Abstract
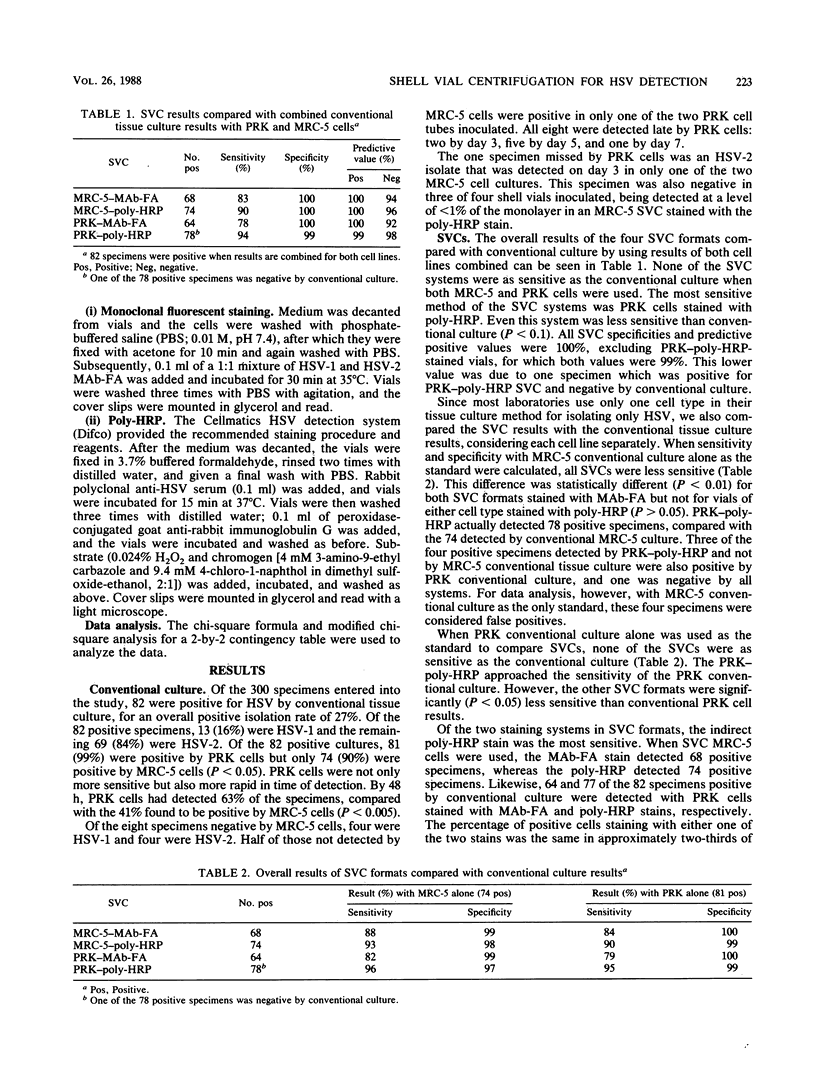
By using a conventional tissue culture method as a standard, four shell vial centrifugation culture (SVC) formats were compared for herpes simplex virus (HSV) detection in 300 clinical samples. Both MRC-5 and primary rabbit kidney (PRK) cells were used in the conventional and SVC systems. In addition, both a direct monoclonal fluorescent antibody to HSV (MAb-FA; Syva Corporation, Palo Alto, Calif.) and an indirect HSV polyclonal antibody-horseradish peroxidase stain (poly-HRP; Difco Laboratories, Detroit, Mich.) were used to stain shell vials of both cell types. Conventional tubes were incubated for up to 7 days with daily examination for cytopathic effect, which was confirmed as HSV by staining with an MAb-FA. Shell vials were inoculated, centrifuged, incubated for 16 to 24 h, and stained directly with MAb-FA or indirectly with a poly-HRP stain. Of the 300 specimens examined, 82 (27%) were HSV positive by conventional tissue culture. PRK cells detected 81 (99%) positive specimens, compared with 74 (90%) specimens detected with MRC-5 cells. Of the 82 positive specimens by conventional culture, the SVC formats detected 68 by MRC-5 and MAb-FA, 74 by MRC-5 and poly-HRP, 64 by PRK and MAb-FA, and 77 by PRK and poly-HRP. Therefore, PRK stained by an indirect method with poly-HRP was the most sensitive of the SVC formats tested, detecting 94% of the positive specimens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callihan D. R., Menegus M. A. Rapid detection of herpes simplex virus in clinical specimens with human embryonic lung fibroblast and primary rabbit kidney cell cultures. J Clin Microbiol. 1984 Apr;19(4):563–565. doi: 10.1128/jcm.19.4.563-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darougar S., Walpita P., Thaker U., Goh B. T., Dunlop E. M. A rapid and sensitive culture test for the laboratory diagnosis of genital herpes in women. Genitourin Med. 1986 Apr;62(2):93–96. doi: 10.1136/sti.62.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayram S. L., Aarnaes S. L., Peterson E. M., de la Maza L. M. Evaluation of five cell types for the isolation of herpes simplex virus. Diagn Microbiol Infect Dis. 1986 Jul;5(2):127–133. doi: 10.1016/0732-8893(86)90114-8. [DOI] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Howell C. L. Rapid detection and identification of herpes simplex virus in cell culture by a direct immunoperoxidase staining procedure. J Clin Microbiol. 1983 Sep;18(3):550–553. doi: 10.1128/jcm.18.3.550-553.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. F. Comparison of human fibroblast cells and primary rabbit kidney cells for isolation of herpes simplex virus. J Clin Microbiol. 1984 Apr;19(4):548–549. doi: 10.1128/jcm.19.4.548-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda R. C., Almanza I. Centrifugation-shell vial technique for rapid detection of herpes simplex virus cytopathic effect in Vero cells. J Clin Microbiol. 1987 Feb;25(2):423–424. doi: 10.1128/jcm.25.2.423-424.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon V. C., Turner R. B., Speranza M. J., Overall J. C., Jr Rapid detection of herpes simplex virus in clinical specimens by centrifugation and immunoperoxidase staining. J Clin Microbiol. 1986 Apr;23(4):683–686. doi: 10.1128/jcm.23.4.683-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Dennis J., Devlin V., Gallo D., Mills J. Comparison of direct immunofluorescence and direct immunoperoxidase procedures for detection of herpes simplex virus antigen in lesion specimens. J Clin Microbiol. 1983 Aug;18(2):445–448. doi: 10.1128/jcm.18.2.445-448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


