Abstract
Inflammation contributes to disease development, and the neuro-immuno-endocrine interface is a potential site of action for inflammatory products like IL-6 to affect health. Although plasma IL-6 can stimulate the activity of the hypothalamo-pituitary-adrenocortical (HPA) axis, the precise role, if any, for IL-6 in the HPA response to non-immunological stressors is unclear. The purpose of this study was to test the hypothesis that IL-6 in the stalk median eminence (SME) can be directly involved in stimulating ACTH secretion in response to acute stress in female swine. This study was undertaken as a result of finding IL-6 localized to the external zone of the stalk median eminence (SME) next to the hypophyseal portal vessels. Results indicate that content of IL-6 in the SME decreases in response to acute stress along with an increase in phosphorylation of STAT3 in the anterior pituitary and a simultaneous increase in plasma concentrations of IL-6 and ACTH. Furthermore, we show that females concomitantly display greater SME content of IL-6 and greater HPA responsiveness to stress, thereby suggesting that IL-6 release from the SME is an integral factor contributing to enhanced stress responsiveness in females. Our results provide evidence for a direct link between IL-6 and ACTH release and reveal a sex difference in this relationship.
INTRODUCTION
Chronic overproduction of IL-6 has been suggested as a core pathway contributing to the health risks associated with aging, inflammation, chronic stress and metabolic disease (1–4). In depression, there is a dysregulation of the circadian rhythm of circulating IL-6 and elevated plasma IL-6 levels have been reported among individuals with sleep disorders (5, 6). It has been proposed that plasma IL-6 affects health through stimulating HPA axis activity thereby contributing to the development of metabolic disorders and cardiovascular disease (3). Despite the correlative evidence between plasma IL-6 and disease the mechanism of action for plasma IL-6 to elicit negative effects on health remains to be elucidated.
IL-6 may affect health through interactions with the HPA axis (2, 7–10). IL-6 has the ability to activate each level of the HPA axis (11–15) and a dose-dependent increase in ACTH and cortisol is seen in response to IL-6 administration in man (16). Studies with rat hemipituitaries provide evidence that IL-6 can increase ACTH secretion and intraventricular injection of IL-6 increases plasma ACTH within 15 min (17). The ability of IL-6 to activate the HPA axis occurs even in the absence of CRH (15, 18, 19). Although the hypothalamus expresses IL-6 (20) and cells of the anterior pituitary express the IL-6 receptor (7, 18, 21), an anatomical pathway providing access for hypothalamic-IL-6 to directly affect anterior pituitary function has not been clearly described.
In rats, IL-6 is co-expressed with vasopressin in the hypothalamic paraventricular and supraoptic nuclei, the internal zone of the SME, and the posterior pituitary (22, 23). In response to dehydration IL-6 mRNA and protein content in the hypothalamus increase and IL-6 protein content in the posterior pituitary is reduced (23). In this report, when we examined IL-6 expression in the pituitary of pigs we discovered IL-6 also localized to the external zone of the stalk median eminence (SME).
The discovery of IL-6 in the external zone of the SME provides an anatomical pathway for IL-6 to be released into the hypophyseal portal vessels, transported to the anterior pituitary, and thereby affect anterior pituitary function. This evidence, along with the evidence that plasma IL-6 increases in response to physical and psychological stressors (24), led us to the primary hypothesis of this study: that IL-6 in the SME can be directly involved in stimulating ACTH secretion in response to acute stress. If this hypothesis is correct, we may expect to find the following in response to acute stress: 1) a decrease in IL-6 content in the SME; 2) activation of the IL-6/gp130 signaling pathway at the level of the anterior pituitary; and 3) a simultaneous increase in plasma concentrations of IL-6 and ACTH. Thus, the purpose of the study reported in this manuscript was to test the functional significance of IL-6 in the SME by determining whether these three predictions of our hypothesis are correct. We chose to use exhaustive exercise as a stressor of female pigs since we had previously observed robust changes in ACTH in response to this stressor in females. In addition, exercise has been reported to increase IL-6 release from the brain in humans (25).
MATERIALS AND METHODS
Animals
Male and female adult Yucatan miniature swine were obtained from a breeder (Sinclair Research Farm, Columbia, MO) and maintained in accordance with standards set forth by the University of Missouri Institutional Animal Care and Use Committee. Pigs were sexually mature, 7–13 months of age and weighed 24–40 kg. Pigs used for blood collection during exercise had indwelling catheters placed in the jugular vein and connected to a vascular access port. Pigs were allowed to recover at least one week prior to inclusion in studies. For tissue harvest, pigs were sedated with ketamine (35 mg/kg im) and xylazine (2.25 mg/kg, im) and anesthetized with thiopental sodium (10 mg/kg im). Following euthanasia, the brain was removed and the pituitary dissected.
Immunohistochemistry
Collected tissues were immersed in 10% neutral buffered formalin, embedded in paraffin, then sectioned serially at 6 μm thickness. Sections were floated onto positively charged slides (Fisher), deparaffinized then steamed in an antigen target retrieval solution (Dako S1699). Endogenous biotin was blocked with an avidin-biotin two-step blocking solution (Vector SP-2001) and endogenous peroxidase was inhibited by placing sections in 3% hydrogen peroxide. Prior to incubation with primary antibody, a non-serum protein block (Dako X909) was applied. Sections were incubated overnight at 4 C with a monoclonal anti-porcine IL-6 antibody (25 ng/ml; MAB686, R&D Systems). Additional sections were incubated overnight with other antibodies including anti-CRF (1:2,000; Peninsula Laboratories), vasopressin antiserum (1:12,000; W3, Rudolf Magnus Institute, Utrecht), pSTAT3 (1:200, Cell Signaling) and POMC (1:300; Santa Cruz). For negative control, sections were incubated overnight with the antibody diluent minus primary antibody. Sections were incubated with a biotinylated link antibody (Dako LSAB+ kit) for 30 min then incubated with peroxidase-labeled streptavidin for 30 min (Dako LSAB+ kit). Diaminobenzidine (DAB; Dako) was applied for visualization of the reaction product followed by counterstaining with Mayer’s hematoxylin stain, dehydration and coverslipping.
For dual immunohistochemistry slides were first incubated with pSTAT3 antibody as described above. After the application of DAB (brown) slides were rinsed then blocked again prior to incubation with POMC antibody. The replacement of DAB with Vector VIP chromogen (Vector Laboratories) provided a violet reaction product for the visualization of POMC immunoreactivity. Photographs were obtained with an Olympus BX40 photomicroscope and Spot Insight Color camera (Diagnostic Instruments).
Western blotting
Whole median eminence was placed in chilled homogenization buffer (50 mM Tris-HCL, 0.1 mM EDTA, 0.1 mM EGTA, 0.5 mM dithiothreitol, 1:200 protease inhibitor cocktail (P8340, Sigma)), homogenized (Omni GLH), and centrifuged at 100,000 × g for 45 min (26). The supernatant was collected and protein concentration was determined (Coomassie Protein Assay, Pierce). A volume of supernatant containing 20 μg of protein was diluted 10:1 with trichloroacetic acid (TCA) solution, placed on ice for 10 min, then centrifuged at 16,000 × g for 10 min. The TCA was suctioned off, replaced with 500 μl acetone, and samples were centrifuged again at 16,000 × g for 10 min. The acetone was removed and the protein pellet was resuspended in 20 μl Laemmli buffer then kept at −80 C until analysis. On the day of analysis, samples were heated to 70 C for 10min, loaded (10 μg/lane) onto 4–12% NuPage Bis-Tris gel (Invitrogen), electrophoresed under reducing conditions and electrotransferred to polyvinylidene difluoride membrane (Hybond-enhanced chemiluminescence, Amersham). The membrane was blocked for 1 h at room temperature with 5% nonfat milk in Tris-buffered saline with Tween and incubated overnight at room temperature with primary antibody against porcine IL-6 (1 μg/ml; MAB686, R&D Systems). After another 1 h wash, the membrane was incubated with secondary antibody (1:2,500, horseradish peroxidase-conjugated anti-mouse; Sigma), washed then detected by enhanced chemiluminescence (Amersham). Exposed films were scanned and band intensity determined (NIH Image). To confirm equal loading, membranes were reprobed for α-Tubulin (1:15,000, Santa Cruz).
Plasma assays
Blood samples were collected in chilled EDTA containers, centrifuged and kept at −80 C until analysis. Lactate was measured (Sigma) spectrophotometrically. IL-6 was measured using an anti-porcine IL-6 ELISA (R&D Systems). ACTH and cortisol were measured by chemiluminescent assay (Immulite, DPC). The intra- and inter-assay coefficients of variation for cortisol were 4.5 and 5.1, and ACTH was 3.4 and 3.6, respectively.
Stress models
The exhaustive exercise bout involved incremental increases in running speed to exhaustion. Caudal electrical stimulation was used. The animals began walking on the treadmill at 3.2 km/h and then speed was increased for 5 min intervals each at 4.8, 8.0, 11.3 and 11.3 km/h with 5% incline. Between each stage the animals were allowed to walk for 5 min at 3.2 km/h at which time blood samples were collected via indwelling catheters. For gender comparisons, additional pigs were acclimated to sub-maximal treadmill jogging in the absence of electrical stimulation. Pigs walked at 3.2 km/h for 5 min then performed fast walking at 4.8 km/h for 5 min and finished with a 5 min jog at 8.0 km/h. Blood samples were drawn during the final minute of each stage via indwelling catheters.
For basal concentrations of ACTH, blood samples were collected by automated blood sampling (Accusampler, DiLab) every hour during the daylight hours. For restraint stress conscious pigs were flipped on to their back and blood samples were obtained via jugular venipuncture within 5 min. Samples for the stress associated with anesthesia and surgery were collected approximately 30 min after the animals had been placed on anesthesia (thiopental sodium, 10 mg/kg, im) and following minor surgery including intubation.
Statistical analysis
One way ANOVA was used to compare ACTH changes during exercise. A Kruskal-Wallis one way ANOVA on ranks was used if the normality test failed. A Pearson correlation was run to compare the relationship between plasma concentrations of ACTH and IL-6 during exercise. A t-test was run to compare groups and, if normality failed Mann-Whitney U-test was employed. Data are presented as mean ± SEM.
RESULTS
Localization of IL-6 to external zone of SME
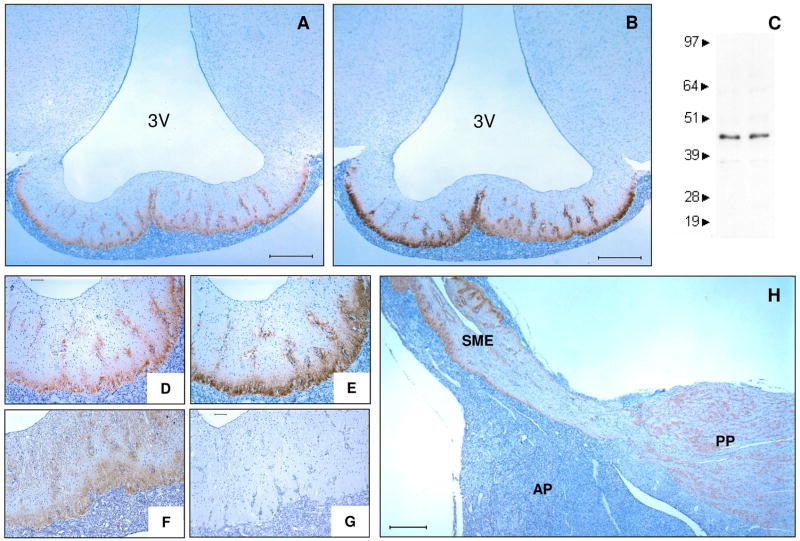
IL-6 was found to be localized to the external zone of the SME demonstrating the same immunoreactive pattern as CRH (Fig. 1). IL-6 was localized to the external zone in both male and female pigs and demonstrated a molecular weight of 45kDa (Fig. 1C). Vasopressin was localized to both the internal and external zones of the SME (Fig. 1F). IL-6 was also found to be localized to the posterior pituitary (Fig. 1H).
Figure 1.
Immunohistochemical detection of IL-6 in the external zone of the SME. Frontal view of IL-6 (A, D), CRH (B, E), VP (F) and non-immune control (G) in the SME (representative images, n ≥ 5 animals). Immunoblot analysis of IL-6 protein isolates from SME displaying immunoreactive bands at 45kDa (C). Sagittal view of pituitary showing IL-6 immunoreactivity in the external zone of the SME and the posterior pituitary (H). 3V, third ventricle; AP, anterior pituitary; PP, posterior pituitary; SME, stalk median eminence. Bar represents 500μm (A, B, H) and 100μm (D–G).
SME IL-6 content changes in response to acute stress
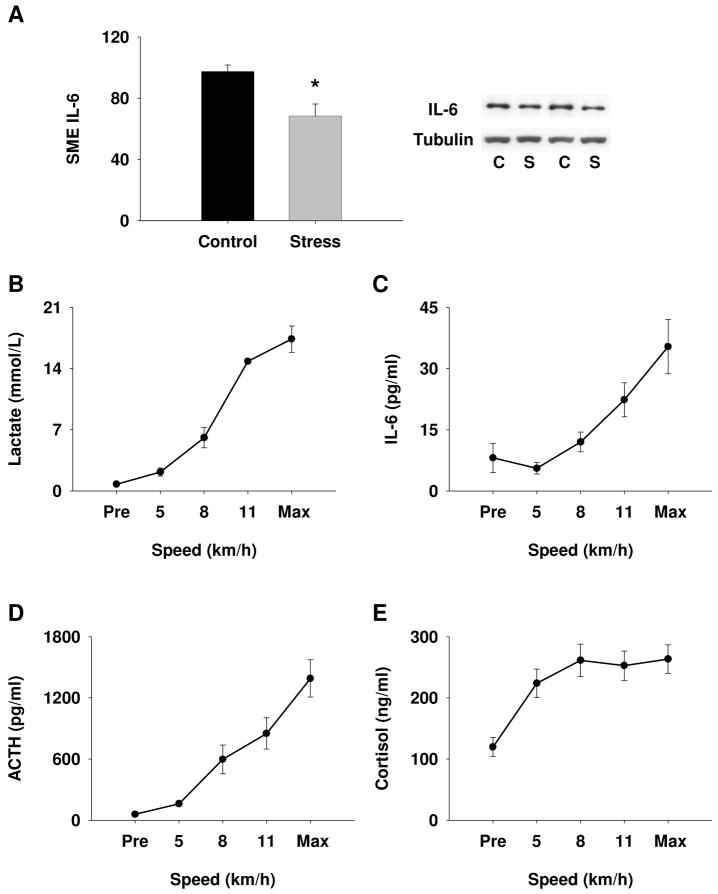
Exhaustive exercise bouts were completed by female pigs using an incremental exercise protocol (Fig. 2). The increasing intensity of this exercise is illustrated by the changes in blood lactate (Fig. 2B). We observed a significant decrease in SME content of IL-6 in response to exhaustive exercise (Fig. 2A) which in part supports our hypothesis that IL-6 is released from the SME in response to acute stress. Plasma concentrations of IL-6 and ACTH increased simultaneously as predicted by our hypothesis (Fig. 2C and D, respectively), demonstrating a significant correlation (r = 0.89; P < 0.001) between the plasma concentrations of these two molecules. Plasma cortisol also increased in response to exercise (Fig. 2E).
Figure 2.
The effects of acute stress on IL-6 content in the SME. (A) In response to exhaustive exercise, IL-6 protein content in the SME decreased as revealed by Immunoblot analysis (*P < 0.01; n ≥ 5). Increase in plasma lactate during exhaustive exercise bout demonstrates increase in intensity (B). Increase in plasma IL-6 (C) ACTH (D), and cortisol (E) in response to exercise (n ≥ 5). C, control; S, stress.
Phosphorylation of STAT3 in response to acute stress
The same animals used to test the change in SME IL-6 content (Fig. 2) were also used to test the hypothesis that IL-6 release from the SME will result in phosphorylation of STAT3 in the anterior pituitary (Fig. 3). In support of our hypothesis, we observed a significant increase in the phosphorylation of STAT3 in the anterior pituitary (Fig. 3C, D), presumably due to activation of the IL-6/gp130 signaling pathway in those tissues (27). The nuclear immunoreactivity of pSTAT3 in the anterior pituitary demonstrates that pSTAT3 has translocated to the nucleus in response to exhaustive exercise (Fig. 3D). Nuclear immunoreactivity of pSTAT3 is seen in POMC positive cells demonstrating activation of the IL-6/gp130 signaling pathway in corticotrophs (Fig. 3E).
Figure 3.
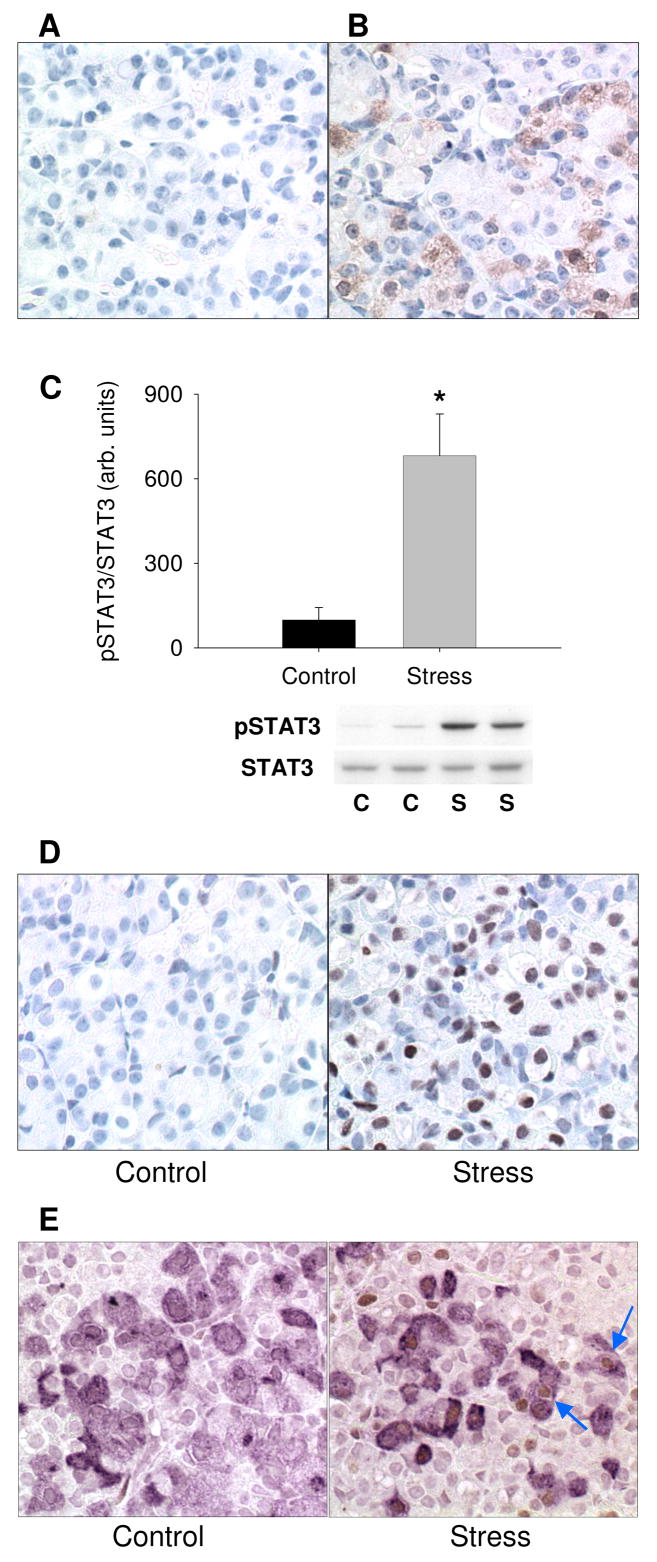
Phosphorylation of STAT3 in the anterior pituitary in response to acute stress. In the anterior pituitary there was lack of immunoreactivity in the non-immune control (A) and positive immunoreactivity for POMC (B). The ratio of pSTAT3 to STAT3 was determined by Immunoblot and demonstrated an increase in phosphorylation of STAT3 in response to exhaustive exercise (C; *P < 0.05; n = 4). The increase in STAT3 phosphorylation is also demonstrated by immunohistochemistry showing increased nuclear pSTAT3 after exercise (B, n = 3). Dual immunohistochemical results for pSTAT3 (brown) and POMC (violet) in control and acute stress animals (E). (E) Blue arrows point to POMC positive cells demonstrating nuclear immunoreactivity for pSTAT3. C, control; S, stress.
Sex difference in SME IL-6 content and ACTH response to stress
When we evaluated the influence of gender on this relationship, we observed that female pigs had greater IL-6 content in their SME (Fig. 4A) and displayed an exaggerated ACTH response to exercise (Fig. 4B) when compared to male pigs. We additionally note that the exercise model used in Fig. 4 involved running at sub-maximal intensities and, unlike Figures 2 and 3, these animals were not exercised to exhaustion. Nonetheless, from pre-exercise to jogging on the treadmill, female pigs responded with a 10-fold increase in mean plasma ACTH while male pigs were unresponsive (Fig. 4B). In addition, there was a corresponding sex difference in cortisol response to exercise as well (Fig. 4C).
Figure 4.
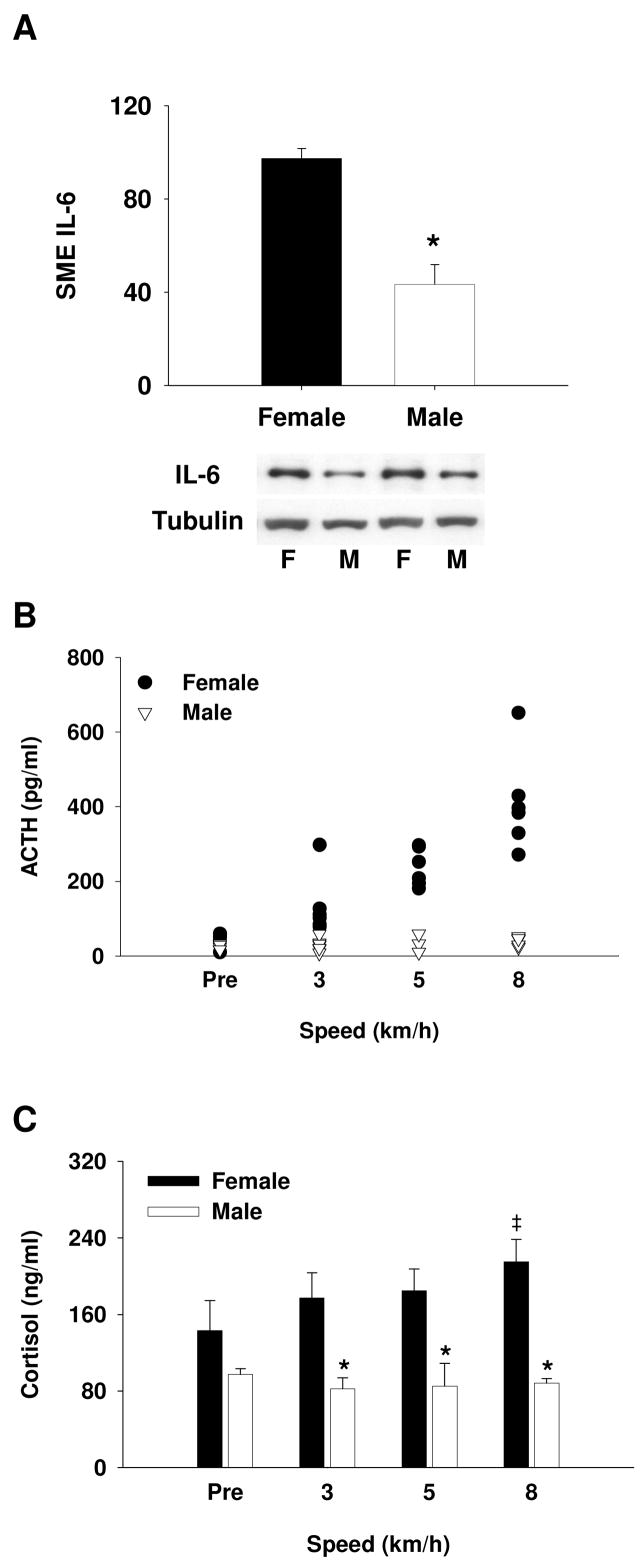
Sex difference in SME IL-6 protein content and ACTH response to stress. (A) Immunoblot analysis showing lesser SME IL-6 protein content in males compared to females (*P < 0.001; n ≥ 5). (B) Females display increased ACTH at all speeds versus pre-exercise (P < 0.05) and compared to males (P < 0.05; n ≥ 6). Results displayed as individual data points at corresponding speeds. There was no sex difference in ACTH concentration prior to initiation of exercise (Pre). (C) There was a significant sex difference in the cortisol response to exercise (*P < 0.05) and only females demonstrated a significant increase in cortisol during exercise (‡P < 0.05, vs. Pre). There was no increase in ACTH or cortisol in males in response to exercise. F, female; M, male.
HPA activity in pigs
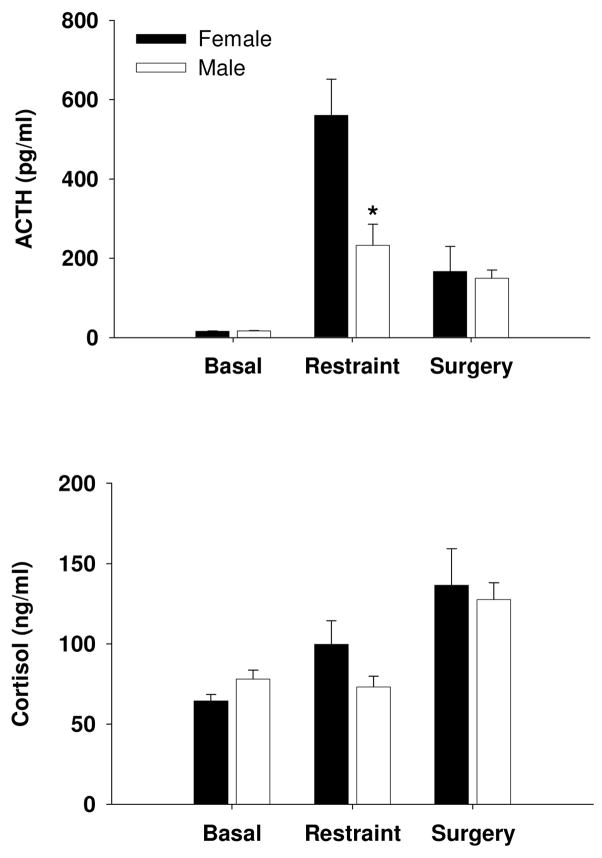
To determine whether the sex difference in ACTH response in pigs was unique to the stressor used, we subsequently incorporated two additional stressors. We used restraint as one of our stressors since previous work in mice demonstrated that the sex difference in HPA response to restraint is IL-6 dependent (18). We report basal concentrations of ACTH for female and male pigs at 15.5 ± 0.5 pg/ml and 16.9 ± 0.9 pg/ml, respectively. Automated blood sampling was used to collect the basal, undisturbed blood samples and there was no sex difference in ACTH or cortisol at rest (Fig. 5). Restraint blood samples were collected within 5 min of placing the pig on its back and female pigs demonstrated a greater ACTH response to restraint than male pigs while there was no difference in the cortisol response (Fig. 5). We found no effect of gender on the ACTH or cortisol response to the stress associated with anesthesia and surgery (Fig. 5).
Figure 5.
HPA activity in male and female pigs. In undisturbed animals there was no sex difference in resting ACTH or cortisol (n ≥ 48). Females exhibited a greater ACTH response to restraint (*P < 0.05; n ≥ 7) but no sex difference was seen in the ACTH response to the stress associated with anesthesia and surgery (female, n = 3; male, n = 6). There were no sex differences in cortisol response to restraint or the cortisol response to the stress associated with anesthesia and surgery.
DISCUSSION
The purpose of the study reported in this manuscript was to test the hypothesis that IL-6 in the SME can be directly involved in stimulating ACTH secretion in response to acute stress in female swine. Our initial observation of IL-6 localized to the external zone of the SME suggested that IL-6 may be released into the portal vessels and directly modulate anterior pituitary function. This hypothesis was supported by two important observations in our results: 1) IL-6 content in the SME decreases in response to acute stress coincident with increases in anterior pituitary STAT3 phosphorylation and a simultaneous increase in plasma IL-6 and ACTH (Figures 2 and 3), and 2) females demonstrate greater SME IL-6 content and greater ACTH response to stress than males (Fig. 4).
Depending on the level of glycosylation a monomer of IL-6 has been reported in the size range of 21–30 kDa (28), and is found to circulate as complexes of high molecular mass of 100–150 kDa and 400–500 kDa (29). A 45 kDa dimer has been reported as the predominant molecular weight species detected in human serum following TNF infusion or endotoxin challenge (30, 31). Also, a 45 kDa form of IL-6 survived under reducing and denaturing conditions and demonstrated both hepatocyte stimulating activity and B cell differentiation activity, two cardinal features of IL-6 (30, 31). Indeed, the previous names for IL-6 (B cell differentiation factor/hepatocyte-stimulating factor) were derived from the biological activities of this cytokine. Consistent with these previous observations in humans, we report the molecular weight of IL-6 present in the porcine SME as 45 kDa.
We believe the uniqueness of the work reported in this manuscript rests not only in the anatomical evidence for IL-6 being directly involved in neuroendocrine activity, but also the evidence of a physiological role for SME IL-6. The observed changes in response to acute stress (1: decrease in IL-6 content in the SME; 2: increase in phosphorylated STAT3 in corticotrophs; and 3: a simultaneous increase in plasma IL-6 and ACTH) along with the observation that the higher SME IL-6 content found in females coincides with greater ACTH responsiveness; strongly supports a physiological role for IL-6 role in augmenting/signaling the ACTH response to stress.
Our observation of a sex difference in HPA response to restraint in swine is of interest since previous work in mice has demonstrated that the sex difference in HPA response to restraint could be eliminated in IL-6 KO animals (18). We did not observe a sex difference in ACTH response to the stress associated with anesthesia and surgery. However, it is possible that lack of time course information hid a sex difference in this response since blood samples were collected at only one time point in this experiment. Previous work, reported that WT female mice had a greater HPA response to restraint stress than male WT mice (18). Of interest, these investigators also reported that female IL-6 KO mice had a 70% reduction in their corticosterone response to restraint compared to female WT, while there was no difference in corticosterone response between male WT and male IL-6 KO mice. These data provide evidence that IL-6 contributes to the greater HPA responsiveness seen in female mice (18). Our data suggest that IL-6 release from the SME and activation of pituitary corticotrophs is the mechanism of action by which IL-6 contributes to sex differences in HPA response.
Previous reports have shown that in the basal state cytokines are expressed in low abundance in the brain and that IL-6 is co-localized with vasopressin (22, 23, 32). Consistent with both these notions, when we examined IL-6 immunoreactivity in the hypothalamus of pigs we observed faint immunoreactivity in vasopressinergic neurons (data not shown). IL-6 mRNA has been detected in response to LPS in the PVN and in the median eminence/arcuate region (32). Additionally, in response to dehydration IL-6 mRNA was detected in the PVN and SON (23). Evidence collected using axonal blockade has also shown IL-6 protein co-expressed with vasopressin in the PVN and SON (22). Although we did not consistently find strong immunoreactivity for IL-6 in the hypothalamus we show that IL-6 immunoreactivity in the external zone of the SME is seen alongside immunoreactivity for both CRH and VP (Fig. 1).
The use of exercise as our stressor to demonstrate that SME IL-6 is involved in the ACTH response has value as it has been shown in humans that the ACTH response to exercise stress is facilitated by factor(s) in addition to CRH (33). Even in the face of dexamethasone suppression an HPA response to high-intensty exercise in humans was seen leading Deuster et al. (34) to suggest that the stress of exercise may be different from other stimuli. In their study using glucocorticoid inhibition they observed a greater VP and cortisol, but not ACTH, response to exercise in females compared with males. In contrast, a greater initial ACTH response to exercise was seen in males compared with females under hypogonadal conditions (35). Roca et al. (35) suggested that sex-differences in HPA response are not dependent on circulating concentrations of gonadal steroids.
Our findings may have clinical relevance as there is a sex difference in the association between plasma IL-6 and risk factors for cardiovascular disease (36). In women, plasma IL-6 was associated with blood pressure, but not insulin sensitivity, whereas in men, plasma IL-6 was associated with insulin sensitivity but not blood pressure (36). Our results describing the interaction between IL-6 and the stress axis may be of particular interest to women’s health and quality of life, as Constanzo et al. (37), reported: “IL-6 may, therefore, be one pathway by which psychosocial factors such as depression and social support could influence disease outcomes in ovarian cancer.”
Despite associations between plasma IL-6 and disease it must be noted that chronic elevation in plasma IL-6 represents a concentration change that is a mere fraction of what plasma IL-6 levels can reach during an infection/inflammatory response. As an example, in patients with sleep apnea mean plasma concentrations of IL-6 increase to 4 pg/ml versus the 1.5 pg/ml level observed in controls (6). In contrast, during the inflammatory response to turpentine injection plasma concentrations of IL-6 can exceed 2,000 pg/ml (38).
In response to injection of IL-6, which elevated plasma IL-6 for 4 h, increased ACTH was observed when IL-6 plasma concentrations were maintained at 290 and 4,050 pg/ml (16). However, when IL-6 injections produced plasma IL-6 concentrations of 8, 22, or 65 pg/ml there was no effect on circulating ACTH (16). Our observation of an increase in concentration of plasma IL-6 to 35 pg/ml suggests that circulating IL-6 was not entirely derived from stores in the SME. The increase in plasma IL-6 to 35 pg/ml also suggests that if it were entirely derived from the periphery this concentration would not be high enough to affect ACTH release. Thus, if peripheral production of IL-6 is the only source of circulating IL-6, the small change in circulating IL-6 that occurs during chronic stress, or in the acute stressor response, would not be expected to affect pituitary function. However, if plasma IL-6 concentrations partially reflect IL-6 release from the SME, then the impact of IL-6 on anterior pituitary function, and thus health, may be greater than is currently appreciated.
In conclusion, we provide evidence here that IL-6 is localized to the external zone of the SME. We also demonstrate that the content of IL-6 protein in the SME decreases in response to acute stress, accompanied by an increase in phosphorylated STAT3 in the nucleus of corticotrophs, and that plasma concentrations of IL-6 and ACTH increase congruently. In addition, the content of IL-6 in the SME, as well as the ACTH response to restraint or exercise stress, is greater in female pigs compared to males. Thus, IL-6 can be involved in augmenting ACTH release in response to acute stress and this relationship may provide a mechanistic link among IL-6, stress, and disease.
Acknowledgments
The authors thank DK Bowles, D Harah, R Johnson and RM McAllister for their assistance, VM Wiegant for his gift of W3 antiserum, and the University of Missouri National Center for Gender Physiology. This work was supported by NIH grants R01HL36088, R24RR018276, P01HL52490 and T32AR048523.
This research was supported by NIH grants R01HL36088, R24RR018276, P01HL52490 and T32AR048523.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Publisher's Disclaimer: “This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.”
References
- 1.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 4.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–30. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 7.Chesnokova V, Melmed S. Minireview: Neuro-immuno-endocrine modulation of the hypothalamic-pituitary-adrenal (HPA) axis by gp130 signaling molecules. Endocrinology. 2002;143:1571–4. doi: 10.1210/endo.143.5.8861. [DOI] [PubMed] [Google Scholar]
- 8.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 9.Kariagina A, Romanenko D, Ren SG, Chesnokova V. Hypothalamic-pituitary cytokine network. Endocrinology. 2004;145:104–12. doi: 10.1210/en.2003-0669. [DOI] [PubMed] [Google Scholar]
- 10.Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–17. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 11.Dunn AJ. Interleukin-6 access to the axis. Endocrinology. 2004;145:3578–9. doi: 10.1210/en.2004-0460. [DOI] [PubMed] [Google Scholar]
- 12.Navarra P, Tsagarakis S, Faria MS, Rees LH, Besser GM, Grossman AB. Interleukins-1 and -6 stimulate the release of corticotropin-releasing hormone-41 from rat hypothalamus in vitro via the eicosanoid cyclooxygenase pathway. Endocrinology. 1991;128:37–44. doi: 10.1210/endo-128-1-37. [DOI] [PubMed] [Google Scholar]
- 13.Spinedi E, Hadid R, Daneva T, Gaillard RC. Cytokines stimulate the CRH but not the vasopressin neuronal system: evidence for a median eminence site of interleukin-6 action. Neuroendocrinology. 1992;56:46–53. doi: 10.1159/000126207. [DOI] [PubMed] [Google Scholar]
- 14.Abraham EJ, Minton JE. Cytokines in the hypophysis: a comparative look at interleukin-6 in the porcine anterior pituitary gland. Comp Biochem Physiol A Physiol. 1997;116:203–7. doi: 10.1016/s0300-9629(96)00278-2. [DOI] [PubMed] [Google Scholar]
- 15.Silverman MN, Miller AH, Biron CA, Pearce BD. Characterization of an interleukin-6- and adrenocorticotropin-dependent, immune-to-adrenal pathway during viral infection. Endocrinology. 2004;145:3580–9. doi: 10.1210/en.2003-1421. [DOI] [PubMed] [Google Scholar]
- 16.Tsigos C, Papanicolaou DA, Defensor R, Mitsiadis CS, Kyrou I, Chrousos GP. Dose effects of recombinant human interleukin-6 on pituitary hormone secretion and energy expenditure. Neuroendocrinology. 1997;66:54–62. doi: 10.1159/000127219. [DOI] [PubMed] [Google Scholar]
- 17.Lyson K, McCann SM. The effect of interleukin-6 on pituitary hormone release in vivo and in vitro. Neuroendocrinology. 1991;54:262–6. doi: 10.1159/000125884. [DOI] [PubMed] [Google Scholar]
- 18.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A. 2000;97:9317–22. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muglia LJ, Bethin KE, Jacobson L, Vogt SK, Majzoub JA. Pituitary-adrenal axis regulation in CRH-deficient mice. Endocr Res. 2000;26:1057–66. doi: 10.3109/07435800009048638. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–32. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Hanisch A, Dieterich KD, Dietzmann K, Ludecke K, Buchfelder M, Fahlbusch R, Lehnert H. Expression of members of the interleukin-6 family of cytokines and their receptors in human pituitary and pituitary adenomas. J Clin Endocrinol Metab. 2000;85:4411–4. doi: 10.1210/jcem.85.11.7122. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Hernandez T, Afonso-Oramas D, Cruz-Muros I, Barroso-Chinea P, Abreu P, del Mar Perez-Delgado M, Rancel-Torres N, del Carmen Gonzalez M. Interleukin-6 and nitric oxide synthase expression in the vasopressin and corticotrophin-releasing factor systems of the rat hypothalamus. J Histochem Cytochem. 2006;54:427–41. doi: 10.1369/jhc.5A6845.2005. [DOI] [PubMed] [Google Scholar]
- 23.Ghorbel MT, Sharman G, Leroux M, Barrett T, Donovan DM, Becker KG, Murphy D. Microarray analysis reveals interleukin-6 as a novel secretory product of the hypothalamo-neurohypophyseal system. J Biol Chem. 2003;278:19280–5. doi: 10.1074/jbc.M209902200. [DOI] [PubMed] [Google Scholar]
- 24.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–30. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 25.Nybo L, Nielsen B, Pedersen BK, Moller K, Secher NH. Interleukin-6 release from the human brain during prolonged exercise. J Physiol. 2002;542:991–5. doi: 10.1113/jphysiol.2002.022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young SF, Smith JL, Figueroa JP, Rose JC. Ontogeny and effect of cortisol on vasopressin-1b receptor expression in anterior pituitaries of fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2003;284:R51–6. doi: 10.1152/ajpregu.00427.2002. [DOI] [PubMed] [Google Scholar]
- 27.Auernhammer CJ, Kopp FB, Vlotides G, Dorn F, Isele NB, Spottl G, Cengic N, Weber MM, Senaldi G, Engelhardt D. Comparative study of gp130 cytokine effects on corticotroph AtT-20 cells--redundancy or specificity of neuroimmunoendocrine modulators? Neuroimmunomodulation. 2004;11:224–32. doi: 10.1159/000078440. [DOI] [PubMed] [Google Scholar]
- 28.May LT, Santhanam U, Sehgal PB. On the multimeric nature of natural human interleukin-6. J Biol Chem. 1991;266:9950–5. [PubMed] [Google Scholar]
- 29.Sehgal PB. Interleukin-6 in vivo. Folia Histochem Cytobiol. 1992;30:195–6. [PubMed] [Google Scholar]
- 30.Fong Y, Moldawer LL, Marano M, Wei H, Tatter SB, Clarick RH, Santhanam U, Sherris D, May LT, Sehgal PB, et al. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989;142:2321–4. [PubMed] [Google Scholar]
- 31.Jablons DM, Mule JJ, McIntosh JK, Sehgal PB, May LT, Huang CM, Rosenberg SA, Lotze MT. IL-6/IFN-beta-2 as a circulating hormone. Induction by cytokine administration in humans. J Immunol. 1989;142:1542–7. [PubMed] [Google Scholar]
- 32.Grinevich V, Harbuz M, Ma XM, Jessop D, Tilders FJ, Lightman SL, Aguilera G. Hypothalamic pituitary adrenal axis and immune responses to endotoxin in rats with chronic adjuvant-induced arthritis. Exp Neurol. 2002;178:112–23. doi: 10.1006/exnr.2002.8022. [DOI] [PubMed] [Google Scholar]
- 33.Smoak B, Deuster P, Rabin D, Chrousos G. Corticotropin-releasing hormone is not the sole factor mediating exercise-induced adrenocorticotropin release in humans. J Clin Endocrinol Metab. 1991;73:302–6. doi: 10.1210/jcem-73-2-302. [DOI] [PubMed] [Google Scholar]
- 34.Deuster PA, Petrides JS, Singh A, Lucci EB, Chrousos GP, Gold PW. High intensity exercise promotes escape of adrenocorticotropin and cortisol from suppression by dexamethasone: sexually dimorphic responses. J Clin Endocrinol Metab. 1998;83:3332–8. doi: 10.1210/jcem.83.9.5110. [DOI] [PubMed] [Google Scholar]
- 35.Roca CA, Schmidt PJ, Deuster PA, Danaceau MA, Altemus M, Putnam K, Chrousos GP, Nieman LK, Rubinow DR. Sex-related differences in stimulated hypothalamic-pituitary-adrenal axis during induced gonadal suppression. J Clin Endocrinol Metab. 2005;90:4224–31. doi: 10.1210/jc.2004-2525. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 37.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–13. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 38.Turnbull AV, Prehar S, Kennedy AR, Little RA, Hopkins SJ. Interleukin-6 is an afferent signal to the hypothalamo-pituitary-adrenal axis during local inflammation in mice. Endocrinology. 2003;144:1894–906. doi: 10.1210/en.2002-220964. [DOI] [PubMed] [Google Scholar]