langton R, Armstrong DT. Follicular steroidogenesis and its control. In: Knobil E, Neill J, editors. The Physiology of Reproduction. vol. 1. New York: Raven Press; 1988. pp. 331–385. [Google Scholar]
- 49.Orly J, Sato G, Erickson GF. Serum suppresses the expression of hormonally induced functions in cultured granulosa cells. Cell. 1980;20:817–827. doi: 10.1016/0092-8674(80)90328-1. [DOI] [PubMed] [Google Scholar]
- 50.Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- 51.Sommersberg B, Bulling A, Salzer U, Frohlich U, Garfield RE, Amsterdam A, Mayerhofer A. Gap junction communication and connexin 43 gene expression in a rat granulosa cell line: regulation by follicle-stimulating hormone. Biol Reprod. 2000;63:1661–1668. doi: 10.1095/biolreprod63.6.1661. [DOI] [PubMed] [Google Scholar]
- 52.Wright CS, Becker DL, Lin JS, Warner AE, Hardy K. Stage-specific and differential expression of gap junctions in the mouse ovary: connexin-specific roles in follicular regulation. Reproduction. 2001;121:77–88. doi: 10.1530/rep.0.1210077. [DOI] [PubMed] [Google Scholar]
- 53.Ackert CL, Gittens JE, O’Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin 43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270. doi: 10.1006/dbio.2001.0216. [DOI] [PubMed] [Google Scholar]
- 54.Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, Hardy K. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72:107–118. doi: 10.1095/biolreprod.104.032003. [DOI] [PubMed] [Google Scholar]
- 55.Thomas FH, Ethier JF, Shimasaki S, Vanderhyden BC. Follicle-stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology. 2005;146:941–949. doi: 10.1210/en.2004-0826. [DOI] [PubMed] [Google Scholar]
- 56.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor-9-deficient ovary. Mol Endocrinol. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 57.Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373–384. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- 58.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]