Abstract
The first isolate of influenza virus in Canada during the winter of 1986 to 1987 was a genetic variant of A/Taiwan/1/86. This genetic variant type was the predominant strain obtained from several of the western provinces. The variant strains were antigenically indistinguishable from A/Taiwan/1/86 but were remarkably distinct by T1 oligonucleotide mapping. T1 mapping of individual genome segments indicated that the variants evolved from an A/Taiwan/1/86-like virus through the accumulation of point mutation or deletion or insertion events and probably do not contain foreign genes. The relative distribution of genetic variation was approximately equal among the individual genes, with the possible exception of segments 1 or 2 that were analyzed in combination and thus could not be individually associated with the observed variation.
Full text
PDF
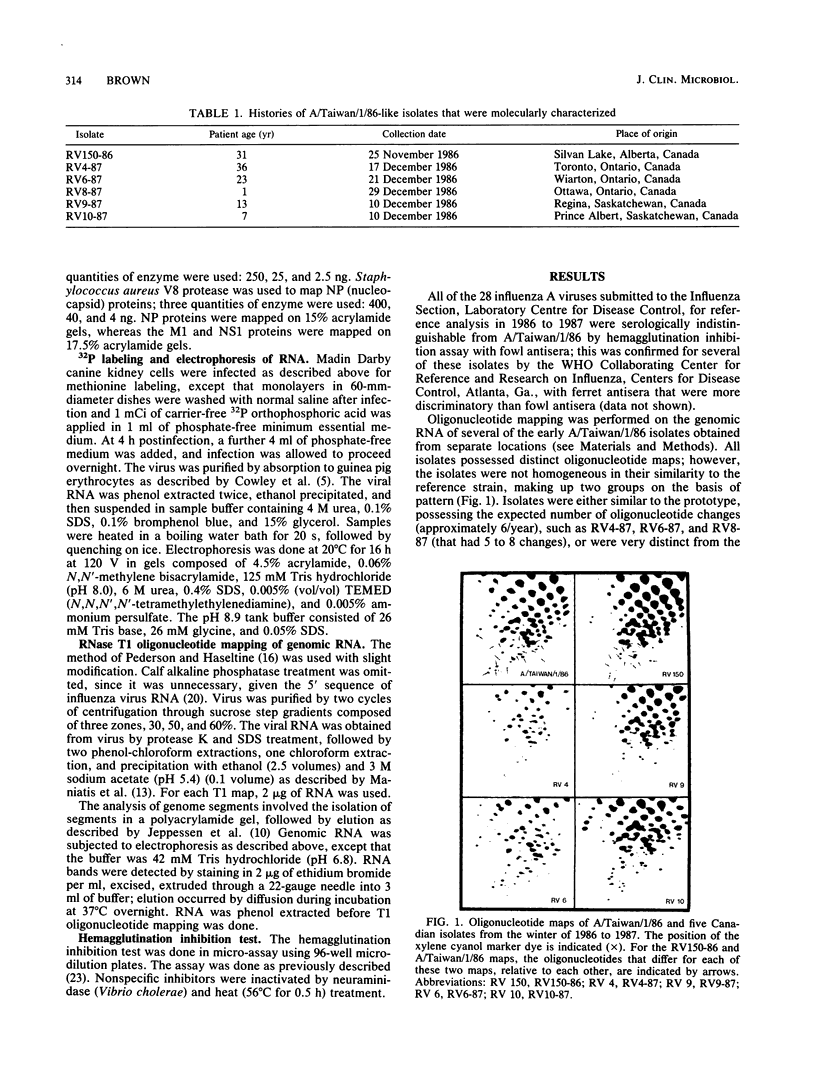
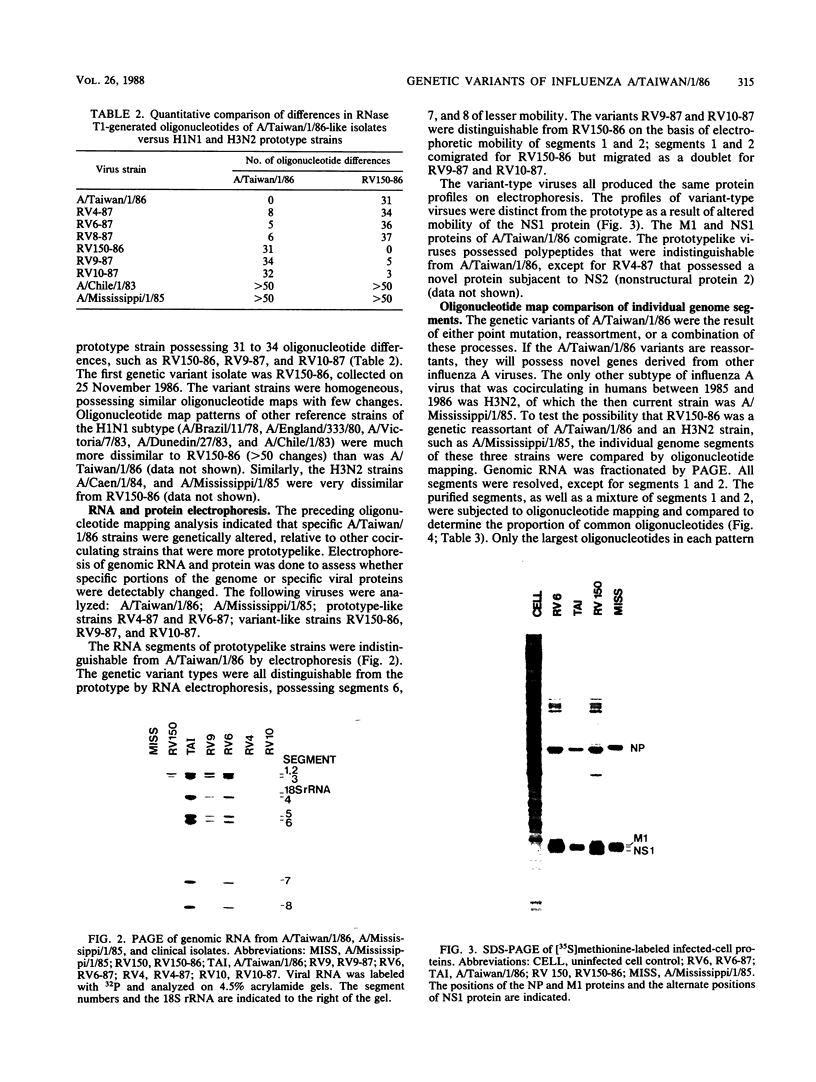
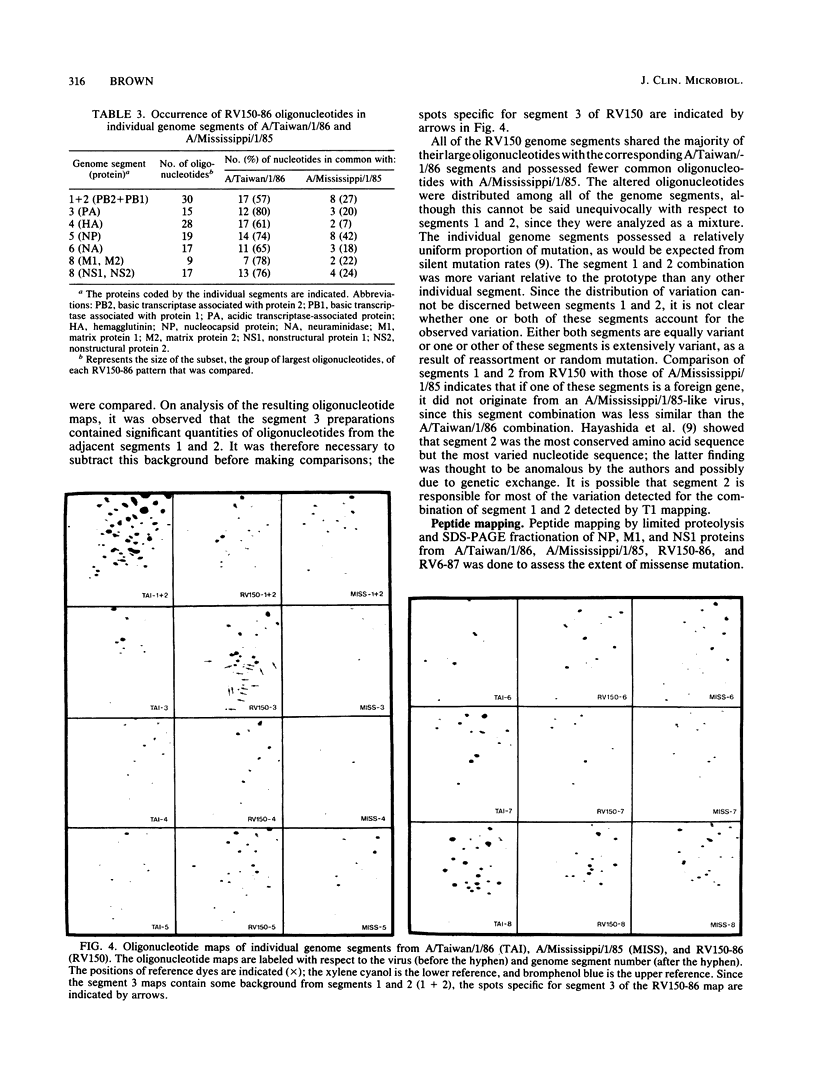


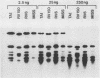
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean W. J., Jr, Cox N. J., Kendal A. P. Recombination of human influenza A viruses in nature. Nature. 1980 Apr 17;284(5757):638–640. doi: 10.1038/284638a0. [DOI] [PubMed] [Google Scholar]
- Both G. W., Sleigh M. J., Cox N. J., Kendal A. P. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J Virol. 1983 Oct;48(1):52–60. doi: 10.1128/jvi.48.1.52-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A. J., Brownlee G. G., Yewdell J. W., Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell. 1982 Dec;31(2 Pt 1):417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cowley J. A., Tannock G. A., Barry R. D. A simple procedure for the analysis of the structural proteins of influenza and parainfluenza viruses involving adsorption to erythrocytes. J Virol Methods. 1984 Feb;8(1-2):9–18. doi: 10.1016/0166-0934(84)90036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Frielle D. W., Huang D. D., Youngner J. S. Persistent infection with influenza A virus: evolution of virus mutants. Virology. 1984 Oct 15;138(1):103–117. doi: 10.1016/0042-6822(84)90151-x. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Markarian K. Anomalous electrophoretic behavior of the membrane (M) protein of influenza virus in polyacrylamide gels. Arch Virol. 1983;76(3):263–267. doi: 10.1007/BF01311110. [DOI] [PubMed] [Google Scholar]
- Hayashida H., Toh H., Kikuno R., Miyata T. Evolution of influenza virus genes. Mol Biol Evol. 1985 Jul;2(4):289–303. doi: 10.1093/oxfordjournals.molbev.a040352. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. G., Barrell B. G., Sanger F., Coulson A. R. Nucleotide sequences of two fragments from the coat-protein cistron of bacteriophage R17 ribonucleic acid. Biochem J. 1972 Aug;128(5):993–1006. doi: 10.1042/bj1280993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Cox N. J. Forecasting the epidemic potential of influenza virus variants based on their molecular properties. Vaccine. 1985 Sep;3(3 Suppl):263–266. doi: 10.1016/0264-410x(85)90119-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Cox N. J., Kendal A. P. Antigenic and genomic analyses of influenza A(H1N1) viruses from different regions of the world, February 1978 to March 1980. Infect Immun. 1981 Apr;32(1):287–294. doi: 10.1128/iai.32.1.287-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. A micromethod for detailed characterization of high molecular weight RNA. Methods Enzymol. 1980;65(1):680–687. doi: 10.1016/s0076-6879(80)65066-6. [DOI] [PubMed] [Google Scholar]
- Raymond F. L., Caton A. J., Cox N. J., Kendal A. P., Brownlee G. G. Antigenicity and evolution amongst recent influenza viruses of H1N1 subtype. Nucleic Acids Res. 1983 Oct 25;11(20):7191–7203. doi: 10.1093/nar/11.20.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S. Sequence analysis of the haemagglutinin of A/Taiwan/1/86, a new variant of human influenza A(H1N1) virus. J Gen Virol. 1987 Apr;68(Pt 4):1205–1208. doi: 10.1099/0022-1317-68-4-1205. [DOI] [PubMed] [Google Scholar]
- Rott R. Molecular basis of infectivity and pathogenicity of myxovirus. Brief review. Arch Virol. 1979;59(4):285–298. doi: 10.1007/BF01317469. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet C., Smith H. Pathogenicity of influenza virus. Microbiol Rev. 1980 Jun;44(2):303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Young J. F., Palese P. Evolution of human influenza A viruses in nature: recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]