Abstract
Rationale: There is conflicting information about the development and resolution of airway inflammation and airway hyperresponsiveness (AHR) after repeated airway exposure to allergen in sensitized mice.
Methods: Sensitized BALB/c and C57BL/6 mice were exposed to repeated allergen challenge on 3, 7, or 11 occasions. Airway function in response to inhaled methacholine was monitored; bronchoalveolar lavage fluid inflammatory cells were counted; and goblet cell metaplasia, peribronchial fibrosis, and smooth muscle hypertrophy were quantitated on tissue sections. Bone marrow–derived dendritic cells were generated after differentiation of bone marrow cells in the presence of growth factors.
Results: Sensitization to ovalbumin (OVA) in alum, followed by three airway exposures to OVA, induced lung eosinophilia, goblet cell metaplasia, mild peribronchial fibrosis, and peribronchial smooth muscle hypertrophy; increased levels of interleukin (IL)-4, IL-5, IL-13, granulocyte-macrophage colony–stimulating factor, transforming growth factor-β1, eotaxin-1, RANTES (regulated on activation, normal T-cell expressed and secreted), and OVA-specific IgG1 and IgE; and resulted in AHR. After seven airway challenges, development of AHR was markedly decreased as was the production of IL-4, IL-5, and IL-13. Levels of IL-10 in both strains and the level of IL-12 in BALB/c mice increased. After 11 challenges, airway eosinophilia and peribronchial fibrosis further declined and the cytokine and chemokine profiles continued to change. At this time point, the number of myeloid dendritic cells and expression of CD80 and CD86 in lungs were decreased compared with three challenges. After 11 challenges, intratracheal instillation of bone marrow–derived dendritic cells restored AHR and airway eosinophilia.
Conclusions: These data suggest that repeated allergen exposure leads to progressive decreases in AHR and allergic inflammation, through decreases in myeloid dendritic cell numbers.
Keywords: airway hyperresponsiveness, chronic asthma, cytokine, dendritic cells, eosinophil
Chronic inflammation of the airways with variable airflow limitation is responsible for the altered airway responsiveness seen in individuals with asthma to a variety of stimuli (1). Elevated serum IgE levels and inflammatory cell infiltration, especially of eosinophils, mast cells, and CD4+ T cells, appear to be involved in the pathogenesis of the disease (2–4). Much of our understanding of asthma points to a complex and multifactorial condition involving interactions between genetic factors and environmental stimuli. It has been argued that repeated exposure to airborne allergens, such as house dust mite or animal dander, contributes to the initiation and persistence of airway inflammation, which is believed to be the central abnormality resulting in airway damage and dysfunction (5, 6). Several studies identified increased muscle mass and airway wall thickening as determinants of airway hyperresponsiveness (AHR) (7, 8). This chronic airway inflammatory response, which is characteristic of asthma, can lead to airway remodeling, and structural alterations that may underlie some of the persistent manifestations of asthma. Indeed, a variety of cells, including epithelial cells, fibroblasts, and smooth muscle cells, in the chronic stages of asthma likely contribute through the secretion of various cytokines and chemokines that result in the irreversible fibrosis and remodeling seen in the airways of individuals with asthma (9, 10).
In allergic diseases, such as atopic dermatitis and allergic asthma, allergen-specific T cells express a helper T-cell type 2 (Th2) phenotype (11). In animal models of acute allergic inflammation, Th2 cell–derived cytokines, notably interleukin (IL)-4, IL-5, IL-9, and IL-13 and C-C chemokine family members such as RANTES (regulated on activation, normal T-cell expressed and secreted) and eotaxin-1, are pivotal in the induction of eosinophilic airway inflammation, antigen-specific IgE production, and AHR (12). Although airway inflammation and Th2 cytokines and chemokines are undoubtedly a cornerstone of asthma, it is clear that the asthmatic response is more complex. The relative importance of these factors may vary according to the stage or duration of asthma. Much less is known about the pathogenesis of chronic airway eosinophilic inflammation and cytokine/chemokine production in long-standing asthma. Because no satisfactory disease-modifying treatment is currently available for chronic asthma, evaluation of the therapeutic effects of the modulation of cytokine/chemokine production in chronic disease is an important area of investigation (13). In this regard, an essential first step is to characterize the profile and kinetics of cytokine/chemokine production in a model system where allergen challenges are incremental, to identify potential therapeutic targets.
Whereas the role of T cells has been fairly well described, much less information is available on the role of dendritic cells (DCs) in allergic airway inflammation. DCs have been thought to be an important cell type in the initiation of T-cell–mediated inflammation by priming naive T cells (14, 15). Several reports have identified a critical role for DCs in allergic airway inflammation (16, 17). DCs are subdivided on the basis of surface antigen expression. Each DC subtype may induce different stimulatory signals on T cells and on the development of allergic inflammation. For example, bone marrow–derived DCs (BMDCs) expressing a myeloid DC (mDC) phenotype prime T cells to specific antigen and induce their expansion (18). On the other hand, plasmacytoid DCs (pDCs) inhibit the development of effector T cells (19). The purpose of this study was to delineate the characteristics of allergic airway responses after repeated allergen challenge and the role of DCs in regulating these responses.
METHODS
Animals
Female BALB/c and C57BL/6 mice were purchased at 8 to 12 wk of age from Jackson Laboratories (Bar Harbor, ME) and housed under specific pathogen–free conditions. The animals were maintained on an ovalbumin (OVA)-free diet. Experiments were conducted under a protocol approved by Institutional Animal Care and Use Committee of the National Jewish Medical and Research Center (Denver, CO).
Protocols for OVA-induced Allergic Airway Inflammation
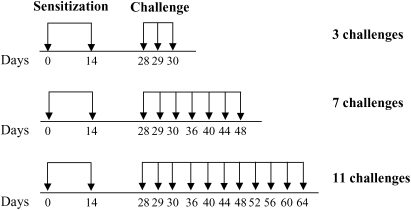
Mice were sensitized on Days 1 and 14 by intraperitoneal injection of 20 μg of OVA (grade V; Sigma-Aldrich, St. Louis, MO) premixed with 2.25 mg of Al(OH)3 (Pierce Biotechnology, Rockford, IL) in 100 μl of phosphate-buffered saline (PBS). After sensitization, animals were exposed to aerosolized OVA (1% in saline) for 20 min/d on Days 28, 29, and 30 (3 challenges, short-term challenge), or continued to receive airway challenges on Days 36, 40, 44, and 48 (7 challenges), as well as on Days 52, 56, 60, and 64 (11 challenges; Figure 1). Forty-eight hours after the last OVA challenge (Day 32, 50, or 66) AHR was assessed and bronchoalveolar lavage (BAL) fluid, serum, and tissues were obtained for further analyses. Control groups consisted of nonsensitized but OVA-challenged animals.
Figure 1.
Experimental protocol for antigen sensitization and challenge. BALB/c and C57BL/6 mice were injected intraperitoneally with ovalbumin (OVA)–alum in 100 μl of phosphate-buffered saline (PBS) on Days 1 and 14 and received aerosol challenges with 1% OVA–saline solution on Days 28, 29, and 30 (short-term group; 2ip+3Neb) or on Days 28, 29, 30, 36, 40, 44, and 48, or on Days 28, 29, 30, 36, 40, 44, 48, 52, 56, 60, and 64 (long-term groups; 2ip+7Neb and 2ip+11Neb). Control mice received 3, 7, or 11 challenges without intraperitoneal sensitization.
Assessment of Airway Function
Airway function was assessed as previously described by measuring changes in lung resistance (Rl) and dynamic compliance (Cdyn) in response to increasing doses of inhaled methacholine (MCh) (20).
BAL
Immediately after assessment of airway function, lungs were lavaged via the tracheal tube with 1 ml of Hanks' balanced salt solution at room temperature. Total leukocyte numbers were measured (Coulter Counter; Beckman Coulter, Fullerton, CA). Cytospin slides were stained with Leukostat (Fisher Diagnostics, Pittsburgh, PA) and differentiated by standard hematologic procedures in a blinded fashion.
Lung Histopathology and Morphometric Analyses
Lungs were fixed in 10% formalin and processed into paraffin. Mucus-containing goblet cells were detected by staining of paraffin sections (5-μm thick) with periodic acid–Schiff (PAS). Airway fibrosis was detected by staining of collagen with Sirius red. Airway smooth muscle cells were stained with immunoperoxidase, using anti–α-smooth muscle actin monoclonal antibody (Sigma-Aldrich). Tissue-infiltrating eosinophils were identified by immunofluorescence staining of major basic protein as previously described using rabbit anti-mouse major basic protein (kindly provided by J. J. Lee, Mayo Clinic, Scottsdale, AZ) (20). CD4+ T cells were detected by immunoperoxidase staining of frozen lung tissue sections with a rat anti-mouse CD4 antibody (L3T4; BD Biosciences Pharmingen, San Diego, CA). As a control, rat IgG was used. Histologic analyses were performed in a blinded manner by light microscopy linked to an image capture system. The data were analyzed by quantitative morphometry, using Scion Image analysis software available as public domain software from the U.S. National Institutes of Health (http://rsb.info.nih.gov/nih-image/). Numbers of PAS-positive goblet cells, airway tissue–infiltrating eosinophils, and collagen (Sirius red staining) were determined only in cross-sectional areas of the airway wall. Tangential sections and airway wall areas adjacent to large blood vessels were excluded from the analysis. For smooth muscle and collagen analysis, the slice density function of the software was used to highlight pixels belonging to the staining, excluding intercellular space and unstained tissue structures, which fall within the stained collagen or smooth muscle areas, and expressed as square micrometers. To allow for comparisons, all measurements were normalized to the length of the basement membrane of the adjacent epithelium for each corresponding airway. Airways 250 to 500 μm in internal diameter were evaluated and 10 different sections were evaluated per animal. The obtained measurements were averaged for each animal and the mean values and standard errors were determined for each group.
Measurement of BAL Fluid Cytokines and Chemokines
Levels of cytokines and chemokines were determined by commercial ELISAs in accordance with the manufacturer instructions. ELISA kits for detection of IL-4, IL-5, IL-10, IL-12 (p70), and IFN-γ in supernatants were obtained from BD Biosciences Pharmingen. IL-13, transforming growth factor (TGF)-β1, granulocyte-macrophage colony–stimulating factor (GM-CSF), eotaxin-1 and RANTES ELISA kits were purchased from R&D Systems (Minneapolis, MN). Before the TGF-β1 assay, BAL fluid samples were acidified to activate any latent TGF-β1 (21). The limits of detection for each assay were as follows: 4 pg/ml for IL-4 and IL-5; 10 pg/ml for IL-10, IL-12, and IFN-γ; 1.5 pg/ml for IL-13 and GM-CSF; 3 pg/ml for eotaxin-1 and RANTES; and 7 pg/ml for TGF-β1.
Measurement of OVA-specific Antibodies
Serum levels of OVA-specific IgE, IgG1, and IgG2a were measured by ELISA as previously described (22).
Lung Cell Isolation
Lungs were dissected into small pieces and exposed to an enzymatic digestion solution containing collagenase V (175 IU/ml; Sigma-Aldrich, St. Louis, MO) in Hanks' balanced salt solution for 60 min. After enzymatic digestion, total lung leukocytes were further enriched by 35% Percoll (Sigma-Aldrich) gradient centrifugation. The pellets were resuspended in 5 ml of red blood cell lysing buffer (Sigma-Aldrich) for 10 min on ice. The cells were then washed twice and resuspended in PBS containing 1% bovine serum albumin.
Flow Cytometry
The surface phenotype of lung cells was analyzed with monoclonal antibodies (mAbs) in conjunction with a three- or four-color immunofluorescence test. To minimize nonspecific binding, 5 × 105 cells were incubated with 0.25 μg of Fc-blocking solution (CD16/CD32; clone 2.4G2) for 10 min and subsequently treated with fluorochrome-labeled mAbs. The mAbs included fluorescein isothiocyanate-, phycoerythrin-, peridinin chlorophyll protein-, or allophycocyanin-conjugated anti-CD11b (M1/70), anti-CD11c (HL3), anti–Ly-6G and anti–Ly-6C (Gr-1 RB6–8C5), anti-CD80 (16–10A1), anti-CD86 (GL1), and anti-CD45R/B220 (RA3–6B2; all obtained from BD Biosciences Pharmingen). For staining pDCs, anti–mPDCA-1 (JF05–1C2.4.1) was obtained from Miltenyi Biotec (Auburn, CA). For control staining, similarly labeled isotype-matched control antibodies were used. After washing, staining was analyzed by flow cytometry on a FACSCalibur using CellQuest software (BD Biosciences, Mountain View, CA).
Generation and Transfer of BMDCs
DCs were generated from bone marrow cells of naive BALB/c mice according to the procedure described by Inaba and coworkers (23), with some modification. In brief, bone marrow cells obtained from femurs and tibias of mice were placed in 75-cm2 flasks at 37°C in DC culture medium (RPMI 1640 containing 10% heat-inactivated fetal calf serum, 50 μM 2-mercaptoethanol, 2 mM l-glutamine, penicillin [100 U/ml], streptomycin [100 μg/ml; GIBCO; Invitrogen, Carlsbad, CA], recombinant mouse GM-CSF [10 ng/ml], and recombinant mouse IL-4 [10 ng/ml; R&D Systems]). Nonadherent cells were collected by aspirating the medium and transferred into fresh flasks. On Day 8, cells were pulsed with OVA (200 μg/ml) for 24 h and washed three times with PBS. More than 90% of the cells were mDCs (CD11c+CD11b+Gr-1–). For DC transfer after sensitization and 11 challenges with OVA, under anesthesia 1 × 106 OVA-pulsed BMDCs in 40 μl of PBS were instilled to BALB/c mice through the trachea, using fiberoptic illumination. Control groups of mice received PBS. Six days after DC transfer, mice were exposed to aerosolized OVA (1% in saline) for 20 min/d for 3 consecutive d. Forty-eight hours after the last challenge, AHR was assessed and BAL fluid and lung tissues were obtained.
Statistical Analysis
Mann-Whitney U tests were used to determine the levels of difference between all groups. Data were pooled from three independent experiments with four mice per group in each experiment (n = 12). Comparisons for all pairs were performed by Kruskal-Wallis test. Significance was assumed at p values less than 0.05. Values for all measurements were expressed as means ± SEM.
RESULTS
Airway Hyperresponsiveness
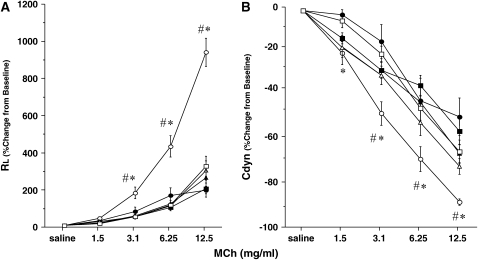
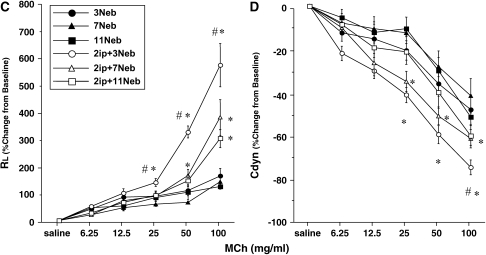
Figure 2 illustrates the changes in lung resistance and dynamic compliance in response to inhaled MCh in both strains of mice after repeated allergen exposure. Exposure of sensitized mice to aerosolized allergen results in AHR to inhaled MCh. In sensitized BALB/c mice, short-term allergen exposure (i.e., three daily challenges) resulted in the development of increased AHR, detected by significant increases in lung resistance (Figure 2A) and decreases in dynamic compliance (Figure 2B), compared with similar challenge of nonsensitized animals. These results were strikingly different when sensitized mice were subjected to additional exposures of aerosolized allergen—in these mice there was little persistent alteration in airway function after seven allergen challenges. In fact, the results were similar to those of nonsensitized mice exposed to seven challenges alone. This absence of AHR was also observed after additional allergen exposure (11 challenges). In sensitized C57BL/6 mice the results were similar, with AHR developing after short-term exposure, but progressively declining with long-term allergen exposures (Figures 2C and 2D). As seen previously (24), the amounts of MCh required to elicit maximum responses in C57BL/6 mice were higher than needed in BALB/c animals.
Figure 2.
Changes in airway resistance (RL) and dynamic compliance (Cdyn) in short- and long-term challenged mice. Increasing concentrations of nebulized methacholine (MCh) were administered 48 h after the last OVA challenge. (A and B) BALB/c mice (n = 12/group); (C and D) C57BL/6 mice (n = 12/group). Data represent means ± SEM. *p < 0.05 compared with nonsensitized OVA-challenged mice; #p < 0.05 compared with sensitized mice exposed to 7 and 11 OVA challenges. 3Neb, 7Neb, and 11Neb = nonsensitized mice exposed to 3, 7, or 11 OVA challenges, respectively; 2ip+3Neb, 2ip+7Neb, and 2ip+11Neb = sensitized mice exposed to 3, 7, or 11 OVA challenges, respectively.
Inflammatory Cell Composition
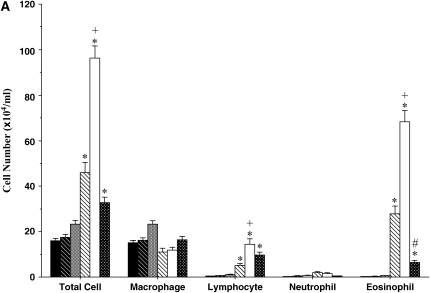
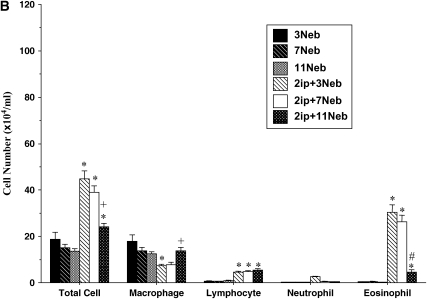
BAL fluid was examined in sensitized mice after 3, 7, and 11 inhalational challenges. In both BALB/c (Figure 3A) and C57BL/6 (Figure 3B) mice, significant increases in numbers of BAL fluid eosinophils were seen after three challenges. These numbers further increased in BALB/c mice exposed to seven challenges. However, after 11 challenges eosinophil numbers were reduced significantly in both strains. In both strains of mice, BAL fluid lymphocyte numbers were also increased after short-term challenge and the increases were sustained even after 11 challenges; in BALB/c mice the increases were also greater after long-term challenge. There were only small changes in neutrophil numbers. These results revealed some dissociation between AHR and the persistence of BAL fluid eosinophilia, at least after seven challenges of sensitized mice.
Figure 3.
Bronchoalveolar lavage (BAL) fluid cellular composition of mice sensitized and challenged with OVA. (A) BALB/c mice; (B) C57BL/6 mice. BAL fluid was obtained from the same groups described in Figure 2. *p < 0.05 compared with nonsensitized OVA-challenged mice; +p < 0.05 compared with sensitized mice exposed to three OVA challenges; #p < 0.05 compared with sensitized mice exposed to three and seven OVA challenges.
Lung Tissue Analysis
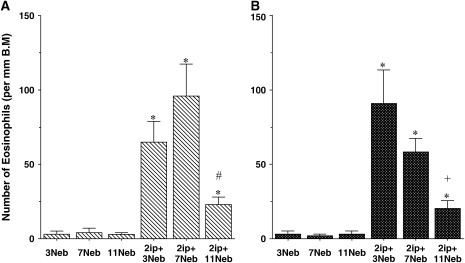
In parallel to the analysis of BAL fluid, lung tissue sections were immunolabeled with antibody to major basic protein to identify eosinophils in the lung tissue. As shown in Figures 4A and 4B, few eosinophils were detected in nonsensitized but challenged mice. In sensitized mice, after both short-term (three) and long-term (seven) challenges, lung eosinophilia developed and persisted. Interestingly, when these mice were monitored for an additional 2 wk (11 challenges), tissue eosinophilia was significantly decreased in the lung.
Figure 4.
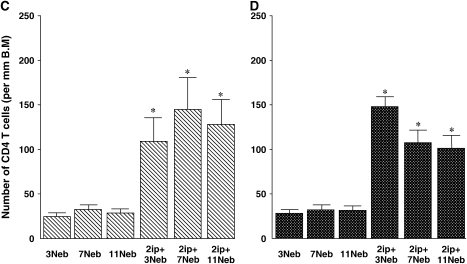
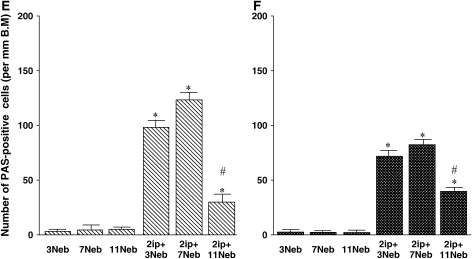
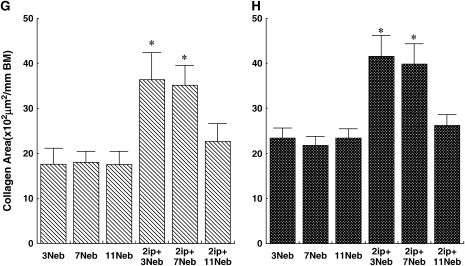
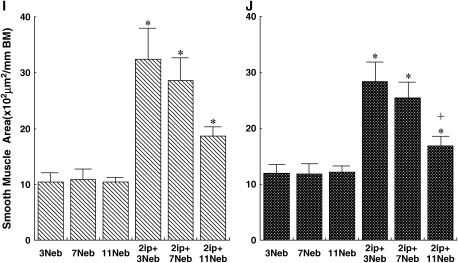
Quantitative analysis of lung eosinophils (A and B), CD4+ T cells (C and D), periodic acid–Schiff (PAS)-positive cells (E and F), Sirius red staining (G and H), and anti–α-smooth muscle actin staining (I and J) in BALB/c mice (A, C, E, G, and I) and C57BL/6 mice (B, D, F, H, and J) sensitized and challenged with OVA. Data represent means + SEM for groups of mice and are representative of two separate experiments. *p < 0.05 compared with nonsensitized OVA-challenged mice; +p < 0.05 compared with sensitized mice exposed to three OVA challenges; #p < 0.05 compared with sensitized mice exposed to three and seven OVA challenges. BM = basement membrane.
When these same tissues were stained for the presence of CD4+ T lymphocytes, both short- and long-term challenge protocols resulted in marked increases in cell numbers, compared with the nonsensitized controls (Figures 4C and 4D). These increases in CD4+ T cells persisted through 11 challenges in both sensitized strains of mice.
Goblet cell metaplasia and mucus hyperproduction were evaluated by PAS staining and quantification of positively stained cells. In nonsensitized mice of either strain, few positive cells were detected (Figures 4E and 4F, and Figure 5A). This contrasted with staining of tissue from sensitized and short- and long-term challenged mice, where the number of PAS-positive cells increased and was maintained, at least after 7 challenges (Figures 4E and 4F, and Figures 5B and 5C); after 11 challenges, the numbers of PAS-positive cells were decreased (Figures 4E and 4F, and Figure 5D). Thus, despite the persistence of BAL fluid and lung tissue eosinophilia, mucus hyperproduction, and infiltration of CD4+ lymphocytes, seven challenges with allergen via the airways was nonetheless associated with reduced AHR.
Figure 5.
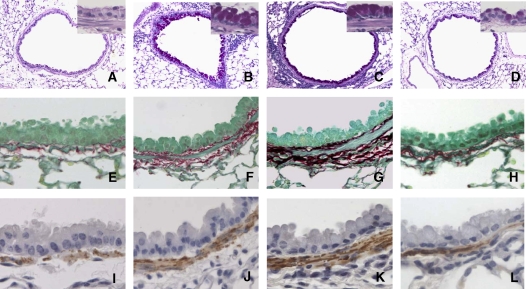
Mucus production and subepithelial collagen in BALB/c lung tissue obtained 48 h after the last challenge. Sections were stained with PAS (A–D; original magnification: ×100, insets, ×400), Sirius red (E–H), and anti–α-smooth muscle actin (I–L; original magnification, ×400). Representative histology from controls (A, E, and I), short-term challenged mice (sensitized mice exposed to 3 OVA challenges; B, F, and J), and long-term challenged mice (sensitized mice exposed to 7 OVA challenges [C, G, and K] and sensitized mice exposed to 11 OVA challenges [D, H, and L]).
Sirius red staining of the same tissues revealed a prominent subepithelial deposition of collagen in sensitized BALB/c and C57BL/6 mice after three and seven OVA challenges. However, after 11 challenges, the areas of collagen detected in the airway wall of sensitized mice were significantly reduced to levels that were comparable to those seen in nonsensitized but challenged control mice (Figures 4G and 4H, and Figures 5E–5H).
After short-term allergen challenge, the area of the peribronchial smooth muscle layer was also increased in both sensitized BALB/c and C57BL/6 mice, when compared with challenged-only mice. However, this increase in airway smooth muscle area declined progressively with increasing allergen challenge (Figures 4I and 4J, and Figures 5I–5L).
Cytokine/Chemokine Levels in BAL Fluid
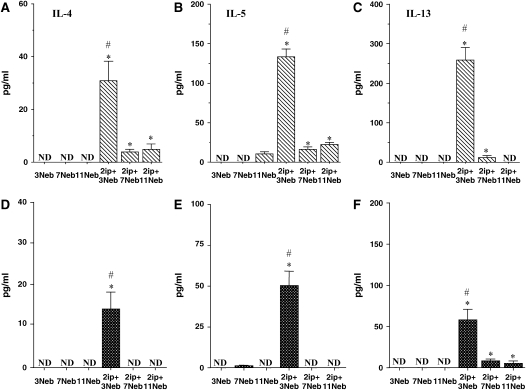
After sensitization and short-term challenge of both strains of mice, levels of the Th2 cytokines IL-4, IL-5, and IL-13 were significantly increased in BAL fluid (Figures 6A–6F). As previously reported (25), the BAL fluid levels of these cytokines were consistently higher in BALB/c mice (Figures 6A–6C) than in C57BL/6 mice (Figures 6D–6F). In contrast to short-term challenge, the levels of these cytokines decreased after additional (7 or 11) challenges.
Figure 6.
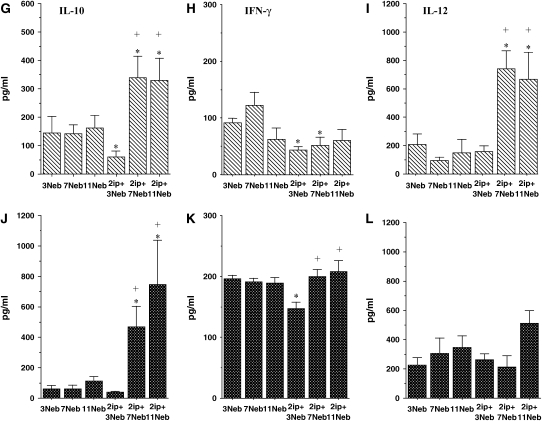
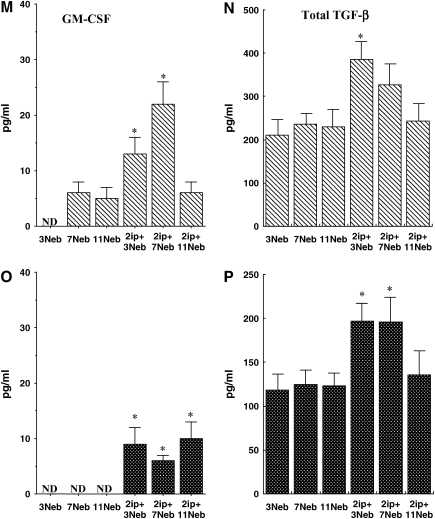
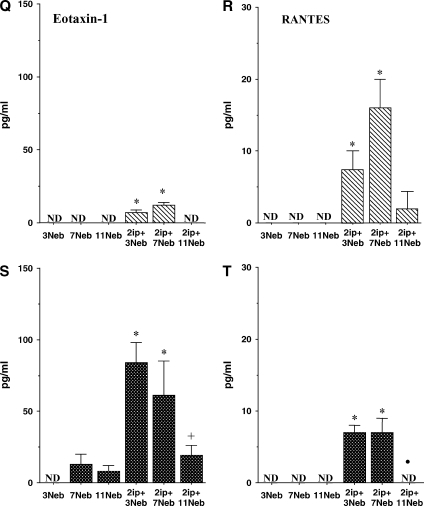
BAL fluid interleukin (IL)-4 (A and D), IL-5 (B and E), IL-13 (C and F), IL-10 (G and J), IFN-γ (H and K), IL-12 (I and L), granulocyte-macrophage colony–stimulating factor (GM-CSF; M and O), transforming growth factor (TGF)-β1 (N and P), eotaxin-1 (Q and S), and RANTES (R and T) levels were determined in short- and long-term challenged mice after OVA challenge by ELISA as described in METHODS. (A–C, G–I, M, N, Q, and R) BALB/c mice; (D–F, J–L, O, P, S, and T) C57BL/6 mice. Results for each group are expressed as means + SEM. *p < 0.05 compared with nonsensitized OVA-challenged mice; #p < 0.05 compared with sensitized mice exposed to 7 and 11 OVA challenges; +p < 0.05 compared with sensitized mice exposed to three OVA challenges; •p < 0.05 compared with sensitized mice exposed to 3 and 7 OVA challenges. ND = not detectable.
As previously reported (26), IL-10 levels in BALB/c mice decreased in the BAL fluid of sensitized animals compared with controls after short-term allergen challenge (Figures 6G and 6J). In contrast, after 7 and 11 challenges, levels of IL-10 increased significantly, to levels above those in controls of both strains. IFN-γ levels showed significant decreases after short-term challenge (Figures 6H and 6K), whereas IL-12 levels increased after the long-term challenges, more markedly in BALB/c mice than in C57BL/6 mice (Figures 6I and 6L). GM-CSF levels increased in both strains and were maintained even after 11 challenges in C57BL/6 mice (Figures 6M and 6O).
Levels of total TGF-β1 in BAL fluid were also elevated after three challenges in BALB/c mice or after three and seven challenges in C57/BL6 mice. However, these levels declined to control baseline levels after 11 challenges (Figures 6N and 6P). Among the chemokines assayed, eotaxin-1 levels increased after three and seven challenges in both strains, although levels were much higher in C57BL/6 mice (Figures 6Q and 6S). After 11 challenges, these levels fell significantly. RANTES levels also increased after 3 and 7 challenges in both strains, but returned to control values after 11 challenges (Figures 6R and 6T).
Serum Antibody Levels
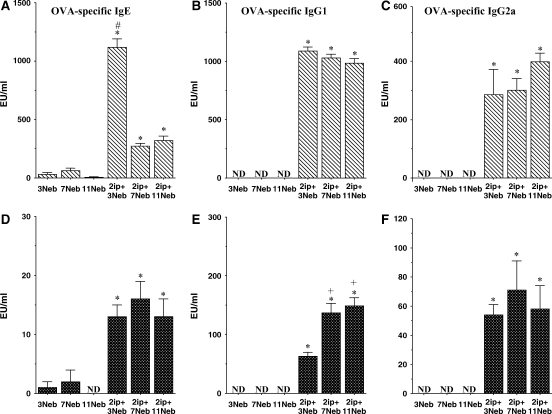
OVA-specific IgE levels paralleled those of the Th2 cytokines, especially in sensitized BALB/c mice, with increases after three challenges followed by a significant decrease after long-term challenge (Figures 7A and 7D). In both strains, levels of OVA-specific IgG1 were maintained through the 11 challenges, as were levels of OVA-specific IgG2a (Figures 7B, 7C, 7E, and 7F).
Figure 7.
(A–F) Serum titers of OVA-specific antibodies were determined in short- and long-term challenged mice as described in METHODS. *p < 0.05 compared with nonsensitized OVA challenged mice; +p < 0.05 compared with sensitized mice exposed to three OVA challenges; #p < 0.05 compared with sensitized mice exposed to 7 and 11 OVA challenges. ND = not detectable.
Lung DCs
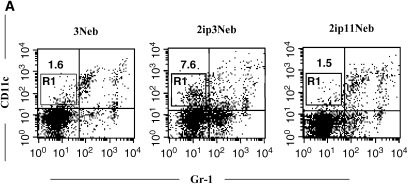
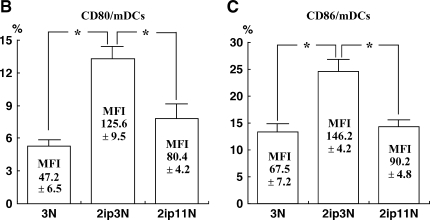
To determine whether repeated allergen challenge altered the number of DCs, lung cells were stained with anti–Gr-1 and anti-CD11c. In mice exposed to 11 challenges, the percentage of mDCs (CD11c+Gr-1–) was significantly decreased compared with that of mice receiving three challenges, returning to control baseline levels (percentage mDCs: 6.5 ± 0.7, 1.4 ± 0.2, and 1.5 ± 0.3 for sensitized mice exposed to 3 challenges, sensitized mice exposed to 11 challenges, and nonsensitized mice exposed to 3 challenges, respectively; n = 6; Figure 8A). Furthermore, expression of CD80 and CD86 on mDCs was significantly lower after 11 challenges, as was the mean fluorescence intensity (Figures 8B and 8C). On the other hand, numbers of pDCs (CD11c+ Gr-1+B220+PDCA-1+) were not significantly different between the groups exposed to 3 and 11 challenges (data not shown).
Figure 8.
Flow cytometric analysis of lung dendritic cells. (A) Anti–Gr-1 and anti-CD11c staining of lung leukocytes from nonsensitized mice exposed to three OVA challenges (3Neb), sensitized mice exposed to three OVA challenges (2ip3Neb), and sensitized mice exposed to 11 OVA challenges (2ip11Neb). Data from a representative experiment are shown. (B and C) Percentage of CD80-expressing cells (B) and percentage of CD86-expressing cells (C) in the myeloid dendritic cell (mDC) fraction; n = 6. Mean fluorescence intensity (MFI) values for the corresponding samples are shown. *p < 0.05 between the groups indicated. 3N = nonsensitized mice exposed to three OVA challenges; 2ip3N = sensitized mice exposed to three OVA challenges; 2ip11N = sensitized mice exposed to 11 OVA challenges.
Transfer of BMDCs
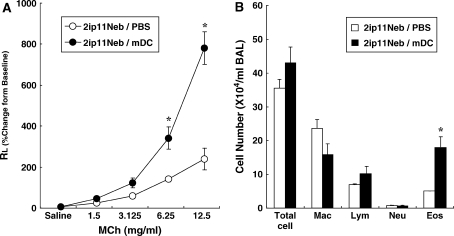
Given that repeated allergen challenge, which led to the attenuation of AHR and Th2-mediated inflammation, was associated with decreased numbers of mDCs and lower expression of costimulatory molecules, we investigated whether intratracheal administration of OVA-pulsed mDCs could restore AHR and airway inflammation in recipient mice previously exposed to 11 challenges with OVA. As shown in Figure 9, the transfer of antigen-pulsed BMDCs restored AHR (Figure 9A) and BAL fluid eosinophilia (Figure 9B) compared with control mice.
Figure 9.
Changes in airway resistance (RL) in response to inhaled MCh (A) and BAL fluid cellular composition (B) after transfer of bone marrow–derived dendritic cells (BMDCs). After 11 challenges, 106 antigen-pulsed BMDCs or PBS was administered to BALB/c mice via the trachea. Six days later, allergen exposure via the airways was performed on 3 consecutive d followed by assessment of airway function and BAL fluid cell analysis 48 h after the last OVA challenge. *p < 0.05 compared with PBS-administered mice (n = 12/group). Eos = eosinophils; Lym = lymphocytes; Mac = macrophages; Neu = neutrophils.
DISCUSSION
Airway inflammation and AHR are hallmarks of allergic asthma. There are many reports in humans and in animal models linking airway inflammation and altered airway function to increases in Th2 cytokine and/or chemokine levels produced by immune cells (27, 28); the common theme is that these factors produce AHR indirectly through the recruitment of inflammatory cells. However, there remains significant controversy as to which cytokine(s) or chemokine(s) are essential to one or both processes. Despite their importance, only limited data are available with respect to the kinetics of development and maintenance or persistence of airway immune/inflammatory responses and AHR after repetitive allergen exposure of sensitized hosts. The primary purpose of the present study was to examine the kinetics of the development and resolution of AHR and airway inflammation after repetitive allergen exposure and relate these changes, when possible, to cytokine and chemokine responses.
A characteristic and consistent feature of the murine response to sensitization and short-term airway allergen challenge is the development of AHR in response to inhaled MCh. Changes in lung function, measured in a variety of ways, have been demonstrated in both BALB/c and C57BL/6 mice. Although there are strain-related differences in the extent, for example, of changes in lung resistance versus compliance (24), or MCh reactivity (24, 25), AHR remains a consistent outcome of sensitization and short-term airway challenge. Extending airway challenge with the addition of four or eight more exposures over an additional 2 to 4 wk resulted in a marked attenuation of AHR in both strains of mice. In BALB/c mice, AHR was virtually abolished after seven challenges, whereas C57BL/6 mice showed a progressive decrease in AHR with increasing challenges. These decreases in AHR were observed throughout the MCh dose–response curve and in measures of both Rl and Cdyn.
These significant decreases in AHR occurred despite the maintenance of a significant increase in numbers of BAL fluid and lung tissue eosinophils through seven inhalation challenges with allergen. At face value, the data suggest the absence of a correlation between lung and BAL fluid eosinophilia and persistence of AHR. By 11 challenges, this dissociation was no longer apparent, as airway function normalized. The role of eosinophils in AHR is controversial in many species, including humans (29, 30) and mice (31, 32). Even after sensitization and short-term challenge, discrepancies have been reported (33). We and others (31, 34) have suggested that eosinophils and IL-5 are linked to AHR, in contrast to another report (35), at least when sensitization is followed by limited airway challenge (one to three intranasal or nebulized challenges). Moreover, with respect to the temporal sequence of these events, lung tissue eosinophilia has been better correlated with AHR than BAL fluid eosinophil numbers (24). Exceptions to the association of AHR and BAL fluid eosinophilia may be attributed to differences in the challenge protocol and readouts of lung function (36), although the exact reason(s) remain unclear. In a previous study, we also demonstrated that after secondary challenge, the role of eosinophils in the development of AHR became more complicated, and, to some extent, eosinophil independent (37).
The persistence of lung and BAL fluid eosinophilia in the absence of AHR after seven challenges could be the result of several possibilities. One possibility is that the data simply reflect differences in the kinetics of individual responses. A second possibility is that eosinophilia is not linked to development of AHR; this is possible but less likely on the basis of the data discussed above. These discrepancies are reinforced by two contradictory reports using “eosinophil-less” mice (38, 39). A third alternative is that the eosinophils persist for longer periods in the lung tissue (34), but are no longer activated so as to result in AHR. As there are no good markers of eosinophil activation, this question remains open, and could explain the shift in kinetics or dissociation of eosinophilia and AHR. Since other factors (eotaxin-1, RANTES, and GM-CSF) affecting eosinophil recruitment, survival, and activation were elevated, at least after seven challenges, this may account for the persistent eosinophilia but an eosinophil activation factor present after short-term challenge, such as IL-5, was no longer available. However, our results showed that the levels of RANTES or GM-CSF in BAL fluid were not correlated to the persistence of AHR in mice after repeated OVA challenges.
It is also striking that other responses implicated in the development of AHR also persisted after repetitive challenges despite the normalization of lung function in response to inhaled MCh. CD4+ T cells are thought to play a central role in the development of AHR and lung inflammation (40). Depletion of CD4+ T cells during the sensitization phase prevented the subsequent development of these responses (41). CD4+ T cells are also responsible for the production, at least in part, of the Th2 cytokines IL-4, IL-5, and IL-13 (11). Virtually all the pathophysiological manifestations of allergen-induced airway inflammation and AHR can be accounted for by these cytokines (42–44). After increasing numbers of challenges, the levels of these Th2 cytokines in BAL fluid were significantly reduced despite persistent elevation of CD4+ T cells in lung tissue. If CD4+ T cells are primarily responsible for Th2 cytokine production, one implication is that after long-term airway challenge, the functional activity of these lung T cells or their phenotype is altered. Although Th2 CD4+ T cells may be essential in the induction phase of allergic responses in the lung, their role over time may become modified (45). It is also possible that with repeated challenges they become “tolerized”—that is, no longer responsive to allergen— although we have no direct data supporting this conclusion.
Despite the decreases in levels of IL-13 after seven challenges, goblet cell metaplasia/mucus hyperproduction persisted. However, after 11 challenges, this increase in PAS-positive cell numbers was also resolving. IL-13 appears essential for goblet cell metaplasia and mucus production (44). Rather than involving an undefined pathway maintaining this response over seven challenges, the results likely reflect a simple shift in kinetics, with levels of IL-13 falling more rapidly than the disappearance of PAS-positive cells. As observed with eosinophils, continued monitoring for an additional 2 wk showed a loss of these PAS-positive cells.
In contrast to the decreases in Th2 cytokine production, increases were seen in other cytokine levels. IL-12 levels were increased in the BAL fluid of BALB/c and C57BL/6 mice, but with different kinetics. IL-12 is an important regulator of the balance between Th1 and Th2 cells (46). IL-12 induces production of IFN-γ (47), but we saw no concomitant increases in the levels of IFN-γ in BAL fluid. In similar murine models, IL-12 administration did inhibit lung eosinophilia and AHR, as well as decrease IL-4 and IL-5, and increase IFN-γ (48). IL-12 effects may also be IFN-γ independent (49). However, it seems unlikely that the dissociation between AHR and lung and BAL fluid eosinophilia after seven challenges was related to IL-12 levels. This dissociation was not seen after 11 challenges in both strains, and elevated IL-12 levels at this time point were associated with the inhibition of AHR as well as the attenuation of lung and BAL fluid eosinophilia.
Common to both strains after long-term challenge was the increase in BAL fluid IL-10 levels. These IL-10 levels were sustained for a further 2 wk (11 challenges). In general, IL-10 is considered to be an antiinflammatory factor and the release of IL-10 in asthma serves to downregulate the inflammatory reaction associated with this disorder (50–52). IL-10 is produced by CD4+CD25+ regulatory T cells, which have been shown to play a critical role in several models of tolerance (53, 54). Although in our study it is not clear that tolerance was induced by long-term challenge, the increases in IL-10 with repeated challenge could have contributed to the loss of AHR, the reduction in lung BAL fluid eosinophilia, and perhaps the prevention of eosinophil activation. We also considered “tolerance” from the point of view of oral tolerance developing as a result of licking of the pelt over time with repeated exposures. Initial experiments using repeated intranasal challenges did not alter the character of the responses, but simply shifted the kinetics by 1 to 2 wk (data not shown).
Considerable efforts have been made by several laboratories to develop a murine model of chronic allergic airway inflammation and AHR (55–60) (see Table E1 in the online supplement). The presumption in these studies is that chronic allergic airway inflammation, expressing an eosinophilic signature, is maintained by long-term (repetitive) airway allergen exposure, which leads to structural airway remodeling and, as a result, persistent AHR. Although the experimental designs varied considerably between the studies (i.e., strains of mice, type of allergen, route of sensitization, and duration or number of airway challenges), the endpoint measurements were common and included AHR, airway eosinophilia, and airway tissue remodeling (goblet cell metaplasia, collagen deposition, and thickness of the airway smooth muscle layer). Of significance in evaluating and comparing these studies is the implication of a specific component in the ability to elicit chronic and persistent changes. Thus, the strain of mouse, the specific allergen, the dose and number of allergen challenges, the route of administration, and the intervals between exposures all have been interpreted to explain a successful outcome. There are also considerable differences in what constitutes a persistent change in, for example, lung function or eosinophilic inflammation, where for the most part, all have shown attenuation of both of these responses with increasing allergen exposure, with rare exceptions (60). The demonstration of increased collagen deposition, smooth muscle hypertrophy, or limited airway eosinophilia with increasing allergen exposure, findings we did not observe, is also subject to interpretation. Careful morphometry, avoidance of tangential sections, and exclusion of areas around blood vessels are absolutely necessary to show such chronic changes, especially in the face of normalization of lung function.
Concern has also been raised that commercial preparations of OVA are contaminated with endotoxin and that endotoxin coadministration with OVA creates a state of tolerance (61). Somewhat to the contrary, endotoxin contamination has also been stated to be necessary for Th2 responses, at least in some strains of mice (62). We have seen little difference in results when using preparations of OVA assayed to be “endotoxin free.”
A number of studies have focused on the functional diversity of DCs in the lung parenchyma and airways (63–66), and the role these cells play in the priming and activation of T lymphocytes (65, 66). The important contribution of DCs in asthma pathogenesis has also been described (16, 17). The role of DCs after chronic or repeated allergen challenge has not been well defined. In the present study, we determined whether alterations in DC function might account for the progressive decreases in AHR and airway inflammation after increasing challenges, while numbers and expression of CD80 and CD86 were significantly decreased after 11 versus 3 challenges. Further, we investigated whether there were changes in DC numbers or phenotype in the peribronchial lymph nodes, and none were detected. It is unclear at present whether the decrease in number and accessory molecule expression of lung mDCs was responsible for the T-cell unresponsiveness to allergen after increased challenges and whether this was linked to the increase in IL-10 seen after 11 challenges. A number of reports suggested that IL-10 attenuates the differentiation and maturation of DCs in vitro, especially mDCs (67–69). Immature mDCs, generated under these high IL-10 conditions, may induce T-cell tolerance (69, 70), thus playing a role in the resolution of AHR and airway allergic inflammation after repeated allergen challenge. de Heer and coworkers reported that pDCs provided intrinsic protection against inflammatory responses to antigen (19). However, in our experiments, pDCs (CD11c+Gr-1+B220+PDCA-1+) were not increased in the lung parenchyma or draining lymph nodes. To directly address the issue of the role of lung mDCs in the resolution of AHR and allergic airway inflammation, transfer experiments were performed. Transfer of antigen-pulsed BMDCs to mice receiving 11 allergen challenges restored responsiveness to allergen, with development of AHR and eosinophilic inflammation. These data suggest that DCs can sustain T-cell–mediated allergic responses in the airways. The mechanism(s) contributing to the restoration of AHR and airway eosinophilia by instillation of mDCs after repeated allergen challenges are not clear. One possibility is that transferred DCs reactivated previously stimulated, antigen-specific T cells. Alternatively, and perhaps more likely, transferred DCs could activate newly primed T cells. BMDCs have the potential to generate primed T cells from naive T cells. Another possibility is that DCs directly cause AHR. Several reports have demonstrated that myeloid DCs have the capacity to produce several cytokines, not only IL-12 and IL-10, but also IL-13, which may directly contribute to airway smooth muscle hypercontractility.
In summary, this study demonstrates that repeated allergen challenge in two strains of mice results in the loss of AHR to inhaled MCh despite the persistence of airway eosinophilia, at least for a period beyond normalization of lung function. Together with the loss of AHR, there were significant decreases in Th2 cytokine production but consistent and sustained increases in IL-10 and IL-12 levels. Repeated challenge was associated with a reduction in the number of lung mDCs, and transfer of antigen-pulsed DCs reversed this attenuation of AHR and eosinophilic inflammation. This decrease in allergic responsiveness with repeated challenge has plagued the development of a true chronic model of allergen exposure in mice. Nonetheless, the data reveal potential avenues of intervention in preventing some of the long-term consequences of repeated allergen exposure.
Supplementary Material
Acknowledgments
The authors thank Diana Nabighian and Ayako Takahashi for their expert help in preparing the manuscript, and Lynn Cunningham for performing the immunolabeling studies.
Supported by NIH grants HL-36577 and HL-61005, and by EPA grant R825702 (to E.W.G.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200505-783OC on September 28, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Busse WW, Lemanske RF. Asthma. N Engl J Med 2001;344:350–362. [DOI] [PubMed] [Google Scholar]
- 2.Peebles RS Jr, Hamilton RG, Lichtenstein LM, Schlsoberg M, Liu MC, Proud D, Togias A. Antigen-specific IgE and IgA antibodies in bronchoalveolar lavage fluid are associated with stronger antigen-induced late phase reactions. Clin Exp Allergy 2001;31:239–248. [DOI] [PubMed] [Google Scholar]
- 3.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol 1994;12:295–335. [DOI] [PubMed] [Google Scholar]
- 4.Kay AB. Asthma and inflammation. J Allergy Clin Immunol 1991;87:893–910. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM, Kumar RK, Thomas PS, Seetoo DO, Herbert C, McKenzie AN, et al. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 6.Ohashi Y, Motpjima S, Fukuda T, Makino S. Airway hyperresponsiveness, increased intracellular spaces of bronchial epithelium, and increased infiltration of eosinophils and lymphocytes in bronchial mucosa in asthma. Am Rev Respir Dis 1992;145:1469–1476. [DOI] [PubMed] [Google Scholar]
- 7.Benayoun L, Druilhe MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 2003;167:1360–1368. [DOI] [PubMed] [Google Scholar]
- 8.Vignola AM, Kips J, Bousquet J. Tissue remodeling as a feature of persistent asthma. J Allergy Clin Immunol 2000;105:1041–1053. [DOI] [PubMed] [Google Scholar]
- 9.Coutts A, Chen G, Stephens N, Hirest S, Douglas D, Eichholtz T, Khalil N. Release of biologically active TGF-β from airway smooth muscle induces autocrine synthesis of collagen. Am J Physiol Lung Cell Mol Physiol 2001;280:L999–L1008. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel SE, Trudeau JB, Barnes S, Zhou X, Cundall M, Westcott JY, McCord K, Chu W. TGF-β and IL-13 synergistically increase eotaxion-1 production in human airway fibrosis. J Immunol 2002;169: 4613–4619. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DR, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol 2002;38:881–885. [DOI] [PubMed] [Google Scholar]
- 13.Kon OM, Kay AB. T cells and chronic asthma. Int Arch Allergy Immunol 1991;118:133–135. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767–811. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Paczesny S, Blanco P, Bennett L, Pascual V, Fay J, Palucka AK. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann N Y Acad Sci 2003;987:180–187. [DOI] [PubMed] [Google Scholar]
- 16.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol 1998;160:4090–4097. [PubMed] [Google Scholar]
- 17.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005;201:981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest 2000;106:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004;200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda K, Hamelmann E, Joetham A, Shultz LD, Laresn GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil N. TGF-β: from latent to active. Microbes Infect 1999;1:1255–1263. [DOI] [PubMed] [Google Scholar]
- 22.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest 1996;97:1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects difference in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:394–402. [DOI] [PubMed] [Google Scholar]
- 25.Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol 2003;285:32–42. [DOI] [PubMed] [Google Scholar]
- 26.Rha YH, Taube C, Haczku A, Joetham A, Takeda K, Duez C, Siegel M, Aydintug MK, Born WK, Dakhama A, et al. Effect of microbial heat shock proteins on airway inflammation and hyperresponsiveness. J Immunol 2002;169:5300–5307. [DOI] [PubMed] [Google Scholar]
- 27.Eum SY, Maghni K, Hamid Q, Eidelman DH, Campbell H, Isogai S, Martin JG. Inhibition of allergic airway inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol 2003;111:1049–1061. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann N, Hershey GK, Foster PS, Rothenberg ME. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J Allergy Clin Immunol 2003;111:227–242. [DOI] [PubMed] [Google Scholar]
- 29.Shi HZ, Xiao CQ, Zhong D, Qin SM, Liu Y, Liang GR, Xu H, Chen YQ, Long XM, Xie ZF. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am J Respir Crit Care Med 1998;157:204–209. [DOI] [PubMed] [Google Scholar]
- 30.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness, and the late asthmatic response. Lancet 2000;356: 2144–2148. [DOI] [PubMed] [Google Scholar]
- 31.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 1996;186:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathur M, Herrmann K, Li X, Qin Y, Weinstock J, Elliott D, Monahan J, Padrid P. TRFK-5 reverses established airway eosinophilia but not established hyperresponsiveness in a murine model of chronic asthma. Am J Respir Crit Care Med 1999;159:580–587. [DOI] [PubMed] [Google Scholar]
- 33.Shen HH, Ochkur SI, McGarru MP, Crosby JR, Hines EM, Borchers MT, Wang H, Biechelle TL, O'Neill KR, Ansay TL, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol 2003;170:3296–3305. [DOI] [PubMed] [Google Scholar]
- 34.Tomkinson A, Cielewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med 2000;163:721–730. [DOI] [PubMed] [Google Scholar]
- 35.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperresponsiveness in BALB/c mice independently of IL-4 and IL-5. J Immunol 1998;161:1501–1509. [PubMed] [Google Scholar]
- 36.Kanehiro A, Takeda K, Joetham A, Tomkinson A, Ikemura T, Irvin CG, Gelfand EW. Timing of administration of anti-VLA-4 differentiates airway hyperresponsiveness in the central and peripheral airways in mice. Am J Respir Crit Care Med 2000;162:1132–1139. [DOI] [PubMed] [Google Scholar]
- 37.Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, Gelfand EW. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med 2000;163:173–184. [DOI] [PubMed] [Google Scholar]
- 38.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 39.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 40.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol 1991;88:935–942. [DOI] [PubMed] [Google Scholar]
- 41.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocyte prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol 1994;10:587–593. [DOI] [PubMed] [Google Scholar]
- 42.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med 1997;185:2143–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rankin JA, Picarella DE, Geba GP, Temann UA, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA 1996;93:7821–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999;103:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS–ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 2002;8:1024–1032. [DOI] [PubMed] [Google Scholar]
- 46.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med 1993;177:1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HA, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of interferon γ production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med 1991;173:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med 1995;182:1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruselle GG, Kips JC, Peleman RA, Joos GF, Devos RR, Tavernier JH, Pauwels RA. Role of IFN-γ in the inhibition of the allergic airway inflammation caused by IL-12. Am J Respir Cell Mol Biol 1997;17:767–771. [DOI] [PubMed] [Google Scholar]
- 50.John M, Lim S, Seybold J, Jose P, Robichaud A, O'Connor B, Barnes PJ, Chung KF. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1α, granulocyte-macrophage colony-stimulating factor, and interferon-γ release from alveolar macrophages in asthma. Am J Respir Crit Care Med 1998;157:256–262. [DOI] [PubMed] [Google Scholar]
- 51.Oh JW, Seroogy CM, Meyer EH, Akbari O, Berry G, Fathman CG, Dekruyff RH, Umetsu DT. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J Allergy Clin Immunol 2002;110:460–468. [DOI] [PubMed] [Google Scholar]
- 52.van Scott MR, Justice JP, Bradfield JF, Enright E, Sigounas A, Sur S. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L667–L674. [DOI] [PubMed] [Google Scholar]
- 53.Kohm AP, Carpentier PA, Anger HA, Miller SD. CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol 2002;169:4712–4716. [DOI] [PubMed] [Google Scholar]
- 54.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000;12:431–440. [DOI] [PubMed] [Google Scholar]
- 55.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Rodriguez M, Lee SY, McElwain K, McElwain S, Raz E, et al. Immunostimulatory DNA inhibits transforming growth factor-β expression and airway remodeling. Am J Respir Cell Mol Biol 2004;30:651–661. [DOI] [PubMed] [Google Scholar]
- 56.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 2004;169:378–385. [DOI] [PubMed] [Google Scholar]
- 57.Shinagawa K, Kojima M. Mouse model of airway remodeling: strain differences. Am J Respir Crit Care Med 2003;168:959–967. [DOI] [PubMed] [Google Scholar]
- 58.Temelkovski J, Hogan SP, Shepherd DP, Foster PS, Kumar RK. An improved murine model of asthma: selective airway inflammation, epithelial lesions and increased methacholine responsiveness following chronic exposure to aerosolized allergen. Thorax 1998;53:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakai K, Yokoyama A, Kohno N, Hamada H, Hiwada K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. Int Arch Allergy Immunol 2001;126:126–134. [DOI] [PubMed] [Google Scholar]
- 60.Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, O'Byrne PM, Inman MD. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol 2002;27:526–535. [DOI] [PubMed] [Google Scholar]
- 61.Gerhold K, Bluemchen K, Franke A, Stock P, Hamelmann E. Exposure to endotoxin and allergen in early life and its effect on allergen sensitization in mice. J Allergy Clin Immunol 2003;112:389–396. [DOI] [PubMed] [Google Scholar]
- 62.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stumbles PA, Thomas JA, Pimm CL, Lee PT, Venaille TJ, Proksch S, Holt PG. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J Exp Med 1998;188: 2019–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest 2002;110:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity 2002;16:271–283. [DOI] [PubMed] [Google Scholar]
- 66.Huh JC, Strickland DH, Jahnsen FL, Turner DJ, Thomas JA, Napoli S, Tobagus I, Stumbles PA, Sly PD, Holt PG. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med 2003;198:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol 1997;27:1229–1235. [DOI] [PubMed] [Google Scholar]
- 68.Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW. Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation 1998;66:1567–1574. [DOI] [PubMed] [Google Scholar]
- 69.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology 2002;107:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol 1997;159:4772–4780. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.