Abstract
The micronutrient selenium is found in proteins as selenocysteine (Sec), the 21st amino acid cotranslationally inserted in response to a UGA codon. In vitro studies in archaea and mouse showed that Sec-tRNASec formation is a 3-step process starting with serylation of tRNASec by seryl-tRNA synthetase (SerRS), phosphorylation of serine to form phosphoserine (Sep)-tRNASec by phosphoseryl-tRNASec kinase (PSTK), and conversion to Sec-tRNASec by Sep-tRNA:Sec-tRNA synthase (SepSecS). However, a complete study of eukaryotic selenoprotein synthesis has been lacking. Here, we present an analysis of Sec-tRNASec formation in the parasitic protozoon Trypanosoma brucei in vivo. Null mutants of either PSTK or SepSecS abolished selenoprotein synthesis, demonstrating the essentiality of both enzymes for Sec-tRNASec formation. Growth of the 2 knockout strains was not impaired; thus, unlike mammals, trypanosomes do not require selenoproteins for viability. Analysis of conditional RNAi strains showed that SerRS, selenophosphate synthase, and the Sec-specific elongation factor, EFSec, are also essential for selenoprotein synthesis. These results with T. brucei imply that eukaryotes have a single pathway of Sec-tRNASec synthesis that requires Sep-tRNASec as an intermediate.
Keywords: phosphoseryl-tRNASec kinase, selenocysteine tRNA, Sep-tRNA:Sec-tRNA synthase, Trypanosoma brucei, selenoprotein
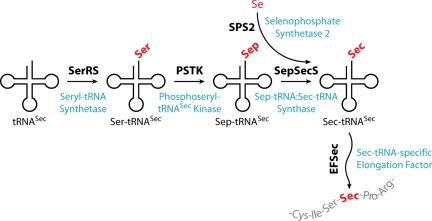
Selenium is an essential dietary trace element. It is present in proteins as selenocysteine (Sec), a cotranslationally-inserted amino acid encoded by UGA. Sec is not attached directly to tRNASec, but is formed by the tRNA-dependent conversion of serine (reviewed in refs. 1–4). In the first step tRNASec is misacylated by seryl-tRNA synthetase (SerRS). The subsequent conversion to Sec proceeds by 2 different pathways in nature. In bacteria Ser-tRNASec is directly transformed to Sec-tRNASec in a pyridoxal-5′-phosphate-dependent reaction by Sec synthase, the selA gene product (5). Archaea and eukaryotes require an additional step, the formation of the intermediate phosphoserine (Sep) by phosphoseryl-tRNASec kinase (PSTK). The resultant Sep-tRNASec is then converted into the Sec-tRNASec by the pyridoxal-5′-phosphate-dependent enzyme Sep-tRNA:Sec-tRNA synthase (SepSecS) (6, 7). The Se donor for this reaction, selenophosphate, is synthesized by selenophosphate synthase (SPS2) (8). Then EFSec, the tRNASec-specific elongation factor, carries the Sec-tRNASec to the ribosome (9) where a translational recoding process allows UGA to be read as Sec (Fig. 1). Selenoproteins are found in organisms from all 3 domains of life. Humans have 25 selenoproteins, many of them are essential for organismal viability (10). Some selenoproteins are predicted to be redox proteins containing catalytic Sec residues (e.g., glutathione peroxidase or thioredoxin reductase).
Fig. 1.
tRNASec-dependent amino acid transformations leading to Sec in eukaryotes as elucidated by using recombinantly produced mammalian components (6, 7).
Most of our knowledge of eukaryotic Sec-tRNASec formation comes from in vitro reconstitution experiments using components of mammalian cells (7). In vivo formation of eukaryotic Sec-tRNASec has been addressed in a study that showed that RNAi-mediated ablation of SepSecS in mammalian cells did not completely abolish selenoprotein expression (11). Thus, the existence of an alternative SepSecS-independent pathway for Sec-tRNASec formation could not be excluded. Moreover, the in vivo role of PSTK has not yet been analyzed.
Here, we present a comprehensive analysis of the in vivo role of the 5 major proteins required for eukaryotic Sec-tRNASec formation and function. The study was done in the insect form of the parasitic protozoon Trypanosoma brucei as double allelic KO cell lines can easily be produced in this system by homologous recombination-directed gene replacements (12). Because T. brucei is diploid, double KO cell lines are null mutants in both alleles. Furthermore, RNAi-based methods for highly efficient inducible ablation of proteins are also available (13). Moreover, T. brucei is an excellent model for exploring eukaryotic diversity. It represents a different branch of the eukaryotic evolutionary tree than the phylogenetically more closely related classical model organisms (e.g., mouse, Drosophila, Caenorhabitits elegans, and yeast) (14).
Results
T. brucei Components Involved in Selenoprotein Formation.
A bioinformatic analysis of the T. brucei genome predicts 3 selenoproteins. They include distant homologs of mammalian SelK and SelT and a selenoprotein, termed SelTryp, that is specific for the kinetoplastid line (15). Moreover, in silico screens by several groups have identified Tb-tRNASec, Tb-SerRS, Tb-PSTK, Tb-SepSecS, Tb-SPS2, and Tb-EFSec, the trypanosomal orthologues of essentially all major components of the Sec-inserting system (16). However, of these only Tb-SerRS, Tb-tRNASec (16–18), and Tb-SPS2 (19) have been subject to preliminary experimental analyses.
To analyze the Sec-tRNASec formation pathway in vivo and establish its physiological importance for T. brucei we used RNAi cell lines allowing inducible ablation of Tb-SerRS, Tb-SPS2, and Tb-EFSec. Moreover, we prepared KO cell lines that lack either Tb-PSTK or Tb-SepSecS, the 2 core components of the eukaryotic Sec-tRNASec formation pathway (Fig. S1). All cell lines were analyzed for selenoprotein synthesis by labeling with radioactive 75Se (Fig. 2). The Tb-SerRS-RNAi cell line had been analyzed before by other methods, and it was shown that Tb-SerRS activity is required to charge the Tb-tRNASec with serine (18). Labeling of uninduced Tb-SerRS-RNAi cells with 75Se and subsequent analysis by Tris-Tricine polyacrylamide gels revealed 3 bands whose molecular mass are consistent with the 3 predicted trypanosomal selenoproteins SelK, SelT, and SelTryp (Fig. 2A). SelTryp migrated in some experiments slightly faster than expected (Fig. 2 B and C). We do not know why but the simplest explanation is proteolytic clipping during sample preparation, which could also account for the simultaneous presence of both bands as seen in Fig. 2B, lane 2d.
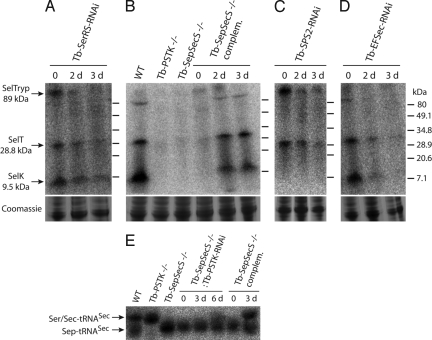
Fig. 2.
Selenoprotein expression in various T. brucei cell lines. Expression was analyzed by 75Se labeling of living cells and subsequent analysis of the labeled proteins by 10–20% polyacryamide Tris-Tricine gels. The following cell lines were analyzed. (A) Uninduced and induced Tb-SerRS-RNAi cells. (B) WT 427 cells, Tb-PSTK KO cells (Tb-PSTK−/−), Tb-SepSecS KO cells (Tb-SepSecS−/−), and a Tb-SepSecS KO cell line allowing inducible ectopic expression of Tb-SepSecS (Tb-SepSecS −/− complem.). (C) Uninduced and induced Tb-SPS2-RNAi cells. (D) Uninduced and induced Tb-EFSec-RNAi cells. For the RNAi and the complemented Tb-SepSecS −/− cell lines days of induction (d) by tetracycline are indicated. The putative identity of the 3 labeled selenoproteins and their molecular mass as predicted in silico are indicated on the left. Molecular mass markers are indicated on the right. Segments of the tubulin region (≈50–80 kDa) of the corresponding Coomassie-stained gels are shown as loading controls. (E) Total RNA isolated for the cell lines analyzed in B and a Tb-SepSecS KO line capable of RNAi-mediated ablation of Tb-PSTK (Tb-SepSecS −/−:Tb-PSTK RNAi) was separated on a long acidic urea gel and analyzed for the presence of the different forms of tRNASec by Northern analysis (18). The positions of the Sep-tRNASec and the comigrating Ser/Sec-tRNASec are indicated.
Induction of Tb-SerRS-RNAi causes, in line with the role of SerRS in serylation of tRNASec, an efficient but incomplete reduction of the selenoprotein labeling (Fig. 2A). This finding is in contrast to the Tb-PSTK KO and Tb-SepSecS KO cell lines in which the labeling of the 3 selenoproteins was abolished (Fig. 2B). Moreover, tetracycline-inducible ectopic expression of Tb-SepSecS in the Tb-SepSecS KO cell line restored 75Se labeling of all 3 proteins. In the case of SelTryp and SelT the restoration was to wild-type level, whereas in the case of SelK the complementation was not quantitative. These experiments show that both Tb-PSTK and Tb-SepSecS are indispensable for selenoprotein synthesis.
Next, we isolated and analyzed aminoacylated tRNAs from the different cell lines by acid urea polyacrylamide electrophoresis (Fig. 2E) (20). This technique allows the separation of the faster-migrating Sep-tRNASec from the slower-migrating Ser-tRNASec and Sec-tRNASec species. Distinction between the latter 2 species is not possible as they comigrate. The Northern blot analysis in Fig. 2E shows 2 tRNASec bands in wild-type cells. The lower one corresponds to Sep-tRNASec and the upper corresponds to Sec-tRNASec (as inferred from the pattern observed in the PSTK and SepSecS KO cell lines). In the Tb-PSTK KO cell line only the upper band is detected, which most likely represents the Ser-tRNASec form, because this species is expected to accumulate in the absence of PSTK activity (see Fig. 1). In the Tb-SepSecS-KO cell line, however, only the Sep-tRNASec band is detected. Moreover, if Tb-PSTK is depleted by inducible RNAi in the Tb-SepSecS-KO cell line, a time-dependent partial recovery of the upper band is observed, which indicates that Ser-tRNASec accumulates as would be expected in the absence of both PSTK and SepSecS. Moreover, the upper band, which is absent in the KO cells, reappears if the KO cell line is complemented by inducible ectopic expression of Tb-SepSecS. Thus, the accumulation of distinct intermediates of the Sec-tRNASec synthesis pathway in the 2 KO cell lines together with the 75Se-labeling experiments show that the Tb-PSTK and Tb-SepSecS act sequentially in the indicated order (Fig. 1).
SPS2 generates selenophosphate, the Se donor required by SepSecS. Mammalian SPS2, which itself is a selenoprotein, is essential for selenoprotein synthesis in vivo (8). Tb-SPS2 an SPS2 orthologue capable of complementing SelA-deficient Escherichia coli has been identified in trypanosomatids (19). Like the E. coli orthologue, but in contrast to its mammalian counterpart, Tb-SPS2 is not a selenoprotein. Fig. 2C shows that RNAi-mediated ablation of the Tb-SPS2 severely impairs selenoprotein synthesis, indicating that SPS2 (with a cysteine in place of the Sec) is catalytically active in trypanosomes. Surprisingly, even before the induction of RNAi we reproducibly see labeling of SelTryp and SelT but not of SelK. It is possible that the SPS2-RNAi cell line is leaky and that even in the absence of tetracycline a fraction of the SPS2 mRNA is partially down-regulated. If SelK labeling is more sensitive to SPS2 levels than labeling of the other 2 trypanosomal selenoproteins its absence could be explained. Indeed selenium labeling of SelK shows a larger variation in the different cell lines than that of the other 2 selenoproteins, indicating that it might be quite sensitive to small changes of the labeling conditions (compare Fig. 2A, lane 0 with Fig. 2B, lane WT). However, without further experiments this explanation must remain speculative at present.
Sec-tRNASec is an elongator tRNA; however, it does not interact with elongation factor 1a but requires its own elongation factor, EFSec. Consequently, ablation of Tb-EFSec by RNAi essentially abolishes selenoprotein synthesis (Fig. 2D).
Selenoprotein Synthesis in T. brucei Is Not Essential.
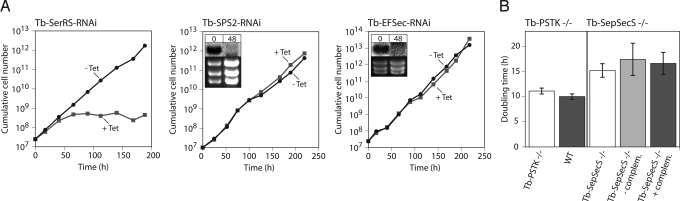
Selenoproteins are found in all 3 domains, indicating that they were acquired early in the evolution of life (4). However, in all domains there are many species that then lost the Sec-inserting system (e.g., fungi and plants), which raises the question of whether organisms that possess a Sec-inserting system require selenoproteins for viability. Clearly, this is the case in mammals, because a tRNASec KO mouse has an embryonic lethal phenotype (21). In other systems, however, this question has not been rigorously addressed. With our T. brucei cell lines described above we now can ask whether selenoproteins are essential for this unicellular eukaryote. Therefore, we analyzed the effects RNAi-mediated ablation of Tb-SerRS, Tb-SPS2, and Tb-EFSec (Fig. 3A) and the effects the complete lack of Tb-PSTK and Tb-SepSecS (Fig. 3B) have on T. brucei growth. Ablation of Tb-SerRS caused a growth arrest, which can be explained by the down-regulation of Ser-tRNASer levels with concomitant reduction and cessation of protein synthesis (18). Growth of all other RNAi and KO cell lines was not or only marginally impaired. For the RNAi cell lines this is not very informative because of the possible residual activity of the ablated enzymes. However, the normal growth of the Tb-PSTK and Tb-SepSecS double KO cell lines clearly demonstrates that T. brucei does not depend on selenoproteins.
Fig. 3.
Trypanosomal selenoproteins are not essential. (A) Representative growth curves in standard culture medium SDM-79 of uninduced and induced (−Tet, +Tet) clonal RNAi cell lines for Tb-SerRS (redrawn from ref. 18), Tb-SPS2, and Tb-EFSec. The Northern blots verifying mRNA ablation are indicated for the cell lines not showing a growth arrest. (B) (Left) Comparisons of doubling times of the Tb-PSTK KO cell line (Tb-PSTK−/−) and the parent WT strain 427 (WT). (Right) Comparisons of doubling times of the Tb-SepSecS KO cell line (Tb-SepSecS−/−) and the corresponding cell line allowing tetracycline-dependent ectopic Tb-SepSecS expression (SepSec −/− plus or minus complem.). The parent cell line for the Tb-SepSecS KO strain is T. brucei 29-13 that has a longer doubling time than T. brucei 427. Standard errors (n = 6) are indicated.
Conserved Sec-tRNASec Formation in Eukaryotes and Archaea.
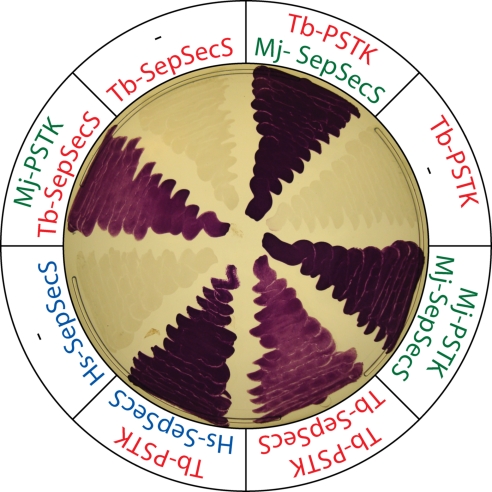
When grown anaerobically E. coli produces selenium-dependent formate dehydrogenase (FDH). Its activity can easily be visualized on plates overlaid with benzyl viologen, because in the presence of formate active FDH reduces benzyl viologen to a blue substance (22). Using this assay it was shown that an E. coli selA deletion strain could be rescued by coexpression of archaeal PSTK and human or archaeal SepSecS (6). We extended these studies and showed that Tb-PSTK in combination with either endogenous T. brucei SepSecS or the corresponding archaeal or human enzymes reconstitute selenoprotein synthesis in an E. coli selA deletion strain (Fig. 4). Likewise, archaeal PSTK and Tb-SepSecS also restored FDH activity in the special E. coli strain. Taken together, these results underscore the highly conserved nature of the PSTK/SepSecS pathway of Sec-tRNASec formation in Archaea and eukaryotes.
Fig. 4.
Expression of SepSecS together with PSTK restores FDH activity in an anaerobically-grown E. coli selA deletion strain. The indicated genes of the following organisms were tested: T. brucei (Tb-PSTK, Tb-SepSecS), the Archeaon Methanocaldococcus jannaschii (Mj-PSTK, Mj-SepSecS), and humans (Hs-SepSecS).
Discussion
In vitro experiments indicate that PSTK and SepSecS are the core components for Sec biosynthesis in eukaryotes (6, 7). However, the in vivo analysis of Sec-tRNASec formation in eukaryotes has been hampered by the lack of a suitable system. Here, we have used T. brucei to produce KO cell lines for the trypanosomal orthologues of PSTK and SepSecS. Analysis of these cell lines has shown that formation of Sec-tRNASec in living T. brucei requires the sequential action of Tb-PSTK and Tb-SepSecS. It remains unknown why eukaryotes use the additional phosphorylation step and thus require 2 enzymes, even though direct conversion of Ser-tRNASec to Sec-tRNASec is possible, as exemplified in bacteria (5).
Our results also show that no alternative pathway for Sec-tRNASec formation exists that is independent of Tb-SepSecS and Tb-PSTK. Interestingly, RNAi-mediated ablation of SepSecS had only a marginal effect on mammalian selenoprotein synthesis (11). However, the results obtained in T. brucei might not be readily comparable with the study in mammalian cells for the following reason. In T. brucei selenoprotein synthesis was assayed in a double KO cell line completely devoid of Tb-SepSecS, whereas in mammalian cells the analysis might have been obscured by the fact that RNAi cannot be relied on to completely deplete protein levels. Thus, it is likely that even in mammalian cells, just as in T. brucei, only a single pathway for Sec-tRNASec formation is operational.
Insect-stage T. brucei lacking selenoproteins grew as well as wild type in standard medium SDM-79, which shows that normal growth of T. brucei does not require selenoproteins and indicates that neither Tb-PSTK nor Tb-SepSecS have a second essential role that is unlinked to Sec-tRNASec formation. The unimpaired growth of selenoprotein-lacking T. brucei was unexpected, because it had been reported that both insect- and bloodstream-stage T. brucei cells were sensitive to nanomolar concentrations of auranofin, a compound suggested to inactivate selenoproteins (15). T. brucei is the causative agent of human sleeping sickness. Based on the reported auranofin experiments it was proposed that selenoprotein synthesis might be a novel target for development of an antiparasitic drug (15). Because auranofin interacts with selenol and thiol groups (23), we suggest that auranofin-induced cell death might have been caused by the drug's interaction with thiols. All of our experiments were carried out in insect-stage T. brucei cells; therefore it is still possible that Sec is essential in T. brucei bloodstream forms, but it seems unlikely because all 3 predicted selenoproteins are already expressed in the insect-stage form. Thus, the prospect of using the pathway of Sec or selenoprotein synthesis as drug targets against T. brucei is questionable. However, because T. brucei in its normal habitat adapts to situations and environments that are difficult to reproduce in the laboratory, selenoproteins may play a role under such conditions.
In summary, this work illustrates that T. brucei is an experimentally highly-tractable system for examining the in vivo formation of eukaryotic Sec-tRNASec. Our analysis revealed the presence of a single pathway for Sec-tRNASec synthesis involving SerRS, PSTK, and SepSecS and strengthens the notion that all eukaryotes have only a single route to Sec. Furthermore, we show that selenoproteins are dispensable for insect-stage T. brucei under standard growth conditions.
Materials and Methods
Cell Culture.
Procyclic T. brucei, strain 427 and strain 29-13, and the corresponding transgenic cell lines were grown at 27 °C in SDM-79 (24) supplemented with 5% and 15% FCS, respectively.
Transgenic Cell Lines.
RNAi cell lines were produced by using pLew-100-based stem loop constructs containing the puromycin resistance gene (25–27). As inserts we used a 451-bp fragment (nucleotides 181–632) for the Tb-SPS2 gene and a 528-bp fragment of the Tb-EFSec gene (nucleotides 1–528). Production and initial characterization of Tb-SerRS RNAi cell line has been described (18). The double allelic replacements of the Tb-PSTK gene, in T. brucei 427, and of the Tb-SepSecS gene, in T. brucei 29–13, are described in Fig. S1. Inducible ectopic expression of Tb-SepSecS was done by using pLew-100 carrying the phleomycin resistance gene (25). Transfection, selection of transformants, and production of clonal cell lines were done by using standard procedures as described (12).
75Selenium Labeling.
A total of 5 × 107 cells was resuspended in 0.5 mL of FCS-supplemented SDM-79. The cultures were labeled with 9.6 μCi of Hepes-neutralized [75Se] selenite (University of Missouri Research Reactor, Columbia) in the presence of 100 μg/mL cysteine at 27 °C for 3 h (for the KO strains) or 1 h (for the RNAi cell lines). After the labeling, the culture was washed with PBS, and the resulting pellet was resuspended in sample buffer and heated to 100 °C for 10 min. Finally, the labeled proteins (≈107 cell equivalents) were analyzed on 10–20% Tris-tricine gels (Ready gel; Bio-Rad) and visualized with a phosphoimager.
Acid Gel Analysis of tRNASec Population.
Total RNA was isolated as described (29) and resupended in 10 mM Na-acetate, pH 4. Isolated RNA corresponding to 8 × 106 cell equivalents was run on a 50-cm-long acidic sequencing gel as described (20). The gel was run at 4 °C in 0.1 M Na-acetate, pH 5 until the xylene blue front migrated to ≈28 cm from the top. The section of the gel containing the RNA was blotted to a Genescreen plus membrane and analyzed by Northern blots using oligonucleotide hybridization as described (28).
Benzyl Viologen Assay for Active FDH.
Benzyl viologen-dependent FDH assays were performed as described (6). The PSTK and SepSecS genes of the different species were cloned into the pACYCDuet-1 and pET15b expression vectors (Novagen), respectively. In strains expressing only 1 gene, the corresponding empty plasmid was cotransformed as a control.
Supplementary Material
Acknowledgments.
We thank G. Cross (The Rockefeller University, New York) for cell lines and plasmids and Isabel Roditi (University of Bern) for helpful discussions. This work was supported by Swiss National Foundation Grant 3100-067906 (to A.S.) and National Institutes of Health Grants AI028798 (to E.U.) and GM22854 (to D.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901575106/DCSupplemental.
References
- 1.Commans S, Böck A. Selenocysteine inserting tRNAs: An overview. FEMS Microbiol Rev. 1999;23:335–351. doi: 10.1111/j.1574-6976.1999.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 2.Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatfield DL, et al. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog Nucleic Acid Res Mol Biol. 2006;81:97–142. doi: 10.1016/S0079-6603(06)81003-2. [DOI] [PubMed] [Google Scholar]
- 4.Su D, et al. How an obscure archaeal gene inspired the discovery of selenocysteine biosynthesis in humans. IUBMB Life. 2009;61:35–39. doi: 10.1002/iub.136. [DOI] [PubMed] [Google Scholar]
- 5.Forchhammer K, Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J Biol Chem. 1991;266:6324–6328. [PubMed] [Google Scholar]
- 6.Yuan J, et al. RNA-dependent conversion of phosphoserine forms selenocysteine in eukaryotes and archaea. Proc Natl Acad Sci USA. 2006;103:18923–18927. doi: 10.1073/pnas.0609703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu XM, et al. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007;5:e4. doi: 10.1371/journal.pbio.0050004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu XM, et al. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem J. 2007;404:115–120. doi: 10.1042/BJ20070165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forchhammer K, Leinfelder W, Böck A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989;342:453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- 10.Kryukov GV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 11.Xu XM, et al. Evidence for direct roles of two additional factors, SECp43 and soluble liver antigen, in the selenoprotein synthesis machinery. J Biol Chem. 2005;280:41568–41575. doi: 10.1074/jbc.M506696200. [DOI] [PubMed] [Google Scholar]
- 12.Beverley SM, Clayton CE. Transfection of Leishmania and Trypanosoma brucei by electroporation. Methods Mol Biol. 1993;21:333–348. doi: 10.1385/0-89603-239-6:333. [DOI] [PubMed] [Google Scholar]
- 13.Ullu E, Tschudi C, Chakraborty T. RNA interference in protozoan parasites. Cell Microbiol. 2004;6:509–519. doi: 10.1111/j.1462-5822.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 14.Dacks JB, Walker G, Field MC. Implications of the new eukaryotic systematics for parasitologists. Parasitol Int. 2008;57:97–104. doi: 10.1016/j.parint.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Lobanov AV, Gromer S, Salinas G, Gladyshev VN. Selenium metabolism in Trypanosoma: Characterization of selenoproteomes and identification of a Kinetoplastida-specific selenoprotein. Nucleic Acids Res. 2006;34:4012–4024. doi: 10.1093/nar/gkl541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassago A, et al. Identification of Leishmania selenoproteins and SECIS element. Mol Biochem Parasitol. 2006;149:128–134. doi: 10.1016/j.molbiopara.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Bouzaidi-Tiali N, et al. Elongation factor 1a mediates the specificity of mitochondrial tRNA import in T. brucei. EMBO J. 2007;26:4302–4312. doi: 10.1038/sj.emboj.7601857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geslain R, et al. Trypanosoma seryl-tRNA synthetase is a metazoan-like enzyme with high affinity for tRNASec. J Biol Chem. 2006;281:38217–38225. doi: 10.1074/jbc.M607862200. [DOI] [PubMed] [Google Scholar]
- 19.Sculaccio SA, et al. Selenocysteine incorporation in Kinetoplastid: Selenophosphate synthetase (SELD) from Leishmania major and Trypanosoma brucei. Mol Biochem Parasitol. 2008;162:165–171. doi: 10.1016/j.molbiopara.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Varshney U, Lee C-P, RajBhandary UL. Direct analysis of aminoacylation levels of tRNAs in vivo. J Biol Chem. 1991;266:24712–24718. [PubMed] [Google Scholar]
- 21.Bösl MR, et al. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci USA. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacourciere GM, Levine RL, Stadtman TC. Direct detection of potential selenium delivery proteins by using an Escherichia coli strain unable to incorporate selenium from selenite into proteins. Proc Natl Acad Sci USA. 2002;99:9150–9153. doi: 10.1073/pnas.142291199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rigobello MP, Scutari G, Boscolo R, Bindoli A. Induction of mitochondrial permeability transition by auranofin, a Gold(I)-phosphine derivative. Br J Pharmacol. 2002;136:1162–1168. doi: 10.1038/sj.bjp.0704823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brun R, Schönenberger M. Cultivation an in vitro cloning of procyclic culture forms of Trypansoma brucei in a semidefined medium. Acta Tropica. 1979;36:289–292. [PubMed] [Google Scholar]
- 25.Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knockouts and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC, et al. Replication of kinetoplast DNA: An update for the new millennium. Int J Parasitol. 2001;31:453–458. doi: 10.1016/s0020-7519(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 27.Bochud-Allemann N, Schneider A. Mitochondrial substrate level phosphorylation is essential for growth of procyclic Trypanosoma brucei. J Biol Chem. 2002;277:32849–32854. doi: 10.1074/jbc.M205776200. [DOI] [PubMed] [Google Scholar]
- 28.Tan THP, et al. tRNAs in Trypanosoma brucei: Genomic organization, expression, and mitochondrial import. Mol Cell Biol. 2002;22:3707–3717. doi: 10.1128/MCB.22.11.3707-3716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.