Abstract
It was previously believed that obesity and osteoporosis were two unrelated diseases, but recent studies have shown that both diseases share several common genetic and environmental factors. Body fat mass, a component of body weight, is one of the most important indices of obesity, and a substantial body of evidence indicates that fat mass may have beneficial effects on bone. Contrasting studies, however, suggest that excessive fat mass may not protect against osteoporosis or osteoporotic fracture. Differences in experimental design, sample structure, and even the selection of covariates may account for some of these inconsistent or contradictory results. Despite the lack of a clear consensus regarding the impact of effects of fat on bone, a number of mechanistic explanations have been proposed to support the observed epidemiologic and physiologic associations between fat and bone. The common precursor stem cell that leads to the differentiation of both adipocytes and osteoblasts, as well the secretion of adipocyte-derived hormones that affect bone development, may partially explain these associations. Based on our current state of knowledge, it is unclear whether fat has beneficial effects on bone. We anticipate that this will be an active and fruitful focus of research in the coming years.
Key words: obesity, osteoporosis, fat mass, correlation
INTRODUCTION
Obesity is a state of excess storage of body fat resulting from a chronic imbalance between energy intake and energy expenditure.(1) About 250 million adults worldwide are considered obese (body mass index [BMI] ≥ 30 kg/m2],(2,3) and recent data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES)(4) showed that almost 65% of the adult population in the United States is overweight. This represents a substantial increase compared with 56% seen in NHANES III, conducted between 1988 and 1994.(4) The direct cost associated with obesity in the United States is ∼$100 billion per year, representing 5.7% of the national health expenditure in 1995,(5) and obesity is associated with many other diseases, such as type 2 diabetes mellitus, hypertension, coronary heart disease, and some cancers.(6,7) Thus, obesity has become a serious national public health problem.
Osteoporosis is another major public health problem, characterized by excessive skeletal fragility and susceptibility to low-trauma fracture among the elderly.(8,9) This excessive skeletal fragility is attributable to intrinsic skeletal factors such as low bone mass, unfavorable geometry at cortical bone sites,(10) poor bone structure at cancellous bone sites,(11) and sluggish or ineffective repair of microdamage.(12) In the United States, ∼10 million women and men already have osteoporosis and another 34 million people are at high risk for developing osteoporosis.(8) Osteoporosis results in >1.5 million osteoporotic fractures (OFs) each year in the United States,(8) including ∼300,000 hip fractures and ∼700,000 vertebral fractures.(13) More than 40% of postmenopausal women, on average, will suffer at least one OF.(14) OF can lead to permanent disabilities, nursing home placement, and even death. The direct cost for osteoporosis has risen rapidly and reached ∼17.5 billion dollars in the United States in 2002.(13)
Similarities between obesity and osteoporosis have been identified for these two complex diseases, suggesting some type of pathophysiologic linkage.(15) Similarities suggesting linkage between obesity and osteoporosis include the following:
Both diseases are affected by genetic and environmental factors, or the interaction between them, and there is some overlap between the genetic and environmental factors influencing both diseases.
Normal aging is associated with both a high incidence of osteoporosis and bone marrow adiposity.(15)
Bone remodeling and adiposity are both regulated through the hypothalamus and sympathetic nervous system.(15)
Adipocytes (the cell for storing energy) and osteoblasts (the bone formation cells) derive from a common progenitor—the mesenchymal stem cell.
An extensive literature has been developed exploring the clinical, epidemiologic, and pathophysiologic linkage between obesity and osteoporosis. The purpose of this article is to review our current understanding of this relatively new area of investigative research.
RELATIONSHIP BETWEEN FAT AND BONE: EPIDEMIOLOGIC AND CLINICAL OBSERVATIONS
It has been well accepted that the most powerful, measurable determinant of fracture risk is the amount of bone in the skeleton, as measured by either BMD or BMC.(16,17) Extensive data have shown that high body weight or BMI is correlated with high BMD or BMC and that a decrease in body weight leads to bone loss.(18–22) These correlations are seen in both men and women, across the entire adult age range, and throughout the skeleton.(18,22–24) This relationship is also found in children and adolescence, although its significance is less clear because of the intensive bone acquisition in this period.(25,26)
Ample evidence supports the view that fat mass, a component of total body weight and one of the most important indices of obesity, has a similar beneficial effect on increasing bone mass, thereby reducing the risk of osteoporosis. In normal pre- and postmenopausal women, total body fat was positively related to BMD throughout the skeleton, and this effect was found in both white(22,23,27) and Japanese subjects.(28,29) Furthermore, a longitudinal study showed that changes in BMD at most sites was positively related to the rate of change in fat mass,(30,31) and the EPIC study also showed that “rapid” bone losers had significantly lower fat mass than the “slow” bone losers.(32) Finally, Lau et al.(33) showed that men with severe vertebral deformity had much lower fat mass and BMD than controls.
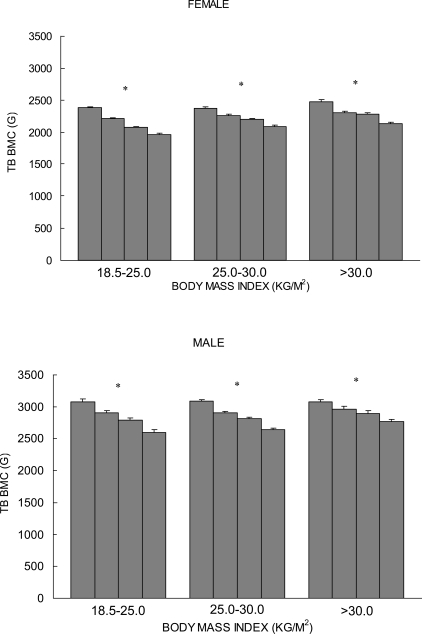
In contrast to the findings above, other independent groups have suggested that excessive fat mass may not protect against decreases in bone mass.(34–39) In a large-scale sample of Chinese and white subjects, our group found that there is a positive correlation between fat mass and bone mass, consistent with the aforementioned studies, when results are not corrected for the mechanical loading effect caused by total body weight. When the mechanical loading effect of total body weight is statistically removed, however, fat mass is negatively correlated with bone mass, suggesting that fat mass actually has a detrimental effect on bone.(40) We further studied the relationship of fat mass to bone mass in subjects matched by BMI. According to the proposed cut-off points by a World Health Organization (WHO) expert committee,(41) we divided 4489 white subjects into normal weight (18.5 < BMI ≤ 25.0 kg/m2), overweight (25.0 < BMI ≤ 30.0 kg/m2), and obese (BMI > 30.0 kg/m2) groups. Figure 1 plots the least-squares means and SEs of total body (TB) BMC for the quartiles of percentage fat mass subgroups from each of the different BMI strata. Significant negative associations (p < 0.001) between percentage fat mass and TB BMC were found in all BMI strata, for both men and women.(40) Consistent with this finding, in a study of a large cohort of Chinese subjects by Hsu et al.,(37) across 5-kg strata of body weight, a negative relationship between fat mass and bone mass was found, and the risks of osteoporosis, osteopenia, and non-spine fractures were significantly higher for subjects with a higher percentage body fat, independent of body weight.(37) Other studies provide further evidence that fat mass may have no beneficial effect on bone. In a cohort of 153 premenopausal women, a higher proportion of fat mass was negatively associated with bone mass.(38) Moreover, high adiposity has been associated with increased risk of bone fragility fracture in children,(35,36) and recent studies with adolescents and young adults indicate that fat mass is not beneficial to bone.(39)
FIG. 1.
Least-squares mean (±SE) of the TB BMC stratified by percentage fat mass in normal weight (18.5 < BMI ≤ 25.0 kg/m2), overweight (25.0 < BMI ≤ 30.0 kg/m2), and obese (BMI > 30.0 kg/m2) white men and women. Each bar in each BMI stratum represents quartiles (Q) 1, 2, 3, and 4 (from left to right) of percentage fat mass. A linear mixed model was used with age, weight, exercise, and menopause status as covariates to adjust TB BMC. Familial relationships were treated as random effects in the model. *p < 0.0001.
Several lines of evidence from environmental factors and medical interventions also support an inverse correlation between fat mass and bone mass. For instance, physical exercise increases bone mass while reducing fat mass.(42) Consumption of milk and tea are believed to be beneficial for the prevention of both osteoporosis and obesity.(43) Milk is a good source of highly absorbable calcium. Increased milk intake has been shown to increase peak bone mass at puberty, slow bone loss, and reduce the incidence of OFs in the elderly.(44) It has also been shown that high calcium intake may promote weight or fat loss,(45) although long-term trials are needed to confirm such observations. Menopause has also been associated with increased bone loss, increased fat mass, and decreased lean mass. Hormone replacement therapy is an effective means of attenuating loss of lean mass and bone(46) and reversing menopause-related obesity(47) in postmenopausal women. Whereas the beneficial interventions identified above reduce the risk of both osteoporosis and obesity, other interventions have been shown to have adverse effects on health, predisposing to both osteoporosis and obesity. For example, osteoporosis and obesity are the two main side effects of treatment with gonadotropin-releasing hormone agonists, agents that are used for treating nonmetastatic prostate cancer.(48) Furthermore, the clinical use of glucocorticoids has been shown to cause decreased bone mass and an increase in central obesity.(49–52) The finding that all of these interventions have opposite effects on fat versus bone mass supports the concept that there is an inverse correlation between fat and bone mass and that fat does not have a protective effect on bone.
Although the studies cited above showed either a clear positive or negative effect of whole body fat mass on bone, regional fat distribution may also influence bone mass, independently of total body fat mass.(53) In epidemiologic studies, the waist/hip circumferences ratio (WHR) is widely used as an index of regional adipose tissue distribution because it is associated with the amount of abdominal visceral adipose tissue measured by CT or MRI.(54) Several lines of evidence have shown that abdominal fat weight and WHR were positively and significantly associated with bone mass.(53,55,56) In contrast, Jankowska et al.(57) reported that WHR was inversely related to bone mass in Polish men. Similarly, Huang et al.(58) reported that increased visceral fat is associated with reduced lumbar spine BMD in HIV-infected men. The inverse association between subcutaneous abdominal fat mass and bone mass has also been reported in healthy children.(59)
These conflicting results suggest a complex effect of fat mass on bone and may be partially attributed to interstudy differences related to sex, sample size, ethnicity, study design, analysis methods, population structure, etc. For example, in a large-scale cross-sectional study of 7137 men, 4585 premenopausal women, and 2248 postmenopausal women, Hsu et al.(37) concluded that the lowest quartile of percentage fat mass has a higher risk of osteoporosis than the highest quartile in both Chinese men and women. However, a smaller study with 68 healthy white premenopausal women and 51 white men showed that BMD is positively related to fat mass in premenopausal women but less so in men.(23) The study from Pluijm et al.(60) confirmed the beneficial effect of fat mass on BMD in white women but not in white men in a small sample (264 women and 258 men). Castro et al.(61) reported that increased obesity is associated with high BMD in white women, but with significantly decreased BMD in black women. Finally, Afghani and Goran(59) reported an inverse correlation between subcutaneous abdominal adipose tissue and BMC in whites, but not in blacks, and an inverse association between intra-abdominal adipose tissue and BMC in blacks but not in whites. These differential findings might imply that results from one ethnic group may not be transferable to another ethnic group or that studies with a large sample size generally have sufficient power to detect associations that may be undetectable in a smaller sample. The qualitatively different relationship between fat mass and bone mass is dependent on whether bone mass is unadjusted or adjusted for total body weight(40,62) indicates that selection of covariates may also contribute to the diverse results presented above. Supporting this perspective is the study by Reid et al.,(42) who proposed that exercise may dissociate the relationship between fat mass and BMD. They found that a positive association between fat mass and BMD was only found in sedentary women but not in the exercising subjects.(42) Finally, the effect of fat mass on bone may also be affected by growth.(63) For example, a longitudinal study of female twins 8–25 years of age indicated that during the “linear growth” period up to 4 years postmenarche, BMD at the lumbar spine, total hip, and femoral neck are independent from changes in fat mass. However, in postlinear growth, changes in fat mass are an important predictor of bone mineral measures.(63) Wearing et al.(64) indicated that the relationship between obesity and the risk of fracture is related to age. High adiposity is considered to be associated with increased risk of distal forearm fracture in children but seems to protect against hip and wrist fracture in the elderly.(64)
Although the reasons listed above may partially explain the inconsistent results from clinical and epidemiologic studies, the relationship between fat mass and bone mass is also confounded by complex genetic backgrounds and interacting metabolic and regulatory pathways that influence both obesity and osteoporosis.
RELATIONSHIP BETWEEN FAT AND BONE: POTENTIAL MECHANISTIC EXPLANATIONS
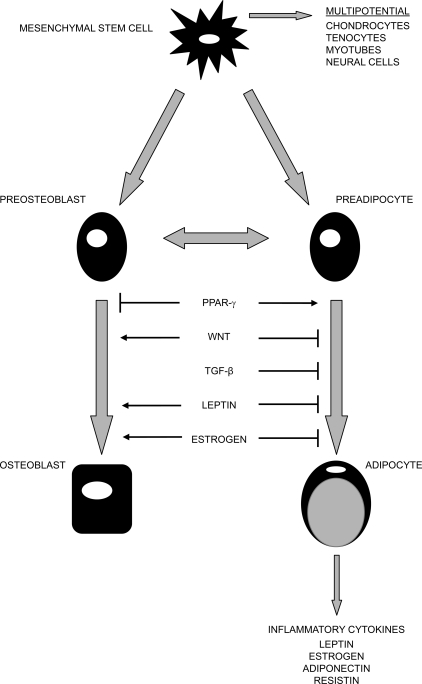
Several potential mechanisms have been proposed to explain the complex relationship between fat and bone mass. One straightforward explanation is that greater fat mass imposes a greater mechanical stress on bone, and in response, bone mass increases to accommodate the greater load. However, only ∼27% and 38% of total body weight in white men and women is attributable to fat mass, respectively.(40) Therefore, weight-associated gravitational forces associated with increased fat mass may be insufficient to explain the impact of fat mass on bone. Studies of adipocyte function have revealed that adipose tissue is not just an inert organ for energy storage. It expresses and secretes a variety of biologically active molecules, such as estrogen, resistin, leptin, adiponectin, and interleukin-6 (IL-6). These molecules affect human energy homeostasis and may be involved in bone metabolism, which may contribute to the complex relationship between fat mass and bone. The secretion of bone-active hormones from the pancreas (including insulin, amylin, and preptin) may also explain part of the relationship between fat mass and bone mass. Finally, adipocytes and osteoblasts originate from a common progenitor, the pluripotential mesenchymal stem cell.(65,66) These stem cells display an equal propensity for differentiation into adipocytes or osteoblasts, and the balance of the differentiation is regulated by several interacting pathways that may contribute to the final effect of fat mass on bone. Figure 2 shows the possible function of common factors shared in osteoblast and adipocyte differentiation.
FIG. 2.
Common factors shared in osteoblast and adipocyte differentiation. Osteoblasts and adipocytes originate from common progenitor-mesenchymal stem cells. The balance of their differentiation is determined by several common factors, such as PPAR-γ, Wnt, TGF-β, leptin, and estrogen. Adipocytes express and secrete a variety of bioactive peptides, such as estrogen, resistin, leptin, adiponectin, and inflammatory cytokines. Some of these peptides affect human energy homeostasis and may be involved in bone metabolism. Adapted from Reference 15.
In the following paragraphs, we will further explore mechanistic explanations for the complex interactions between bone and fat.
Adipocyte-derived peptides
Aromatase:
The enzyme aromatase, which is found in gonadal tissue and adipocytes, uses androstene or testosterone to synthesize estrogen, an important hormone to protect against osteoporosis. Estrogen inhibits bone turnover by reducing osteoclast-mediated bone resorption and stimulating osteoblast-mediated bone formation. In postmenopausal women, because ovaries no longer secrete estrogens, extragonadal estrogen synthesis in fat tissue becomes the dominant estrogen source. Therefore, the role of adipocytes as estrogen producers may become more important to bone metabolism in postmenopausal women.(24) In obese postmenopausal women, increased extragonadal estrogen synthesis caused by high fat mass has been suggested as one of the potential mechanisms for the protective effect of fat mass on bone.
The identification of humans lacking aromatase or estrogen receptor-α, and the recent development of knockout mice lacking aromatase or estrogen receptor-α or -β, supports the concept that estrogen is an inhibitor of obesity and protects against cancellous bone loss.(67–72) This is consistent with findings that estrogen deficiency in postmenopausal women is associated with an increase in central body fat,(73) increased bone turnover, and acceleration of bone loss.(74) Furthermore, decreased endogenous estrogen levels in postmenopausal women were recently shown to be accompanied by an increase in adipocyte numbers and decreased osteoblast counts in bone marrow.(75) Estrogen replacement therapy has also been shown to prevent menopause-induced gains in fat mass(73,76,77) and reduce the incidence of OFs in postmenopausal women.(78) Finally, high and early increases of estrogen concentrations in bone marrow mesenchymal stem cells have been shown to directly stimulate bone formation and inhibit adipocyte differentiation.(79)
Hydroxyl steroid dehydrogenase:
Glucocorticoids are used as therapy for a wide range of inflammatory diseases, and the clinical use of oral glucocorticoids has been shown to have an adverse impact on bone(49,50) and central obesity.(51,52) Two isozymes of 11-β-hydroxysteroid dehydrogenases (11b-HSDs) regulate the peripheral action of glucocorticoids. 11b-HSD1 converts inactive cortisone into active cortisol (the most important human glucocorticoid) in intact cells and enhances glucocorticoid receptor activation.(80,81) In contrast, 11b-HSD2 potently inactivates glucocorticoids. 11b-HSD1, but not 11b-HSD2, is expressed in human adipocytes.(82)
11b-HSD1 is involved in adipocyte differentiation and plays an important role in the regulation of obesity.(83) In vivo, 11b-HSD1 mRNA is elevated in adipose tissue in obese humans and rodents.(80,84,85) Consistently, 11b-HSD1−/− mice have been found to have low intracellular glucocorticoid levels and are protected from obesity.(80,86) In addition, 11b-HSD1−/− mice are resistant to hyperglycemia normally seen with stress or high fat feeding.(87) Finally, 11b-HSD1 activity in fat cells from obese patients was markedly enhanced compared with its activity in fat cells from nonobese individuals.(88)
Although glucocorticoids are essential for human osteoblast differentiation and formation of a mineralized extracellular matrix,(89,90) they are generally considered to be negative regulators of bone formation. The risk of glucocorticoid-induced osteoporosis is widely recognized and this risk increases with age and depends on autocrine actions of the enzyme 11b-HSD1.(91) In addition to its expression in adipocytes, 11b-HSD1 is also present in osteoblasts and osteoclasts.(92,93) The expression of osteoblastic 11b-HSD1 affects the synthesis of active glucocorticoids with consequent effects on osteoblast proliferation and differentiation.(91) Osteoblastic 11b-HSD1 is considered to be a good predictor of the detrimental effects of glucocorticoids on bone.(94,95) Because of the important role of 11b-HSD1 on both bone and obesity, it has been suggested that antagonists of 11b-HSD1 could be used to reduce the adverse effects of glucocorticoids.(88)
Leptin:
Leptin is the most widely recognized adipocyte-derived hormone. It is mainly known for its function of suppressing appetite and increasing energy expenditure. Leptin-deficient ob/ob mice and leptin receptor–deficient db/db mice are extremely obese.(96)
The effect of leptin on obesity is mediated by a series of integrated neuronal pathways including the catabolic pathway represented by proopiomelanocortin (POMC) neurons and the anabolic pathway represented by neuropeptide Y (NPY).(97) Leptin stimulates POMC neurons to secrete α-melanocyte stimulating hormone (α-MSH), a post-translational processing product of POMC.(98) The combination of α-MSH and its receptors MC3R and MC4R (melanocortin receptor 3 and 4) results in reduced food intake and increased energy expenditure.(98) In addition, leptin inhibits the activity of agouti-related peptide (AgRP), an endogenous MC3R and MC4R antagonist. All these produce increases in MC3R and MC4R signaling, which leads to decreased appetite.(98) In addition to the effect on obesity, melanocortin-signaling pathways may contribute to bone resorption(99) but not bone formation.(100) Mc4r knockout mice displayed an increase in hypothalamic cocaine amphetamine-regulated transcripts (CART) expression and high bone mass(99) because of a decrease in bone resorption.(99)
NPY is a hypothalamus-derived peptide, essential for the regulation of food consumption, energy homeostasis, and bone remodeling.(101) Leptin inhibits NPY gene expression in the hypothalamus, and knocking out the NPY gene in ob/ob mice resulted in a reduction of body weight.(102) On the other hand, decreases in plasma leptin levels enhanced NPY expression, stimulated food intake, inhibited energy expenditure, and lead to the development of obesity and its related phenotypes.(103) The effect of NPY is regulated by the Y-receptor system, which is expressed in the hypothalamus and consists of five different Y receptors (Y1, Y2, Y4, Y5, and Y6), Among them, the NPY Y2 receptor has been most widely studied. Hypothalamus-specific Y2 receptor conditional knockout mice exhibited a significant decrease in body weight and a significant increase in food intake.(104) Moreover, the trabecular bone volume in germline Y2–/– mice was increased 2-fold compared with wildtype animals.(105) Studies showed that simultaneous hypothalamus-specific Y2 and Y4 receptor conditional knockout mice exhibited greater reductions in adiposity and greater increases in cancellous bone volume than mice with deficiencies of either the Y2 or Y4 receptor alone.(106) These findings suggest a potential synergy between Y2 and Y4 receptors in the regulation of adiposity and bone mass.(106) Subsequent studies on Y2–/– mice suggested distinct actions of the Y2 and leptin pathways in their regulation of osteoblast activity, with Y2 or Y2 receptor deletion consistently activating osteoblast activity.(107)
In addition to regulating the appetite for food consumption, leptin is also a major regulator of bone remodeling.(96,100,108,109) The effect of leptin on bone is complex. Both negative(38,110) and positive relationships(111–113) between serum leptin levels and BMD have been reported in humans. Ob/ob mice have a complex bone phenotype, displaying increased trabecular bone volume in the spine but short femora with reduced cortical thickness and reduced trabecular volume.(114,115) In vitro studies found that leptin can act directly on bone marrow–derived mesenchymal stem cells to enhance their differentiation to osteoblasts and to inhibit their differentiation to adipocytes.(116,117) In vivo studies indicate that the effect of leptin may depend on its site and mode of function.(118–120) It has been proposed that peripheral administration of leptin could increase bone mass by inhibiting bone resorption(121) and increasing bone formation.(117,122) However, leptin inhibits bone formation through a central nervous effect.(109) The seminal study by Ducy et al.(109) found that intracerebroventricular infusion of leptin causes bone loss in leptin-deficient and wildtype mice. Further studies indicated that leptin regulates both bone formation and bone resorption through the sympathetic nervous system (SNS) and CART.(99,100) The sympathetic nervous system neurotransmitter, noradrenalin, activates β2 adrenergic receptors (β2-AR) on osteoblasts, which activates two distinct molecular cascades downstream of this receptor. One promotes RANKL expression and increases bone resorption mediated by protein kinase A and phosphorylation of ATF4, an essential regulator of osteoblast biology.(99,100,123) The other inhibits osteoblast proliferation through the molecular clock regulation of c-myc and Cyclin-D expression.(124,125) In contrast to the favorable bone resorption effect through the SNS, leptin inhibits bone resorption through CART,(99) a neuropeptide expressed in the hypothalamus that also regulates body weight in mice.(126) β2-AR−/− mice, whose osteoblasts lack adrenergic receptors, exhibit reduced bone resorption effects, leading to a net increase of bone formation.(99,127) In contrast, absence of CART increases bone resorption in ob/ob mice.(99)
Adiponectin:
Adiponectin is another adipocyte-derived hormone that regulates energy homeostasis and has anti-inflammatory and anti-atherogenic effects.(128,129) Unlike leptin, plasma adiponectin levels are generally reduced in obesity and diabetic subjects(130) and may increase with moderate weight loss.(131) Adiponectin and the corresponding receptors are expressed in primary human osteoblasts,(132) suggesting a link between adiponectin and bone. Generally an inverse relationship between serum adiponectin level and BMD are found in association studies.(131,133) Reduced levels of adiponectin associated with obesity, in conjunction with inverse correlations between adiponectin levels and BMD, may provide a partial explanation for the protective effects of fat on bone. Complicating this perspective, however, are in vivo and in vitro studies indicating that adiponectin actually increases bone mass by suppressing osteoclastogenesis and by activating osteoblastogenesis.(132,134,135) Based on these latter studies, it would be reasonable to anticipate that a rise in adiponectin levels caused by fat reduction should have a beneficial effect on BMD. Clearly, further work is needed to improve our understanding of the adiponectin effects on bone.
Resistin:
Resistin, also known as adipocyte-secreted factor, was discovered recently while screening for substances that are downregulated in response to insulin-sensitizing anti-diabetic drugs.(136) Resistin has been proclaimed to be associated with obesity and diabetes,(136,137) and circulating serum resistin levels were found to be elevated proportionally to the degree of obesity.(137,138) Few studies have been performed on the effect of resistin on bone. Thommesen et al.(139) showed that resistin may play a role in bone remodeling. Their study indicates that resistin is expressed in mesenchymal bone marrow stem cells, osteoblasts, and osteoclasts and that resistin increases osteoblast proliferation and cytokine release, as well as osteoclast differentiation.(139) The associations between serum resistin levels and BMD have recently been studied. Oh et al.(140) found an inverse association (r = −0.237) between serum resistin levels and lumbar spine BMD in adult men, but the variance was small.
IL-6:
IL-6, a pluripotent inflammatory cytokine, is released from adipocytes, the adipose tissue matrix, and elsewhere.(141) Adipose tissue accounts for one third of the circulating levels of IL-6. As with leptin, overweight and obese children and adults generally have elevated serum levels of IL-6,(142,143) and genetic polymorphism of IL-6 (−174G/C) is associated with indices of obesity.(144) Peripheral administration of IL-6 induces hyperlipidemia, hyperglycemia, and insulin resistance in rodents and humans.(145) In contrast to the peripheral effect of IL-6 in increasing obesity, administration of IL-6 in the central nervous system increases energy expenditure and decreases body fat in rodents.(145) Therefore, IL-6 has different effects on energy homeostasis depending on its site of production or administration. It has been reported that IL-6 stimulates osteoclastogenesis in cell culture systems,(146) and IL-6 is generally recognized as an osteoresorptive factor.(147) IL-6 mRNA is expressed in pre-osteoblasts and osteoblasts,(148) and IL-6 has been shown to stimulate osteoblast proliferation or differentiation,(149) possibly indirectly, by controlling the production of local factors.(150) Interestingly, IL-6 knockout (−/−) mice are generally healthy and experience no specific bone phenotype, which may indicate that IL-6 is not required for normal bone resorption and homeostasis.(150) However, Franchimont et al.(150) and Sims et al.(151) showed that IL-6 is essential for bone formation in conditions of high bone turnover.
Pancreatic hormones
Insulin:
Insulin resistance is highly correlated with obesity, and several studies support the concept that insulin is a potential regulator of bone metabolism. Fasting insulin levels were significantly and positively associated with BMD of the radius and spine in middle-aged women,(152) and a similar effect was shown in both men and women in another study.(153) Additionally, Tuominen et al.(154) showed that individuals with type 1 and type 2 diabetes mellitus generally have lower BMD compared with normal controls. In contrast, other studies revealed that insulin may contribute to the bone protective effect of obesity. For example, hyperinsulinemia found in obesity is associated with overproduction of androgen and estrogen and reduced production of sex hormone-binding globulin in the liver.(24) These phenomena lead to increased concentrations of free sex hormones, resulting in reduced osteoclast activity and, possibly, increased osteoblast activity, leading to high bone mass.(24) The complex effects of insulin on bone are similar to the complicated relationship between fat and bone. It should be noted that, although obesity is strongly and significantly correlated with type 2 diabetes, only a fraction (10%) of obese people suffer diabetes. Obesity phenotypes represent continuous traits, and the cut-off for the definition of obesity is arbitrary. Our lack of knowledge on the continuum of obesity may account for the discrepancies in the literature with respect to the correlation between fat and bone.
Amylin:
Co-secreted with insulin, amylin, a 37-amino acid peptide, is a member of the calcitonin family of hormones. The mean basal amylin concentration is generally higher in obese than in lean, human subjects.(155) Amylin administration seems to decrease food intake through both central and peripheral mechanisms and has potential for reducing body weight and body fat.(155,156) The elevated amylin levels associated with obesity, however, may lead to downregulation of amylin receptors and lessen the impact of postprandial amylin secretion on satiety and gastric emptying.(155) Similar to calcitonin, amylin has been shown to reduce osteoclast development and thus inhibit bone resorption.(157) In addition, amylin stimulates the proliferation of osteoblasts in vitro and increases indices of bone formation when administered either locally or systemically in vivo.(158) Consequently, high amylin levels associated with obesity leads to high bone mass.
Preptin:
Preptin is a 34-amino-acid peptide hormone from the pancreatic β cells, corresponding to Asp(69)-Leu(102) of the proinsulin-like growth factor II (proIGF-II) E-peptide.(159) Cosecreted with insulin and amylin from the pancreatic β cells, circulating preptin levels are elevated in obesity.(159) Cornish et al.(160) assessed preptin's activities on bone and showed that preptin is anabolic to bone in vitro and in vivo but that it does not affect osteoclast activity.
Osteoblast and adipocyte differentiation
Our current understanding of the skeleton and differentiation of adipose tissue in the bone marrow supports a negative relationship between fat mass and bone mass. For example, activation of peroxisome proliferators activated receptor-γ (PPAR-γ) favors the differentiation of mesenchymal stem cells into adipocytes over osteoblasts.(161) In contrast, the Wnt signaling pathway inhibits adipogenesis(162,163) while promoting osteogenesis.(164–166) Based on these findings, an inverse relationship model, called the “see-saw paradigm” has been proposed to describe the relationship between fat mass and bone mass in bone marrow.(167) It should be noted that the above relationship is limited to the marrow microenvironment. As discussed in the epidemiologic and clinical observations, the effect of fat on bone may be site-specific. For example, similar to bone marrow mesenchymal stem cells, adipose-derived stromal cells have osteogenic potential. Their osteogenic capacity may vary by anatomical site, with visceral adipose-derived cells exhibiting higher osteogenic potential than those isolated from subcutis.(168) In addition, prospective studies indicate that excess of visceral adipose tissue has higher risk of morbidity than the excess of subcutaneous adipose tissue.(169) This may be related to higher expression of glucocorticoid, interleukin-6, and 11b-HSD1 and lower leptin and adiponectin levels in visceral adipose tissue compared with subcutaneous adipose tissue.(169)
PPAR-γ pathway:
The PPAR-γ pathway plays a key regulatory role in initiating adipogenesis.(170) In the bone marrow, PPAR-γ2 regulates osteoblast development and bone formation negatively and regulates marrow adipocyte differentiation positively. PPAR-γ ligands not only induce murine bone marrow stem cell adipogenesis but also inhibit osteogenesis.(171) Kirkland et al.(172) indicated that the PPAR-γ pathway is also associated with fat redistribution and bone loss related with aging. Indeed, the expression of PPAR-γ in subcutaneous fat tissue is lower in old than young monkeys, and mutations of the PPAR-γ gene are associated with an altered balance between bone and fat formation in the marrow.(166,172,173) In advanced age, subcutaneous and visceral fat depot size declines, whereas fat depots in bone marrow increase, and it has been suggested that PPAR-γ accounts for increased bone marrow fat and decreased production of osteoblasts related to aging.(174) Decreased fat depot size is associated with decreased production of the transcription factors, CCAAT/enhancer binding α (C/EBPα) and PPAR-γ, which results in reduced expression of differentiation-dependent genes.(172) The expression of adipocyte-specific transcription factor is also increased in old marrow compared with adult marrow.(174) Altered production of these transcription factors leads to dysdifferentiation of nonadipose, mesenchymal cells into mesenchymal adipocyte-like default cells (MAD cells), which contributes to accumulation of lipid in locations outside of fat tissue, such as the bone marrow.(173)
The effect of PPAR-γ on bone and obesity suggests that treatment with PPAR-γ antagonists might be therapeutically beneficial for age-related bone loss intervention and high marrow fat, although this has not yet been studied.(174,175) Combined treatment of bone marrow–derived mesenchymal stem cells with PPAR-γ and cytokines (IL-1 or TNF-α) in vitro, however, inhibited adipogenesis and induced osteogenesis.(175)
Wnt signaling pathway:
Recent studies have indicated that the Wnt signaling pathway plays an important role in inhibiting adipogenesis(162,176) and enhancing osteogenesis.(164,165) The inhibitory effect of the Wnt signaling pathway on adipogenesis is mediated, in part, through β-catenin, which inhibits expression of select PPAR-γ target genes.(177) The low-density lipoprotein receptor–related protein 5 (LRP5) acts as a Wnt co-receptor,(178) and recent data indicate that Wnt-mediated signaling through LRP5 affects bone formation.(164,165) Clinical studies indicate that mutations in LRP5 are associated with changes in BMD.(165,179) For example, loss-of-function LRP5 knockout mice showed an osteopenic/osteoporotic phenotype,(180) and osteoporosis-pseudoglioma syndrome, an autosomal recessive disorder, was found in a patient with mutations in LRP5.(164) In contrast, broader genetic studies have shown that other point mutations in the LRP5 gene are associated with enhanced high bone mass in humans(165) and that transgenic mice expressing this LRP5 mutation have a similar phenotype.(181,182)
TGF-β signaling pathway:
A third, rather complex, regulatory pathway for mesenchymal stromal cell differentiation is the TGF-β signaling pathway. TGF-β, a secreted factor that is present at high levels in bone, has been shown to inhibit adipocyte differentiation.(183) With osteoblasts, the effects of TGF-β on proliferation and differentiation vary, depending on the extracellular milieu and the stage of cell differentiation at the time of exposure. TGF-β stimulates proliferation and early osteoblast differentiation while inhibiting terminal osteoblast differentiation.(184,185) The TGF-β signaling pathway interacts with the PPAR-γ pathway.(186) TGF-β has been shown to inhibit PPAR-γ expression in human marrow stem cells and downregulate several PPAR-γ target genes.(186) TGF-β has also been shown to influence the Wnt signaling pathway(187) by enhancing expression of Wnt and the Wnt co-receptor LRP5 and stabilizing β-catenin. These TGF-β–induced changes stimulated chondrocyte differentiation and inhibited adipocyte differentiation in human marrow stromal cells.(187)
It should be noted that, in addition to adipocytes and osteoblasts, pluripotential mesenchymal stem cells have the potential to differentiate into chondrocytes, tenocytes, myotubules, and neural cells (Fig. 2).(188) The PPAR-γ, Wnt, and TGF-β signaling pathways may also contribute to differentiation into other mesenchymal lineages, besides adipocytes and osteoblasts.(187) Clearly, the modulation role of PPAR-γ, Wnt, and TGF-β pathways in adipogenesis and osteogenesis are complex, and further studies are needed to clarify their role in cell differentiation.
SUMMARY
The majority of epidemiologic studies have highlighted the existence of the interaction between adiposity and bone. The results of these studies have supported contrasting conclusions, however, and based on data generated to date, it is unclear whether fat mass has a beneficial effect on bone. These inconsistent findings reflect the inherently complicated nature of this relationship and call for new approaches and strategies to accommodate the confounding effects of fat mass on bone. It should be noted that our knowledge of the clinical association between fat mass and bone mass is derived primarily from cross-sectional studies, which have limited capacity to dissect out the relationship between these two variables. Longitudinal studies with large sample size, powerful design, and careful data analysis will be needed to conclusively show the true effect of fat mass on bone. Elucidation of this relationship will generate substantial research interest in the coming years, and studies using molecular and genetic methodology should help identify regulatory pathways that lead to the development of therapeutic interventions that can be used to treat osteoporosis and obesity simultaneously.
ACKNOWLEDGMENTS
This study was partially supported by grants from the NIH (K01 AR02170-01, R01 AR050496-01, and R01 GM60402-01A1). The study also benefited from grants from the CNSF, Huo Ying Dong Education Foundation, Hunan Province, and the Ministry of Education of China. We thank Dr Yong-Jun Liu for reading the manuscript.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999;2:17–31. doi: 10.1016/s1098-3597(99)90002-9. [DOI] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Seidell JC. Obesity: A growing problem. Acta Paediatr Suppl. 1999;88:46–50. doi: 10.1111/j.1651-2227.1999.tb14350.x. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. National Center for Health Statistics Survey. [Accessed May 2007];2004 Available online at www.cdc.gov/nchs/products/pubs/pubd/hestats/obese/obse99.htm.
- 5.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 6.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 7.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Melton LJ., III Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res. 2003;18:1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ., III The prevalence of osteoporosis: Gender and racial comparison. Calcif Tissue Int. 2001;69:179–181. doi: 10.1007/s00223-001-1043-9. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. J Bone Miner Res. 1993;8:1211–1217. doi: 10.1002/jbmr.5650081008. [DOI] [PubMed] [Google Scholar]
- 11.Recker RR, Kimmel DB. Changes in trabecular microstructure in osteoporosis occur with normal bone remodeling dynamics. J Bone Miner Res. 1991;6(S1):S225. [Google Scholar]
- 12.Schaffler MB, Choi K, Milgrom C. Aging and matrix microdamage accumulation in human compact bone. Bone. 1995;17:521–525. doi: 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 13.Ray NF, Chan JK, Thamer M, Melton LJ., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: Report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 14.Spencer H, Kramer L. NIH Consensus Conference: Osteoporosis. Factors contributing to osteoporosis. J Nutr. 1986;116:316–319. doi: 10.1093/jn/116.2.316. [DOI] [PubMed] [Google Scholar]
- 15.Rosen CJ, Bouxsein ML. Mechanisms of Disease: Is osteoporosis the obesity of bone. Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 17.Melton LJ, III, Atkinson EJ, O'Fallon WM, Wahner HW, Riggs BL. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8:1227–1233. doi: 10.1002/jbmr.5650081010. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: The Framingham study. J Bone Miner Res. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 19.Marcus R, Greendale G, Blunt BA, Bush TL, Sherman S, Sherwin R, Wahner H, Wells B. Correlates of bone mineral density in the postmenopausal estrogen/progestin interventions trial. J Bone Miner Res. 1994;9:1467–1476. doi: 10.1002/jbmr.5650090920. [DOI] [PubMed] [Google Scholar]
- 20.Mazess RB, Barden HS, Ettinger M, Johnston C, Dawson-Hughes B, Baran D, Powell M, Notelovitz M. Spine and femur density using dual-photon absorptiometry in US white women. Bone Miner. 1987;2:211–219. [PubMed] [Google Scholar]
- 21.Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–1627. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]
- 22.Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, Lim TM, Cundy TF. Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab. 1992;75:45–51. doi: 10.1210/jcem.75.1.1619030. [DOI] [PubMed] [Google Scholar]
- 23.Reid IR, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 24.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 25.Ilich JZ, Skugor M, Hangartner T, Baoshe A, Matkovic V. Relation of nutrition, body composition and physical activity to skeletal development: A cross-sectional study in preadolescent females. J Am Coll Nutr. 1998;17:136–147. doi: 10.1080/07315724.1998.10718739. [DOI] [PubMed] [Google Scholar]
- 26.Sabatier JP, Guaydier-Souquieres G, Benmalek A, Marcelli C. Evolution of lumbar bone mineral content during adolescence and adulthood: A longitudinal study in 395 healthy females 10-24 years of age and 206 premenopausal women. Osteoporos Int. 1999;9:476–482. doi: 10.1007/s001980050173. [DOI] [PubMed] [Google Scholar]
- 27.Khosla S, Atkinson EJ, Riggs BL, Melton LJ., III Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 28.Douchi T, Oki T, Nakamura S, Ijuin H, Yamamoto S, Nagata Y. The effect of body composition on bone density in pre- and postmenopausal women. Maturitas. 1997;27:55–60. doi: 10.1016/s0378-5122(97)01112-2. [DOI] [PubMed] [Google Scholar]
- 29.Douchi T, Yamamoto S, Oki T, Maruta K, Kuwahata R, Nagata Y. Relationship between body fat distribution and bone mineral density in premenopausal Japanese women. Obstet Gynecol. 2000;95:722–725. doi: 10.1016/s0029-7844(99)00663-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean tissue mass: Which one is the major determinant of bone mineral mass in healthy postmenopausal women. J Bone Miner Res. 1997;12:144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 31.Reid IR, Ames RW, Evans MC, Sharpe SJ, Gamble GD. Determinants of the rate of bone loss in normal postmenopausal women. J Clin Endocrinol Metab. 1994;79:950–954. doi: 10.1210/jcem.79.4.7962303. [DOI] [PubMed] [Google Scholar]
- 32.Riis BJ, Rodbro P, Christiansen C. The role of serum concentrations of sex steroids and bone turnover in the development and occurrence of postmenopausal osteoporosis. Calcif Tissue Int. 1986;38:318–322. doi: 10.1007/BF02555743. [DOI] [PubMed] [Google Scholar]
- 33.Lau EM, Chan YH, Chan M, Woo J, Griffith J, Chan HH, Leung PC. Vertebral deformity in chinese men: Prevalence, risk factors, bone mineral density, and body composition measurements. Calcif Tissue Int. 2000;66:47–52. doi: 10.1007/s002230050009. [DOI] [PubMed] [Google Scholar]
- 34.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, III, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 35.Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: A dual-energy x-ray absorptiometry study. J Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- 36.Mobley SL, Ha E, Landoll JD, Badenop-Sevens NE, Clairmont A, Goel P, Matkovic V. Children with bone fragility fractures have reduced bone mineral areal density at the forarm and hip and higher percent body fat. J Bone Miner Res. 2005;20:S34. [Google Scholar]
- 37.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 38.Blum M, Harris SS, Must A, Naumova EN, Phillips SM, Rand WM, Dawson-Hughes B. Leptin, body composition and bone mineral density in premenopausal women. Calcif Tissue Int. 2003;73:27–32. doi: 10.1007/s00223-002-1019-4. [DOI] [PubMed] [Google Scholar]
- 39.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 40.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–1646. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Oraganization. Genvea, Switzerland: World Health Organization; 1997. Obesity: Preventing and Managing the Global Epidemic. [Google Scholar]
- 42.Reid IR, Legge M, Stapleton JP, Evans MC, Grey AB. Regular exercise dissociates fat mass and bone density in premenopausal women. J Clin Endocrinol Metab. 1995;80:1764–1768. doi: 10.1210/jcem.80.6.7775619. [DOI] [PubMed] [Google Scholar]
- 43.St Onge MP. Dietary fats, teas, dairy, and nuts: Potential functional foods for weight control. Am J Clin Nutr. 2005;81:7–15. doi: 10.1093/ajcn/81.1.7. [DOI] [PubMed] [Google Scholar]
- 44.Reid IR. Therapy of osteoporosis: Calcium, vitamin D, and exercise. Am J Med Sci. 1996;312:278–286. doi: 10.1097/00000441-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Zemel MB. Role of calcium and dairy products in energy partitioning and weight management. Am J Clin Nutr. 2004;79:907S–912S. doi: 10.1093/ajcn/79.5.907S. [DOI] [PubMed] [Google Scholar]
- 46.Manson JE, Martin KA. Postmenopausal hormone-replacement therapy. N Engl J Med. 2001;345:34–40. doi: 10.1056/NEJM200107053450106. [DOI] [PubMed] [Google Scholar]
- 47.Sorensen MB, Rosenfalck AM, Hojgaard L, Ottesen B. Obesity and sarcopenia after menopause are reversed by sex hormone replacement therapy. Obes Res. 2001;9:622–626. doi: 10.1038/oby.2001.81. [DOI] [PubMed] [Google Scholar]
- 48.Smith MR. Osteoporosis and obesity in men receiving hormone therapy for prostate cancer. J Urol. 2004;172:S52–S56. doi: 10.1097/01.ju.0000141820.17959.2f. [DOI] [PubMed] [Google Scholar]
- 49.de Gregorio LH, Lacativa PG, Melazzi AC, Russo LA. Glucocorticoid-induced osteoporosis. Arq Bras Endocrinol Metabol. 2006;50:793–801. doi: 10.1590/s0004-27302006000400024. [DOI] [PubMed] [Google Scholar]
- 50.Steinbuch M, Youket TE, Cohen S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos Int. 2004;15:323–328. doi: 10.1007/s00198-003-1548-3. [DOI] [PubMed] [Google Scholar]
- 51.Gaillard D, Wabitsch M, Pipy B, Negrel R. Control of terminal differentiation of adipose precursor cells by glucocorticoids. J Lipid Res. 1991;32:569–579. [PubMed] [Google Scholar]
- 52.Livingstone DE, Jones GC, Smith K, Jamieson PM, Andrew R, Kenyon CJ, Walker BR. Understanding the role of glucocorticoids in obesity: Tissue-specific alterations of corticosterone metabolism in obese Zucker rats. Endocrinology. 2000;141:560–563. doi: 10.1210/endo.141.2.7297. [DOI] [PubMed] [Google Scholar]
- 53.Tarquini B, Navari N, Perfetto F, Piluso A, Romano S, Tarquini R. Evidence for bone mass and body fat distribution relationship in postmenopausal obese women. Arch Gerontol Geriatr. 1997;24:15–21. doi: 10.1016/s0167-4943(96)00723-6. [DOI] [PubMed] [Google Scholar]
- 54.Wajchenberg BL. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 55.Heiss CJ, Sanborn CF, Nichols DL, Bonnick SL, Alford BB. Associations of body fat distribution, circulating sex hormones, and bone density in postmenopausal women. J Clin Endocrinol Metab. 1995;80:1591–1596. doi: 10.1210/jcem.80.5.7745005. [DOI] [PubMed] [Google Scholar]
- 56.Stewart KJ, Deregis JR, Turner KL, Bacher AC, Sung J, Hees PS, Tayback M, Ouyang P. Fitness, fatness and activity as predictors of bone mineral density in older persons. J Intern Med. 2002;252:381–388. doi: 10.1046/j.1365-2796.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 57.Jankowska EA, Rogucka E, Medras M. Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia. 2001;33:384–389. doi: 10.1046/j.1439-0272.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 58.Huang JS, Rietschel P, Hadigan CM, Rosenthal DI, Grinspoon S. Increased abdominal visceral fat is associated with reduced bone density in HIV-infected men with lipodystrophy. AIDS. 2001;15:975–982. doi: 10.1097/00002030-200105250-00005. [DOI] [PubMed] [Google Scholar]
- 59.Afghani A, Goran MI. Racial differences in the association of subcutaneous and visceral fat on bone mineral content in prepubertal children. Calcif Tissue Int. 2006;79:383–388. doi: 10.1007/s00223-006-0116-1. [DOI] [PubMed] [Google Scholar]
- 60.Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P. Determinants of bone mineral density in older men and women: Body composition as mediator. J Bone Miner Res. 2001;16:2142–2151. doi: 10.1359/jbmr.2001.16.11.2142. [DOI] [PubMed] [Google Scholar]
- 61.Castro JP, Joseph LA, Shin JJ, Arora SK, Nicasio J, Shatzkes J, Raklyar I, Erlikh I, Pantone V, Bahtiyar G, Chandler L, Pabon L, Choudhry S, Ghadiri N, Gosukonda P, Muniyappa R, von-Gicyzki H, McFarlane SI. Differential effect of obesity on bone mineral density in White, Hispanic and African American women: A cross sectional study. Nutr Metab (Lond) 2005;2:9. doi: 10.1186/1743-7075-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao LJ, Liu YJ, Recker RR, Deng HW. Genes decrease osteoporosis risk also decrease risk of obesity. J Bone Miner Res. 2005;20:S339. [Google Scholar]
- 63.Young D, Hopper JL, Macinnis RJ, Nowson CA, Hoang NH, Wark JD. Changes in body composition as determinants of longitudinal changes in bone mineral measures in 8 to 26-year-old female twins. Osteoporos Int. 2001;12:506–515. doi: 10.1007/s001980170097. [DOI] [PubMed] [Google Scholar]
- 64.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: A biomechanical perspective. Obes Rev. 2006;7:239–250. doi: 10.1111/j.1467-789X.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 65.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 67.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes Relat Metab Disord. 1985;9(Suppl 1):83–92. [PubMed] [Google Scholar]
- 68.Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001;178:147–154. doi: 10.1016/s0303-7207(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 69.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: Effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab. 2004;89:61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 71.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 72.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 73.Tchernof A, Calles-Escandon J, Sites CK, Poehlman ET. Menopause, central body fatness, and insulin resistance: Effects of hormone-replacement therapy. Coron Artery Dis. 1998;9:503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 74.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: Estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 75.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 76.Gambacciani M, Ciaponi M, Cappagli B, De Simone L, Orlandi R, Genazzani AR. Prospective evaluation of body weight and body fat distribution in early postmenopausal women with and without hormonal replacement therapy. Maturitas. 2001;39:125–132. doi: 10.1016/s0378-5122(01)00194-3. [DOI] [PubMed] [Google Scholar]
- 77.Jensen LB, Vestergaard P, Hermann AP, Gram J, Eiken P, Abrahamsen B, Brot C, Kolthoff N, Sorensen OH, Beck-Nielsen H, Nielsen SP, Charles P, Mosekilde L. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: A randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003;18:333–342. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 78.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 79.Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology. 2002;143:2349–2356. doi: 10.1210/endo.143.6.8854. [DOI] [PubMed] [Google Scholar]
- 80.Seckl JR. 11beta-hydroxysteroid dehydrogenases: Changing glucocorticoid action. Curr Opin Pharmacol. 2004;4:597–602. doi: 10.1016/j.coph.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 81.Seckl JR, Morton NM, Chapman KE, Walker BR. Glucocorticoids and 11beta-hydroxysteroid dehydrogenase in adipose tissue. Recent Prog Horm Res. 2004;59:359–393. doi: 10.1210/rp.59.1.359. [DOI] [PubMed] [Google Scholar]
- 82.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”. Lancet. 1997;349:1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, Sun WL, Sun Y, Hu G, Ding GX. Role of 11-beta-hydroxysteroid dehydrogenase type 1 in differentiation of 3T3-L1 cells and in rats with diet-induced obesity. Acta Pharmacol Sin. 2006;27:588–596. doi: 10.1111/j.1745-7254.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- 84.Kannisto K, Pietilainen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Jarvinen H. Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: Studies in young adult monozygotic twins. J Clin Endocrinol Metab. 2004;89:4414–4421. doi: 10.1210/jc.2004-0153. [DOI] [PubMed] [Google Scholar]
- 85.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J, Luft FC, Sharma AM. Regulation of 11beta-HSD genes in human adipose tissue: Influence of central obesity and weight loss. Obes Res. 2004;12:9–17. doi: 10.1038/oby.2004.3. [DOI] [PubMed] [Google Scholar]
- 86.Draper N, Stewart PM. 11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186:251–271. doi: 10.1677/joe.1.06019. [DOI] [PubMed] [Google Scholar]
- 87.Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA. 1997;94:14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 89.Eijken M, Koedam M, van Driel M, Buurman CJ, Pols HA, van Leeuwen JP. The essential role of glucocorticoids for proper human osteoblast differentiation and matrix mineralization. Mol Cell Endocrinol. 2006;248:87–93. doi: 10.1016/j.mce.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 90.Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996;61:182–193. doi: 10.1002/(sici)1097-4644(19960501)61:2<182::aid-jcb3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 91.Cooper MS, Rabbitt EH, Goddard PE, Bartlett WA, Hewison M, Stewart PM. Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- 92.Cooper MS, Walker EA, Bland R, Fraser WD, Hewison M, Stewart PM. Expression and functional consequences of 11beta-hydroxysteroid dehydrogenase activity in human bone. Bone. 2000;27:375–381. doi: 10.1016/s8756-3282(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 93.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25:831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 94.Cooper MS, Blumsohn A, Goddard PE, Bartlett WA, Shackleton CH, Eastell R, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1 activity predicts the effects of glucocorticoids on bone. J Clin Endocrinol Metab. 2003;88:3874–3877. doi: 10.1210/jc.2003-022025. [DOI] [PubMed] [Google Scholar]
- 95.Eijken M, Hewison M, Cooper MS, de Jong FH, Chiba H, Stewart PM, Uitterlinden AG, Pols HA, van Leeuwen JP. 11beta-hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol Endocrinol. 2005;19:621–631. doi: 10.1210/me.2004-0212. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 97.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 98.Wisse BE, Schwartz MW. Role of melanocortins in control of obesity. Lancet. 2001;358:857–859. doi: 10.1016/S0140-6736(01)06037-8. [DOI] [PubMed] [Google Scholar]
- 99.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 100.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 101.Herzog H. Neuropeptide Y and energy homeostasis: Insights from Y receptor knockout models. Eur J Pharmacol. 2003;480:21–29. doi: 10.1016/j.ejphar.2003.08.089. [DOI] [PubMed] [Google Scholar]
- 102.Liu YJ, Araujo S, Recker RR, Deng HW. Molecular and genetic mechanisms of obesity: Implications for future management. Curr Mol Med. 2003;3:325–340. doi: 10.2174/1566524033479735. [DOI] [PubMed] [Google Scholar]
- 103.Lin S, Boey D, Herzog H. NPY and Y receptors: Lessons from transgenic and knockout models. Neuropeptides. 2004;38:189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hokfelt T, Herzog H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci USA. 2002;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109:915–921. doi: 10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sainsbury A, Baldock PA, Schwarzer C, Ueno N, Enriquez RF, Couzens M, Inui A, Herzog H, Gardiner EM. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol Cell Biol. 2003;23:5225–5233. doi: 10.1128/MCB.23.15.5225-5233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Allison SJ, Baldock PA, Herzog H. The control of bone remodeling by neuropeptide Y receptors. Peptides. 2007;28:320–325. doi: 10.1016/j.peptides.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 108.Mantzoros CS. The role of leptin in human obesity and disease: A review of current evidence. Ann Intern Med. 1999;130:671–680. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 109.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 110.Kontogianni MD, Dafni UG, Routsias JG, Skopouli FN. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res. 2004;19:546–551. doi: 10.1359/JBMR.040107. [DOI] [PubMed] [Google Scholar]
- 111.Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86:1884–1887. doi: 10.1210/jcem.86.5.7417. [DOI] [PubMed] [Google Scholar]
- 112.Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, Ozuru R, Sugishita T, Chihara K. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 2001;55:341–347. doi: 10.1046/j.1365-2265.2001.01361.x. [DOI] [PubMed] [Google Scholar]
- 113.Goulding A, Taylor RW. Plasma leptin values in relation to bone mass and density and to dynamic biochemical markers of bone resorption and formation in postmenopausal women. Calcif Tissue Int. 1998;63:456–458. doi: 10.1007/s002239900557. [DOI] [PubMed] [Google Scholar]
- 114.Reid IR. Leptin deficiency–lessons in regional differences in the regulation of bone mass. Bone. 2004;34:369–371. doi: 10.1016/j.bone.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 115.Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 116.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 117.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 118.Thomas T. Leptin: A potential mediator for protective effects of fat mass on bone tissue. Joint Bone Spine. 2003;70:18–21. doi: 10.1016/s1297-319x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 119.Thomas T. The complex effects of leptin on bone metabolism through multiple pathways. Curr Opin Pharmacol. 2004;4:295–300. doi: 10.1016/j.coph.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 120.Thomas T, Burguera B. Is leptin the link between fat and bone mass. J Bone Miner Res. 2002;17:1563–1569. doi: 10.1359/jbmr.2002.17.9.1563. [DOI] [PubMed] [Google Scholar]
- 121.Martin A, de Vittoris R, David V, Moraes R, Begeot M, Lafage-Proust MH, Alexandre C, Vico L, Thomas T. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology. 2005;146:3652–3659. doi: 10.1210/en.2004-1509. [DOI] [PubMed] [Google Scholar]
- 122.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 123.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 124.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 125.Karsenty G. Convergence between bone and energy homeostases: Leptin regulation of bone mass. Cell Metab. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 126.Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Koster A. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–4400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- 127.Cohen MM., Jr Role of leptin in regulating appetite, neuroendocrine function, and bone remodeling. Am J Med Genet A. 2006;140:515–524. doi: 10.1002/ajmg.a.31099. [DOI] [PubMed] [Google Scholar]
- 128.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 130.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 131.Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–651. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 132.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 133.Jurimae J, Rembel K, Jurimae T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res. 2005;37:297–302. doi: 10.1055/s-2005-861483. [DOI] [PubMed] [Google Scholar]
- 134.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 135.Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 136.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 137.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, Simon I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: Relationships in obesity. Obes Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 138.Yannakoulia M, Yiannakouris N, Bluher S, Matalas AL, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88:1730–1736. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]
- 139.Thommesen L, Stunes AK, Monjo M, Grosvik K, Tamburstuen MV, Kjobli E, Lyngstadaas SP, Reseland JE, Syversen U. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem. 2006 doi: 10.1002/jcb.20915. [DOI] [PubMed] [Google Scholar]
- 140.Oh KW, Lee WY, Rhee EJ, Baek KH, Yoon KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol (Oxf) 2005;63:131–138. doi: 10.1111/j.1365-2265.2005.02312.x. [DOI] [PubMed] [Google Scholar]
- 141.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 142.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 143.Das UN. Is obesity an inflammatory condition. Nutrition. 2001;17:953–966. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 144.Berthier MT, Paradis AM, Tchernof A, Bergeron J, Prud'homme D, Despres JP, Vohl MC. The interleukin 6-174G/C polymorphism is associated with indices of obesity in men. J Hum Genet. 2003;48:14–19. doi: 10.1007/s100380300002. [DOI] [PubMed] [Google Scholar]
- 145.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 146.Richards CD, Langdon C, Deschamps P, Pennica D, Shaughnessy SG. Stimulation of osteoclast differentiation in vitro by mouse oncostatin M, leukaemia inhibitory factor, cardiotrophin-1 and interleukin 6: Synergy with dexamethasone. Cytokine. 2000;12:613–621. doi: 10.1006/cyto.1999.0635. [DOI] [PubMed] [Google Scholar]
- 147.Rodan GA. Introduction to bone biology. Bone. 1992;13(Suppl 1):S3–S6. doi: 10.1016/s8756-3282(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 148.Dodds RA, Merry K, Littlewood A, Gowen M. Expression of mRNA for IL1 beta, IL6 and TGF beta 1 in developing human bone and cartilage. J Histochem Cytochem. 1994;42:733–744. doi: 10.1177/42.6.8189035. [DOI] [PubMed] [Google Scholar]
- 149.Taguchi Y, Yamamoto M, Yamate T, Lin SC, Mocharla H, DeTogni P, Nakayama N, Boyce BF, Abe E, Manolagas SC. Interleukin-6-type cytokines stimulate mesenchymal progenitor differentiation toward the osteoblastic lineage. Proc Assoc Am Physicians. 1998;110:559–574. [PubMed] [Google Scholar]
- 150.Franchimont N, Wertz S, Malaise M. Interleukin-6: An osteotropic factor influencing bone formation. Bone. 2005;37:601–606. doi: 10.1016/j.bone.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 151.Sims NA, Jenkins BJ, Quinn JM, Nakamura A, Glatt M, Gillespie MT, Ernst M, Martin TJ. Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest. 2004;113:379–389. doi: 10.1172/JCI19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone. Diabetes Care. 1996;19:1388–1392. doi: 10.2337/diacare.19.12.1388. [DOI] [PubMed] [Google Scholar]
- 153.Stolk RP, Van Daele PL, Pols HA, Burger H, Hofman A, Birkenhager JC, Lamberts SW, Grobbee DE. Hyperinsulinemia and bone mineral density in an elderly population: The Rotterdam Study. Bone. 1996;18:545–549. doi: 10.1016/8756-3282(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 154.Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–1200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 155.Reda TK, Geliebter A, Pi-Sunyer FX. Amylin, food intake, and obesity. Obes Res. 2002;10:1087–1091. doi: 10.1038/oby.2002.147. [DOI] [PubMed] [Google Scholar]
- 156.Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Anti-obesity effects of the {beta}-cell hormone amylin in diet induced obese rats: Effects on food intake, body weight, composition, energy expenditure and gene expression. Endocrinology. 2006;147:5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 157.Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164:509–514. doi: 10.1083/jcb.200312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cornish J, Naot D. Amylin and adrenomedullin: Novel regulators of bone growth. Curr Pharm Des. 2002;8:2009–2021. doi: 10.2174/1381612023393341. [DOI] [PubMed] [Google Scholar]
- 159.Buchanan CM, Phillips AR, Cooper GJ. Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet beta-cells and enhances insulin secretion. Biochem J. 2001;360:431–439. doi: 10.1042/0264-6021:3600431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cornish J, Callon KE, Bava U, Watson M, Xu X, Lin JM, Chan VA, Grey AB, Naot D, Buchanan CM, Cooper GJ, Reid IR. Preptin, another peptide product fo the pancreatic {beta}-cell, is osteogenic in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;292:E117–E122. doi: 10.1152/ajpendo.00642.2005. [DOI] [PubMed] [Google Scholar]
- 161.Pei L, Tontonoz P. Fat's loss is bone's gain. J Clin Invest. 2004;113:805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ross SE, Erickson RL, Gerin I, DeRose PM, Bajnok L, Longo KA, Misek DE, Kuick R, Hanash SM, Atkins KB, Andresen SM, Nebb HI, Madsen L, Kristiansen K, MacDougald OA. Microarray analyses during adipogenesis: Understanding the effects of Wnt signaling on adipogenesis and the roles of liver X receptor {alpha} in adipocyte metabolism. Mol Cell Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 164.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 165.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 167.Gimble JM, Nuttall ME. Bone and fat: Old questions, new insights. Endocrine. 2004;23:183–188. doi: 10.1385/ENDO:23:2-3:183. [DOI] [PubMed] [Google Scholar]
- 168.Peptan IA, Hong L, Mao JJ. Comparison of osteogenic potentials of visceral and subcutaneous adipose-derived cells of rabbits. Plast Reconstr Surg. 2006;117:1462–1470. doi: 10.1097/01.prs.0000206319.80719.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- 170.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087–1094. [PubMed] [Google Scholar]
- 171.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 172.Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: Does aging make fat go MAD. Exp Gerontol. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 173.Hotta K, Bodkin NL, Gustafson TA, Yoshioka S, Ortmeyer HK, Hansen BC. Age-related adipose tissue mRNA expression of ADD1/SREBP1, PPARgamma, lipoprotein lipase, and GLUT4 glucose transporter in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 1999;54:B183–B188. doi: 10.1093/gerona/54.5.b183. [DOI] [PubMed] [Google Scholar]
- 174.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takada I, Suzawa M, Kato S. Nuclear receptors as targets for drug development: Crosstalk between peroxisome proliferator-activated receptor gamma and cytokines in bone marrow-derived mesenchymal stem cells. J Pharmacol Sci. 2005;97:184–189. doi: 10.1254/jphs.fmj04008x5. [DOI] [PubMed] [Google Scholar]
- 176.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 177.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 178.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 179.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 180.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, II, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 182.Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- 183.Locklin RM, Oreffo RO, Triffitt JT. Effects of TGFbeta and bFGF on the differentiation of human bone marrow stromal fibroblasts. Cell Biol Int. 1999;23:185–194. doi: 10.1006/cbir.1998.0338. [DOI] [PubMed] [Google Scholar]
- 184.Bonewald LF, Dallas SL. Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem. 1994;55:350–357. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]
- 185.Centrella M, Horowitz MC, Wozney JM, McCarthy TL. Transforming growth factor-beta gene family members and bone. Endocr Rev. 1994;15:27–39. doi: 10.1210/edrv-15-1-27. [DOI] [PubMed] [Google Scholar]
- 186.Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-beta/Smad3 signaling. J Biol Chem. 2005;280:22688–22696. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhou S, Eid K, Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 188.Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood) 2001;226:507–520. doi: 10.1177/153537020122600603. [DOI] [PubMed] [Google Scholar]