Abstract
We previously determined the structure of the Pasteurella multocida Heddleston type 1 lipopolysaccharide (LPS) molecule and characterized some of the transferases essential for LPS biosynthesis. We also showed that P. multocida strains expressing truncated LPS display reduced virulence. Here, we have identified all of the remaining glycosyltransferases required for synthesis of the oligosaccharide extension of the P. multocida Heddleston type 1 LPS, including a novel α-1,6 glucosyltransferase, a β-1,4 glucosyltransferase, a putative bifunctional galactosyltransferase, and two heptosyltransferases. In addition, we identified a novel oligosaccharide extension expressed only in a heptosyltransferase (hptE) mutant background. All of the analyzed mutants expressing LPS with a truncated main oligosaccharide extension displayed reduced virulence, but those expressing LPS with an intact heptose side chain were able to persist for long periods in muscle tissue. The hptC mutant, which expressed LPS with the shortest oligosaccharide extension and no heptose side chain, was unable to persist on the muscle or cause any disease. Furthermore, all of the mutants displayed increased sensitivity to the chicken antimicrobial peptide fowlicidin 1, with mutants expressing highly truncated LPS being the most sensitive.
Pasteurella multocida is a gram-negative bacterial pathogen that is the causative agent of fowl cholera in wild and domestic birds. It is also responsible for a range of diseases in mammals, including bovine hemorrhagic septicemia and swine atrophic rhinitis (4). Serological classification of P. multocida strains is based primarily on the capsular type, which divides strains into serogroups A, B, D, E, and F (7, 26), and secondly on lipopolysaccharide (LPS) antigens that allow further classification into 16 Heddleston serotypes (16). Fowl cholera is an economically important disease of poultry that occurs in most countries. The pathogenesis of infection is not well understood at the molecular level, but initial colonization is thought to occur via the host mucosa and may proceed to a rapid and severe systemic disease that is generally fatal (4). Most fowl cholera outbreaks in the poultry industry are associated with strains belonging to serogroup A or F (4).
We showed previously that LPS is an important virulence factor and that a complete LPS structure is required by P. multocida strain VP161 for full virulence in chickens (14, 15). However, it is unclear at what point during infection LPS is critical for survival or how the length of the LPS structure affects bacterial survival in vivo. The complete structures of the LPSs expressed by VP161 (Heddleston type 1) and two other fowl cholera-causing strains, X-73 (Heddleston type 1) and Pm70 (Heddleston type 3), have been determined (29-31). Like other pathogens, such as Haemophilus spp. and Neisseria spp., P. multocida expresses LPS that lacks a polymeric O side chain. The outer-core sugars of the LPS molecule, consisting of the oligosaccharide extension and side branches, are thus the most distal and exposed region of the LPS molecule. Comparison of the LPS structure isolated from each of the three P. multocida strains mentioned above revealed that the inner-core sugars were highly conserved, but there was significant variation in the outer sugars (29-31).
Unusually, all three of the P. multocida strains examined to date simultaneously express LPS molecules with two different inner-core structures, one containing a single phosphorylated 3-deoxy-d-manno-octulosonate (Kdo) residue and the other containing two Kdo residues (shown for strain VP161 in Fig. 1B). In a previous study, we identified the transferases required for the assembly of the two inner-core glycoforms, A and B (12). These comprised a Kdo transferase (KdtA) required for addition of the Kdo molecules to lipid A, a Kdo kinase (KdkA) required to phosphorylate Kdo in glycoform A, and two heptosyl-I-transferases (HptA and HptB), each of which exclusively recognized different acceptor molecules (Fig. 1B) (12). Furthermore, we showed that despite the expression of both glycoforms by wild-type P. multocida during growth in vivo and in vitro, mutants expressing LPS composed of only glycoform B were able to cause systemic disease in chickens. Additionally, we have recently identified a locus that is required for the addition of the terminal phosphocholine (PCho) residues to the LPS molecule (14). Mutants lacking the terminal PCho residues were less virulent than the parent strain but were still able to cause disease (14).
FIG. 1.
Structures of the LPS molecules expressed by P. multocida strains Pm70 (A) and VP161 (B) and the glycoform C observed only in the VP161 hptE mutant (C). Two inner-core LPS glycoforms were observed in Pm70 and VP161 wild-type strains, and the variable sections are boxed in panel B and designated glycoform A and B. The gene known (B) or predicted (A and C) to be required for each biosynthetic step in the synthesis of each molecule is shown below the linkages. The residues are Glc, glucose; Hep, heptose; Gal, galactose; GlcNAc, N-acetylglucosamine; and PEtn, phosphoethanolamine.
In this study, we have completed the identification and characterization of the transferases required for the assembly of the oligosaccharide extension of the P. multocida VP161 LPS molecule. Each of the glycosyltransferase genes predicted to be involved in LPS assembly was inactivated, and the structure of the LPS expressed by each of the mutants was determined. These analyses identified a novel α-1,6 glucosyltransferase and a putative bifunctional galactosyltransferase capable of addition of a galactose to both the 4 and 6 positions of the fourth heptose. Furthermore, we tested each mutant for virulence in the natural chicken host. Lastly, we assessed the roles of specific LPS components in conferring resistance to the avian antimicrobial peptide fowlicidin 1, which is predicted to be an important component of the chicken innate immune system.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli was grown routinely in Luria-Bertani broth. P. multocida and Mannheimia haemolytica were grown in brain heart infusion (BHI) broth. Solid media were obtained by the addition of 1.5% agar. When required, the media were supplemented with spectinomycin (100 μg/ml), streptomycin (50 μg/ml), kanamycin (50 μg/ml), or tetracycline (2.5 μg/ml for routine culturing or 8 μg/ml for selection of P. multocida transconjugants). For structural studies of LPS isolated from in vitro-grown P. multocida and M. haemolytica, strains were grown in 2 liters of BHI, which was incubated at 37°C with shaking until early log phase was reached. This culture was then used to inoculate 24 liters of BHI in a 28-liter New Brunswick Scientific fermentor. The cultures were grown at 37°C, with 24-liter min−1 aeration and stirring at 200 rpm for 18 h with 20% O2 saturation, and then the P. multocida strains were incubated with hyaluronidase (1 g; Sigma) for 1 h at 37°C to remove the capsule. The cells were killed by addition of phenol to 2% for 4 h and harvested using a Sharples continuous centrifuge.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λ− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Bethesda Research Laboratories |
| Sm10 λ pir | Strain for propagation of pUA826 and its derivatives | 27 |
| M. haemolytica | ||
| O1A | M. haemolytica wild-type strain | 20 |
| AA100 | gctA mutant of M. haemolytica O1A | This study |
| AA101 | Complemented M. haemolytica gctA mutant | This study |
| P. multocida | ||
| VP161 | Serotype A:1; Vietnamese isolate from chickens | 33 |
| AL435 | VP161 carrying a Tn916 insertion in gene pm1417; fully virulent (Tetr) | 12 |
| AL523 | hptE mutant of AL435 | This study |
| AL543 | AL523 containing pAL99 | This study |
| AL544 | Complemented hptE mutant; AL523 containing pAL291 | This study |
| AL554 | gctB mutant of AL435 | This study |
| AL574 | gctA mutant of AL435 | This study |
| AL669 | PM0442 mutant of AL435 | This study |
| AL696 | hptC mutant of AL435 | This study |
| AL725 | gatA mutant of AL435 | This study |
| AL804 | Complemented gatA mutant; AL725 containing pAL445 | This study |
| AL807 | AL725 containing pAL99 | This study |
| AL878 | AL574 containing pAL99 | This study |
| AL879 | Complemented gctA mutant; AL574 containing pAL466 | This study |
| AL880 | AL554 containing pAL99 | This study |
| AL881 | Complemented gctB mutant; AL554 containing pAL468 | This study |
| AL882 | AL696 containing pAL99 | This study |
| AL883 | Complemented hptC mutant; AL696 containing pAL470 | This study |
| Plasmids | ||
| pAL99 | P. multocida expression plasmid (Kanr) | 15 |
| pAL291 | Complete hptE gene cloned into pAL99 | This study |
| pAL445 | Complete gatA gene cloned into pAL99 | This study |
| pAL466 | Complete gctA gene cloned into pAL99 | This study |
| pAL468 | Complete gctB gene cloned into pAL99 | This study |
| pAL470 | Complete hptC gene cloned into pAL99 | This study |
| pBluescript | E. coli cloning vector | Stratagene |
| pNF2176 | M. haemolytica/E. coli shuttle plasmid (Ampr) | 10 |
| pNF2176-MH1116 | Complete M. haemolytica gctA cloned into pNF2176 | This study |
| pUA826 | Mob+; R6K replicon; Apr Strr Spcr; single-crossover insertional mutagenesis vector | 6 |
| pUA826tpi | pUA826 derivative with a 240-bp PstI fragment containing the P. multocida tpiA promoter; nonpolar mutagenesis vector | This study |
DNA manipulations.
Restriction digestions and ligations were performed according to the manufacturers' instructions using enzymes obtained from NEB (Beverley, MA) or Roche Diagnostics GmbH (Mannheim, Germany). Plasmid DNA was prepared using alkaline lysis (3) and further purified using Qiagen columns (Qiagen GmbH, Germany), while genomic DNA was prepared using the cetyltrimethylammonium bromide method (2). PCR amplification of DNA was performed using Taq DNA polymerase or the Expand High Fidelity PCR System (Roche Diagnostics) and purified using the Qiagen PCR Purification Kit. The oligonucleotides used in this study are listed in Table S1 in the supplemental material. DNA sequences were determined on an Applied Biosystems 3730S Genetic Analyzer and analyzed with Sequencher version 3.1.1 (GenCodes, Ann Arbor, MI) or Vector NTI Advance version 10 (Invitrogen, Carlsbad, CA).
Construction of P. multocida mutants.
For inactivation of each of the candidate glycosyltransferase genes in P. multocida strain VP161, we used the previously described single-crossover insertional-mutagenesis method, which utilizes the λ pir-dependent plasmid pUA826 (Table 1). For inactivation of all genes except gatA, internal DNA fragments of each gene were amplified by PCR, using the primers listed in Table S1 in the supplemental material; digested with SalI; and ligated to SalI-digested pUA826. The correct recombinant plasmids (Table 1) were then mobilized into the recipient P. multocida strain AL435 (Table 1) by conjugation from E. coli Sm10 λ pir (Table 1) as described previously (12). Single-crossover insertion of the recombinant plasmid into each of the target genes was confirmed by PCR using a genomic flanking primer in combination with the primer BAP2782 located within the vector sequence (see Table S1 in the supplemental material). For inactivation of gatA, bioinformatics analysis of the region suggested that a single-crossover insertion within gatA may have polar effects on the downstream gene hptE. Therefore, the SalI-digested internal gatA fragment was ligated into the vector pUA826tpi (Table 1), which contains a constitutive P. multocida promoter. Insertion of this construct into the genome allowed transcription of the downstream hptE gene from the tpiA promoter carried within the plasmid.
Construction of an M. haemolytica gctA mutant.
An ortholog of the P. multocida gatA (PM1116) gene was identified in the M. haemolytica PHL213 genome using BLAST analysis (11). The protein encoded by M. haemolytica MHA_2524 displayed 74% amino acid identity to P. multocida GatA. The MHA_2524 gene was amplified by PCR from chromosomal DNA of the M. haemolytica strain O1A (Table 1) using the flanking primers BamHI-1F and EcoRI-1R (see Table S1 in the supplemental material), which amplified a 1,267-bp fragment. Following cloning into pBluescript (Table 1), inverse PCR was performed using the plasmid as a template and primers that correspond to sequences within the PCR product (Inv1R and Inv1F [see Table S1 in the supplemental material]). A kanamycin (aph3) resistance cassette (19) was ligated to the gel-purified inverse-PCR product to generate the plasmid pMHαglucosyltransferase::kan. This plasmid was transformed into M. haemolytica by electroporation as described previously (9), and transformants were selected on kanamycin. PCR was used to confirm the insertional inactivation of gatA in the mutant, designated AA100, using the primers 2F and 2R, specific to sequence flanking the gatA gene (see Table S1 in the supplemental material).
In trans complementation of mutants.
For complementation of the P. multocida mutants, the complete open reading frame corresponding to each of the mutated genes, with the exception of hptE, was amplified from P. multocida VP161 genomic DNA using flanking oligonucleotides (see Table S1 in the supplemental material). The amplified DNA fragments were then ligated into SalI- and BamHI-digested pAL99 (Table 1) so that transcription of the gene would be driven by the constitutive P. multocida tpiA promoter. For cloning of the hptE gene, an EcoRV/SmaI deletion plasmid was generated from a previously constructed pAL99-derived plasmid containing both gatA and hptE genes (the original fragment was amplified using oligonucleotides BAP3329 and BAP3330) so that only the hptE gene remained intact. The nucleotide sequence of each of the recombinant plasmids was determined to check the fidelity of the cloned gene, and then each plasmid was transformed into the corresponding P. multocida mutant, generating the complemented strains listed in Table 1. As a control, the vector pAL99 was transformed separately into each mutant (Table 1). The M. haemolytica gatA mutant was complemented by cloning the wild-type copy of gatA into the M. haemolytica shuttle plasmid pNF2176, generating plasmid pNF2176-MH1116 (Table 1). This plasmid was then used to transform the M. haemolytica gatA mutant, generating the complemented mutant AA101.
Structural analyses of LPS.
For analyses requiring small quantities of LPS, plate-grown cells were killed with phenol (2% [wt/vol] final), washed four times with H2O, lyophilized, resuspended in 200 μl of H2O containing 5 μg of proteinase K, and incubated at 37°C for 5 h. The sample was heated to 70°C for 10 min, lyophilized, dissolved in 200 μl of ammonium acetate buffer (20 mM, pH 7.4) containing 1 μg of RNase and 2 μg of DNase, incubated at 37°C for 5 h, and then lyophilized. The crude LPS-containing samples were O deacylated by dissolving them in 200 μl of anhydrous hydrazine and incubating them with stirring at 37°C for 1.5 h. Excess hydrazine was destroyed by the addition of 5 volumes of ice-cold acetone to the chilled samples, followed by repeated acetone washes. The O-deacylated LPS (LPS-OH) pellet was redissolved in H2O and lyophilized.
For analyses requiring larger quantities, LPS was isolated and purified as described previously (15). Briefly, freeze-dried killed cells were washed once with ethanol and then twice each with acetone and light petroleum ether to remove lipids and other lipophilic components. The washed cells were extracted by the hot-phenol/water method, and following dialysis, the retentate was freeze-dried. A 2% solution of this crude LPS was treated with DNase and RNase at 37°C for 4 h, followed by proteinase K treatment at 37°C for 4 h. Small peptides were removed by dialysis. After being freeze-dried, the retentate was made up to a 2% solution in water and centrifuged at 8,000 × g for 15 min, yielding a pellet (8K pellet), followed by further centrifugation of the supernatant at 100,000 × g for 5 h. The pellet from this high-speed centrifugation, containing purified LPS, was redissolved in water and freeze-dried. O-deacylated LPS and core oligosaccharide were prepared as described previously (30). Sugars were identified as their alditol acetate derivatives, and linkage analysis was determined following methylation analysis by gas-liquid chromatography-mass spectrometry (MS), as described previously (15). Electrospray-MS and nuclear magnetic resonance (NMR) analyses were performed as described previously (15).
Competitive-growth assays and assessment of virulence.
Competitive-growth assays were used to quantify the relative growth rates of the P. multocida strains in vivo as described previously (13). Mutants were identified as attenuated for in vivo growth if the relative competitive index (rCI) value was significantly less than 1.0 (P < 0.05 using a one-sided z test). Direct virulence trials in chickens were performed as described previously (12). To check the identities of strains recovered from infected chickens, muscle swabs and blood samples were plated on BHI agar and BHI agar containing spectinomycin. To confirm the genotypes of recovered strains, colony PCR was performed using a primer specific for DNA flanking the mutated gene and one specific for the plasmid pUA826. To determine the LPS phenotypes of the recovered strains, proteinase K-treated whole-cell lysate samples of pooled bacteria from each site were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by carbohydrate silver staining as described previously (32).
Antimicrobial peptide sensitivity assays.
The chicken antimicrobial peptide fowlicidin 1 (RVKRVWPLVIRTVIAGYNLYAIKKK) (34) was synthesized at 96% purity by Auspep (Parkville, Australia). The MICs of fowlicidin 1 for the P. multocida parent and mutant strains were determined using a broth microdilution assay. Briefly, 100 μl of fowlicidin 1 in 10 mM phosphate buffer, pH 7.4, was added to 100 μl of BHI containing approximately 1 × 105 CFU of exponential-phase P. multocida. This concentration of BHI and phosphate buffer was found to be optimal for both the overnight growth of P. multocida and the activity of the fowlicidin 1. The assay mixtures were incubated overnight at 37°C, and the absorbance was measured at 570 nm. The MIC was determined by averaging the concentration of fowlicidin 1 that completely inhibited bacterial growth and the next-lowest concentration that allowed some growth. Standard deviations were calculated assuming that the MIC for each assay was equally likely to lie at any value within this interval.
RESULTS
Bioinformatics analysis and nomenclature of LPS biosynthesis genes in P. multocida VP161.
The transferase genes involved in the assembly of the oligosaccharide extension of the P. multocida Pm70 LPS have been previously predicted based on homology to known transferases, using BLAST and the CAZy database of glycosyltransferases (Fig. 1A) (31). However, the functions of these putative transferases have not been confirmed experimentally. A comparison of the Pm70 and VP161 LPS structures (Fig. 1) allowed us to predict those glycosyltransferases likely to be conserved between the two strains. We showed previously that the Pm70 genes PM1138 to PM1141 are not present in VP161 and that the corresponding genomic region in strain VP161 contains the genes pcgABCD, encoding proteins required for the addition of PCho to LPS (14). This region also contained a gene that encoded a glycosyltransferase displaying 70% identity to Pm70 LosA (predicted to be a glucosyltransferase) and a gene encoding a protein with 94% identity to the Pm70 protein PM1144, predicted to be a putative heptosyl (IV) transferase. As predicted from the LPS structural data, which showed that both Pm70 and VP161 expressed LPSs with identical inner-core sugars, sequence analysis of the VP161 glycosyltransferase genes PM1306 and rfaF (Fig. 1) revealed that these genes were highly conserved between VP161 and Pm70. Using BLAST (1), we were unable to predict a glycosyltransferase involved in the addition of the α-1,6-linked glucose residue onto glycoform A (Fig. 1). However, using the CAZy database of glycosyltransferases, two candidate glycosyltransferase genes were identified in the Pm70 genome sequence (8). Each of these genes (PM1116 and PM1562) encoded glycosyltransferases with homologs in the genomes of the closely related veterinary pathogens M. haemolytica and Actinobacillus pleuropneumoniae, which express a similar LPS structure with an α-1,6-linked glucose residue (5, 28). Moreover, there were no homologues of either of these genes in Haemophilus influenzae, which does not contain an α-1,6-linked glucose in its LPS structure.
To standardize the nomenclature of the P. multocida enzymes involved in LPS biosynthesis, we have assigned gene and protein names that specifically imply enzyme function. We previously characterized two acceptor-specific heptosyltransferase genes (hptA and hptB) and a Kdo kinase gene (kdkA) and named each with the first two letters designating the sugar substrate and the third letter designating the enzyme action. Thus, heptosyltransferase genes were designated hpt and the Kdo kinase gene kdk (12). In this work, we have confirmed by directed mutagenesis and structural analysis (see below) the function of each of the transferases required for synthesis of the outer oligosaccharide extension. Accordingly, PM1306, encoding a β-1,4 glucosyltransferase, has been named gctB; PM1116, encoding an α-1,6 glucosyltransferase, has been named gctA; rfaF, encoding a heptosyltransferase responsible for the addition of the second heptose (Hep II), has been renamed hptC; the gene encoding the novel galactosyltransferase has been named gatA; and PM1144, encoding the heptosyltransferase required for the addition of the fourth heptose (Hep IV), has been named hptE. Following this new nomenclature, the heptosyltransferase required for the addition of the third heptose (Hep III) to the LPS, previously identified as WaaQPM (annotated as PM1294 in Pm70), has been renamed HptD (Fig. 1B) (15).
To confirm the function of the predicted transferases HptC, GctA, GctB, HptE, and GatA, the gene encoding each transferase was mutated in P. multocida strain VP161 by allelic exchange. The PM1562 gene was also inactivated in VP161, as this gene was a possible candidate to encode the α-1,6-linked glucosyltransferase. However, MS analysis showed that the mutant expressed wild-type LPS (data not shown), and it was not investigated further. Each of the constructed mutants was complemented with the appropriate full-length gene cloned into the P. multocida expression vector pAL99 (Table 1). The structure of the LPS expressed by each of the mutants and complemented strains was then determined by combined MS and NMR analyses.
Structural analysis of the LPS expressed by the gctB mutant.
Capillary electrophoresis-MS (CE-MS) analysis of the LPS-OH derived from the gctB mutant confirmed a set of truncated glycoforms that contained three heptose residues but elaborated only one hexose residue (Table 2). Similarly, MS analysis of the isolated core oligosaccharide identified only two glycoforms consisting of Kdo, 3Hep and Kdo, 3Hep, Hex. The majority of the Kdo-phosphate (P)-containing glycoforms (glycoform A) elaborated a single hexose residue, which was confirmed by methylation analysis to be the α-1,6-linked glucose. Methylation analysis revealed a terminal residue and a 2-substituted l-glycero-d-manno-heptose (LD heptose) residue in approximately equimolar amounts, with smaller amounts of terminal glucose, 3-substituted heptose, and 3,6-disubstituted heptose, consistent with the MS data. NMR data were obtained on a purified core oligosaccharide fraction (data not shown) and confirmed the MS and methylation data. The α-glucose residue was identified by its characteristic spin system in a total correlation spectroscopy experiment and was shown to substitute the first heptose residue at the 6 position in a nuclear Overhauser effect spectroscopy experiment. The characteristic inter-nuclear Overhauser effect connectivity between the anomeric protons of the two glucose residues substituting heptose I in the wild type was absent from this mutant, consistent with the absence of the β-glucose residue. Complementation with an intact copy of gctB restored wild-type LPS expression (see Table S2 in the supplemental material). Therefore, these data confirm the function of GctB as the β-1,4-glucosyltransferase responsible for transfer of a glucose residue to the 4 position of the proximal heptose residue.
TABLE 2.
Negative-ion CE-ES-MS data and proposed compositions of LPS-OH from the P. multocida VP161 and M. haemolytica O1A wild-type and mutant strains
| Strain and descriptiona | Observed ions (m/z)
|
Molecular ion
|
Proposed compositionb | |||
|---|---|---|---|---|---|---|
| (M-4H)4− | (M-3H)3− | (M-2H)2− | Observed | Calculated | ||
| AL435(parent) | 743.8 | 991.9 | 1,488.6 | 2,979.1 | 2,977.6 | 2PCho, 3Hex, 4Hep, 2Kdo, lipid A-OH |
| 749.4 | 999.2 | 1,499.4 | 3,001.0 | 2,999.5 | 2PCho, 4Hex, 4Hep, Kdo-P, lipid A-OH | |
| 780.2 | 1040.5 | 3,124.6 | 3,122.6 | 2PCho, 4Hex, 4Hep, Kdo-P-PEtn, lipid A-OH | ||
| AL696 (hptC mutant) | 463.8 | 1,392.4 | 2Kdo, lipid A-OH | |||
| 481.1 | 721.9 | 1,445.8 | 1,444.3 | Hep, Kdo-P, lipid A-OH | ||
| 803.0 | 1,608.0 | 1,606.5 | Hex, Hep, Kdo-P, lipid A-OH | |||
| 864.1 | 1,731.0 | 1,729.5 | Hex, Hep, Kdo-P-PEtn, lipid A-OH | |||
| AL554 (gctB mutant) | 608.8 | 913.8 | 1,829.5 | 1,828.6 | 3Hep, Kdo-P, lipid A-OH | |
| 649.7 | 975.1 | 1,952.2 | 1,951.7 | 3Hep, Kdo-P-PEtn, lipid A-OH | ||
| 655.6 | 983.9 | 1,969.8 | 1,968.8 | 3Hep, 2Kdo, lipid A-OH | ||
| 663.1 | 994.9 | 1,992.0 | 1,990.8 | Hex, 3Hep, Kdo-P, lipid A-OH | ||
| 704.1 | 1,056.3 | 2,114.9 | 2,113.8 | Hex, 3Hep, Kdo-P-PEtn, lipid A-OH | ||
| AL574 (gctA mutant) | 708.8 | 945.0 | 1,418.1 | 2,838.1 | 2,837.4 | 2PCho, 3Hex, 4Hep, Kdo-P, lipid A-OH |
| 739.5 | 986.3 | 1,479.6 | 2,961.7 | 2,960.4 | 2PCho, 3Hex, 4Hep, Kdo-P-PEtn, lipid A-OH | |
| 743.9 | 992.0 | 1,488.6 | 2,979.3 | 2,977.6 | 2PCho, 3Hex, 4Hep, 2Kdo, lipid A-OH | |
| AL523 (hptE mutant) | - | 709.8 | 1,064.8 | 2,132.4 | 2,131.0 | Hex, 3Hep, 2Kdo, lipid A-OH |
| 537.3 | 716.8 | 1,075.9 | 2,153.9 | 2,154.0 | 2Hex, 3Hep, Kdo-P, lipid A-OH | |
| 568.2 | 758.1 | 1,137.7 | 2,276.8 | 2,277.0 | 2Hex, 3Hep, Kdo-P, PEtn, lipid A-OH | |
| - | 953.3 | - | 2,862.9 | 2,861.7 | 2HexNAc, 3Hex, 3Hep, 2Kdo, lipid A-OH | |
| AL725 (gatA mutant) | 773.8 | 1,161.0 | 2,324.2 | 2,323.1 | Hex, 4Hep, 2Kdo, lipid A-OH | |
| 781.1 | 1,172.0 | 2,346.1 | 2,345.1 | 2Hex, 4Hep, Kdo-P, lipid A-OH | ||
| 821.6 | 1,233.8 | 2,468.7 | 2,468.2 | 2Hex, 4Hep, Kdo-P-PEtn, lipid A-OH | ||
| M. haemolytica O1A (wild type) | 704.7 | 940.1 | 1,410.6 | 2,823.1 | 2,822.5 | 3Hex, 5Hep, Kdo-P-PEtn, lipid A-OH |
| M. haemolytica AA100 (gctA mutant) | 633.5 | 844.9 | 1,268.0 | 2,537.9 | 2,537.3 | 2Hex, 5Hep, Kdo-P, lipid A-OH |
| 664.1 | 886.0 | 1,329.4 | 2,660.8 | 2,660.3 | 2Hex, 5Hep, Kdo-P-PEtn, lipid A-OH | |
All mutants plus vector controls gave the same spectra as the corresponding mutants, and all complemented mutants gave the same spectra as the AL435 parent. The LPS structural data for these strains have been omitted but are shown in full in Table S2 in the supplemental material.
Average mass units were used for calculation of molecular weight based on proposed compositions as follows: lipid A, 952.00; Hex, 162.15; Hep, 192.17; Kdo, 220.18; Kdo-P, 300.13; PEtn (phosphoethanolamine), 123.05; PCho, 165.05.
Structural analysis of the LPS expressed by the hptC mutant.
CE-MS analysis of the LPS-OH isolated from the hptC mutant confirmed the presence of a set of highly truncated glycoforms (Table 2). Similarly, MS analysis of the isolated core oligosaccharide identified a major glycoform Kdo-Hep with lesser amounts of a Kdo-Hep-Hex glycoform. No LPS glycoforms were identified that contained more than a single heptose. This is consistent with HptC being the transferase required for the addition of the branched heptose II to heptose I. There was also an increase in the amount of the 2Kdo-lipid A glycoform expressed by this mutant, which we attribute to a polar effect of the mutation on the downstream gene encoding HptA (the heptosyl-I-transferase required for glycoform A assembly) (12). Interestingly, although the first heptose residue was added to the Kdo-P inner-core glycoform (glycoform A), only small amounts of a single hexose were able to be added to this acceptor molecule, and there was no oligosaccharide extension beyond this point. This single hexose was confirmed by methylation analysis to be the α-1,6-linked glucose. Thus, there is no addition of the β-1,4-glucose residue in this mutant and no further assembly of the main chain oligosaccharide, indicating that GctB is highly dependent on the correct conformation of the acceptor molecule. When the hptC mutant was complemented with intact hptC, wild-type LPS expression was restored (see Table S2 in the supplemental material). Therefore, these data confirm that HptC is the heptosyl II transferase responsible for addition of the second heptose to the first heptose residue of both glycoforms A and B.
Structural analysis of the LPS expressed by P. multocida and M. haemolytica gctA mutants.
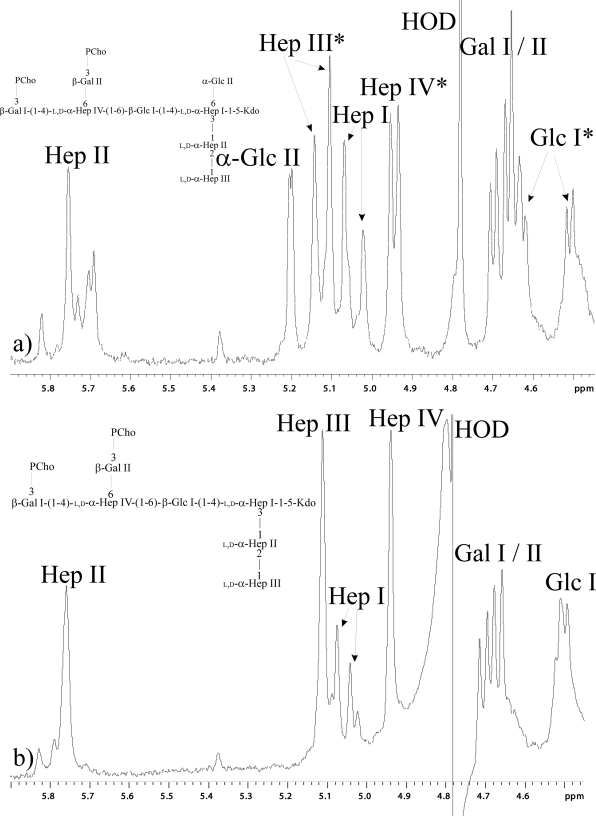
CE-MS analysis of LPS-OH derived from the P. multocida gctA mutant strain revealed a fully extended glycoform B, but glycoform A was 162 atomic mass units smaller than that expressed by the parent, consistent with the absence of a single hexose residue (see Fig. S1 and Table S2 in the supplemental material). Methylation analysis of the LPS expressed by the mutant revealed the appearance of a 3,4-disubstituted heptose residue with the concomitant loss of the 3,4,6-trisubstituted heptose residue, confirming and extending the MS data. Finally, NMR analysis of the purified core oligosaccharide revealed that signals diagnostic for the α-glucose residue were absent. Examination of the anomeric region of the 1H-NMR spectrum (Fig. 2) clearly revealed that the signal diagnostic for the anomeric proton of the α-glucose residue at 5.21 ppm in the parent spectrum (Fig. 2a) was absent in the gctA mutant spectrum (Fig. 2b). Signals in the parent spectrum that are due to the variable presence of the α-glucose residue (present in glycoform A but not glycoform B) affecting their chemical shifts in the parent are shown in Fig. 2a. Therefore, in the gctA mutant spectrum, there is only one signal for each of these residues, as the heterogeneous α-glucose residue of the parent is now completely absent. Complementation in trans restored the wild-type LPS glycoform (see Table S2 in the supplemental material). Therefore, these data confirmed that P. multocida GctA is an α-1,6 glucosyltransferase.
FIG. 2.
Anomeric regions of the 1H-NMR spectra of core oligosaccharide from the VP161 wild-type parent strain (a) and the gctA mutant AL574 (b). The anomeric resonances are as indicated. The asterisks refer to heterogeneity in the parent strain caused by the variable presence of the α-glucose residue, which affects chemical shifts of the marked anomeric resonances and which is absent in the mutant strain, where the α-glucose residue is absent. The insets show the structures for the wild-type and mutant strains. The spectra were recorded in D2O at 25°C and referenced to the deuterated water signal at 4.78 ppm.
The identification of this novel α-1,6-linked glucosyltransferase was corroborated in the related veterinary pathogen M. haemolytica (formerly Pasteurella haemolytica), which also expresses LPS with a similar α-1,6-linked glucose residue on the first heptose. We inactivated the homologous gene (MHA_2524), in the M. haemolytica strain O1A to generate the gctA mutant AA100. As was observed in P. multocida, the M. haemolytica gctA mutant also expressed LPS that was 162 atomic mass units smaller than the LPS expressed by the parent strain, consistent with the absence of a single hexose residue (Table 2). This hexose was confirmed by methylation and NMR analysis to be the α-1,6-linked glucose (data not shown). Wild-type LPS expression was restored when the M. haemolytica gctA mutant was complemented in trans with a functional copy of gctA (see Table S2 in the supplemental material). Therefore, these data confirm the function of GctA as the α-glucosyltransferase responsible for transfer of a glucose residue to the 6 position of the proximal heptose residue in LPS expressed by both P. multocida and M. haemolytica.
Structural analysis of the LPS expressed by the hptE mutant.
CE-MS analysis of the LPS-OH derived from the hptE mutant strain revealed ions consistent with a set of truncated LPS molecules lacking the terminal (PCho-Gal)2-Hep IV moiety (Table 2). Methylation analysis of the mutant strain revealed the presence of terminal glucose, terminal LD-heptose, 2-subsituted heptose, 3,4-disubstituted LD-heptose, and 3,4,6-trisubstituted LD-heptose residues in an approximate ratio of 4:2:2:1:1, confirming and extending the MS data. Complementation of the hptE mutant restored the production of wild-type LPS (see Table S2 in the supplemental material). These data confirmed the function of HptE as the heptosyl (IV) transferase.
Interestingly, the methylation analysis also revealed lesser amounts (approximately 15%) of a 4-substituted glucose residue, along with 3-substituted and terminal galactose residues and 3- and 4-substituted N-acetyl-glucosamine residues. Examination of the MS data revealed small amounts of ions consistent with the presence of a novel P. multocida LPS glycoform consisting of lipid A-OH, 2Kdo, 3Hep, 3Hex, 2HexNAc from an LPS-OH sample and Kdo, 3Hep, 3Hex, 2HexNAc from a core oligosaccharide sample. These data indicate that in the hptE mutant, approximately 15% of the LPS molecules contain a novel LPS extension from the β-d-glucose comprising β-Gal-(1-4)-β-GlcNAc-(1-3)-β-Gal-(1-3)-β-GlcNAc-(1-4) (Fig. 1C).
Structural analysis of the LPS expressed by the gatA mutant.
CE-MS analysis of the LPS-OH derived from the gatA mutant revealed ions consistent with the loss of the two terminal PCho residues and two galactose residues (Table 2). Methylation analysis indicated the presence of terminal glucose, 6-substituted glucose, terminal LD-heptose, 3,4-disubstituted heptose, and 3,4,6-trisubstituted heptose residues, consistent with the MS data. Thus, both the 1,4- and 1,6-linked galactose sugars are absent from the LPS expressed by the gatA mutant. Complementation with a functional gatA gene restored expression of the wild-type LPS (see Table S2 in the supplemental material).
Virulence of the P. multocida mutants expressing modified LPS.
All mutants were shown to grow normally in vitro, indicating that the LPS truncations did not affect cell viability or the growth rate. The ability of each of the mutants to grow in vivo was assessed using competitive-growth assays. All mutants except the gctA mutant (AL574) were strongly attenuated for growth in chickens following intramuscular injection (Table 3).
TABLE 3.
Relative in vivo growth rate (rCI), as determined by competitive-growth assays, and virulence, as determined by direct challenge, of parent and mutant strains
| Strain | Competitive-growth assay rCIa | Direct virulence challenge
|
||||
|---|---|---|---|---|---|---|
| Dose (CFU) | Deathsb | Time to death (h) | Bacteria recovered
|
|||
| Muscle | Blood | |||||
| AL696 (hptC) | 0.0 | 60 | 0/7c | NAd | NDe | NDf |
| 0.0 | ||||||
| AL554 (gctB) | 0.0 | 90 | 7/7 | >66f | WT-rg and AL554 | WT-r and trace amounts of AL554 |
| 0.0 | ||||||
| AL574 (gctA) | 1.19 | 50 | 7/7 | 24 ± 3 | AL574 | AL574 |
| 1.16 | ||||||
| AL523 (hptE) | 0.0 | 110 | 7/7 | >66f | AL523 | WT-r and AL523 |
| 0.09 | ||||||
| AL725 (gatA) | 0.0 | 50 | 7/7 | >66f | AL725 | WT-r and trace amounts of AL725 |
| 0.0 | ||||||
| AL435 (parent) | 0.88 | 60 | 7/7 | 28 ± 6 | AL435 | AL435 |
| 1.33 | ||||||
| 1.29 | ||||||
| 0.83 | ||||||
The values represent the rCIs calculated for individual animals, determined by dividing the in vivo competitive index (CI) by the in vitro CI. Each CI value was determined by dividing the output mutant/wild-type ratio by the input mutant/wild-type ratio. All rCI values under 0.1 have a P value of < 0.001 as calculated using a one-tailed z test. All other values have P values of >0.05.
All birds showing terminal signs of fowl cholera were euthanized in accordance with animal ethics requirements.
The survival of chickens infected with AL696 was statistically different from that of all other groups (Fisher's exact test; P < 0.001).
NA, not applicable.
ND, not done.
Some of the birds in this group displayed only early signs of fowl cholera infection at the designated end of the experiment. Therefore, an accurate time to death cannot be calculated.
WT-r, wild-type revertant.
The mutants were then assessed by direct challenge for the ability to cause fowl cholera in chickens. Each P. multocida strain was injected into the pectoral muscles of chickens, and each bird was monitored at regular intervals for signs of disease (Table 3). All birds injected with the parent strain AL435 showed signs of disease within 16 h and were euthanized by 36 h (average time to death, 28 ± 6 h). Similarly, all birds injected with the gctA mutant were euthanized by 28 h (average time to death, 24 ± 3 h), demonstrating that expression of GctA is not required for P. multocida to cause fowl cholera. None of the birds injected with the hptC mutant showed any clinical signs over the 66 h of the experiment, and no bacteria were recovered from the blood of these birds. Thus, mutants expressing LPS truncated at the first heptose residue are avirulent. However, all of the birds injected with the gctB, hptE, and gatA mutants eventually showed clinical signs of fowl cholera, with the first signs appearing at approximately 40 h (Table 3). Both blood and muscle (injection site) samples were recovered from a minimum of four birds within each group at the time of euthanasia. Within these groups, the mutant strain could be recovered from all muscle samples, and the majority of birds examined at necropsy had visible muscle lesions at the site of injection. Therefore, the gctB, hptE, and gatA mutants can all survive and grow within the muscles of infected chickens for at least 66 h.
Blood samples were taken from chickens injected with either the gctB or gatA mutant at the time of euthanasia and plated out onto both nonselective and selective media. Only very small numbers of spectinomycin-resistant P. multocida bacteria were recovered from some birds within each group, but in all cases, spectinomycin-sensitive P. multocida could be recovered, suggesting the presence of wild-type revertants. Similarly, SDS-PAGE analysis of proteinase K-treated whole-cell lysates of the pooled bacteria recovered from the blood showed only the presence of wild-type LPS (data not shown). Finally, PCR analysis of bacteria isolated from the blood of these birds detected only wild-type P. multocida (data not shown). Taken together, these data indicate that, although trace levels of the gctB and gatA mutants were detected in the blood, the primary causative agent of disease observed in birds within these groups was wild-type revertant bacteria.
Blood samples were taken from chickens infected with the hptE mutant and plated onto nonselective and selective agar. Both spectinomycin-resistant and spectinomycin-sensitive bacteria were identified. PCR and SDS-PAGE analysis revealed that both mutant and wild-type bacteria were present in approximately equal numbers (data not shown). Taken together, these data indicate that the gctB, hptE, and gatA mutants can survive and grow in muscle for long periods. Furthermore, the hptE mutant was able to grow to a high level in the blood of chickens, but the gctB and gatA mutants were highly attenuated for growth in blood.
Sensitivities of LPS mutants to the antimicrobial peptide fowlicidin 1.
Antimicrobial peptides are a critical component of the innate immune system of higher organisms (18). A number of chicken antimicrobial peptides have been characterized, including members of the β-defensin (17) and cathelicidin (34) families. A critical first step in the action of cationic antimicrobial peptides is the interaction of the peptide with LPS; alteration of LPS structure can therefore affect susceptibility to antimicrobial peptides (21, 25). We have shown recently that the terminal PCho residues on the P. multocida LPS contribute to resistance to fowlicidin 1 (14). Accordingly, we determined the sensitivity of the P. multocida parent strain AL435 and each of the mutants constructed in this study to the bactericidal action of fowlicidin 1 (Fig. 3).
FIG. 3.
Sensitivities of P. multocida strains to killing by the action of fowlicidin 1. The MIC of fowlicidin 1 was determined by growth of each strain in 50% BHI containing increasing concentrations of fowlicidin 1. The strains tested were the parent (AL435), PM0442 mutant (strain AL669 with an insertion in a gene unrelated to LPS biosynthesis), pcgC mutant (PCho− strain [14]), gatA mutant (AL725), hptE mutant (AL523), gctB mutant (AL554), gctA mutant (AL574), and hptC mutant (AL696). The MIC was determined by averaging the concentration of fowlicidin 1 that completely inhibited bacterial growth and the next-lowest concentration that allowed some growth. Standard deviations were calculated assuming that the MIC for each assay was equally likely to lie at any value within this interval. The reported values are the MICs for three replicates, and the error bars show ±1 standard deviation.
All of the mutants expressing truncated LPS were more sensitive to the bactericidal action of fowlicidin 1, although the difference for the gctA mutant was slight. The hptC mutant was the most sensitive strain, while the hptE, gctB, and gatA mutants showed intermediate levels of sensitivity, comparable to that of a mutant (pcgC) lacking the terminal PCho substituents (Fig. 3) (14). These data indicate that the terminal PCho residues are important for maximal resistance, while the other main-chain oligosaccharides have a minor effect on sensitivity to fowlicidin 1. However, loss of the heptose side chain significantly increased susceptibility.
DISCUSSION
The structures of the P. multocida strain VP161 (Heddleston LPS type 1) and Pm70 (Heddleston LPS type 3) LPS molecules were determined previously (30, 31). We predicted the genes required for synthesis of the VP161 LPS and inactivated each of the genes in VP161. Each of the mutants expressed modified LPS, with clear differences observed in the ratios and types of sugars present.
The most subtle difference was observed in the LPS expressed by the gctA mutant (AL574), which differed from wild-type LPS only in the expression of a glycoform A lacking the α-d-glucose. Directed mutagenesis of the homologous gene (MHA_2524) in M. haemolytica and structural analysis of the LPS expressed by the mutant also confirmed GctA as the α-1,6 glucosyltransferase in this species. The presence of the α-d-glucose on the first heptose of the LPS is only observed in veterinary pathogens within the Pasteurellaceae, including P. multocida, M. haemolytica, Haemophilus parasuis, and A. pleuropneumoniae (28), and is not observed in human pathogens of the family, such as H. influenzae. The reason for this specificity is currently unclear. The P. multocida gctA mutant grew as well as the wild type in our fowl cholera model, but the possibility remains that this sugar is required for survival in some other as-yet-undefined niche.
The hptC mutant expressed the most highly truncated LPS, with a structure not extended beyond HepI except for addition of the α-1,6 glucose on glycoform A (Fig. 1 and Table 2). Structural analysis indicated that this severe truncation was due to the failure of another transferase, GctB, to add glucose to the main-chain oligosaccharide. Previously, we showed that inactivation of HptD (Fig. 1) also affected GctB activity, resulting in approximately 90% of the LPS lacking extension beyond the first heptose residue (15). Therefore, GctB can only function efficiently when the triheptose side chain is intact, confirming the critical reliance of this transferase on the correct conformation of the acceptor molecule.
HptE was identified as the heptosyltransferase required for the addition of the fourth heptose to glycoforms A and B. Interestingly, this mutant expressed a small amount of a novel LPS glycoform (glycoform C) that differed from glycoforms A and B in the outer-core region. In many wild-type strains of Neisseria spp. and H. influenzae, LPS glycoforms have been detected that contain the same terminal trisaccharide, β-Gal-(1-4)-β-GlcNAc-(1-3)-β-Gal, as that observed in glycoform C, which mimics paragloboside and other glycosphingolipids expressed in mammalian tissues (22, 23). The glycosyltransferases required for the assembly of this trisaccharide in H. influenzae were identified when genes from the lsg locus were expressed in E. coli (24). A search of the P. multocida Pm70 genome sequence identified five genes within a single locus (PM0507 to -0513) that shared significant identity with genes within the H. influenzae lsg locus. The H. influenzae LsgA, LsgB, LsgD, LsgE, and LsgF proteins showed identity to PM0507 (82%), PM0508 (59%), PM0509 (79%), PM0511 (76%), and PM0512 (88%), respectively. The genes encoding these proteins are also present in P. multocida strain VP161, and based on homology with known transferases, we predict that PM509, PM0511, and PM0512 are the glycosyltransferases required for assembly of the terminal trisaccharide in glycoform C (Fig. 1C). We were unable to show definitively the involvement of the PM507-PM513 locus in the expression of glycoform C in the hptE mutant, as it is currently not possible to make multiple directed mutants in P. multocida.
The glycosyltransferase responsible for addition of the galactose residues onto glycoforms A and B was identified as GatA. Inactivation of gatA resulted in a mutant strain that expressed LPS lacking both the terminal galactose sugars and PCho residues. Therefore, either GatA is a galactosyltransferase that is capable of adding both galactose residues to the terminal (IV) heptose or, alternatively, GatA is responsible for addition of one of the galactose residues and failure to add this sugar precludes the action of an as-yet-unidentified galactosyltransferase encoded elsewhere on the VP161 genome. In the sequenced P. multocida Pm70 strain, a similar addition to the LPS structure is predicted, except that two glucose residues are added to the fourth heptose. However, the LPS locus in Pm70 encodes only one glucosyltransferase, LosA, and a search of the entire genomic sequence failed to identify a second glucosyltransferase predicted to have the specificity required for this addition. Therefore, we predict that both LosA and GatA are bifunctional transferases, capable of adding galactose (GatA) or glucose (LosA) to both the 4 and 6 positions of the distal heptose residue on the LPS structure.
Mutants expressing altered LPS were tested both for virulence in chickens and resistance to fowlicidin 1. All mutants, with the exception of gctA, were severely attenuated for in vivo growth. The hptC mutant, which expressed the most highly truncated LPS, caused no disease in birds at the dose tested and was highly sensitive to the action of fowlicidin 1, indicating that the heptose side chain is critical for the ability to cause disease and resistance to fowlicidin 1. As the triheptose residues are uncharged, we predict that they form a steric barrier against the initial interaction of fowlicidin 1 with lipid A.
The gctB and gatA mutants were highly attenuated for in vivo growth, as determined by competitive-growth assays, and although birds directly challenged with the gctB or gatA mutant developed fowl cholera late in the trial, the data indicate that the systemic infection was caused by wild-type revertant P. multocida. Interestingly, gctB and gatA mutants could survive for long periods (several days) in the muscle but were unable to persist in the blood. While the single-crossover insertional mutants constructed in this work have a very low level of instability in vitro (and are completely stable in the presence of antibiotic selection), the ability of these mutants to survive and multiply in the muscles of birds in the absence of antibiotic selection for many days allows wild-type revertants to arise. Once these revertants are generated, they have a significant selective advantage in vivo and can enter the bloodstream, multiply rapidly, and cause systemic disease. Similar instability leading to delayed disease has been observed previously with a hptD (WaaQPM) transposon mutant (15). While it would have been of interest to test the virulence of more stable double-crossover mutants within each of these genes, we have been unable to construct these mutants. Indeed, there are no published reports of double-crossover mutants in this P. multocida strain.
We have shown previously that the terminal PCho residues on P. multocida LPS are important for maximal resistance to the chicken antimicrobial peptide fowlicidin 1, and it is likely that the positive charge on PCho is critical in repelling the cationic fowlicidin 1 (14). The gctB and gatA mutants were slightly more susceptible to the action of fowlicidin 1 than mutants lacking just the terminal PCho residues, suggesting that these sugars in the main-chain oligosaccharide extension contribute marginally to antimicrobial peptide resistance.
All birds infected with the hptE mutant showed signs of fowl cholera, but again, at later times than birds infected with the parent strain. However, birds infected with the hptE mutant had significant amounts of both mutant and wild-type revertant P. multocida in the bloodstream. While we cannot completely exclude the possibility that the survival of the hptE mutant was assisted by the presence of wild-type bacteria, we believe this is unlikely. A number of different LPS mutants were tested in chickens, and in all cases wild-type revertants were present, yet not all mutants were able to survive in the blood of infected birds. This finding suggests that the hptE mutant has a unique component that contributes to its survival in the host, and we propose that it is the expression of glycoform C LPS.
In conclusion, we have identified all of the transferases required for synthesis of the main outer-core oligosaccharide extension of the P. multocida strain VP161 LPS. We have identified a novel α-1,6 glycosyltransferase (GctA) and a predicted bifunctional galactose transferase (GatA). The hptC mutant was completely attenuated and significantly more susceptible to fowlicidin 1 than any of the other mutants tested, indicating that the heptose side chain is critical for survival in the host. All mutants expressing a truncated main oligosaccharide extension (gctB, hptE, and gatA) could survive in the muscle for long periods but were more susceptible than the wild-type strain to the action of fowlicidin 1. While the gctB and gatA mutants displayed significantly reduced growth within the blood, the hptE mutant, which expresses the novel glycoform C, was able to survive and multiply within the blood of chickens until late in infection and may be capable of causing disease. It is interesting to speculate that glycoform C may be critical in some as-yet-undefined host environments; we are currently investigating this possibility.
Supplementary Material
Acknowledgments
We thank Perry Fleming for growing bacteria, Jacek Stupak for CE-MS, Paola Vaz for construction of the gctB mutant, Jason Steen for assistance with the animal experiments, and Paul Harrison for statistical calculations and advice.
This work was funded by grants from the Australian Research Council, Canberra, Australia.
Editor: F. C. Fang
Footnotes
Published ahead of print on 21 January 2009.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped Blast and Psi-Blast - a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. Wiley Interscience, New York, NY.
- 3.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 71513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce, J. D., R. Y. C. Lo, I. Wilkie, and B. Adler. 2004. Pasteurella and Mannheimia, p. 385-396. In C. Gyles, C. Thoen, J. Prescott, and G. Songer (ed.), Pathogenesis of bacterial infections of animals. Blackwell Publishing, Ames, IA.
- 5.Brisson, J.-R., E. Crawford, D. Uhrin, N. H. Khieu, M. B. Perry, W. B. Severn, and J. C. Richards. 2002. The core oligosaccharide component from Mannheimia (Pasteurella) haemolytica serotype A1 lipopolysaccharide contains l-glycero-d-manno-and d-glycero-d-manno-heptoses: analysis of the structure and conformation by high-resolution NMR spectroscopy. Can. J. Chem. 80949. [Google Scholar]
- 6.Cardenas, M., A. R. F. de Henestrosa, S. Campoy, A. M. P. de Rozas, J. Barbe, I. Badiola, and M. Llagostera. 2001. Virulence of Pasteurella multocida recA mutants. Vet. Microbiol. 8053-61. [DOI] [PubMed] [Google Scholar]
- 7.Carter, G. R. 1967. Pasteurellosis: Pasteurella multocida and Pasteurella hemolytica. Adv. Vet. Sci. 11321-379. [PubMed] [Google Scholar]
- 8.Coutinho, P. M., and B. Henrissat. 1999. Carbohydrate-active enzymes: an integrated database approach, p. 3-12. In H. J. Gilbert, G. Davies, B. Henrissat, and B. Svensson (ed.), Recent advances in carbohydrate bioengineering. Royal Society of Chemistry, Cambridge, United Kingdom.
- 9.Craig, F. F., J. G. Coote, R. Parton, J. H. Freer, and N. J. Gilmour. 1989. A plasmid which can be transferred between Escherichia coli and Pasteurella haemolytica by electroporation and conjugation. J. Gen. Microbiol. 1352885-2890. [DOI] [PubMed] [Google Scholar]
- 10.Fedorova, N. D., and S. K. Highlander. 1997. Plasmids for heterologous expression in Pasteurella haemolytica. Gene 186207-211. [DOI] [PubMed] [Google Scholar]
- 11.Gioia, J., X. Qin, H. Jiang, K. Clinkenbeard, R. Lo, Y. Liu, G. E. Fox, S. Yerrapragada, M. P. McLeod, T. Z. McNeill, L. Hemphill, E. Sodergren, Q. Wang, D. M. Muzny, F. J. Homsi, G. M. Weinstock, and S. K. Highlander. 2006. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. J. Bacteriol. 1887257-7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harper, M., J. D. Boyce, A. D. Cox, F. St Michael, I. Wilkie, P. Blackall, and B. Adler. 2007. Pasteurella multocida expresses two LPS glycoforms simultaneously both in vitro and in vivo but only a single form is required for virulence: identification of two acceptor specific heptosyl I transferases. Infect. Immun. 753885-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harper, M., J. D. Boyce, I. Wilkie, and B. Adler. 2003. Signature-tagged mutagenesis of Pasteurella multocida identifies mutants displaying differential virulence characteristics in mice and chickens. Infect. Immun. 715440-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harper, M., A. D. Cox, F. St Michael, H. Parnas, I. Wilkie, P. J. Blackall, B. Adler, and J. D. Boyce. 2007. Decoration of Pasteurella multocida LPS with phosphocholine is important for virulence. J. Bacteriol. 1897384-7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper, M., A. D. Cox, F. St Michael, I. W. Wilkie, J. D. Boyce, and B. Adler. 2004. A heptosyltransferase mutant of Pasteurella multocida produces a truncated lipopolysaccharide structure and is attenuated in virulence. Infect. Immun. 723436-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heddleston, K. L., J. E. Gallagher, and P. A. Rebers. 1972. Fowl cholera: gel diffusion precipitin test for serotyping Pasteurella multocida from avian species. Avian Dis. 16925-936. [PubMed] [Google Scholar]
- 17.Higgs, R., D. J. Lynn, S. Gaines, J. McMahon, J. Tierney, T. James, A. T. Lloyd, G. Mulcahy, and C. O'Farrelly. 2005. The synthetic form of a novel chicken beta-defensin identified in silico is predominantly active against intestinal pathogens. Immunogenetics 5790-98. [DOI] [PubMed] [Google Scholar]
- 18.Jenssen, H., P. Hamill, and R. E. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 1701704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan, S. M., W. Chen, A. Aubry, M. A. Gidney, S. Lacelle, F. St Michael, R. Kuolee, M. Higgins, S. Neufeld, and A. D. Cox. 2006. Production of a d-glycero-d-manno-heptosyltransferase mutant of Mannheimia haemolytica displaying a veterinary pathogen specific conserved LPS structure; development and functionality of antibodies to this LPS structure. Vet. Microbiol. 116175-186. [DOI] [PubMed] [Google Scholar]
- 21.Loutet, S. A., R. S. Flannagan, C. Kooi, P. A. Sokol, and M. A. Valvano. 2006. A complete lipopolysaccharide inner core oligosaccharide is required for resistance of Burkholderia cenocepacia to antimicrobial peptides and bacterial survival in vivo. J. Bacteriol. 1882073-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandrell, R. E. 1992. Further antigenic similarities of Neisseria gonorrhoeae lipooligosaccharides and human glycosphingolipids. Infect. Immun. 603017-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandrell, R. E., J. M. Griffiss, and B. A. Macher. 1988. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J. Exp. Med. 168107-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips, N. J., T. J. Miller, J. J. Engstrom, W. Melaugh, R. McLaughlin, M. A. Apicella, and B. W. Gibson. 2000. Characterization of chimeric lipopolysaccharides from Escherichia coli strain JM109 transformed with lipooligosaccharide synthesis genes (lsg) from Haemophilus influenzae. J. Biol. Chem. 2754747-4758. [DOI] [PubMed] [Google Scholar]
- 25.Ramjeet, M., V. Deslandes, F. St Michael, A. D. Cox, M. Kobisch, M. Gottschalk, and M. Jacques. 2005. Truncation of the lipopolysaccharide outer core affects susceptibility to antimicrobial peptides and virulence of Actinobacillus pleuropneumoniae serotype 1. J. Biol. Chem. 28039104-39114. [DOI] [PubMed] [Google Scholar]
- 26.Rimler, R. B. 1987. Cross-protection factor(s) of Pasteurella multocida: passive immunization of turkeys against fowl cholera caused by different serotypes. Avian Dis. 31884-887. [PubMed] [Google Scholar]
- 27.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 28.St. Michael, F., J. R. Brisson, S. Larocque, M. A. Monteiro, J. Li, M. Jacques, M. B. Perry, and A. D. Cox. 2004. Structural analysis of the lipopolysaccharide derived core oligosaccharides of Actinobacillus pleuropneumoniae serotypes 1, 2, 5a and the genome strain 5b. Carbohydr. Res. 3391973-1984. [DOI] [PubMed] [Google Scholar]
- 29.St. Michael, F., J. Li, and A. D. Cox. 2005. Structural analysis of the core oligosaccharide from Pasteurella multocida strain X73. Carbohydr. Res. 3401253-1257. [DOI] [PubMed] [Google Scholar]
- 30.St. Michael, F., J. Li, E. Vinogradov, S. Larocque, M. Harper, and A. D. Cox. 2005. Structural analysis of the lipopolysaccharide of Pasteurella multocida strain VP161: identification of both Kdo-P and Kdo-Kdo species in the lipopolysaccharide. Carbohydr. Res. 34059-68. [DOI] [PubMed] [Google Scholar]
- 31.St. Michael, F., E. Vinogradov, J. Li, and A. D. Cox. 2005. Structural analysis of the lipopolysaccharide from Pasteurella multocida genome strain Pm70 and identification of the putative lipopolysaccharide glycosyltransferases. Glycobiology 15323-333. [DOI] [PubMed] [Google Scholar]
- 32.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119115-119. [DOI] [PubMed] [Google Scholar]
- 33.Wilkie, I. W., S. E. Grimes, D. O'Boyle, and A. J. Frost. 2000. The virulence and protective efficacy for chickens of Pasteurella multocida administered by different routes. Vet. Microbiol. 7257-68. [DOI] [PubMed] [Google Scholar]
- 34.Xiao, Y., Y. Cai, Y. R. Bommineni, S. C. Fernando, O. Prakash, S. E. Gilliland, and G. Zhang. 2006. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 2812858-2867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.