Abstract
Atypical protein kinase C (aPKC) is a central component of the evolutionarily conserved Par3-Par6-aPKC complex, one of the fundamental regulators of cell polarity. We recently demonstrated that these proteins interact with Neph-nephrin molecules at the slit diaphragm of the glomerular filtration barrier. Here, we report that podocyte-specific deletion of aPKCλ/ι in mice results in severe proteinuria, nephrotic syndrome, and death at 4 to 5 wk after birth. Podocyte foot processes of knockout mice developed structural defects, including mislocalization of the slit diaphragm. In the glomerulus, aPKCλ/ι was primarily expressed in developing glomerular epithelial cells and podocyte foot processes. Interestingly, under physiologic conditions, aPKCλ/ι translocated from the apical surface to the basolateral side of developing podocytes, and this translocation preceded the development of foot processes and formation of slit diaphragms. Supporting a critical role for aPKCλ/ι in the maintenance of slit diaphragms and podocyte foot processes, aPKCλ/ι associated with the Neph-nephrin slit diaphragm complex and localized to the tips of filopodia and leading edges of cultured podocytes. These results suggest that aPKC signaling is fundamental to glomerular maintenance and development.
Polarity is a common feature of many different cell types and a prerequisite for the development of multicellular organisms. The evolutionarily conserved Par3-Par6-aPKC complex is required for the establishment of cell polarity. The core of this complex consists of the atypical protein kinase C (aPKC) and Par6 together with the scaffold protein Par3. Both Par6 and Par3 belong to the PDZ family of adaptor proteins. Par6 seems to be necessary for activation of aPKC. The interaction with the GTP-bound form of the small GTPases Rac and Cdc42 results in a conformational change of Par6,1,2 facilitating the phosphorylation and subsequent activation of aPKC.3 Par3 is thought to act as a scaffolding protein necessary to recruit Par6/aPKC to sites where its activity is required.4 We recently demonstrated that the glomerular slit diaphragm molecules Neph1 and nephrin directly bind to Par3, suggesting that Par3 recruits the Par polarity complex to the slit diaphragm.5
The aPKC is a key player in the generation of almost all forms of polarity.6–8 Two types of aPKC are known, aPKCζ and aPKCλ/ι, which are highly related but encoded by separate genes.9 It is unclear whether both aPKCs are functionally redundant and thus mediate the various aPKC functions or distinct roles are performed by each aPKC. In Drosophila, loss of aPKC results in polarity defects in different cell types and lethality of the embryo.10 In mammalian epithelia, aPKC plays a critical role in the development of the junctional structures, and in neurons the Par3-Par6-aPKC complex seems to specify axon development.11
The glomerular filtration barrier is a unique structure characterized by a precise three-dimensional framework of podocytes that elaborate long, regularly spaced, interdigitating foot processes, enveloping the glomerular capillaries. All podocytes are connected through the slit diaphragm, a specialized cell junction. The slit diaphragm is the only cell–cell contact of mature podocytes, representing a signaling platform that regulates podocyte survival, endocytosis, and cytoskeletal organization.12 Mutations in genes encoding slit diaphragm proteins result in severe loss of protein in the urine (nephrotic syndrome) in both animal models and patients. Among these slit diaphragm proteins, Neph1 and nephrin have been shown to be indispensable for the maintenance of the architecture of podocytes—both proteins participate in intracellular signaling networks to support cytoskeletal organization, cell adhesion, and cell polarity.12 It is intriguing to speculate that the cellular programs used to maintain the complex podocyte architecture are similar to the programs that drive podocyte morphogenesis and repair after foot process effacement, a condition that can be rapidly reversible after some forms of glomerular injury.
Here we demonstrate that podocyte-specific deletion of aPKCλ/ι in mice results in slit diaphragm displacement, foot process effacement, proteinuria, and accelerated renal failure. We document a unique transition of the Par polarity complex in glomerular podocytes from an apical to a basal localization. During glomerular development, this polarity conversion seems to precede podocyte foot process development. In agreement with a critical role of aPKCλ/ι for foot process development and maintenance, aPKC associates with the Neph1-nephrin complex and localizes to the leading edge and filopodia tips of cultured podocytes. We propose that aPKC signaling is a fundamental mechanism for glomerular maintenance and development.
RESULTS
Podocyte-Specific Deletion of aPKCλ/ι Results in Severe Glomerular Disease
Recently we identified the Par3-Par6-aPKC polarity complex as novel components of the glomerular slit diaphragm.5 To define further the function of aPKCs in podocytes, we first examined the expression of the two aPKC isoforms in isolated murine glomeruli and podocytes by reverse transcriptase–PCR (RT-PCR). We found that both isoforms are expressed in a glomerular-enriched fraction as well as in cultured murine podocytes (Figure 1A). Because aPKCζ-deficient mice display no renal phenotype,13 we examined the role of aPKCλ/ι in more detail. The complete knockout is embryonic lethal14; therefore, we crossed aPKCλ/ι-floxed mice to podocin-cre mice to induce a podocyte-specific deletion in these animals.15,16
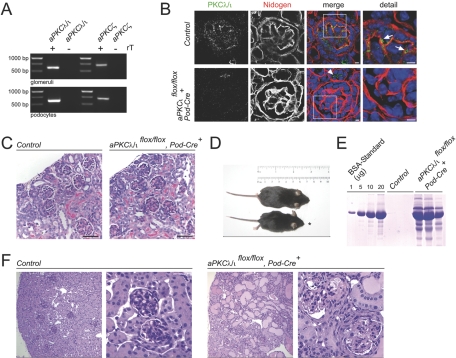
Figure 1.
Podocyte-specific deletion of aPKCλ/ι results in severe glomerular disease. (A) Glomerular podocytes express both aPKC isoforms PKCλ/ι and PKCζ as shown by RT-PCR from mouse glomeruli and differentiated mouse podocytes. (B) Podocyte-specific loss of aPKCλ/ι protein can be detected as early as in capillary loop stage, whereas tubular expression of aPKCλ/ι is unaffected (arrowhead) in podocyte-specific aPKCλ/ι knockout animals. Arrows indicate expression of aPKCλ/ι in podocytes of control mice. Bars = 5 μm. (C) At approximately 1 wk of age, podocyte-specific aPKCλ/ι knockout mice display no significant histologic phenotype. Kidneys from 6-d-old WT (left) and from 6-d-old knockout (right) mice were analyzed by Periodic acid-Schiff (PAS) staining. Six-day-old aPKCλ/ι knockout mice show no obvious histologic abnormalities. (D and E) At approximately 4 wk of age, podocyte-specific aPKCλ/ι knockout mice (*) exhibit growth retardation and significant proteinuria (E). (F) Kidneys from 4-wk-old WT (left) and from 4-wk-old knockout (right) mice were analyzed by PAS staining. Four-week-old aPKCλ/ι knockout mice developed segmental glomerulosclerosis, kidney tubule dilation, and proteinuric casts in the tubule system (right).
To confirm the podocyte-specific loss of aPKCλ/ι protein, we used isotype-specific aPKCλ/ι antibodies on kidney sections of 6-d-old knockout and wild-type (WT) mice, respectively. Podocin Cre-mediated aPKCλ/ι deletion could be demonstrated as early as in the capillary loop developmental stage (Figure 1B), which is in agreement with the start of podocin expression in developing glomeruli (see also Figure 4E). Lysates of 293T cells expressing either aPKCλ/ι or aPKCζ were used to test for the isotype specificity of aPKCλ/ι antibodies. As demonstrated in Supplemental Figure 1, the indicated antibodies specifically detected aPKCλ/ι but not aPKCζ. The aPKCλ/ι conditional knockout mice were born healthy and indistinguishable from littermates up to 2 wk after birth. After this point, growth retardation became evident in these mice, and all aPKCλ/ι conditional knockout mice died within 4 to 5 wk of birth. Six-day-old aPKCλ/ι conditional knockout mice did not display obvious morphologic alterations on histology (Figure 1C). In contrast, at approximately 4 wk of age, aPKCλ/ι conditional knockout mice exhibited significant growth retardation and significant proteinuria as confirmed by Coomassie gel (Figure 1, D and E). In agreement with these clinical findings, histology revealed generalized glomerulosclerosis, kidney tubule dilation, and proteinuric casts in the tubule system (Figure 1F).
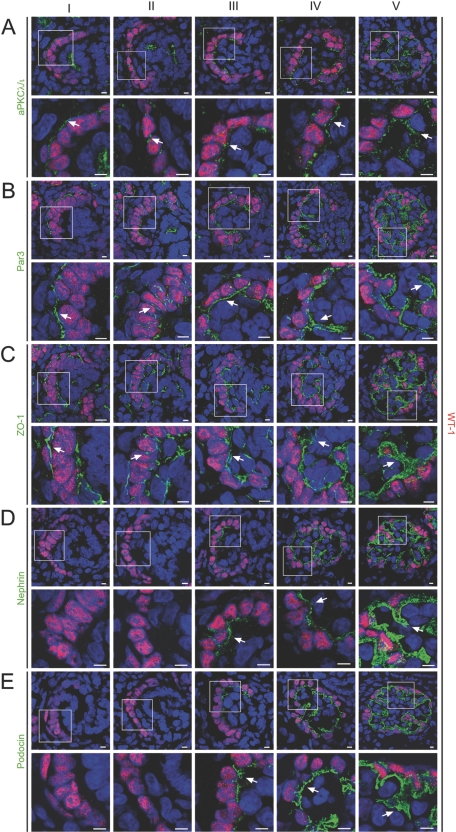
Figure 4.
Translocation of the polarity proteins aPKCλ/ι and Par3 precedes slit diaphragm formation and podocyte foot process development. (A through E) Frozen kidney sections of newborn Wistar rat (day 1) were stained using antibodies against aPKCλ/ι, Par3, ZO-1, nephrin, podocin, and the podocyte marker WT-1 and subjected to confocal laser microscopy. Newborn rats display various stages of glomerular development because glomerular development is asynchronous. Each panel displays the expression pattern of the accordant proteins during glomerular development (from left to right): Developmental stages ranging from comma-shaped body (I), s-shaped body (II), capillary loop stage (III to IV), to a maturing glomerulus (V). (A through C) Whereas aPKCλ/ι, Par3, and ZO-1 are apically expressed during early developmental stages (I, arrows) and translocate to the basal side of glomerular podocytes at later glomerular development (II to III, arrows), nephrin (D) and podocin (E) expression begins at the late s-shaped body stage/early capillary loop stage (III, arrows) on the basolateral side of podocytes. Bars = 5 μm.
Loss of aPKCλ/ι in Podocytes Causes Disruption of Regular Foot Process Architecture
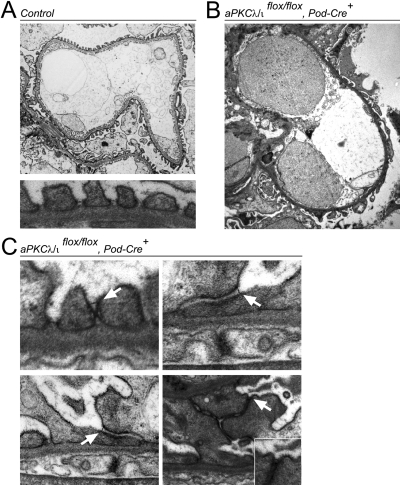
To examine the effects of aPKCλ/ι disruption on ultrastructural glomerular morphology, we performed electron microscopic analysis on 4-wk-old proteinuric PodCre-aPKCλ/ιflox/flox mice and control littermates (Figure 2, A and B). In knockout mice, foot processes were globally effaced (Figure 2B). Intact foot processes were detectable in some areas at higher magnifications, although neighboring foot processes often displayed significant slit diaphragm displacement as well as severe junctional abnormalities (Figure 2C).
Figure 2.
Electron microscopic analysis of aPKCλ/ι knockout glomeruli. (A) Ultrastructural analysis of a glomerular capillary wall from a 4-wk-old WT mouse demonstrates the normal morphology of the glomerular filtration barrier (top) with regular foot process architecture (bottom). (B) Analysis of glomerular filtration barrier from 4-wk-old PKCλ/ι knockout mice shows global foot process effacement. (C) In some areas, foot processes can still be detected but display significant slit diaphragm displacement (arrows) and junction abnormalities.
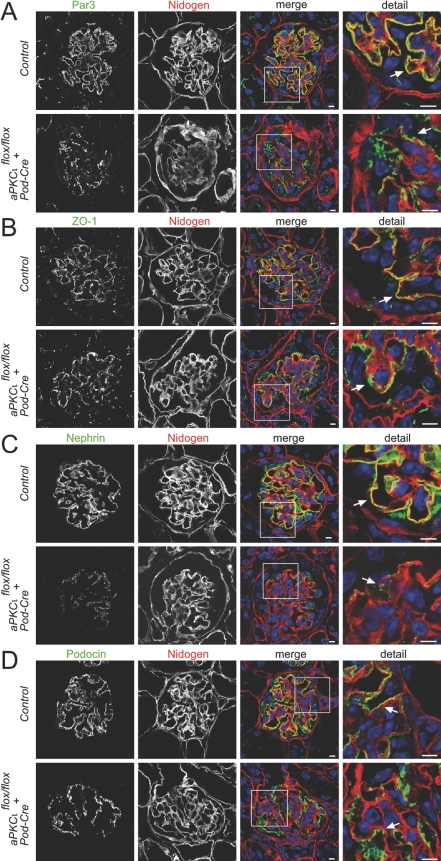
Altered Distribution of Slit Diaphragm Molecules in Podocyte-Specific aPKCλ/ι Knockout Mice
By confocal laser microscopy, we observed the expression intensity and distribution pattern of the polarity protein Par3, the tight junction marker ZO-1, and the slit diaphragm molecules nephrin and podocin. The staining of these markers revealed a linear pattern along the glomerular capillary wall in control mice. In contrast, expression of Par3, ZO-1, nephrin, and podocin was significantly impaired in 4-wk-old PodCre-aPKCλ/ιflox/flox mice with a mainly granular distribution along the glomerular basement membrane (Figure 3). In addition, nephrin expression seemed considerably reduced in PodCre-aPKCλ/ιflox/flox mice (Figure 3C).
Figure 3.
Immunofluorescence studies of WT and aPKCλ/ι knockout glomeruli. Frozen mouse kidney sections from 4-wk-old WT mice (control, top) versus knockout mice (bottom) were stained using antibodies against the Par complex protein Par3, the tight junction marker ZO-1, the slit diaphragm molecules nephrin and podocin, and nidogen, a glomerular basement membrane marker, and subjected to confocal laser microscopy. (A through D) In contrast to the linear staining of Par3, ZO-1, nephrin, and podocin in WT mice, these molecules exhibited a significantly impaired, granular distribution along the glomerular basement membrane in knockout mice. In addition, nephrin expression seemed to be reduced in knockout mice (C). Bars = 5 μm.
Translocation of the Polarity Proteins aPKCλ/ι and Par3 Precedes Slit Diaphragm Formation and Podocyte Foot Process Development
As the glomerulus matures, podocytes undergo a remarkable transformation. They lose their lateral cell contacts, except at the very basolateral aspect of immature podocytes adjacent to the basal membrane. Subsequently, when the podocyte cell bodies have become isolated from each other, they start to extend large projections. These projections divide into intermediate branches, which then divide into multiple smaller foot processes to interdigitate with the foot processes of adjacent podocytes.17 Although these early changes have morphologically been described in detail, little is known about the molecular pathways and mechanisms accompanying these podocyte transformations. It is conceivable to speculate that the morphogenetic switch from a simple polygonal cell to a very complex three-dimensional architecture with the octopus-like cell shape of mature podocytes relies at least in part on evolutionarily conserved polarity signaling. To examine the spatiotemporal expression of the Par polarity complex and slit diaphragm molecules during glomerular developmental stages, we stained frozen kidney sections of newborn Wistar rat (day 1) using antibodies against aPKCλ/ι, Par3, ZO-1, nephrin, podocin, and the podocyte marker WT-1 and subjected them to confocal laser microscopy. Because newborn rats simultaneously display all stages of glomerular development, they have been extensively used as a model to study glomerular development.18 The polarity proteins aPKCλ/ι and Par3 and the tight junction protein ZO-1 were localized at the apical membrane during early stages of glomerular development (Figure 4, A through C, I, comma-shaped body). Interestingly, during the s-shaped body stage, Par3, aPKCλ/ι, and ZO-1 moved along the lateral side to the cell basis (Figure 4, A through C, II, s-shaped body). In contrast, nephrin and podocin expression started at the late s-shaped body stage and early capillary loop stage and immediately localized to the basolateral side of podocytes (Figure 4, D and E, III, early capillary loop stage). Subsequently, the polarity proteins Par3 and aPKCλ/ι as well as ZO-1 and the slit diaphragm proteins nephrin and podocin co-localized at the developing podocyte foot processes (Figure 4, IV, capillary loop stage, and V, immature glomerulus). Supplemental Figure 2 further illustrates the timing of the “podocyte polarity conversion” with a complete translocation of typical apical epithelial markers such as the Par3 to the basal side of developing podocytes (Supplemental Figure 2A, I, comma-shaped body, II, s-shaped body, III, early capillary loop stage). Interestingly, this polarity conversion seems to precede the targeting of the slit diaphragm molecule nephrin to the basal side of podocytes (Supplemental Figure 2, II, s-shaped body, III early capillary loop stage, IV, capillary loop stage).
aPKCλ/ι Associates with the Neph-Nephrin Complex and Localizes to the Leading Edge of Podocytes
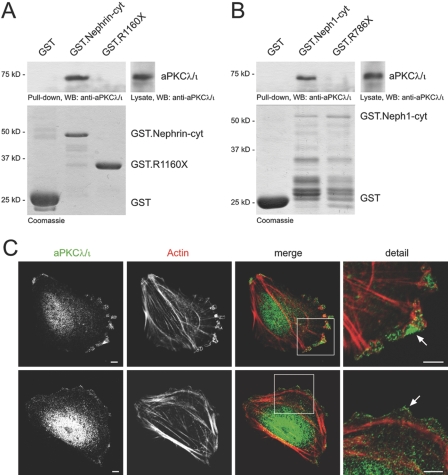
We recently demonstrated the interaction of the PDZ domain–containing Par3 with the Neph-nephrin complex at the slit diaphragm.5 Pulldown assays now revealed the interaction of endogenous aPKCλ/ι with the recombinant glutathione S-Sepharose (GST)-tagged cytoplasmic tail of nephrin and Neph1 but not with the truncations nephrin R1160X and Neph1 R786X lacking the PDZ-binding motif, supporting our hypothesis that aPKCλ/ι associates with the Par3/slit diaphragm molecule complex (Figure 5, A and B).
Figure 5.
aPKCλ/ι associates with the Neph-nephrin complex and localizes to the leading edge of podocytes. (A and B) Interaction of endogenous aPKCλ/ι with recombinant nephrin and Neph proteins. Lysates from mouse kidneys were subjected to a pulldown assay with recombinant affinity-purified cytoplasmic domains of nephrin (A) or Neph1 (B) fused to GST. Whereas GST-nephrin-cyt and GST-Neph1-cyt specifically interacted with aPKCλ/ι, truncations lacking the PDZ-binding domains of nephrin or Neph1 failed to bind to aPKCλ/ι. The bottom panel shows expression levels of GST fusion proteins on a Coomassie Blue stained gel. (C) aPKCλ/ι specifically localizes to the leading edge and the tip of filopodia of differentiated podocytes in culture (arrows).
In neurons, aPKCλ/ι promotes axonal process formation and stabilization.11 The complete loss of a regular foot process architecture in aPKCλ/ι-deficient mice suggests that aPKCλ/ι is actively involved in actin- and microtubule-based cytoskeletal reorganization in podocytes. To determine the role of aPKCλ/ι in podocytes, we analyzed the localization in cultured podocytes. Remarkably, aPKCλ/ι co-localized to the leading edge of migrating podocytes and was significantly enriched at the tip of filopodia-like projections (Figure 5C).
DISCUSSION
The establishment and maintenance of cell polarity is important for the development and function of many cell types. The podocyte as part of the glomerular filtration barrier is one of the most highly differentiated and polarized cell types, divided morphologically and functionally into three distinct parts: The cell body and primary and secondary foot processes.19 The polarity signaling pathways that establish and maintain the specialized three-dimensional podocyte foot-process network of the filtration barrier, however, are largely unknown. These mechanisms not only seem important for the understanding of glomerular development and homeostasis, but also it is likely that repair mechanisms in response to glomerular damage use these programs. Here we analyzed a conditional aPKCλ/ι knockout mouse to define the critical role of polarity and aPKCλ/ι signaling for glomerular maintenance in vivo.
We identified the presence of both known aPKC isoforms in podocytes. Because aPKCζ-deficient mice display no renal phenotype and constitutive knockouts of aPKCλ/ι are embryonic lethal,13,14 we generated a podocyte-specific deletion of aPKCλ/ι for our studies. Mice with podocytes lacking aPKCλ/ι developed renal failure and proteinuria and lost their regular podocyte architecture at an early age. To our knowledge, this is the first genetic model to demonstrate the critical importance of known regulators of apicobasolateral polarity for glomerular maintenance. Remarkably, at birth, these mice displayed no proteinuria and relatively normal glomeruli, suggesting a maintenance rather than a developmental defect underlying this mouse model. Similar phenotypes have been described, for example, for CD2AP-deficient mice.20 One explanation for such a phenotype could be the challenge of the postnatal changes in hemodynamics and the sudden exposure to enormous mechanical stress, requiring the efficient and constant remodeling of podocyte foot processes21; however, there are alternative reasons to explain the lack of a developmental phenotype in our mouse model: First, podocytes seem to express both functionally redundant aPKC isoforms, and deletion of aPKCλ/ι could be compensated by aPKCζ during development. This functional redundancy has previously been observed and is an indicator for the evolutionary conservation of the functional aPKC domains.15 Second and perhaps more likely, the podocin promoter activates Cre expression relatively late during glomerular development,16,22 masking a developmental phenotype of podocyte-specific deletion of aPKCλ/ι.
During development, podocytes undergo extensive morphologic changes necessary to form the glomerular filtration barrier.17 By studying the localization of Par proteins during these morphologic changes, we identified an unprecedented apical-to-basal translocation of the Par polarity complex during glomerular development. This polarity conversion seems to be a unique feature of glomerular podocytes; as in other epithelial cells, Par3, aPKC, and apical junction markers such as ZO-1 always mark the apical membrane of the epithelial cell. Interestingly, this polarity conversion seemed to precede the targeting of the nephrin/podocin complex to the slit diaphragm and podocyte foot process development. In agreement with these data, an apical-to-basal migration of podocyte junctions preceding the formation of slit diaphragms was reported several decades ago.18 On the basis of our observations in the newborn rat, we propose the following model of sequential molecular steps of podocyte transformation: (1) Simple polygonal podocytes form a monolayer connected by tight junctions with an apicobasolateral polarity maintained by the localization of the Par3-Par6-aPKC complex at the apical cell surface; (2) the Par3-Par6-aPKC complex and tight junction proteins such as ZO-1 migrate and translocate to the basal side of the premature podocyte; (3) the Par complex subsequently controls the basal targeting of slit diaphragm molecules such as nephrin; (4) together, the Par complex and the slit diaphragm molecules initiate the formation of foot processes.
Indeed, aPKC localized to the leading edge and the tip of filopodia-like projections, suggesting an involvement of aPKC in foot process dynamics and process elongation during development. In neurons, aPKC has been shown to promote axonal process stabilization and formation by negatively regulating microtubule-regulating kinase, which in turn dephosphorylates τ, leading to increased microtubule assembly and elongation of axons.23
Recent studies have changed our conception of the podocyte from a relatively static to a dynamic epithelial cell, whose complex three-dimensional structure depends on signaling mechanisms at the filtration barrier. The coordinated translocation of the Par3-Par6-aPKC complex during podocyte development could potentially be a prerequisite for the development of foot processes and targeting of molecules to the slit diaphragm. Our findings demonstrate that aPKCλ/ι is essential for a normal podocyte homeostasis and suggest that aPKCλ/ι function is critical for differentiation and remodeling of podocyte foot processes.
CONCISE METHODS
Reagents and Plasmids
Mouse Neph1 and human nephrin were described previously.24,25 To create N-terminally GST-tagged constructs of the cytoplasmic domains, Nephrin (bp 3259 to 3723 and bp 3259 to 3480) and Neph1 (bp 1657 to 2367 and bp 1657 to 2358) were cloned in pGEX-4T-3 by standard cloning procedures. Polyclonal antibody specific for aPKCλ/ι was raised in rabbit by injection of aPKCλ/ι amino acids 184 to 234 and was described previously.26 Antibodies were obtained from Acris (anti-Nephrin guinea pig pAb, BP5030; Acris, Herford, Germany), Sigma (anti-Podocin rabbit pAb, P0372; anti-FLAG mouse mAb, M2, F4049, Sigma, St. Louis, MO), Invitrogen (anti-ZO-1 mouse mAb, ZO1–1A12, 33-9100; anti–ZO-1 rabbit pAb, Z-R1, 61-7300, Invitrogen, Carlsbad, CA), Upstate Biotechnology (anti-Par3 rabbit pAb, 07-330; anti-WT1 mouse mAb, 6F-H2, 05-753, Upstate Biotechnology, Lake Placid, NY), Santa Cruz Biotechnology (anti-aPKCλ/ι mouse mAb, H-12, sc-17837; anti-aPKCλ/ι rabbit pAb, H-76, sc-11399, Santa Cruz Biotechnology, Santa Cruz, CA), Chemicon (anti-Nidogen rat mAb, MAB1946, Chemicon, Temecula, CA), and Abcam (anti-WT1 rabbit pAb, ab15249, Abcam, Cambridge, MA). Secondary antibodies, actin, and nuclear staining reagents were obtained from Invitrogen (To-Pro-3, T3605; Alexa Fluor 546 phalloidin, A22283; Alexa Fluor 488 goat anti-guinea pig IgG, A11073; Alexa Fluor 555 goat anti-rat IgG, A21434; Alexa Fluor 555 donkey anti-rabbit IgG, A31572; Alexa Fluor 488 donkey anti-rabbit, A21206; Alexa Fluor 555 donkey anti-mouse IgG, A31570; Alexa Fluor 488 donkey anti-mouse IgG, A21202).
Generation of Podocyte-Specific aPKCλ/ι Knockout Mice
loxP sites flanking the nucleotides 110 through 233 were inserted and embryonic stem cells containing the floxed PKCλ/ι allele were used to generate mice with germline-transmitted floxed PKCλ/ι, and these mice were crossed to mice harboring a podocin-regulated Cre-recombinase16,27 to generate heterozygous and homozygous podocyte-specific PKCλ/ι-deficient mice and littermates. The functionality of this approach using the same aPKCλ/ι floxed mouse in combination with a different cre-mouse line was previously reported.15 The podocin Cre mice and the aPKC λ/ι floxed mice have been published previously.15,16 All mice, the podocin Cre mice as well as the floxed aPKC λ/ι mice, were crossed on a pure C57/Bl6 background.
RT-PCR
mRNA was isolated from mouse glomeruli, from adult C57BL/6 mice by using the dynabead method,28 or from differentiated immortalized mouse podocytes using RNA easy kit (Qiagen, Hilden, Germany).29 mRNA was transcribed in cDNA by reverse transcriptase. The following PCR primers were used: aPKCλ/ι fp CGGCATGTGTAAGGAAGGAT, aPKCλ/ι rp GGCAAGCAGAATCAGACACA, aPKCζ fp AAGTGGGTGGACAGTGAAGG, aPKCζ rp TCGTGGACAAGCTCCTTCTT. No reverse transcriptase was added in the negative control.
Urinary Protein Measurements
BSA standard (1, 5, 10, and 20 μg) and 1 μl of urine of aPKCλ/ι WT and podocyte-specific knockout mice were separated by 10% SDS-PAGE. The gel was stained by Coomassie Blue.
Histology
Kidneys of aPKCλ/ι WT and podocyte-specific knockout mice were fixed in 4% paraformaldehyde and embedded in paraffin or in Lowicryl K4M resin (Electron Microscopy Sciences; Hatfield, PA) and further processed for periodic acid-Schiff staining or electron microscopy, respectively.
Immunofluorescence Staining of Kidney Sections
Because newborn rats display various stages of glomerular development,18 rat kidneys of 1-d-old Wistar rats or mouse kidneys of aPKCλ/ι WT and podocyte-specific knockout mice were frozen in OCT compound and sectioned at 6 μm (Leica Kryostat; Leica, Wetzlar, Germany). The sections were fixed with 4% paraformaldehyde, blocked in PBS containing 5% BSA, and incubated for 1 h with primary antibodies as indicated. After PBS rinse several times, fluorophore-conjugated secondary antibodies were applied for 30 min. Confocal images were taken using a Zeiss laser scan microscope equipped with a ×63 water immersion objective.
Pulldown Assay
Mouse kidneys were lysed in a 1% Triton X-100 lysis buffer containing 20 mM CHAPS and glass homogenized. After centrifugation (15,000 × g, 15 min, 4°C), ultracentrifugation at 100,000 × g (30 min, 4°C), and preclearing with glutathione Sepharose beads (GE Healthcare, Amersham, United Kingdom), the supernatant was incubated for 2 h at 4°C with 4 to 8 μg of recombinant purified GST protein or fusion proteins of GST and the full-length cytoplasmic domain of Nephrin (amino acids 1087 through 1241), a disease-causing mutant truncation (amino acids 1087 through 1160),24 the full-length cytoplasmic domain of Neph1 (amino acids 553 through 789), or a truncation lacking the PDZ domain–binding motif (amino acids 553 through 786), respectively, prebound to glutathione Sepharose beads. Bound proteins were separated by 7% SDS-PAGE, and precipitated aPKCλ/ι was visualized by anti-aPKCλ/ι antibody. Equal loading of recombinant proteins was confirmed by Coomassie Blue staining of the gels.
Immunofluorescence Staining of Podocytes
Immortalized human podocytes split on Collagen A–coated cover glasses were fixed with 4% paraformaldehyde,30 permeabilized with 0.1% Triton X-100 in PBS, blocked in PBS containing 5% BSA, and incubated for 1 h with primary antibodies as indicated. After PBS rinse several times, fluorophore-conjugated secondary antibodies were applied for 30 min. Confocal images were taken using a Zeiss laser scan microscope equipped with a ×63 water immersion objective.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This study was supported by Deutsche Forschungsgemeinschaft grants to T.B.H. (HU 1016/2-1 and SFB 592) and DFG grants to M.S. (SCHI 587/2-2 and SFB 566).
We thank Charlotte Meyer for excellent technical assistance. We thank Dr. Peter Mundel (Miami, FL) and Dr. Moin Saleem (Bristol, UK) for cultured mouse and human podocytes. We thank Dr. Larry Holzman (Ann Arbor, MI) for providing the podocin Cre mice.
Published online ahead of print. Publication date available at www.jasn.org.
T.B.H. and B.H. contributed equally to this work.
See related editorial, “Initial Insight on the Determinants of Podocyte Polarity,” on pages 683–685.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Joberty G, Petersen C, Gao L, Macara IG: The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T: A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2: 540–547, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR: Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. EMBO J 22: 1125–1133, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D: The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 20: 3738–3748, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henrique D, Schweisguth F: Cell polarity: The ups and downs of the Par6/aPKC complex. Curr Opin Genet Dev 13: 341–350, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Ohno S: Intercellular junctions and cellular polarity: The PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol 13: 641–648, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Macara IG: Par proteins: Partners in polarization. Curr Biol 14: R160–R162, 2004 [PubMed] [Google Scholar]
- 9.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S: Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol 152: 1183–1196, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wodarz A, Ramrath A, Grimm A, Knust E: Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol 150: 1361–1374, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, Chen F, Tao Y, Hu HS, Wang T, Luo ZG: Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol 9: 743–754, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Huber TB, Benzing T: The slit diaphragm: A signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J: Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell 8: 771–780, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Soloff RS, Katayama C, Lin MY, Feramisco JR, Hedrick SM: Targeted deletion of protein kinase C lambda reveals a distribution of functions between the two atypical protein kinase C isoforms. J Immunol 173: 3250–3260, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M: Muscle-specific knockout of PKC-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest 117: 2289–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Reeves W, Caulfield JP, Farquhar MG: Differentiation of epithelial foot processes and filtration slits: Sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978 [PubMed] [Google Scholar]
- 19.Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788–793, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Chen YM, Wang QJ, Hu HS, Yu PC, Zhu J, Drewes G, Piwnica-Worms H, Luo ZG: Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/atypical PKC complex in regulating hippocampal neuronal polarity. Proc Natl Acad Sci U S A 103: 8534–8539, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber TB, Kottgen M, Schilling B, Walz G, Benzing T: Interaction with podocin facilitates nephrin signaling. J Biol Chem 276: 41543–41546, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Sellin L, Huber TB, Gerke P, Quack I, Pavenstadt H, Walz G: NEPH1 defines a novel family of podocin interacting proteins. FASEB J 17: 115–117, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Helfrich I, Schmitz A, Zigrino P, Michels C, Haase I, le Bivic A, Leitges M, Niessen CM: Role of aPKC isoforms and their binding partners Par3 and Par6 in epidermal barrier formation. J Invest Dermatol 127: 782–791, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.