Abstract
Summary
We examined BMC and body composition in 1,209 black, Hispanic, and white men. Weight, BMI, waist circumference, and fat mass were associated with BMC only up to certain thresholds, whereas lean mass exhibited more consistent associations. The protective influence of increased weight appears to be driven by lean mass.
Introduction
Reduced body size is associated with decreased bone mass and increased fracture risk, but associations in men and racially/ethnically diverse populations remain understudied. We examined bone mineral content (BMC) at the hip, spine, and forearm as a function of body weight, body mass index (BMI), waist circumference, fat mass (FM), and nonbone lean mass (LM).
Methods
The design was cross-sectional; 363 non-Hispanic black, 397 Hispanic, and 449 non-Hispanic white residents of greater Boston participated (N=1,209, ages 30–79 y). BMC, LM, and FM were measured by DXA. Multiple linear regression was used to describe associations.
Results
Weight, BMI, waist circumference, and FM were associated with BMC only up to certain thresholds. LM, by contrast, displayed strong and consistent associations; in multivariate models, femoral neck BMC exhibited a 13% increase per 10 kg cross-sectional increase in LM. In models controlling for LM, positive associations between BMC and other body composition measures were eliminated. Results did not vary by race/ethnicity.
Conclusions
The protective effect of increased body size in maintaining bone mass is likely due to the influence of lean tissue. These results suggest that maintenance of lean mass is the most promising strategy in maintaining bone health with advancing age.
Keywords: Aging, Bone, Bone densitometry, Epidemiology, Population studies
Introduction
Osteoporosis and related fractures have long been recognized as substantial public health problems facing white women. Recent years have witnessed their emergence as concerns for male and racially/ethnically diverse populations as well [1–4], and emerging evidence suggests that racial/ethnic variation in bone material and its rate of age-related decline may partly explain corresponding differences in fracture risk [5–9]. Despite these advances, however, the epidemiology of male osteoporosis and bone fragility remains understudied [1, 10–12], and there remain few comprehensive assessments in diverse male populations [13–16].
Abundant epidemiological evidence links body composition to bone mass in older adults [17–22], and increased BMI has been linked to lower rates of osteoporosis and fracture [23]. Likewise, prospective investigations have demonstrated associations between declines in body size and both bone loss and increases in fracture risk [24–26]. These investigations, however, are for the most part limited either to racially/ethnically homogenous populations, to specific skeletal sites, or to specific functions of body size such as body mass index (BMI). In addition, closer examination of the components of body composition has not produced consensus; it remains unclear whether fat mass or lean mass is more strongly associated with bone mass. While several studies imply that lean mass is of greater importance [27–35], others are more equivocal, or indicate that fat mass plays the more prominent role, especially in women [22, 32, 33, 36–41].
To shed further light on these issues, we conducted a study of the association between hip, spine, and forearm bone mineral content (BMC) and several measures of body composition (total weight, BMI, waist circumference, total fat mass, and total and proportionate lean mass) among Hispanic, non-Hispanic black, and non-Hispanic white men living in Boston, MA, USA. The objective of the analysis was to determine whether associations between body composition and BMC were consistent at different skeletal sites, for subjects of differing races/ethnicities, and across the full range of body size parameters.
Methods
Study sample
We analyzed data on men enrolled in the BACH/Bone study, a cross-sectional investigation of bone health in aging men, with data obtained on subjects previously enrolled in the parent Boston Area Community Health (BACH) survey. The BACH survey employed a randomly selected cohort of 5,506 male and female residents of greater Boston, MA, with data collected between April 2002 and June 2005. Subjects’ ages ranged from 30 to 79 y.
BACH required that subjects be living in the community, be of self-identified Hispanic ethnicity and/or black or white race, and be fluent in English or Spanish. Potential BACH subjects were identified using year 2000 U.S. federal census records and contacted by phone screen and field visits to determine eligibility and willingness to participate. Data were collected during early-morning in-home study visits. A total of 24,063 households were contacted; approximately 275,400 phone calls and 74,300 field attempts were made by bilingual study staff. Additional details have been previously published [42].
BACH enrollment was divided into five groups or “batches” of subjects [42]; male enrollees in all but the first batch were considered for enrollment in BACH/Bone, yielding a pool of 1,948 potential BACH/Bone subjects. Enrollment in BACH/Bone required that subjects weigh no more than 300 lbs, be able to travel to the Boston University School of Medicine (BUSM), consent to a dual energy X-ray absorptiometry (DXA) scan and additional measurements, and be able to lift themselves onto the scan table.
Forty subjects were excluded because their weight exceeded 300 lbs., 17 because they were unable to lift themselves onto the scan table, six because they had recently moved, and an additional eight for other reasons. Of the remaining 1,877 eligible subjects, 1,219 (65%) agreed to enroll in BACH/Bone; DXA scans were obtained on the 1,209 subjects whose data are presented here. Additional details have been published elsewhere [13]. Written informed consent was obtained from each subject (independently for both BACH and BACH/Bone), and study subjects received $100 and $75 remuneration for participation in BACH and BACH/Bone, respectively. The BACH protocol was approved by the Institutional Review Board (IRB) of New England Research Institutes (NERI), and the BACH/Bone protocol was approved by IRBs at NERI and BUSM.
Data collection
During BACH data collection, each subject’s age, self-assessed general health, smoking history and alcohol consumption were obtained through self-report. Physical activity was measured using the Physical Activity Scale for the Elderly (PASE) [43]. Waist circumference was obtained using a 200 cm measuring tape.
Data collection for BACH/Bone occurred at the BUSM General Clinical Research Center (GCRC). Subjects executed a written informed consent and GCRC staff administered a brief questionnaire concerning general bone health. Subjects’ height (to the nearest 0.1 cm) and weight (to the nearest 0.1 kg) were obtained using a stadiometer and digital scale. Strength and physical function were assessed using the seven-item physical performance test (PPT) score [44] and the repeated chair stands test [45], and grip strength was measured using a hydraulic hand dynamometer (Sammons Preston, Bolingbrook, Illinois).
The median time between BACH and BACH/Bone data collection visits was 36 days.
DXA
BMC, bone mineral density (BMD), and total body (excluding head) fat and nonfat mass were measured by DXA using a QDR 4500 W densitometer (Hologic, Inc., Waltham, MA). For the purposes of this report, we focus on the femoral neck, L1–L4, and 1/3 distal radius, although results for other sites are similar. The DXA system was calibrated daily. Total lean mass was obtained by subtracting total body BMC from total body nonfat mass (each excluding the head). Proportionate lean mass was defined as the total lean mass divided by the sum of total lean and total fat mass.
Race/ethnicity
Race/ethnicity was determined via self-identification according to U.S. Office of Management and Budget guidelines [46].
Analysis sample and statistical methodology
Nine forearm, 16 hip, and 21 lumbar spine measurements could not be obtained for reasons such as the presence of artifacts or subject discomfort, leaving 1,200 forearm, 1,193 hip, and 1,188 lumbar spine values for study. The BACH survey employed a multistage stratified cluster random sampling scheme [42], with sampling probabilities adjusted to insure nearly equal numbers of subjects by race/ethnicity and four age groups (30–39 y, 40–49 y, 50–59 y, 60–79 y). Each subject’s data were, therefore, assigned analytic weight in inverse proportion to that subject’s combined probability of sampling and subsequent enrollment in BACH/Bone, so that summary statistics, such as means, standard deviations (SD), and sample proportions, as well as regression results, are representative of the greater Boston population. (Though we did not hypothesize that the associations under study would be influenced by sampling weights, we include them here for consistency with the study design and to enhance the applicability of the findings to the community-dwelling population [42]. We note, in addition, that this treatment of the data enforces a higher bar for statistical significance of results, as acknowledging sampling weights increases estimates of variance [47]). Weighted statistical analyses were conducted using SUDAAN 9.0.1 (Research Triangle Institute, Research Triangle Park, NC, USA). Visual data displays presented here were constructed using Splus version 7.0 (Insightful Corp., Seattle, WA, USA).
Because the distribution of BMC displayed mild right-skew, we employed linear regression analysis of log (base e) transformed BMC on covariates, yield weighted regression estimates β*. Percent differences in BMC associated with differences in covariates were estimated using the monotonic transformation [exp (β*) − 1] × 100, and with corresponding 95% confidence intervals (CI). Results were considered statistically significant if appropriate null hypotheses could be rejected at the 0.05 level and/or CIs indicated that data were incompatible with null values. The statistical significance of regression effects was determined using CIs and Wald-type test statistics.
Results
Sample characteristics are presented in Table 1. The mean (SD) subject age was 47.7 (12.8) years; the sample was comprised of 363 black men, 397 Hispanic men, and 449 white men. Overall, white subjects displayed the greatest mean weight, height, waist circumference, and total fat mass, whereas black subjects displayed the greatest mean total and proportionate lean mass.
Table 1.
BACH/Bone study sample characteristics, overall and by race/ethnicity (N=1,209)
| Mean ± standard deviationa, or percenta | ||||
|---|---|---|---|---|
| Variable | Overall | Black (N = 363; 30%b) | Hispanic (N = 397; 33%b) | White (N = 449; 37%b) |
| Age, y | 47.7±12.8 | 48.0±12.4 | 44.6±11.0 | 48.3±13.2 |
| Education, y | 15.1±4.1 | 13.4±3.0 | 12.1±4.9 | 16.4±3.7 |
| Household income | ||||
| <$10 k | 14.7 | 24.8 | 23.5 | 8.9 |
| $10k–29,9 k | 22.5 | 25.6 | 28.9 | 19.9 |
| $30 k–69,9 k | 32.6 | 33.3 | 32.5 | 32.4 |
| ≥$70 k | 30.2 | 16.4 | 15.2 | 38.8 |
| Marital status | ||||
| Married | 43.4 | 35.4 | 55.0 | 44.1 |
| Live with partner | 7.6 | 6.6 | 11.4 | 7.3 |
| Divorced/separated | 14.1 | 19.9 | 18.8 | 10.8 |
| Widowed | 2.8 | 2.4 | 0.9 | 3.3 |
| Single, never married | 32.1 | 35.8 | 14.0 | 34.5 |
| Height, cm | 175.8±7.4 | 174.9±7.3 | 169.6±6.1 | 177.4±6.9 |
| Weight, kg | 87.8±15.6 | 87.8±17.0 | 81.5±14.5 | 89.2±14.9 |
| Body mass index, kg/m2 | 28.4±4.7 | 28.7±5.1 | 28.3±4.8 | 28.3±4.5 |
| Waist circumference, cm | 97.4±12.3 | 95.8±13.1 | 94.9±11.7 | 98.6±11.9 |
| Fat mass, kg | 22.0±8.6 | 20.6±8.9 | 19.7±7.4 | 23.1±8.5 |
| Lean mass, kg | 55.2±7.7 | 56.3±8.8 | 51.8±7.2 | 55.4±7.1 |
| Proportionate lean mass, % | 72.3±6.8 | 74.3±7.6 | 73.1±6.1 | 71.3±6.5 |
| General health, self-assessed | ||||
| Excellent | 20.2 | 16.0 | 16.5 | 22.6 |
| Very good | 35.7 | 28.5 | 22.5 | 41.4 |
| Good | 30.1 | 37.9 | 34.9 | 25.9 |
| Fair / Poor | 14.1 | 17.7 | 26.1 | 10.1 |
| Cigarette smoking, pack yr | 17.3±24.2 | 14.1±19.1 | 13.3±28.1 | 19.5±25.2 |
| Alcohol, drinks/day | ||||
| None | 25.8 | 33.8 | 34.7 | 20.7 |
| <1 | 38.0 | 31.4 | 39.0 | 40.5 |
| 1–3 | 27.0 | 21.0 | 19.3 | 31.0 |
| >3 | 9.2 | 13.9 | 7.0 | 7.8 |
| Physical performance test (PPT) | 25.5±2.7 | 24.8±3.0 | 24.9±2.7 | 25.9±2.5 |
| Physical activity scale for the elderly (PASE) | 186.1±109.0 | 188.4±113.4 | 189.9±110.1 | 184.3±107.2 |
| Time to 5 chair stands, s | ||||
| ≤11.1 | 20.5 | 14.6 | 18.4 | 23.3 |
| 11.2–13.5 | 26.2 | 29.6 | 31.2 | 23.7 |
| 13.6–16.7 | 30.1 | 27.6 | 24.5 | 32.3 |
| >16.7 | 20.1 | 22.0 | 22.6 | 18.8 |
| Unable to complete | 3.1 | 6.2 | 3.3 | 1.8 |
| Grip strength, lbs | 37.0±10.9 | 38.0±11.9 | 34.9±9.2 | 37.0±10.7 |
Estimates weighted to be representative of greater Boston population (see Methods)
Hispanic and Black subpopulations oversampled. Weighted (representative) proportions: Black 25%, Hispanic 13%, White 62%
BMC is displayed by age and race/ethnicity in Table 2. As has been reported elsewhere [13], we observed substantial age-associated decreases in BMC at the femoral neck and distal radius, and while black male subjects exhibited the highest mean BMC at all sites, the steepest age-related trends were observed in the Hispanic subsample.
Table 2.
Summary bone mineral content by age, race/ethnicity, and skeletal site
| Mean ± standard deviationa | |||
|---|---|---|---|
| Femoral neck | Lumbar spine | 1/3 distal radius | |
| Age | |||
| 30–39 y | 5.04±0.80 | 70.04±12.47 | 2.39±0.42 |
| 40–49 y | 4.83±0.75 | 70.96±13.82 | 2.38±0.27 |
| 50–59 y | 4.68±0.90 | 71.02±15.44 | 2.37±0.31 |
| 60–69 y | 4.55±0.69 | 72.05±14.47 | 2.32±0.31 |
| 70–79 y | 4.44±0.75 | 75.12±16.42 | 2.29±0.31 |
| Race/Ethnicity | |||
| Black men | 5.05±0.88 | 73.52±14.60 | 2.51±0.31 |
| Hispanic men | 4.65±0.79 | 63.80±11.94 | 2.20±0.26 |
| White men | 4.76±0.78 | 71.62±13.63 | 2.35±0.36 |
Estimates weighted to be representative of greater Boston population (see Methods)
Exploratory analyses
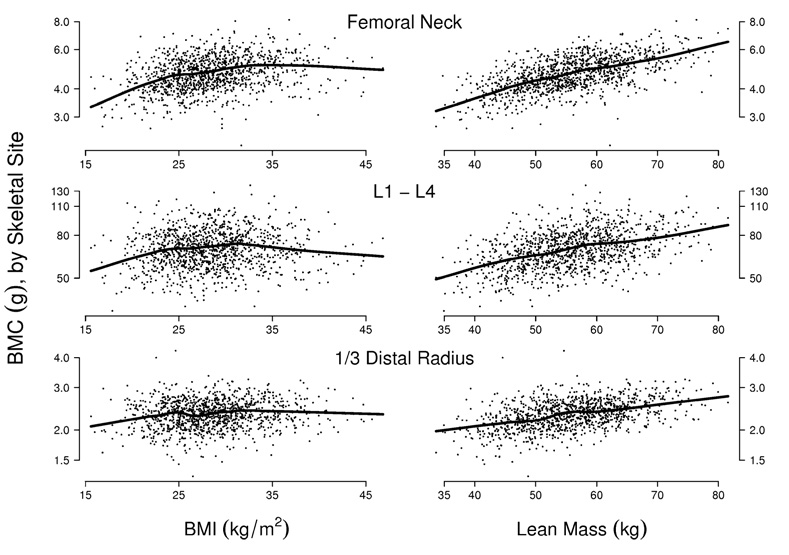
Graphical displays were employed to assess associations between BMC and the body composition variables. Results for BMI and total lean mass are presented in Fig. 1. We observe positive associations between BMI and BMC among the majority of subjects (left panels). However, among the subjects with the greatest BMI, the association is attenuated, so that a nonparametric fit [48] drawn through the data indicates no positive association between BMC and BMI above approximately 35 kg/m2. This threshold phenomenon was consistent across race/ethnicity (not shown). By contrast, there was a consistent linear association between BMC and lean mass (right panels); this too was consistent across race/ethnicity.
Fig. 1.
Bone mineral content (BMC) versus body mass index (BMI) and lean mass, by skeletal site. Semiparametric scatter-plot smooths (black lines) indicate that BMI displays a positive association with BMC up to approximately 35 kg/m2, whereas the association between total lean mass and BMC is positive over the entire range of lean mass values. The association between BMC and body composition is weaker at the wrist than at other sites
Exploratory results for all other parameters were similar to those for BMI. The association between BMC and body size was attenuated above approximately 105 kg (weight), 110 cm (waist circumference), 30 kg (total fat mass), and 70% (proportionate lean mass). We also observed that the association between lean mass and other parameters tended to be stronger below the relevant thresholds than above; for instance, the age-adjusted partial correlation between lean mass and BMI was approximately 0.64 among subjects with BMI < 35 kg/m2, but only 0.33 for subjects with BMI ≥ 35 kg/m2.
Multiple regression analyses
Because, in prior BACH/Bone analyses, age-related declines in bone mass appeared to be steeper among Hispanic than non-Hispanic subjects [13], we included main effects for age and race/ethnicity as well as their two-way interactions in all regression models.
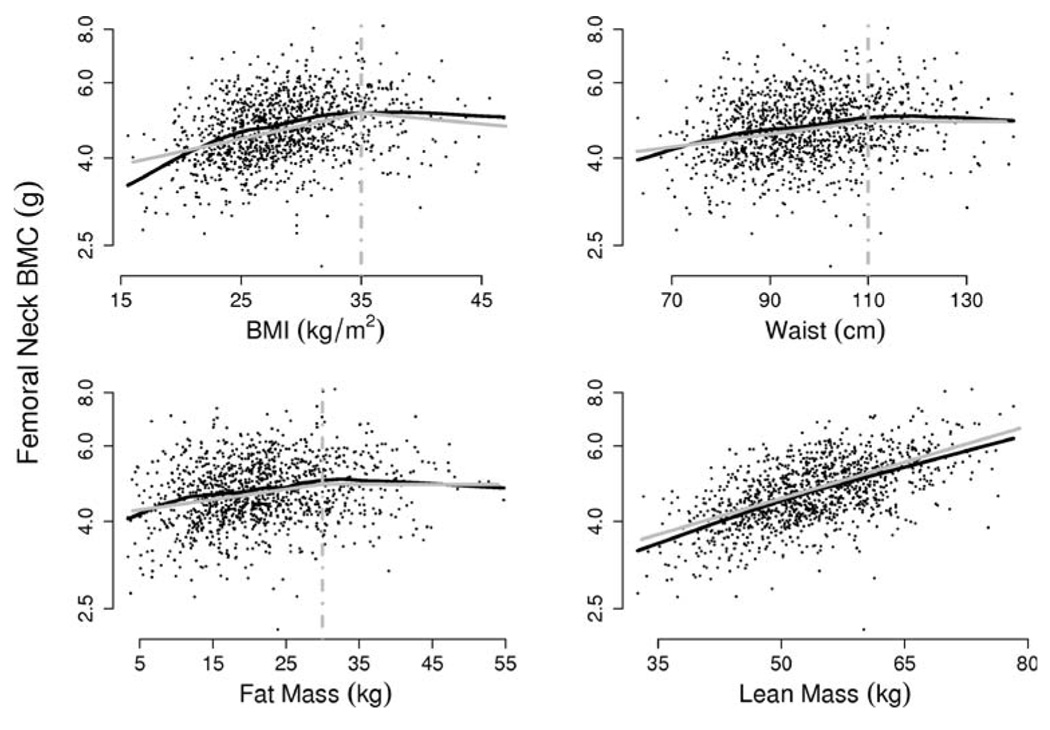
We approximated the trends in mean BMC versus body size variables with linear splines, using single knots at the threshold values given above. Figure 2 indicates that, for femoral neck BMC, the resulting estimated trends agree closely with nonparametric estimates, while a linear regression (with no knot) is appropriate to describe the association between BMC and total lean mass. Results for other sites were similar.
Fig. 2.
Femoral neck BMC versus body shape and composition. Unadjusted associations (black lines, estimated using semiparametric scatterplot smooths) are well approximated by linear spline models (gray lines) with single knots at 35 kg/ m2, 110 cm, 30 kg for BMI, waist circumference, and fat mass, respectively. A single linear trend accurately describes the association between total lean mass and BMC (lower right panel)
The left-hand columns of Table 3 provide the resulting estimated trends in mean BMC as a function of body shape and composition, adjusted for age, race/ethnicity, and all other covariates. Taking the first row of the table as an example, we observe that models estimate a 16.5% positive cross-sectional trend in mean femoral neck BMC per 10 kg/m2 increase in BMI up to 35 kg/m2, while above 35 kg/m2 there is a statistically significant negative trend of −9.2% femoral neck BMC per 10 kg/m2 increase in BMI (the significance of the latter estimate is indicated by the corresponding confidence interval’s disinclusion of the zero value). The data constitute highly significant evidence of a difference in cross-sectional BMI / femoral neck BMC associations between subjects whose BMI is below the 35 kg/m2 threshold and those whose BMI is above that value, as indicated by the significance test and associated p-value reported to the right of the regression estimates.
Table 3.
Multiple regression: Cross-sectional estimates of associations between BMC and body composition
| Predictor | Site | Models controlling for covariatesa |
Models controlling for covariatesa and total lean mass |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Below thresholdc(%) |
95% CI | Above thresholdc(%) |
95% CI | p - vald | Below thresholdc (%) |
95% CI | Above thresholdc(%) |
95% CI | p - vald | ||
| BMI (10 kg/m2)b | Femoral neck | 16.5 | 12.6, 20.5 | −9.2 | −11.7, −6.7 | <0.001 | −0.6 | −4.4, 3.4 | −16.1 | −23.7, −7.6 | 0.003 |
| Lumbar spine | 7.8 | 3.6, 12.2 | −16.7 | −19.0, −14.4 | <0.001 | −11.5 | −15.9, −6.9 | −23.5 | −32.3, −13.6 | 0.04 | |
| 1/3 distal radius | 3.8 | 1.0, 6.7 | −2.3 | −4.4, −0.1 | 0.12 | −7.3 | −10.6, −3.8 | −8.8 | −15.0, −2.1 | 0.72 | |
| Weight (10 kg)b | Femoral neck | 6.1 | 5.1, 7.1 | 0.6 | −2.1, 3.4 | 0.001 | −0.2 | −2.1, 0.7 | −3.3 | −6.0, −0.7 | 0.05 |
| Lumbar spine | 5.2 | 4.0, 6.5 | −4.2 | −6.8, −1.5 | <0.001 | −2.1 | −4.3, −0.1 | −8.5 | −11.2, −5.8 | <0.001 | |
| 1/3 distal radius | 2.2 | 1.2, 3.2 | −0.5 | −2.6, 1.7 | 0.05 | −3.6 | −5.8, −1.4 | −4.9 | −7.1, −2.6 | 0.39 | |
| Waist circumference (10 cm)b | Femoral neck | 5.2 | 3.6, 6.9 | 0.3 | −2.4, 3.1 | 0.01 | −0.2 | −1.9, 1.4 | −5.1 | −8.0, −2.0 | 0.01 |
| Lumpar spine | 3.7 | 1.9, 5.5 | −4.7 | −8.0, −1.3 | <0.001 | −1.9 | −4.0, 0.3 | −9.6 | −13.3, −5.8 | 0.001 | |
| 1/3 distal radius | 0.7 | −0.9, 2.4 | 0.1 | −2.4, 2.7 | 0.75 | −3.0 | −4.9, −1.1 | −4.1 | −6.3, −1.8 | 0.55 | |
| Fat mass (10 kg)b | Femoral neck | 8.1 | 5.7, 10.6 | −2.8 | −5.8, 0.3 | <0.001 | 0.8 | −1.3, 2.5 | −7.7 | −10.5, −4.7 | <0.001 |
| Lumpar spine | 5.8 | 3.4, 8.3 | −8.6 | −11.8, −5.3 | <0.001 | −1.4 | −3.9, 1.0 | −13.5 | −16.7, −10.2 | <0.001 | |
| 1/3 distal radius | 1.0 | −1.2, 3.4 | −2.3 | −4.9, 0.4 | 0.15 | −3.9 | −6.5, −1.2 | −5.5 | −8.1, −2.9 | 0.45 | |
| Lean mass (10 kg)b | Femoral neck | 12.9 | 10.8, 15.0 | − | − | − | |||||
| Lumpar spine | 10.2 | 8.0, 12.3 | − | − | − | ||||||
| 1/3 distal radius | 6.2 | 4.8, 7.6 | − | − | − | ||||||
Linear spline regression models with single knots at threshold values, controlling for age, race/ethnicity, age-by-race/ethnicity interaction, education, marital status, household income, smoking status, alcohol consumption, grip strength, PPT score, PASE score, time to five chair stands, and self-rated health. Models are weighted to accommodate sampling design (see Methods.)
Regression coefficients estimate the percentage trend in BMC associated with 10 kg/m2 increase in BMI, a 10 kg increase in weight, a 10 cm increase in waist circumference, or a 10 kg increase in total fat or total lean mass.
Estimates summarize associations below and above 35 kg/m2 (BMI), 105 kg (weight), 110 cm (waist circumference), 30 kg (total fat mass). Estimate for total lean mass applies over the entire range of lean mass values.
From Wald tests of null hypotheses that true regression coefficients below thresholds equal those above thresholds.
In examining the left-hand side of Table 3, some overarching trends may be noted. First, unadjusted trends produced in exploratory analyses (Fig. 1) are consistent with estimates obtained in models controlling for all covariates (Table 3). Second, cross-sectional increases in BMC associated with increasing body size measures are consistently strongest at the femoral neck and weakest at the forearm; moreover, evidence suggesting differences in trends above versus below threshold values is insignificant at the forearm but significant at the load-bearing sites. Third, site-specific cross-sectional increases in BMC associated with 10 kg increases in total body lean mass are more than double those associated with 10 kg increases in overall weight (below the 105 kg threshold), while increases in BMC associated with increasing total fat mass are substantially smaller that those associated with lean mass. Collectively, these results suggest the primary importance of total lean mass in determining BMC.
Further illustrations of the relative importance of lean mass are provided in comparisons of the left-hand to the right-hand columns of Table 3. When the effects of total lean mass are taken into account, increases in BMC associated with increases in other body shape variables are sharply reduced, and in most cases reversed, so that no other body shape measure retains a significant positive association with BMC once lean mass is taken into account. On the other hand, the cross-sectional associations between lean mass and BMC are largely robust to control for the other parameters. In Table 3, we observe that while the cross-sectional increase in BMC associated with a 10 kg/m2 increase (up to 35 kg/m2) in BMI is 16.5% when lean mass is not considered, it is −0.6% when lean mass is taken into account. Thus controlling for lean mass constitutes a total removal of the apparent effect of BMI on femoral neck BMC. By contrast, the cross-sectional trend in BMC associated with a 10 kg increase in lean mass is 12.9% when BMI is not considered, and is 14.3% when BMI is controlled for (not shown in the table), an 11% increase in the apparent effect of total lean mass on BMC when BMI is taken into account.
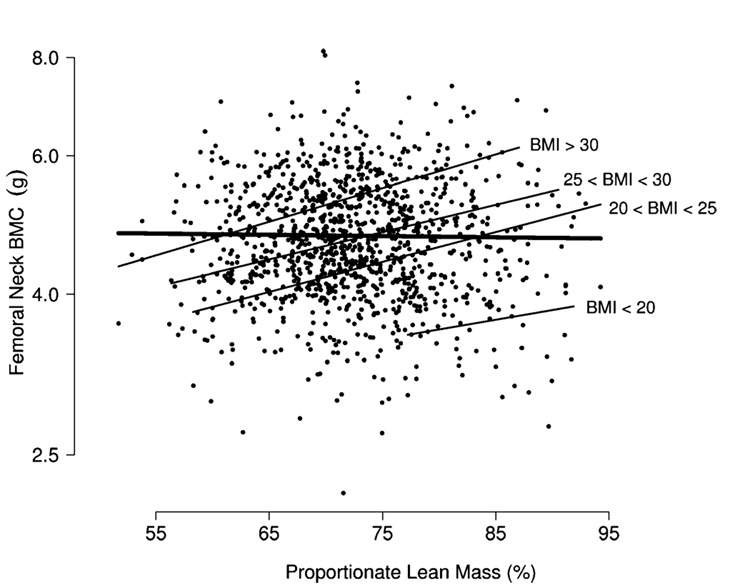
The finding that lean mass is strongly associated with BMC when BMI is held fixed is consistent with the hypothesis that proportionate lean mass is associated will be positively associated with BMC among subjects of similar body size. Additional analyses confirm this conjecture. For instance, when attention is restricted to subjects with BMI in a specific range, one observes a strong positive association between BMC and proportionate lean mass, even though, overall, the marginal relation between BMC and proportionate lean mass (unadjusted for height or any other measure of body size) is relatively weak (Fig. 3). Results are similar when weight or waist circumference is substituted for BMI, and hold at other skeletal sites.
Fig. 3.
Femoral neck BMC versus proportionate lean mass. There is little marginal association (thick line). However, when subjects are grouped according to BMI (kg/m2), the sharper association between lean mass and BMC becomes clear (thin lines)
To assess whether the observed associations were robust to considerations of skeletal size, we conducted two sensitivity analyses. The first, suggested by Reid et al. [32], replaced BMC with the ratio of BMC to height, as indices of height-independent bone mass, and obtained results similar to those reported above. The second examined lean and fat mass independently and jointly as predictors, treating height as a covariate in multiple regression (in addition to the other covariates described above) and obtained results very close to those reported in Table 3.
Bone mineral density
We replicated exploratory and formal analyses described above substituting BMD for BMC, and found both similar threshold associations to those reported above and that the relative importance of lean mass was preserved. These analyses indicated that the proportionate trend in BMD associated with cross-sectional increases in total lean mass was of slightly smaller magnitude than the corresponding trend in BMC.
We observed that controlling for lean mass was sometimes less decisive in removing associations of other body shape parameters with BMD than with BMC. For instance, when lean mass was controlled for, the increase in femoral neck BMD associated with an increase in BMI (below the 35 kg/m2 threshold) was sharply reduced (results not shown), but not completely removed, as was the case with BMC (Table 3). Additionally, controlling for BMI was sufficient to induce a mild reduction in the magnitude of association between lean mass and BMD, whereas the association between lean mass and BMC increased with BMI held constant. As with BMC, there was little evidence that the association between lean or fat mass and BMD differed by race/ethnicity.
Discussion
The results presented here confirm that body size parameters, such as weight, BMI, waist circumference, and total fat mass, are positively associated with bone mineral content at the hip, lumbar spine, and forearm, but indicate that these associations hold only up to certain body size thresholds. Total lean mass, by contrast, displays a consistent association with BMC at all sites and over the entire range of mass values, an association that is particularly strong at load-bearing sites. When the effects of lean mass are taken into account, the positive associations between other body size parameters and BMC are removed while, conversely, the effect of lean mass is robust to statistical control of other factors such as BMI. The contribution of lean mass to other body size parameters, such as BMI, appears to be greater at values below the relevant thresholds than above. Additionally, among men of similar overall weight or BMI, proportionate lean mass is strongly and positively associated with BMC. Results are similar when BMC is replaced by BMD and consistent across race/ethnicity. These results indicate that lean mass is likely the most important factor among those considered in mediating the biomechanical association between body size and bone mass.
The degree to which total lean mass acts as the driver of other body size parameters may indicate the degree to which increased body size can be thought to confer a protective effect on bone and fracture risk. The observation that bone content is strongly adapted to lean mass is not a novel one, but it is not without controversy; debates over whether lean or fat mass is more important in determining BMC and whether those associations are primarily hormonally or mechanically determined are not yet fully resolved. The fact that our results are robust to control for height, and are similar for both BMC and BMD, would appear to contradict the assertion that associations between BMD and lean mass in men represent only a artifactual correlation dictated by skeletal size [22]. Our finding that, in men, lean mass has greater importance than fat mass as a correlate of BMC is seemingly in agreement with others indicating that total fat mass may play a lesser role in maintenance of bone in men than in women [19, 21, 22]. However, existing research has also indicated that the importance of total lean mass may exceed that of fat mass in women as well [40], while at the same time the relative importance of lean versus fat mass in cross-sectional evaluations may not mirror those in longitudinal follow-up [49].
That the BACH/Bone data support the primary importance of lean mass in predicting BMC and indicate that the strength of the association between body size parameters and BMC is strongest at load-bearing sites coincides with the well-established mechanostat hypothesis of negative feedback between strain and bone mass [50, 51]. However, while black subjects exhibited both the greatest BMC and total and proportionate lean mass values, analyses indicate that observed differences in body shape and composition are insufficient to completely explain racial/ethnic differences in BMC [13]. Further investigation is necessary to determine whether these differences may be explained by nutritional or hormonal factors [2, 3, 11]; detailed evaluations of lean mass and muscle strength in the context of physical activity, physical function, and socioeconomic status will also be informative.
Our results indicate that, above certain thresholds, the associations between body size parameters (e.g., BMI) and BMC are reduced and may even be negative. Because of the interrelatedness of lean mass, fat mass, and the other parameters, these results must be interpreted with care. It should be noted, for instance, that when lean mass is held constant, increases in BMI directly imply increased fat mass. The model may, therefore, be interpreted as implying that BMC decreases in relation to that portion of weight that is donated to the total by lean tissue. This interpretation is consistent with the curvilinear (unadjusted) trend of BMC with increasing BMI, as, in general, a greater proportion of BMI is donated by lean mass among subjects whose BMI is lower.
Some limitations of this work should be acknowledged. DXA measures areal and not volumetric bone density, and as such is subject to certain shortcomings [52], such as artifactual increases in apparent BMD with body size as a function of the increased BMC in bones of greater depth. Although the sensitivity analyses described above indicate that our results were not spurious associations induced through variation in subjects’ height, the possibility of artifactual associations [53, 54] between aspects of body composition (particularly fat mass) and DXA measurements cannot be discounted.
If lean and not fat mass is most important in predicting BMC and fracture risk, then the supposed benefits conferred in the form of decreased fracture risk cannot be interpreted as a silver lining accompanying increased adiposity. At the same time, to the degree that weight loss is harmful to older men [55], conferring, with other ill effects, lower bone mass and consequent increased risk of fracture, these results support the contention that sarcopenia and loss of lean muscle mass is a critical factor. Endeavoring to maintain absolute and proportional lean mass appears to be the best strategy in preserving bone mass and density in aging men.
Acknowledgements
The BACH/Bone study was supported by grant AG 20727 from the National Institute on Aging (NIA). The parent study (BACH) was supported by grant DK 56842 from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. National Institutes of Health Consensus Development Conference Statement. Jama. 2001;285:785–795.
- 2.Amin S. Male osteoporosis: epidemiology and pathophysiology. Curr Osteoporos Rep. 2003;1:71–77. doi: 10.1007/s11914-003-0012-9. [DOI] [PubMed] [Google Scholar]
- 3.Amin S, Felson DT. Osteoporosis in men. Rheum Dis Clin North Am. 2001;27:19–47. doi: 10.1016/s0889-857x(05)70186-1. [DOI] [PubMed] [Google Scholar]
- 4.Wright VJ. Osteoporosis in men. J Am Acad Orthop Surg. 2006;14:347–353. doi: 10.5435/00124635-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 5.George A, Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in bone mineral density in older men. J Bone Miner Res. 2003;18:2238–2244. doi: 10.1359/jbmr.2003.18.12.2238. [DOI] [PubMed] [Google Scholar]
- 6.Henry YM, Eastell R. Ethnic and gender differences in bone mineral density and bone turnover in young adults: effect of bone size. Osteoporos Int. 2000;11:512–517. doi: 10.1007/s001980070094. [DOI] [PubMed] [Google Scholar]
- 7.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 8.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 9.Tracy JK, Meyer WA, Grigoryan M, Fan B, Flores RH, Genant HK, Resnik C, Hochberg MC. Racial differences in the prevalence of vertebral fractures in older men: the Baltimore Men’s Osteoporosis Study. Osteoporos Int. 2006;17:99–104. doi: 10.1007/s00198-005-1919-z. [DOI] [PubMed] [Google Scholar]
- 10.Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E. Bone fragility in men - where are we? Osteoporos Int. 2006 doi: 10.1007/s00198-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 11.Heaney RP. Bone mass, the mechanostat, and ethnic differences. J Clin Endocrinol Metab. 1995;80:2289–2290. doi: 10.1210/jcem.80.8.7629221. [DOI] [PubMed] [Google Scholar]
- 12.Cauley JA. The determinants of fracture in men. J Musculoskelet Neuronal Interact. 2002;2:220–221. [PubMed] [Google Scholar]
- 13.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007 doi: 10.1007/s00198-006-0321-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 16.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, Ensrud K, Lau EM, Orwoll ES. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16:1525–1537. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 18.Coin A, Sergi G, Beninca P, Lupoli L, Cinti G, Ferrara L, Benedetti G, Tomasi G, Pisent C, Enzi G. Bone mineral density and body composition in underweight and normal elderly subjects. Osteoporos Int. 2000;11:1043–1050. doi: 10.1007/s001980070026. [DOI] [PubMed] [Google Scholar]
- 19.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–169. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 20.Huuskonen J, Vaisanen SB, Kroger H, Jurvelin C, Bouchard C, Alhava E, Rauramaa R. Determinants of bone mineral density in middle aged men: a population-based study. Osteoporos Int. 2000;11:702–708. doi: 10.1007/s001980070069. [DOI] [PubMed] [Google Scholar]
- 21.Kirchengast S, Peterson B, Hauser G, Knogler W. Body composition characteristics are associated with the bone density of the proximal femur end in middle- and old-aged women and men. Maturitas. 2001;39:133–145. doi: 10.1016/s0378-5122(01)00205-5. [DOI] [PubMed] [Google Scholar]
- 22.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 23.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 24.Bakhireva LN, Barrett-Connor E, Kritz-Silverstein D, Morton DJ. Modifiable predictors of bone loss in older men: a prospective study. Am J Prev Med. 2004;26:436–442. doi: 10.1016/j.amepre.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, Lewis CE, Orwoll E. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 26.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 27.Bakker I, Twisk JW, Van Mechelen W, Kemper HC. Fat-free body mass is the most important body composition determinant of 10-yr longitudinal development of lumbar bone in adult men and women. J Clin Endocrinol Metab. 2003;88:2607–2613. doi: 10.1210/jc.2002-021538. [DOI] [PubMed] [Google Scholar]
- 28.Capozza RF, Cointry GR, Cure-Ramirez P, Ferretti JL, Cure-Cure C. A DXA study of muscle-bone relationships in the whole body and limbs of 2512 normal men and pre- and postmenopausal women. Bone. 2004;35:283–295. doi: 10.1016/j.bone.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Ferretti JL, Capozza RF, Cointry GR, Garcia SL, Plotkin H, Alvarez Filgueira ML, Zanchetta JR. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone. 1998;22:683–690. doi: 10.1016/s8756-3282(98)00046-5. [DOI] [PubMed] [Google Scholar]
- 30.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 31.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–1646. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid IR, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 33.Lim S, Joung H, Shin CS, Lee HK, Kim KS, Shin EK, Kim HY, Lim MK, Cho SI. Body composition changes with age have gender-specific impacts on bone mineral density. Bone. 2004;35:792–798. doi: 10.1016/j.bone.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, Bauer DC, Harris TB. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16:1343–1352. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 35.Van Langendonck L, Claessens AL, Lefevre J, Thomis M, Philippaerts R, Delvaux K, Lysens R, Vanden Eynde B, Beunen G. Association between bone mineral density (DXA), body structure, and body composition in middle-aged men. Am J Hum Biol. 2002;14:735–742. doi: 10.1002/ajhb.10090. [DOI] [PubMed] [Google Scholar]
- 36.Khosla S, Atkinson EJ, Riggs BL, Melton LJ., 3rd Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 37.Stewart KJ, Deregis JR, Turner KL, Bacher AC, Sung J, Hees PS, Tayback M, Ouyang P. Fitness, fatness and activity as predictors of bone mineral density in older persons. J Intern Med. 2002;252:381–388. doi: 10.1046/j.1365-2796.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 38.Pluijm SM, Visser M, Smit JH, Popp-Snijders C, Roos JC, Lips P. Determinants of bone mineral density in older men and women: body composition as mediator. J Bone Miner Res. 2001;16:2142–2151. doi: 10.1359/jbmr.2001.16.11.2142. [DOI] [PubMed] [Google Scholar]
- 39.Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–1627. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]
- 40.Wang MC, Bachrach LK, Van Loan M, Hudes M, Flegal KM, Crawford PB. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37:474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 41.Wu F, Ames R, Clearwater J, Evans MC, Gamble G, Reid IR. Prospective 10-year study of the determinants of bone density and bone loss in normal postmenopausal women, including the effect of hormone replacement therapy. Clin Endocrinol (Oxf) 2002;56:703–711. doi: 10.1046/j.1365-2265.2002.01534.x. [DOI] [PubMed] [Google Scholar]
- 42.McKinlay JB, Link CL. Measuring the Urologic Iceberg: Design and Implementation of The Boston Area Community Health (BACH) Survey. Eur Urol. 2007 doi: 10.1016/j.eururo.2007.03.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 44.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 45.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 46.Wallman K. Data on race and ethnicity: Revising the federal standard. Am Stat. 1997:31–35. [Google Scholar]
- 47.Kish L. Sampling Organizations And Groups Of Unequal Sizes. Am Sociol Rev. 1965;30:564–572. [PubMed] [Google Scholar]
- 48.Hastie TJ, Tibshurani RJ. Generalized additive models. Boca Raton: Chapman and Hall/CRC; 1990. [Google Scholar]
- 49.Chen Z, Lohman TG, Stini WA, Ritenbaugh C, Aickin M. Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res. 1997;12:144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 50.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 51.Frost HM. Perspectives: a proposed general model of the "mechanostat" (suggestions from a new skeletal-biologic paradigm) Anat Rec. 1996;244:139–147. doi: 10.1002/(SICI)1097-0185(199602)244:2<139::AID-AR1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 52.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 53.Bolotin HH. Inaccuracies inherent in dual-energy X-ray absorptiometry in vivo bone mineral densitometry may flaw osteopenic/osteoporotic interpretations and mislead assessment of antiresorptive therapy effectiveness. Bone. 2001;28:548–555. doi: 10.1016/s8756-3282(01)00423-9. [DOI] [PubMed] [Google Scholar]
- 54.Bolotin HH, Sievanen H, Grashuis JL. Patient-specific DXA bone mineral density inaccuracies: quantitative effects of nonuniform extraosseous fat distributions. J Bone Miner Res. 2003;18:1020–1027. doi: 10.1359/jbmr.2003.18.6.1020. [DOI] [PubMed] [Google Scholar]
- 55.Morley JE. Is weight loss harmful to older men? Aging Male. 2006;9:135–137. doi: 10.1080/13685530600765409. [DOI] [PubMed] [Google Scholar]