Abstract
The direct observation of dynamic ligand exchange beween Pt-N coordination-driven self-assembled supramolecular polygons (triangles and rectangles) has been achieved using stable isotope labeling (1H/2D) of the pyridyl donors and electrospray ionization mass spectrometry (ESI-MS) together with NMR spectroscopy. Both the thermodynamic and kinetic aspects of such exchange processes have been established based on quantitative mass spectral results. Further investigation showed that the exchange is highly dependent on experimental conditions such as temperature, solvent, and the counter anions.
During the last two decades, coordination-driven self-assembly has become a well-established methodology in supramolecular chemistry for constructing ensembles of varying structural motifs, as witnessed by the development of diverse metallo-supramolecular helicates, polygons, and polyhedra.1 While most investigations largely focus on the structural features of these supramolecules, reports about the dynamic characteristics of such structures are limited.2 Yet the dynamic nature of self-assembly is widely recognized as a significant feature of the supramolecular assemblies.3 Detailed mechanistic studies are not only important for the understanding of self-assembly processes, but also crucial to the use of supramolecular assemblies in applications such as the construction of constitutional dynamic libraries,4 the self-assembly of supramolecular polymers,5 and the ability to control supramolecular transformations.6
One of the most fundamental features of the dynamic nature of coordination-driven and indeed all supramolecular self-assembly, is the dynamic component exchange—the mutual exchange of the molecular components between the supramolecular assemblies under thermodynamic control.3b,c Although such constitutional dynamic features have been routinely used in explaining experimental results and developing theoretical understandings of supramolecular self-assembly,7 very few examples have been reported that directly and quantitatively characterized such exchanges in self-assembled supramolecules.8 Very recently, Rebek and coworkers demonstrated an innovative way to characterize such dynamic features of supramolecular capsules using the fluorescent resonance energy transfer (FRET) technique, but these studies have so far been limited to hydrogen bonding driven self-assembly.8 Such reports are rare,2f for coordination-driven self-assembly, likely because of lack of a suitable characterizing method.
Isotope labeling is an appropriate tool to characterize dynamic exchanges in metallo-supramolecular assemblies. Indeed, isotope labeling has been widely used in biological studies, such as proteomics, used in the quantitative study of proteins based on mass spectral characterization.9 Herein, we demonstrate an isotope labeling-based method capable of direct and quantitative characterization of the constitutional dynamic feature of coordination-driven self-assembly, using electrospray ionization mass spectrometry (ESI-MS). This method allows for a thorough study of the poorly understood dynamic nature of Pt-N coordination-driven self-assembly.1b,e,10 With this technique, the dynamic ligand exchange process, as well as the subsequent equilibration under thermodynamic control can be directly characterized. Moreover, based on quantitative mass spectral results, the kinetics of the exchange process has also been investigated.
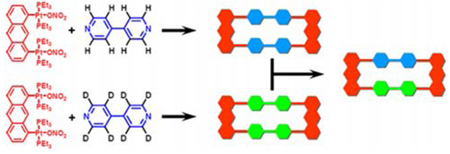
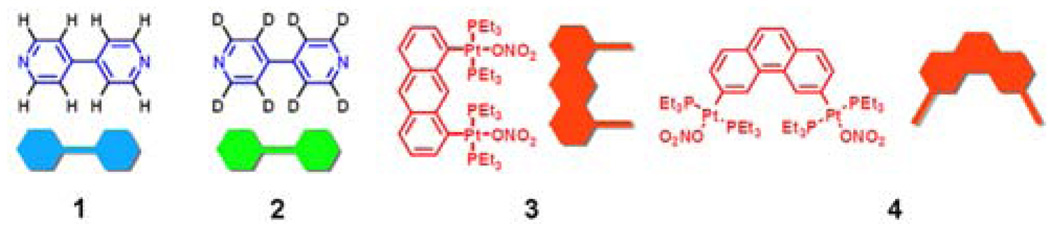
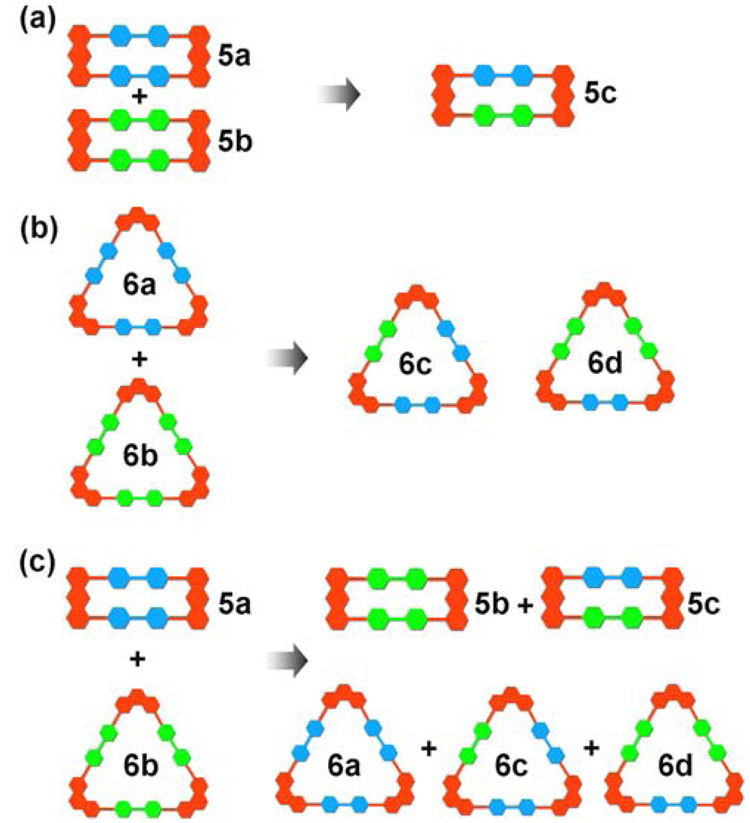
We have employed the isotope labeled (1H/2D) pyridyl ligands 1 and 2 (Figure 1) and electro-spray ionization mass spectrometry to observe and characterize the dynamic ligand exchange of supramolecular polygons 511 and 612 assembled by Pt-N coordination bonding interactions. Combining 1 or 2 with organoplatinum acceptors 3 or 4 results in the self-assembly of isotopically pure supramolecular polygons 5a/b or 6a/b. As shown in Scheme 1, mixing homoisotopic rectangles (5a or 5b) and/or homoisotopic triangles (6a or 6b) leads, due to ligand exchange, to heteroisotopic polygons 5c or 6c and 6d, which can be easily distinguished by mass spectral analysis. More importantly, the relative distribution of these isotopically varying supramolecular entities in the mixture can be quantitatively calculated based on the different intensities of their corresponding spectral signals.
Figure 1.
Schematic and molecular structures of linear dipyridyl donors 1 and 2 as well as 0° and 60° organoplatinum acceptors 3 and 4, respectively.
Scheme 1.
Representation of the dynamic ligand exchange between the same (a and b) and different types (c) of supramolecular polygons (rectangles: 5a and 5b; triangles: 6a and 6b) with isotope label (1H / 2D).
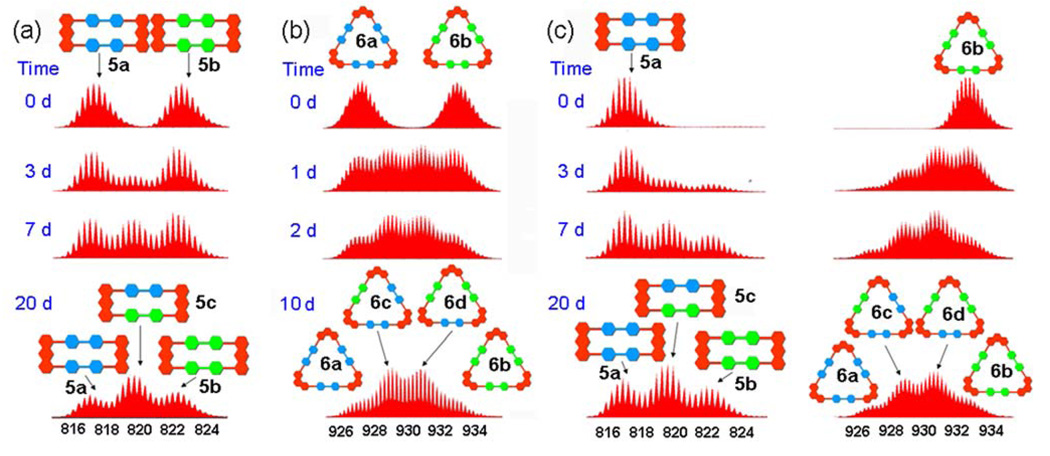
In order to explore the dynamic ligand exchange between the supramolecular polygons sharing the same motif, individually prepared 5a/b or 6a/b were mixed in a 1:1 raio and heated up to 64 ± 1 °C in an aqueous acetone solution (v/v 1:1) (Scheme 1a and b). ESI-MS was used to monitor ligand exchange over a period of days (Figure 2a and 2b as well as Figure S1–Figure S2 in Supporting Information). Initially, in each mixture, only those signals corresponding to homoisotopic polygons (5a: 817.3; 5b: 822.7; 6a: 927.3; 6b: 933.3) were observed and in similar intensity. Upon heating at 64 ± 1 °C, signals corresponding to polygons 5c (820.0), 6c (929.3), and 6d (931.3) could be resolved, clearly indicating dynamic ligand exchange between the supramolecules. After 10–20 d the dynamic ligand exchange processes reached equilibrium as indicated by the unchanging ESI-MS signals. The resulting equilibrium mixtures represented, in each case (Figure S1–2), statistical product distributions: rectangles: 5a: 5c: 5b = 0.518: 1.00: 0.580 (theoretical value: 1: 2: 1); triangles: 6a: 6c: 6d: 6b = 0.334: 1.00: 0.962: 0.326 (theoretical value: 1: 3: 3: 1)13.
Figure 2.
ESI-MS spectra of dynamic ligand exchange between (a) supramolecular rectangles (5a and 5b), (b) supramolecular triangles (6a and 6b), and (c) both rectangle 5a and triangle 6b recorded at different time intervals (d: day).
Similar mass spectral studies were also carried out in a system containing supramolecular polygons 5a and 6b in different motifs capable of self-sorting.10a Dynamic ligand exchange between these different polygons was observed, as indicated by the increase of mass spectral signals corresponding to 5b, 5c, 6a, 6c, and 6d (Figure 2c and Figure S3) besides the peaks for 5a and 6b, upon heating the mixture at 64 ± 1 °C in an aqueous acetone solution (v/v 1:1) for 20 d.
In addition to the mass spectral characterization, supportive NMR spectral studies were also employed to follow these exchange processes. For ligand exchange between structures in the same motif (5a/b or 6a/b), as expected, no significant change could be found in either the 31P{1H} or 1H NMR spectra (Figure S4 and S5) during the exchange. Dynamic ligand exchange between 5a and 6b causes an increase in the signals at 8.52 ppm, 8.71 ppm, and 9.18 ppm and a decrease of signals at 8.32 ppm and 9.08 ppm in the 1H NMR spectra (Figure S6) and no change in the 31P{1H} NMR spectra as the result of the formation of triangles (6a, 6c, and 6d) and rectangles (5b and 5c). These results indicate that only component exchange between the discrete supramolecules occurred without generating any disordered byproduct.
These combined results from ESI mass spectrometry and NMR spectroscopy not only unambiguously support the dynamic ligand exchange of these metallosupramolecular polygons, but also directly demonstrate the subsequent equilibration under thermodynamic control. For example, for the exchange reaction of rectangles (5a + 5b ⇆ 2 5c), constitutional dynamic exchange between these rectangles allows for the redistribution of the isotopically different 5a, 5b, and 5c from the initial input ratio of 1: 1: 0 towards the equilibrium value of 1: 1: 2, which agrees well with the thermodynamic equilibrium constant of K = 4 based on statistical factors.14 Due to the similar intrinsic stabilities of 5a, 5b, and 5c, such a dynamic redistribution process is mainly entropy-driven.
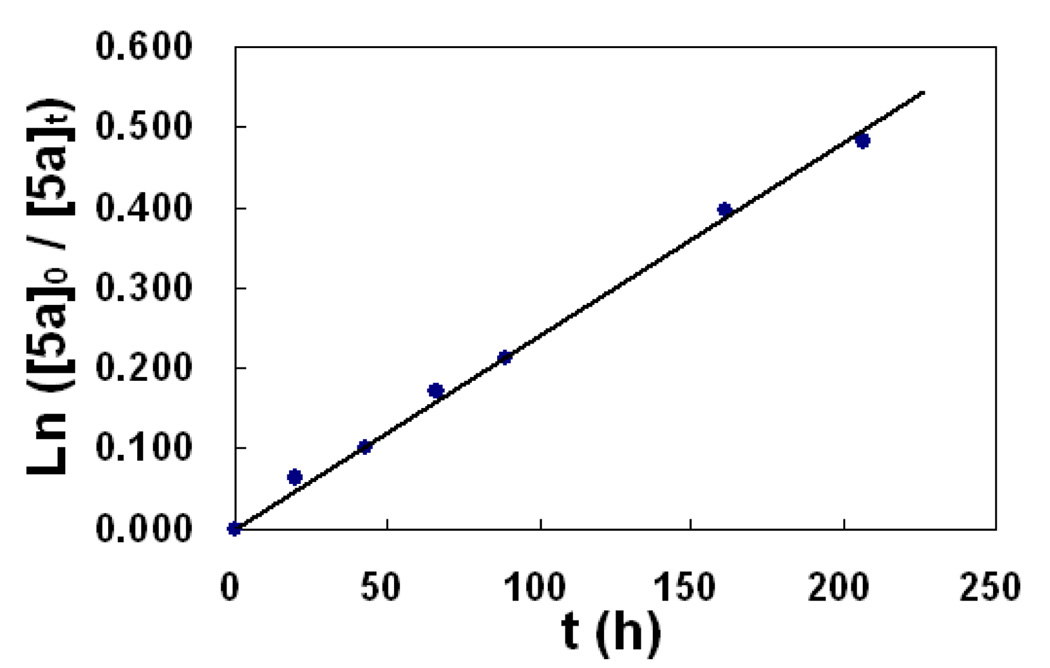
Furthermore, quantitative mass spectral results acquired over time allow for the kinetics of supramolecular dynamic ligand exchange to be determined. Analysis of the data (Figure S7 and Table S1 in Supporting Information) shows a first-order kinetic process with a rate of k = 0.0024 h−1 for the dynamic ligand exchange between 5a and 5b as shown in Figure 3 (R2 = 0.99). Presumably, the first-order exchange kinetics corresponds to the ring-opening of the rectangular supramolecules upon nucleophilic attack on the Pt-N coordination bond by a nitrate anion as the rate determining step. Nitrate anions and the cationic rectangle are likely to form an ion pair in the acetone solution,15 and the ring-opening step could be consequently considered as an intramolecular process of the ion pair, whereby the first-order kinetics can be established.
Figure 3.
Plot of ESI-MS data obtained over 240 h displaying the first-order exchange kinetics for supramolecular rectangles 5a and 5b.
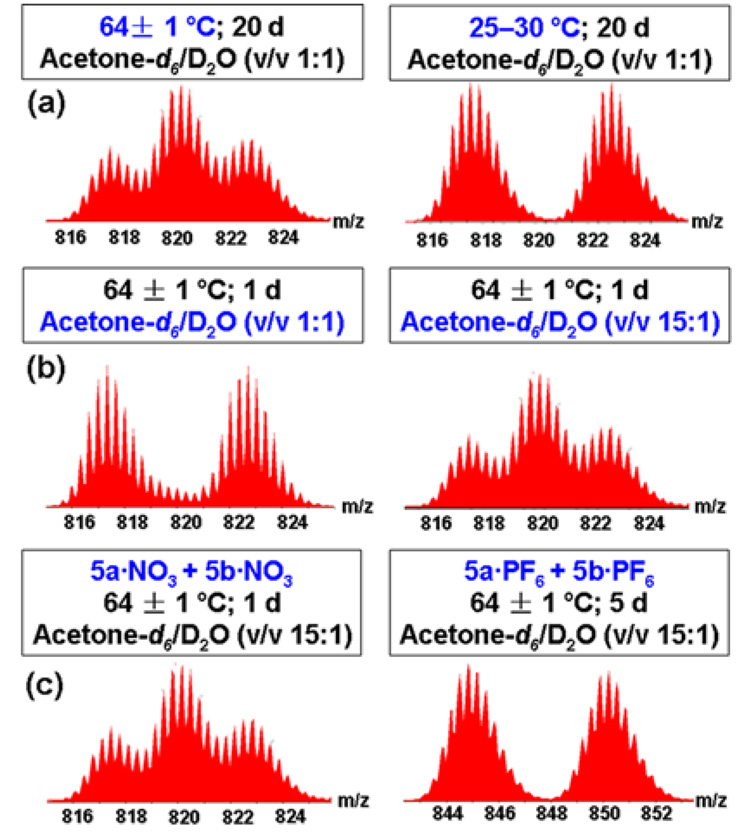
We have also explored the influence of temperature, solvent, and counter anion on the ligand exchange process between 5a and 5b. As may be expected, decreasing the temperature significantly slows the exchange process. At 25–30 °C the exchange is no longer observed (Figure 4a), even after 20 d. Interestingly, as seen in Figure 4b, decreasing the percentage of water in the solution can significantly accelerate the rate of the exchange process. Upon heating at 64 ± 1 °C in an aqueous acetone solution (v/v 15:1), the exchange process reaches equilibrium within 1 d. Additionally, changing the counter anion from nitrate (NO3−) to hexafluorophosphate (PF6−) anions results in no exchange as observed by ESI-MS spectra (Figure 4c).The influence of solvent and counter anion further substantiates the role of the nitrate anions in the rate-determining step of the ligand exchange process: nitrate anions are more nucleophilic when fewer water molecules are present, and when the counter anion is exchanged from NO3− to PF6−, the dynamic exchange does not proceed as PF6− is a less nucleophilic anion.
Figure 4.
ESI-MS spectra of ligand exchange between 5a and 5b influenced by differences in (a) temperature, (b) solvent, and (c) counter anion.
In conclusion, we have directly demonstrated the constitutional dynamic exchange of Pt-N coordination-driven self-assembled supramolecular polygons (triangles and rectangles) using stable isotope labeling (1H/2D) of the pyridyl donors and ESI mass spectrometry together with NMR spectroscopy. Both the thermodynamic and kinetic aspects of such exchange processes have been established based on quantitative mass spectral results. Further investigation showed that, as expected, the exchange is highly dependent on experimental conditions such as temperature, solvent, and the counter anions. The isotope labeling-based mass spectral technique described here represents a new way of the direct and quantitative study of supramolecular dynamics.
Supplementary Material
Supporting Information Available: Experimental details, ESI-MS trace spectra, and NMR spectra of the dynamic exchange process; details of the kinetic experiments. This material is available free of charge via the Internet at http://pubs.acs.org/.
Acknowledgments
P.J.S. thanks the NIH (GM-057052) for financial support. We thank Dr. Brian H. Northrop and Dr. Liang Zhao for helpful discussions, and Dr. James G. Muller for assistance with mass spectral characterization.
References
- 1.(a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502. [Google Scholar]; (b) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (c) Holliday BJ, Mirkin CA. Angew. Chem. Int. Ed. 2001;40:2022. [PubMed] [Google Scholar]; (d) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509. [Google Scholar]; (e) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (f) (b) Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem. Int. Ed. 2004;43:3644. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]; (g) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:351. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; (h) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (i) Lukin O, Voegtle F. Angew. Chem. Int. Ed. 2005;44:1456. doi: 10.1002/anie.200460312. [DOI] [PubMed] [Google Scholar]; (j) Severin K. Chem. Commun. 2006:3859. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (k) Nitschke JR. Acc. Chem. Res. 2007;40:103. doi: 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]; (l) Pitt MA, Johnson DW. Chem. Soc. Rev. 2007;36:1441. doi: 10.1039/b610405n. [DOI] [PubMed] [Google Scholar]; (m) Northrop BH, Yang H-B, Stang PJ. Chem. Commun. 2008:5896. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Schmittel M, Mahata K. Angew. Chem. Int. Ed. 2008;47:5284. doi: 10.1002/anie.200800583. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hori A, Akasaka A, Biradha K, Sakamoto S, Yamaguchi K, Fujita M. Angew. Chem. Int. Ed. 2002;41:3269. doi: 10.1002/1521-3773(20020902)41:17<3269::AID-ANIE3269>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]; (b) Yamamoto T, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:12309. doi: 10.1021/ja0302984. [DOI] [PubMed] [Google Scholar]; (c) Davis AV, Raymond KN. J. Am. Chem. Soc. 2005;127:7912. doi: 10.1021/ja051037s. [DOI] [PubMed] [Google Scholar]; (d) Davis AV, Fiedler D, Seeber G, Zahl A, Van Eldik R, Raymond KN. J. Am. Chem. Soc. 2006;128:1324. doi: 10.1021/ja056556+. [DOI] [PubMed] [Google Scholar]; (e) Pluth MD, Raymond KN. Chem. Soc. Rev. 2006;36:161. doi: 10.1039/b603168b. [DOI] [PubMed] [Google Scholar]; (f) Claessens CG, Vicente-Arana MJ, Torres T. Chem. Commun. 2008:6378. doi: 10.1039/b815898c. [DOI] [PubMed] [Google Scholar]; (g) Levin MD, Stang PJ. J. Am. Chem. Soc. 2000;122:7428. [Google Scholar]
- 3.(a) Lehn J-M. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4763. doi: 10.1073/pnas.072065599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Davis AV, Yeh RM, Raymond KN. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4793. doi: 10.1073/pnas.052018299. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lehn J-M. Chem. Soc. Rev. 2007;36:151. doi: 10.1039/b616752g. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wheaton CA, Jennings MC, Puddephatt RJ. J. Am. Chem. Soc. 2006;128:15370. doi: 10.1021/ja066450u. [DOI] [PubMed] [Google Scholar]; (b) Chow C-F, Fujii S, Lehn J-M. Angew. Chem. Int. Ed. 2007;46:5007. doi: 10.1002/anie.200700727. [DOI] [PubMed] [Google Scholar]; (c) Friese VA, Kurth DG. Coord. Chem. Rev. 2008;252:199. [Google Scholar]
- 5.(a) Barboiu M, Vaughan G, Graff R, Lehn J-M. J. Am. Chem. Soc. 2003;125:10257. doi: 10.1021/ja0301929. [DOI] [PubMed] [Google Scholar]; (b) Giuseppone N, Schmitt J-L, Lehn J-M. J. Am. Chem. Soc. 2006;128:16748. doi: 10.1021/ja0666452. [DOI] [PubMed] [Google Scholar]
- 6.(a) Heo J, Jeon Y-M, Mirkin CA. J. Am. Chem. Soc. 2007;129:7712. doi: 10.1021/ja0716812. [DOI] [PubMed] [Google Scholar]; (b) Hiraoka S, Sakata Y, Shionoya M. J. Am. Chem. Soc. 2008;130:10058. doi: 10.1021/ja803115j. [DOI] [PubMed] [Google Scholar]; (c) Zhao L, Northrop BH, Stang PJ. J. Am. Chem. Soc. 2008;130:11886. doi: 10.1021/ja805770w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Ercolani G, Mandolini L, Mencarelli P, Roelens S. J. Am. Chem. Soc. 1993;115:3901. [Google Scholar]; (b) Ercolani G. J. Phys. Chem. B. 1998;102:5699. [Google Scholar]; (c) Ercolani G. J. Phys. Chem. B. 2003;107:5052. [Google Scholar]; (d) Ercolani G. J. Am. Chem. Soc. 2003;125:16097. doi: 10.1021/ja038396c. [DOI] [PubMed] [Google Scholar]
- 8.(a) Barrett ES, Dale TJ, Rebek J., Jr J. Am. Chem. Soc. 2007;129:3818. doi: 10.1021/ja0700956. [DOI] [PubMed] [Google Scholar]; (b) Barrett ES, Dale TJ, Rebek J., Jr J. Am. Chem. Soc. 2007;129:8818. doi: 10.1021/ja071774j. [DOI] [PubMed] [Google Scholar]; (c) Barrett ES, Dale TJ, Rebek J., Jr J. Am. Chem. Soc. 2008;130:2344. doi: 10.1021/ja078009p. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ong S-E, Foster LJ, Mann M. Methods. 2003;29:124. doi: 10.1016/s1046-2023(02)00303-1. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Ong S-E, Mann M. Nat. Chem. Biol. 2005;1:252. doi: 10.1038/nchembio736. and references therein. [DOI] [PubMed] [Google Scholar]
- 10.(a) Addicott C, Das N, Stang PJ. Inorg. Chem. 2004;43:5335. doi: 10.1021/ic049326p. [DOI] [PubMed] [Google Scholar]; (b) Yang H-B, Ghosh K, Northrop BH, Stang PJ. Org. Lett. 2007;9:1561. doi: 10.1021/ol070371l. [DOI] [PubMed] [Google Scholar]; (c) Zheng Y-R, Yang H-B, Northrop BH, Ghosh K, Stang PJ. Inorg. Chem. 2008;47:4706. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]; (d) Northrop BH, Yang H-B, Stang PJ. Inorg. Chem. 2008;47:11257. doi: 10.1021/ic801711q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehl CJ, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]
- 12.Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:5193. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- 13.We thank a reviewer for pointing out that the exchange ratio should be 1: 3: 3: 1, and not 1: 2: 2: 1, considering the symmetry number method in Ref. 14. For equilibrium: (2/3) 6a + (1/3) 6b ⇆ 6c, the symmetry number of both 6a and 6b is 6 (C3 and C2 axis), and that of 6c is 2 (C2 axis), and the statistical equilibrium constant is given by 6(2/3) × 6(1/3) / 2 = 3. Accordingly if the equilibrium concentration of 6a and 6b is taken as equal to 1, the equilibrium concentration of 6c would be 3. The same consideration holds for equilibrium: (1/3) 6a + (2/3) 6b ⇆ 6d. Thus, a theoretical distribution of 6a: 6c: 6d: 6b = 1: 3: 3: 1 is established.
- 14.Ercolani G, Piguet C, Borkovec M, Hamacek J. J. Phys. Chem. B. 2007;111:12195. doi: 10.1021/jp0740705. [DOI] [PubMed] [Google Scholar]
- 15.Vacek J, Caskey DC, Horinek D, Shoemaker RK, Stang PJ, Michl J. J. Am. Chem. Soc. 2008;130:7629. doi: 10.1021/ja801341m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental details, ESI-MS trace spectra, and NMR spectra of the dynamic exchange process; details of the kinetic experiments. This material is available free of charge via the Internet at http://pubs.acs.org/.