Abstract
The question of whether ovarian hormone therapy can prevent or reduce age-related memory decline in menopausal women has been the subject of much recent debate. Although numerous studies have demonstrated a beneficial effect of estrogen and/or progestin therapy for certain types of memory in menopausal women, recent clinical trials suggest that such therapy actually increases the risk of cognitive decline and dementia. Because rodent models have been frequently used to examine the effects of age and/or ovarian hormone deficiency on mnemonic function, rodent models of age-related hormone and memory decline may be useful in helping to resolve this issue. This review will focus on evidence suggesting that estradiol modulates memory, particularly hippocampal-dependent memory, in young and aging female rats and mice. Various factors affecting the mnemonic response to estradiol in aging females will be highlighted to illustrate the complications inherent to studies of estrogen therapy in aging females. Avenues for future development of estradiol-based therapies will also be discussed, and it is argued that an approach to drug development based on identifying the molecular mechanisms underlying estrogenic modulation of memory may lead to promising future treatments for reducing age-related mnemonic decline.
Keywords: Estradiol, aging, hippocampus, rat, mouse, menopause, hormone therapy
Introduction
Can estrogen therapy reduce cognitive decline in menopausal women? This seemingly simple question has sparked considerable debate in recent years due to reports from the Women’s Health Initiative Memory Study (WHIMS) indicating that treatment with estrogens, either alone or in combination with progestin, failed to prevent age-related memory decline in menopausal women and increased the risk of cognitive decline and dementia (Espeland et al., 2004; Rapp et al., 2003b; Shumaker et al., 2004; Shumaker et al., 2003). Although a follow-up study from the WHI Study of Cognitive Aging (WHISCA) revealed a trend for a positive effect of estrogen plus progestin treatment on figural memory, it also reported that treatment impaired verbal memory and had no effect on tests of attention, working memory, spatial ability, fine motor speed, affect, and depression (Resnick et al., 2006). The findings of the Women’s Health Initiative (WHI) stand in sharp contrast to previous studies linking ovarian hormone loss to an increased risk of Alzheimer’s disease (Launer et al., 1999; Sherwin, 1999; Wolf and Kirschbaum, 2002; Yaffe et al., 2000b; Yaffe et al., 1998; Zandi et al., 2002a), and estrogen therapy to a decreased risk of Alzheimer’s disease (Hogervorst et al., 2000; Tang et al., 1996; Yaffe et al., 1998; Zandi et al., 2002b). The WHI reports also conflict with reports from studies enrolling fewer subjects indicating that estrogen therapy in some menopausal women with (Asthana et al., 2001; Asthana et al., 1999; Yaffe et al., 2000a) and without (Caldwell and Watson, 1952; Duff and Hampson, 2000; Duka et al., 2000; Hogervorst et al., 2000; Maki et al., 2001; Sherwin, 1999; Smith et al., 2001; Yaffe et al., 1998) Alzheimer’s disease can reduce multiple types of memory decline (although see (Henderson, 2006; Mulnard et al., 2000; Resnick and Henderson, 2002; Wang et al., 2000)). Indeed, the negative findings from WHIMS and WHISCA, combined with the increased risks of breast cancer, stroke, and heart disease reported by the larger WHI studies (Chlebowski et al., 2003; Rossouw et al., 2002; Wassertheil-Smoller et al., 2003), were a surprise to many scientists, physicians, and patients. The WHI trial, the largest of its kind to date, was designed to examine effects of the commonly prescribed Premarin® and PremPro® hormone treatments on many aspects of women’s health (e.g., cancer, vascular function, osteoporosis, cognitive function), and its outcome precipitated both a rapid reduction in the number of women taking hormone therapy and substantially altered recommendations for hormone doses and duration of treatment.
Upon reflection, however, the WHI findings are not terribly surprising for numerous reasons, many of which have been articulated elsewhere (Craig et al., 2005; Maki, 2004; Sherwin and Henry, 2008). Among the criticisms leveled against the WHI study design are the fact that subject were too old to benefit from treatment and were not healthy prior to study enrollment. Further, the conjugated equine estrogen formulation of Premarin® is not as potent as estrogen treatments used in other studies (Sherwin and Henry, 2008), and the progestin in PremPro® (medroxyprogesterone acetate) is less neuroprotective than natural progesterone (Nilsen and Brinton, 2003). Premarin®, prescribed to relieve symptoms of menopause, first entered the market in 1942, well before the publication of data supporting an effect of sex-steroid hormones on cognitive function (Caldwell and Watson, 1952). Research using rodents and non-human primates (Hao et al., 2003; Hao et al., 2006; Rapp et al., 2003a; Tang et al., 2004; Tinkler et al., 2004) has since revealed that estrogens and progestins can significantly alter the physiology of “cognitive” regions of the brain, such as the hippocampus and prefrontal cortex, but because this basic research is in its relative infancy, it cannot yet provide the critical information necessary for the design of hormone-based therapies that maximize cognitive benefit. As such, many important questions remain to be addressed. This review aims to identify the issues most crucial to understanding the importance of ovarian hormones to modulating memory in aging females and to provide an overview of data from animal models of cognitive aging which may help shed light on these issues. Because research on the effects of ovarian hormones on memory in aging female rodents (rats and mice) has not previously been reviewed, rodents will be the focus of this discussion. In addition, because the vast majority of this work to date has examined effects of estrogens on types of memory that involve the hippocampus, hippocampal-dependent memory will be discussed most extensively. However, other brain regions and ovarian hormones (e.g., progesterone) will be discussed as appropriate. Finally, directions for future research will be discussed.
Estrogens and the brain
Understanding how estrogens modulate memory can be challenging for numerous reasons, not the least of which is that many brain regions subserve memory formation. With regard to estrogenic modulation of memory, types of memory involving the hippocampus have been most extensively studied due to the numerous effects of estrogens on this structure (see (Spencer et al., 2008; Woolley, 2007) for recent reviews) and the importance of this brain region in multiple types of memory. The hippocampus, a bilateral medial temporal lobe structure, is critical for various types of memories involving spatial, relational, and contextual information, and is necessary only for consolidation of such memories, not their long-term storage (Eichenbaum, 1997; Eichenbaum, 2002; Squire, 1992). Further, the vulnerability of the hippocampus to aging and Alzheimer’s disease (deToledo-Morrell et al., 2007; Driscoll and Sutherland, 2005) makes this brain region of particular interest to the study of estrogens and age-related cognitive function. The basal forebrain, through which the hippocampus receives subcortical information, and temporal cortices adjacent to the hippocampus (e.g., entorhinal and perirhinal cortices), through which the hippocampus receives cortical information, are also particularly vulnerable to the detrimental effects of aging and Alzheimer’s disease (Hof and Morrison, 2004). Other brain regions play important roles in different types of learning and memory, for example, the amygdala in emotional memory, the striatum in response learning, and the prefrontal cortex in working memory and executive function (Eichenbaum, 2002; Squire, 2004). Although these brain regions form intricate networks that include the hippocampus, each has distinct memory functions separate from the hippocampus (Eichenbaum, 2002; Squire, 2004).
Estrogen receptors are located throughout the brain, including most of the aforementioned brain regions. The two nuclear estrogen receptors, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), can be found throughout the cerebral cortex, hippocampus, basal forebrain, and amygdala of the mouse, rat, primate, and human (Milner et al., 2005; Milner et al., 2001; Osterlund et al., 2000; Shughrue et al., 1997a; Shughrue et al., 1997b; Shughrue and Merchenthaler, 2000; Shughrue et al., 2000). In the neocortex, ERα mRNA expression has been weakly detected in laminae IV–V, whereas ERβ mRNA was strongly detected throughout the cortex, particularly in the frontal, parietal, and entorhinal cortices (Osterlund et al., 2000; Shughrue et al., 1997b). ERα and ERβ are expressed in the amygdala in the medial, cortical, and amygdalohippocampal subdivisions (Osterlund et al., 2000; Shughrue et al., 1997b), and ERα expression has also been reported in the central nucleus of the rat (Shughrue et al., 1997b). In the basal forebrain, both receptors colocalize with cholinergic neurons (most of which project to the hippocampus and neocortex), although this is true more for ERα than ERβ (Shughrue et al., 2000). Both receptors are also expressed throughout the dorsal and ventral extent of the hippocampus, particularly in pyramidal neurons of the CA1 and CA3 regions (Shughrue and Merchenthaler, 2000). More recent evidence suggests that these receptors are not limited to the cell nucleus and are, in fact, located throughout neurons in the hippocampus. Ultrastructural evidence demonstrates that ERα is present in the nuclei and cytoplasm of GABAergic interneurons, and in the cytoplasm of pyramidal and granule cells (Milner et al., 2001). In pyramidal neurons, both receptors are also located in dendritic spines, axons, and axon terminals, where ERβ is more widely expressed at extranuclear sites (Milner et al., 2005; Milner et al., 2001). Collectively, the available data demonstrate that both ERs are expressed in brain regions that are critical for learning and memory, thereby providing an opportunity for estrogens to modulate the functioning of these brain regions and the memory processes they subserve. As detailed later in this review, the fact that ERα and ERβ are located at extranuclear sites within hippocampal neurons provides a multitude of potential mechanisms through which these receptors can modulate hippocampal function and hippocampal memory.
Estrogens comprise a class of steroid hormones that includes three biologically significant members: estradiol, estrone, and estriol. All three estrogens are synthesized from the androgens testosterone and androstenedione by the enzyme aromatase. Because androgens are synthesized from progestins, such as progesterone, progestins are obligatory precursors to both androgens and estrogens. In females, the primary sources of estrogens and progestins are the ovaries, although members of both classes of hormones can also be synthesized in the brain (Hojo et al., 2004b; Kretz et al., 2004; Robel et al., 1995). At puberty, the ovaries begin to produce these hormones in a cyclic fashion in response to hormone signals from the brain. Of most importance to the present discussion is the timing of hormone peaks and troughs. At the beginning of the menstrual cycle, estrogen and progestin levels are low. As ovarian follicles mature, levels of estrogens increase and reach peak levels just prior to ovulation, after which levels decrease to baseline just before the next cycle. Progesterone levels begin to rise just after ovulation, remain elevated through the second half of the cycle, and then decrease to baseline just prior to the next cycle (unless fertilization and implantation occur). In laboratory rodents, such as rats (Rattus norvegicus) and mice (Mus musculus), this cycle is termed an “estrous cycle”, which differs from the menstrual cycle in several ways, including the lack of a true luteal phase and the absence of uterine wall sloughing (Wise, 2000). However, cyclic hormone fluctuations are similar in many respects among rats, mice, and humans, including the surges of estradiol and progesterone just prior to ovulation (McCarthy and Becker, 2002). The rodent estrous cycle is just 4–5 days long, each day corresponding roughly to one of four phases. Of these phases, the adjacent proestrus and estrus phases are particularly noteworthy, where proestrus is characterized by peak estradiol and progesterone levels, and estrus is characterized by trough estradiol and progesterone levels (Allen, 1922; Long and Evans, 1922; McCarthy and Becker, 2002).
Incredibly, the drop in estradiol and progesterone levels that occurs within the approximately 24 hours between proestrus and estrus gives rise to extraordinary alterations in the morphology and physiology of the hippocampus. Indeed, the current study of estrogenic modulation of memory can primarily trace its origins to the seminal discovery that dendritic spine density in the CA1 subregion of the hippocampus is approximately 30% higher during proestrus than during estrus (Woolley et al., 1990; Woolley and McEwen, 1992). Subsequent studies have demonstrated that other aspects of hippocampal physiology fluctuate in a cyclic manner; for example, both CA1 long-term potentiation (Warren et al., 1995) and dentate gyrus neurogenesis (Tanapat et al., 1999) are enhanced during proestrus relative to estrus. Bilateral removal of the ovaries (ovariectomy) also significantly decreases CA1 dendritic spine density, and treatment with the potent estrogen 17β-estradiol (E2; two injections spaced 24 hours apart) prevents this decrease (Gould et al., 1990; Woolley and McEwen, 1992). Progesterone injection 48 hours after the last E2 injection initially increases CA1 dendritic spine density, but then sharply decreases spine density more than is observed with E2 alone (Gould et al., 1990; Woolley and McEwen, 1993). Similar increases in CA1 dendritic spine density have been observed in young and aged rhesus monkeys after cyclic estradiol cypionate treatment (Hao et al., 2003). Despite the fact that both hormones so profoundly affect spine density, the vast majority of subsequent research into hormonal modulation of the hippocampus (and of hippocampal-dependent memory) has focused on E2. Among the numerous effects of exogenous E2 on hippocampal function (reviewed in (Woolley, 2007)) are enhancements in baseline synaptic excitability and the magnitude of long-term potentiation (Foy et al., 1999; Woolley, 2007), and suppression of long-term depression (Vouimba et al., 2000). This increased plasticity may result from the activation of N-methyl-D-aspartate (NMDA) receptors on CA1 pyramidal neurons (Woolley and McEwen, 1994; Woolley et al., 1997), made possible, in part, by E2-induced inhibition of GABA synthesis in the inhibitory interneurons that regulate pyramidal neuron function (Hart et al., 2001; Murphy et al., 1998).
Further, E2 can influence hippocampal and neocortical plasticity indirectly by enhancing cholinergic input from hippocampal- and cortically-projecting cholinergic basal forebrain neurons (e.g., Gibbs and Aggarwal, 1998; Wu et al., 1999). Among the many effects of E2 on these neurons, basal forebrain mRNA levels of the cholinergic synthetic enzyme choline acetyltransferase (ChAT) fluctuate during the estrous cycle and are increased in response to E2 and progesterone after ovariectomy (Gibbs, 1996; Gibbs et al., 1994; Luine, 1985). Neocortical, hippocampal, and basal forebrain ChAT activity and acetylcholine release are also enhanced by E2 (Frick et al., 2002a; Gibbs, 2000a; Gibbs et al., 1997), as is high affinity choline uptake (O’Malley et al., 1987; Singh et al., 1994). This modulation of hippocampal and neocortical function by basal forebrain cholinergic neurons is critical with respect to aging, given that pathological changes in these neurons are associated with memory dysfunction in Alzheimer’s disease (Auld et al., 2002; Pappas et al., 2000; Perry et al., 1978; Whitehouse et al., 1982).
In addition to the basal forebrain, E2 influences levels of neurotransmitter systems in other mnemonic brain regions in rodents. For example, in the amygdala, E2 reduces levels of monoamine oxidase and ChAT (Luine et al., 1975), but increases levels of dopamine and metabolites for norepinephrine and serotonin (Bowman et al., 2002). In the hippocampus, E2 decreases levels of the serotonin metabolite 5-HIAA, but increases norepinephrine levels (Bowman et al., 2002; Renner and Luine, 1986). In the prefrontal cortex, levels of dopamine, norepinephrine, and serotonin are reportedly decreased after chronic E2 treatment in ovariectomized rats (Luine et al., 1998). Cyclic E2 treatment also increases spine density in the dorsolateral prefrontal cortex, but not primary visual cortex, of young rhesus monkeys (Tang et al., 2004), indicating that E2 influences synaptic plasticity in specifically in cortical regions critical for mnemonic functioning.
Accumulating evidence suggests that many E2-induced alterations in neural plasticity may be mediated by rapid signal transduction mechanisms. Estrogens have traditionally been thought to act via a “genomic” mechanism, by binding to ERα and ERβ, which act as nuclear transcription factors when the hormone-receptor complex binds to an estrogen response element on DNA. Although many effects of E2 on the brain are likely the result of genomic action on estrogen response elements, many “non-genomic” mechanisms of estradiol action have recently been identified, including activation of various intracellular signaling cascades. For example, the fact that E2’s effects on baseline hippocampal synaptic transmission and LTP are blocked by protein kinase inhibitors (Gu et al., 1999) suggests that activation of intracellular signaling cascades is necessary for E2-induced enhancements of hippocampal excitability. Subsequent work has indicated that E2 can activate signaling cascades that are critical for memory (Adams and Sweatt, 2002), such as the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) (Fitzpatrick et al., 2002; Kuroki et al., 2000; Wade and Dorsa, 2003) and phosphatidylinositol 3-kinase (PI3K) cascades (Mannella and Brinton, 2006; Yokomaku et al., 2003). Further, E2-induced enhancement of basal forebrain cholinergic function (Pongrac et al., 2004) and CA1 spines (Ogiue-Ikeda et al., 2008) has been shown to depend on ERK activation. These findings are supported by evidence placing ERα and ERβ at extra-nuclear sites throughout hippocampal neurons, including dendritic spines and presynaptic terminals (Milner et al., 2005; Milner et al., 2001). Traditional ERα and ERβ have even been shown to promote gene expression by binding directly to the Fos/Jun complex, thereby entirely bypassing estrogen response elements (Webb et al., 1995). The presence of estrogen receptors in the cell membrane has also been reported, and although the nature of these receptors remains unclear (Toran-Allerand, 2004), it appears as if E2 can activate signaling cascades by binding to these putative receptors (Fernandez et al., 2008; Kuroki et al., 2000). Interestingly, the enzymes necessary to synthesize E2 are expressed in the hippocampus, and hippocampal slices can produce E2 when stimulated by NMDA (Hojo et al., 2004a), suggesting that locally synthesized E2 may mediate the rapid effects of E2 on hippocampal physiology. The implications of such rapid changes in hippocampal signaling for future hormone therapy development will be discussed later in this review.
Estradiol and memory in young females
The extant literature generally supports the conclusion that E2 promotes hippocampal function, which leads to the obvious hypothesis that E2 should facilitate hippocampal-dependent memory. Although this is a reasonable hypothesis, directly linking estradiol-induced hippocampal alterations to memory modulation has proven to be a challenge. For example, the increases in spine synapses, LTP, and neurogenesis observed during proestrus relative to estrus during the estrous cycle should lead to enhanced memory during proestrus relative to estrus. However, only one study testing spatial reference memory, a type of long-term memory that is critically dependent on the hippocampus (Morris et al., 1982; Moser et al., 1993) has found this to be so. In this study, young female mice in proestrus learned to find a hidden escape platform in the Morris water maze faster and more accurately than those in estrus (Frick and Berger-Sweeney, 2001). This finding is supported by other work showing that rats in proestrus are more likely than those in estrus to use a spatial learning strategy in dry-land mazes (Korol et al., 2004) and that infusions of E2 directly into the dorsal hippocampus increase spatial strategy use in ovariectomized rats (Zurkovsky et al., 2007). Nevertheless, the superior spatial performance of proestrus mice in the water maze is inconsistent with studies of female rats using other water maze protocols that reported enhanced spatial reference memory in estrus relative to proestrus (Frye, 1995; Warren and Juraska, 1997) or no effect of cyclic ovarian hormone fluctuations on task performance (Berry et al., 1997). Further, conflicting effects of the cycle have been reported in tests of spatial memory using an object recognition task (Frye et al., 2007; Sutcliffe et al., 2007) and from studies using novel object or social recognition tasks in which rodents must detect the presence of a new object, conspecific, or food (Markham and Juraska, 2007; Sanchez-Andrade et al., 2005; Sutcliffe et al., 2007; Walf et al., 2006). Although the estrous cycle literature, on the whole, is inconclusive, the inconsistencies among estrous cycle studies should not necessarily be interpreted as a lack of effect of circulating estrogens and progestins on memory. Rather, the lack of agreement among these studies may be more indicative of how difficult it can be to pinpoint the behavioral effects of hormones that are in a constant state of flux. Indeed, the lack of consensus about the effects of the estrous cycle on memory in young females complicates the issue of how estrous cycle cessation should affect memory in aging females. If estrous cycling, particularly high E2 levels, are beneficial for memory, then age-related reductions in E2 levels should be detrimental to memory. If, however, low circulating E2 levels are more beneficial for memory, then memory may be only minimally affected by age-related reductions in this hormone. In aging females, the influence of acyclicity on memory may best be understood by examining interactions among age, ovarian deterioration, and duration of hormone deprivation prior to E2 treatment.
In both young and aging females, one way to isolate the effects of a single hormone on memory is to administer exogenous hormones to ovariectomized rodents. In young ovariectomized female rodents, exogenous E2 generally improves a short-term form of spatial memory called spatial working memory (Bimonte and Denenberg, 1999; Bohacek and Daniel, 2007; Bowman et al., 2002; Daniel and Dohanich, 2001; Daniel et al., 1997; Fader et al., 1998; Fader et al., 1999; Garza-Meilandt et al., 2006; Gibbs, 1999; Holmes et al., 2002; Luine et al., 1998; O’Neal et al., 1996; Sandstrom and Williams, 2001; Sandstrom and Williams, 2004; Wide et al., 2004), non-spatial working memory (Wide et al., 2004), memory for both the location (Frye et al., 2007; Luine et al., 2003) and identity (Vaucher et al., 2002) of objects, inhibitory avoidance (Frye and Rhodes, 2002; Singh et al., 1994)(but see (Foster et al., 2003)), and trace eyeblink conditioning (Leuner et al., 2004). However, as is true for exogenous E2 administration in aging females, improvements in young females can depend on numerous methodological variables such as dose (Holmes et al., 2002; Wide et al., 2004), duration of treatment (Luine et al., 1998), route of administration (Garza-Meilandt et al., 2006), extent of daily handling (Bohacek and Daniel, 2007), cognitive demand of the task (Bimonte and Denenberg, 1999), and whether was E2 was administered prior to training (Daniel et al., 1997; Gresack and Frick, 2004). Additional variables related to the aging process further complicate the design of E2 treatment studies in aging females, as will be discussed extensively later in this review.
Aging and ovarian hormones
In women, menopause is an inevitable consequence of aging, and this transition occurs, on average, at about age 51. Menopause is a gradual process of change (typically over the course of 2–7 years) resulting in the cessation of menses, profound reductions in ovarian hormone levels, and irreversible ovarian failure (Bellantoni and Blackman, 1996). The impact of menopause, and the consequent ovarian hormone loss, on memory has been the subject of considerable recent study. Numerous reports have linked menopause with memory loss, particularly those studies of surgically menopausal women, most of whom experience significant verbal memory decline after removal of the ovaries (reviewed in (Sherwin, 2006; Sherwin and Henry, 2008)). Among naturally menopausal women, those with low endogenous estrogen levels display worse verbal memory and an increased risk of cognitive decline relative to those with high estrogen levels (Wolf and Kirschbaum, 2002; Yaffe et al., 2000b). Women are also reportedly at increased risk for developing Alzheimer’s disease relative to men (Launer et al., 1999; Yaffe et al., 1998; Zandi et al., 2002a), which suggests that estrogen and/or progestin deficiency during middle age may be a critical factor in the development of dementia. Indeed, some studies have shown that hormone therapy can decrease the risk of developing Alzheimer’s by nearly one third (Yaffe et al., 1998) and delay the onset of the disease (Tang et al., 1996). Because the female hippocampus relies on hormones such as estrogens as trophic factors during adulthood (Brinton, 2001), estrogen deficiency during menopause may render these neurons more vulnerable to deterioration and exacerbate emerging age-related memory deficits. Studies in rodents lend support to this hypothesis.
Rodents are typically considered “aged” when they are approximately 2 years old. “Middle-aged” rodents average about 16–18 months of age, whereas “young” rodents used for memory experiments are typically 3–4 months of age. With regard to reproductive aging, there are several key differences between rodents and humans. For example, unlike in women, where menopause leads to a total loss of primordial follicles (Richardson et al., 1987), rats and mice do not experience complete follicle loss (Lu et al., 1979; Wise, 2000). In addition, whereas the negative feedback effects of E2 on gonadotrophins are decreased in menopausal women, which leads to elevated gonadotrophin levels (Crowley et al., 1985; Yen, 1999), such feedback remains intact in aged acyclic pseudopregnant rats, leading to relatively normal gonadotrophin levels (Lu, 1983; Wise, 2000). Nevertheless, reproductive senescence in rodents is similar to menopause in several critical respects, including similar alterations in pulsatile LH release and the LH surge, variability of cycle length prior to acyclicity, and ultimate cessation of hormone cycling (LeFevre and McClintock, 1988; Nelson et al., 1995). In addition, impending reproductive decline in both middle-aged rodents and humans is characterized by increases in FSH and circulating E2 levels (Downs and Wise, in press; Lu, 1983). Circulating E2 levels ultimately decline in women and rodents (Lu et al., 1979; Nelson et al., 1995), although they tend to remain elevated in middle-aged rats for quite some time (Morrison et al., 2006). In rats, reproductive decline begins at 9–12 months of age (Finch et al., 1984); by 12 months, approximately 70% of female rats cycle irregularly or are acyclic, and nearly 75% of females become acyclic by 24 months (Markowska, 1999). In mice, reproductive alterations begin at 13–14 months of age (Nelson et al., 1995); by 17 months, approximately 80% of female mice cycle irregularly or are acyclic, and all females mice become acyclic by 25 months (Frick et al., 2000). Although reproductive senescence in non-human primates is more similar to menopause than that in rodents (Morrison et al., 2006), practical considerations, including small size and short lifespan, make rodents an important model system in which to test the effects of E2 loss and exogenous E2 on age-related memory decline.
However, a few important caveats are important to keep in mind when extrapolating from rodents to humans. First, differences in the types of estrogens used in many human and rodent studies may limit the applicability of the rodent data to menopausal women. Whereas many clinical studies, including the WHIMS and WHISCA studies, have administered conjugated equine estrogens (a cocktail of estrogens containing mainly estrone sulfate), rodent studies have typically administered some form of estradiol (either E2 or estradiol benzoate). In randomized clinical trials of postmenopausal women, E2 administered intramuscularly or transdermally improved verbal and working memory, whereas oral conjugated equine estrogens did not (see (Sherwin and Henry, 2008) for recent review), suggesting superior efficacy of E2 over conjugated equine estrogens. Indeed, estrone has a considerably lower binding affinity for ERα and ERβ than E2 (Kuiper et al., 1997). As such, findings from rodent or human studies using E2 may not generalize well to conjugated equine estrogens. Second, tests used to measure cognitive function, including memory, differ considerably in rodents and humans, which may also limit application of data from rodents to menopausal women. For example, many clinical studies report an effect of estrogens on verbal memory (whether an improvement as reviewed in (Sherwin and Henry, 2008) or an impairment as shown by (Resnick et al., 2006)), whereas rodents do not have a verbal memory to test, per se. Further, many clinical studies of hormone therapy employ general tests of cognitive function (e.g., the 3MSE) (Rapp et al., 2003b; Shumaker et al., 2003) for which there is no rodent equivalent. When more specific neuropsychological test batteries are used in clinical studies (e.g., digit span, card rotations, California Verbal Learning Test) (Resnick et al., 2006), the investigator can typically tap into more aspects of cognitive function than is possible in a rodent. In addition, most rodent studies utilize tasks based on navigating through the environment (e.g., Morris water maze, radial arm maze, T-maze), or otherwise interacting with stimuli in the environment in a physical way (e.g., investigating an object, moving to avoid a shock), whereas tests used in humans generally involve no physical movement throughout the environment. Although these differences may call into question the applicability of rodent data to humans, the remarkable parallels between the effects of brain lesions and aging on tests designed in rodents and humans to measure the same type of memory suggest a considerable degree of commonality among tests meant to measure similar mnemonic processes in rodents and humans (Rosenzweig and Barnes, 2003; Squire, 1992). In addition, the recent development of virtual computer mazes that simulate movement through mazes such as the Morris water maze allow for better parallels between humans and rodents. Although these virtual mazes are sensitive to sex differences in performance (males outperform females), age (young subjects outperform older subjects), and testosterone levels (high levels correlate with better performance) (Astur et al., 1998; Burkitt et al., 2007; Driscoll et al., 2005), such mazes have not yet been used to study effects of hormone therapy on cognitive function in menopausal women. Adoption of such virtual tools for studies of menopausal women would greatly aid in bridging the methodological gaps between rodents and humans.
The aging brain and estradiol
Certain brain regions, such as the hippocampus, basal forebrain, entorhinal cortex, and prefrontal cortex, are exceptionally vulnerable to the detrimental effects of aging. Age-related deterioration of these brain regions has been extensively documented in several mammalian species, including humans, non-human primates, rats, and mice (e.g. (Burke and Barnes, 2006; Decker, 1987; Erickson and Barnes, 2003; Hof and Morrison, 2004; Morrison and Hof, 2002; Rosenzweig and Barnes, 2003)). Numerous age-related alterations in the rodent hippocampus have been associated with impaired spatial memory including place cell rigidity (Wilson et al., 2003), elevated neurotrophin and protein kinase levels (Bimonte et al., 2003; Columbo et al., 1997), reduced postsynaptic density area (Nicholson et al., 2004) and synaptic proteins (Frick and Fernandez, 2003; Smith et al., 2000), and impaired long-term potentiation (Bach et al., 1999). Elevated protein kinase levels in the prefrontal cortex have also been associated with impaired working memory in aged rats and monkeys (Ramos et al., 2003), and decreased prefrontal dendritic spine density has been related to impaired object recognition in memory in aged female rats (Wallace et al., 2007). Spatial memory deficits in aged gonadally intact female rats have also been correlated with deterioration of basal forebrain cholinergic neurons (Fischer et al., 1992; Fischer et al., 1989).
Of these brain regions, the hippocampus has been the primary focus of rodent studies examining the mnemonic response to E2 in aging females due to the extensive literature on estrogenic effects in the hippocampus among young females. In general, the hippocampus of aging female rodents remains responsive to E2. E2 treatment in the hippocampus of middle-aged and/or aged females increases levels of synaptophysin and nerve growth factor, augments dentate gyrus dendritic spine density, activates protein kinases, normalizes intracellular calcium homeostasis, and phosphorylates NMDA receptors (Bi et al., 2003; Fernandez and Frick, 2004; Foster, 2005; Frick et al., 2002b; Miranda et al., 1999). The E2-induced increase in synaptophysin protein levels in aged females has been associated with improved spatial reference memory in the Morris water maze (Frick et al., 2002b). The hippocampus of aging rodents is also susceptible to long-term depression, and the fact that E2 treatment can block induction of this phenomenon in middle-aged female rats (Foster et al., 2003), may suggest a potential synaptic mechanism through which E2 improves memory in aging females.
Nevertheless, it is important to remember that the effects of E2 treatment in the aging hippocampus are dictated by the myriad of age-related alterations to this structure. Among these alterations are reductions in ERα and ERβ immunoreactivity, mRNA levels, and protein levels in the aged female hippocampus (Adams et al., 2002; Mehra et al., 2005; Yamaguchi-Shima and Yuri, 2007), which may alter responsiveness to E2 in aging females relative to young females. Indeed, several studies have found differing effects of E2 treatment on the hippocampus in young and aged (22–24 months) females. For example, CA1 pyramidal neurons in ovariectomized aged rats do not respond to E2 with an increase in dendritic spine density as do those in young females (Adams et al., 2001). Rather, dendritic spine density is increased by E2 in the dentate gyrus of ovariectomized aged (16–20 months) rats (Miranda et al., 1999). Also, whereas E2 reduces the amount of ERα immunoreactivity per CA1 synapse in young females, it has no such effect in aged females (Adams et al., 2002). Interestingly, although CA1 spine density is not affected by E2 in aged females, E2 does increase the numbers of NMDA receptors per aged CA1 synapse (Adams et al., 2001). E2 also restores the synaptic distribution of NR2B NMDA receptor subunits in the aged CA1 region to that seen in young females (Adams et al., 2004). Collectively, these studies indicate that the CA1 region in aged females does not respond to E2 by increasing spine synapses, but rather by modifying the number and distribution of NMDA receptors in existing synapses. Although it is tempting to speculate that alterations in hippocampal NMDA receptors underlie the beneficial effects of E2 treatment on memory in aging females, E2-induced changes in glutamatergic plasticity have not been directly linked to E2-induced memory modulation in young or aging females. In fact, only one study has measured both the neural and mnemonic response to E2 in the same aged animals. In this study, an E2-induced increase in synaptophysin protein levels in 27–28 month-old females was associated with improved spatial reference memory in the Morris water maze (Frick et al., 2002b), which provides support for a link between enhancement of synaptic plasticity and memory in aging females. Nevertheless, dearth of studies in aging rodents directly associating E2-induced changes in the brain and memory underscore the fact that the specific neural mechanisms underlying estrogenic modulation of memory in aging females are very poorly understood. This important issue deserves much more attention in future studies.
Effects of estradiol on memory in aging female rodents
Memory decline has been associated with the loss of estrous cycling in both rats and mice. This relationship has been particularly well described for spatial reference memory tested in the Morris water maze, which declines at an earlier age in females than in males. Significant deficits in females are observed by 12 months in rats and 17 months in mice, whereas such deficits are not apparent in male rats until 18 months and in male mice until 25 months (Frick et al., 2000; Markowska, 1999). Moreover, the onset of this premature spatial memory decline in females coincides with the cessation of ovarian hormone cycling, as illustrated by the fact that the age at which spatial memory deficits first appear in both species is marked by a sharp decline in regular estrous cycling (Frick et al., 2000; Markowska, 1999) (Fig. 1). In both the Markowska, 1999 and Frick et al., 2000 studies, the low numbers of rodents in each cycling category (regular, irregular, or acyclic) precluded statistically meaningful correlations between cycling status and spatial memory. However, the study by Markowska, 1999 did observe an interesting trend among 12 month-old female rats, whereby performance in a daily probe trial was best in regularly cycling females, intermediate in irregularly cycling females, and worst in acyclic females. Similar trends were reported in females at 18 and 24 months of age (Markowska, 1999), suggesting that disruption of estrous cycling is detrimental to spatial memory throughout the aging process.
Figure 1.
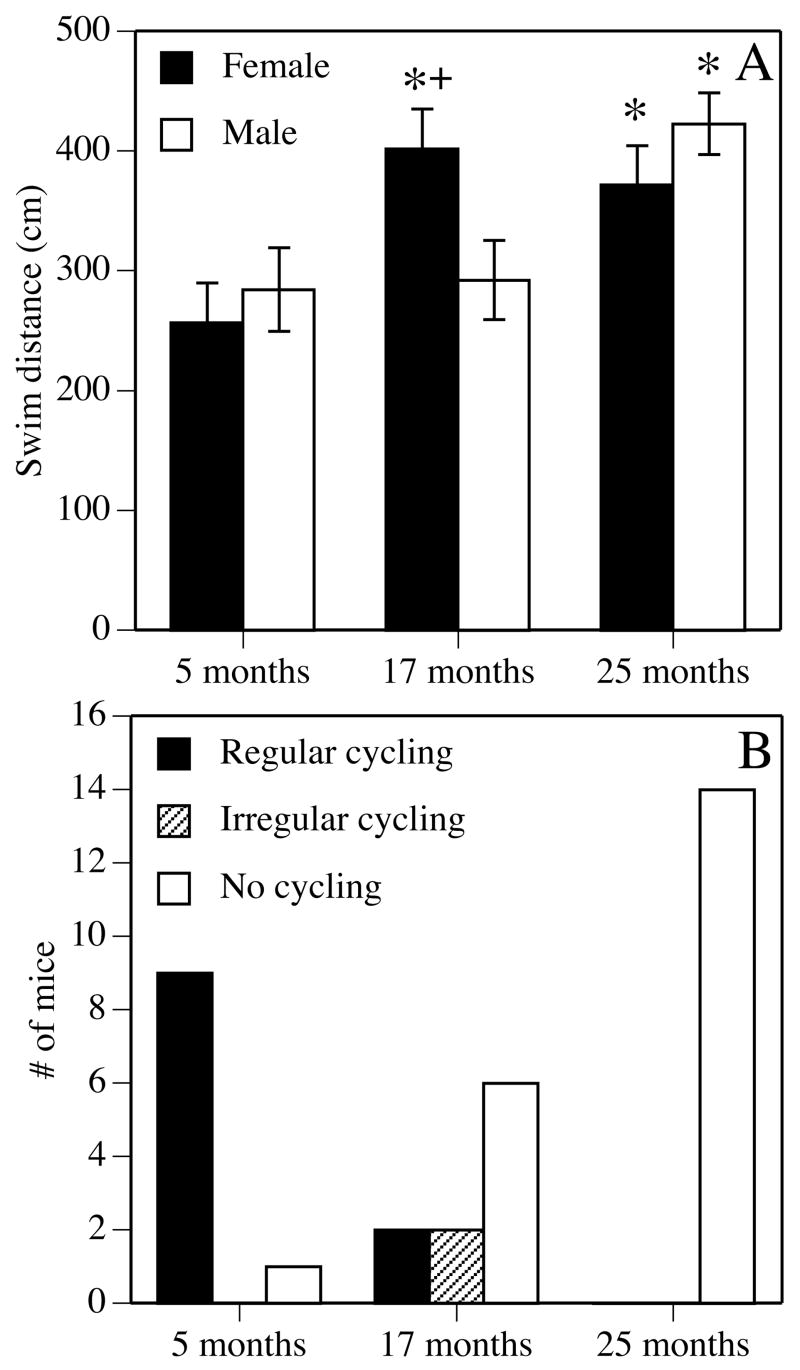
Effects of age on spatial reference memory in the Morris water maze (A) and estrous cycling (B). (A) Gonadally intact male and female mice were tested for 5 days in a spatial Morris water maze task at 5 (young), 17 (middle-aged), or 25 (aged) months of age. Each bar represents the mean ± standard error of the mean (SEM) swim distance for all 5 days of testing; lower numbers indicate better performance. Middle-aged and aged females were significantly impaired relative to young females, whereas only aged males were impaired relative to young males (*p < 0.05). Middle-aged females were also significantly impaired relative to middle-aged males (+p < 0.05). This pattern of data indicates that the onset of spatial reference memory decline in females occurs at an earlier age in females than in males. Adapted from (Frick et al., 2000; Frick et al., 2002a). (B) Estrous cycling was measured using daily vaginal lavage. The incidence of regular 4–5 day estrous cycles declined with age, such that no aged females were observed cycling regularly, whereas the number of mice failing to cycle increased with age. Irregular cycling, consisting of prolonged cycles, was observed among some middle-aged females. Adapted from (Frick et al., 2000).
Interestingly, mRNA for the nerve growth factor receptor trkA decreases significantly in the medial septal nucleus of the basal forebrain between 13 and 25 months of age in gonadally intact female, but not male, rats (Gibbs, 1998). Nerve growth factor is an important trophic factor for cholinergic neuron survival, and over 90% of medial septal cholinergic neurons express trkA (Gibbs, 1998). A relationship between basal forebrain cholinergic dysfunction and spatial memory loss in aging is supported by studies in 24–29 month-old male rats demonstrating significant correlations between poor spatial reference memory in the water maze and reduced ChAT activity in the hippocampus (Dunbar et al., 1993), basal forebrain, and frontal cortex (Gallagher et al., 1990). Because the majority of trkA-expressing medial septal cholinergic neurons project to the hippocampus, the loss of trkA expression in aging females may suggest a disruption of subcortical cholinergic inputs to the hippocampus, which could contribute to sex differences in spatial memory decline.
One way to test the hypothesis that reproductive aging contributes to memory decline in females is to determine if replacement of estrogens and/or progestins can reverse the observed memory dysfunction. On the face of it, this seems like a relatively simple proposition. However, truly restoring the cycle at any age is exceedingly difficult, given the complex timing of hormone fluctuations during the estrous cycle. Further, the added dimension of aging raises complicated issues with respect to experimental design. Some issues are characteristic of nearly any pharmacological experiment. For example, effects of E2 on memory may differ based on dose and type of memory tested. For instance, Bimonte-Nelson and colleagues (2006) found that a low dose of E2 time-release pellets (0.25 mg, 60–day release) administered to 14 month-old ovariectomized rats for four weeks improved spatial reference memory in the water maze, whereas high dose pellets (0.5 mg, 60-day release) had no effect. Among slightly older (17–18 months old) ovariectomized rats, silastic capsules containing a low or high dose of estradiol benzoate had no effect on spatial water maze acquisition, but the high dose improved task retention (Foster et al., 2003). Also among middle-aged females, one of three doses of E2 administered to 18 month-old ovariectomized mice in the drinking water for 5 weeks impaired spatial reference memory tested in a water-escape motivated verision of the radial arm maze, but robustly improved novel object recognition (Fig. 2). In aged females, daily injections of 5 μg, but not 1 μg, estradiol benzoate to 27–28 month-old intact mice prior to training improved spatial water maze acquisition and increased hippocampal synaptophysin levels (Frick et al., 2002b). Although there is unlikely to be a single dose of E2 that improves memory on all tasks at all ages, this sampling of studies that have utilized multiple doses of E2 in aging females illustrates how dose, task, and/or type of memory tested can influence the outcome of E2 treatment. Unfortunately, many studies to date have been limited in the scope of doses, ages, and tasks used, and therefore, more studies must include multiple doses and multiple tasks to better understand how various types of memory are affected by E2 treatment. Such information would be relevant to understanding the specific aspects of cognitive function likely to be affected by estrogen therapy in menopausal women.
Figure 2.
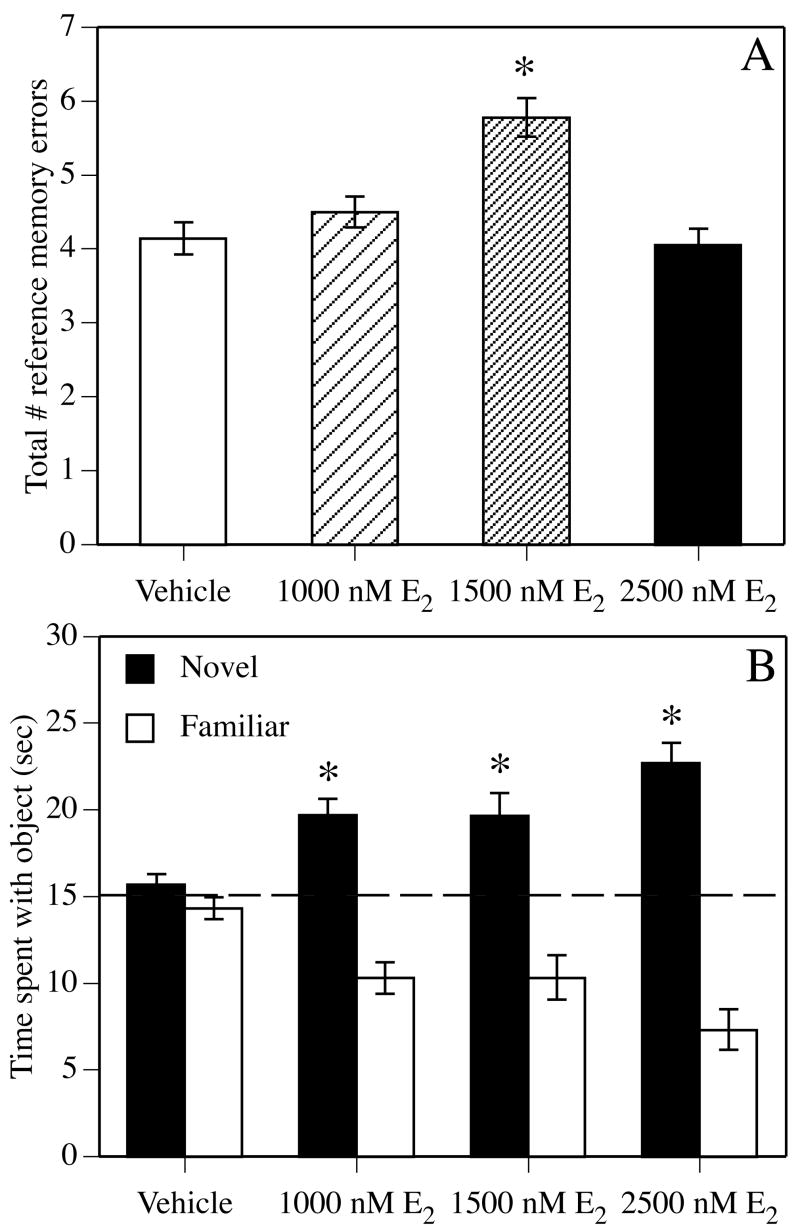
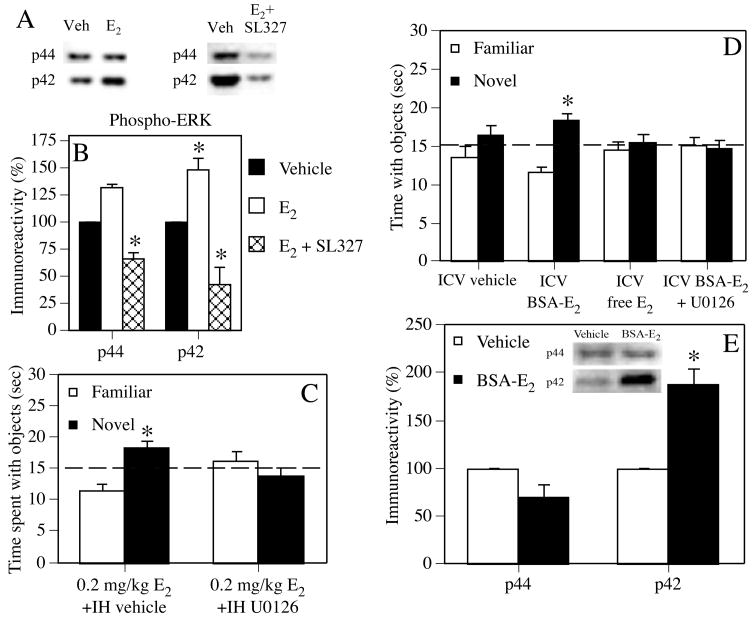
Vehicle or E2 were dissolved in ethyl alcohol and delivered via the home cage drinking water for 5 weeks prior to, and then during, testing in water-escape motivated radial arm maze and novel object recognition tasks. (A) In the radial arm maze, 1500 nM E2 significantly increased the number of spatial reference memory errors made during testing (*p < 0.05 relative to vehicle controls). Each bar represents the mean (± SEM) of 15 days of testing. (B) During novel object recognition training, mice accumulated 30 seconds exploring two identical objects and then were immediately injected with vehicle or E2. Forty-eight hours later, all doses of E2 significantly increased the time spent with the novel object relative to chance (dashed line at 15 seconds; *p < 0.05 relative to chance), indicating intact memory for the familiar object. Each bar represents the group mean (± SEM) for the retention trial. Adapted from (Fernandez and Frick, 2004).
In addition to the more obvious issues of dose, task, and type of memory tested, other important factors must be taken into consideration in studies of hormones and aging, including the age of the subjects, presence or absence of the ovaries during treatment, duration of hormone deprivation prior to treatment, timing of treatment relative to testing, cyclic or continuous nature of the treatment, and the influence of progestin co-administration and environmental factors on the mnemonic response to E2. Most studies manipulate more than one of these factors simultaneously, which provides a challenge to understanding how each factor contributes to the mnemonic effects of E2. Nevertheless, the sections below will provide a synthesis of this literature to date and suggest avenues for future research. A table detailing most of these studies has been previously published; please see Table 2 in Gresack and Frick, 2006a for more specific methodological information on many of the studies described below.
Age at treatment
A common approach used in many aging studies is to examine effects of E2 on memory at a single age (i.e. either middle-aged or aged), with treatment effects measured relative to an age-matched vehicle group and/or a young control group. Among middle-aged (14–18 months) female rats and mice tested in such studies, chronic (1–5 weeks) treatment with E2 via silastic capsules, pellets, or the drinking water improves spatial reference memory in the water maze (Bimonte-Nelson et al., 2006; Markham et al., 2002), spatial working memory in the radial arm maze (Daniel et al., 2006), and novel object recognition (Fernandez and Frick, 2004) (Fig. 2). It is important to note for the Daniel et al., 2006 study that improvements in spatial working memory were observed only in 17 month-old rats receiving treatment immediately after ovariectomy, and not in those whose treatment commenced 5 months after ovariectomy (the issue of duration of ovariectomy prior to treatment will be discussed in detail below). Inconsistent with the positive results of E2 treatment in middle-aged females are the aforementioned data from 18 month-old ovariectomized mice showing that spatial reference memory tested in the radial arm maze can be impaired by 5 weeks of E2 administered in the drinking water prior to training (Fernandez and Frick, 2004) (Fig. 2).
Many studies also report beneficial effects of estradiol in aged female rodents. Among aged (22–28 months) female mice, spatial reference memory in the water maze is improved by daily injections (for 9 days) of estradiol benzoate (Frick et al., 2002b) and spatial reference memory consolidation is enhanced by a single injection of E2 given immediately after training (post-training) (Frye et al., 2005; Harburger et al., 2007). Spatial reference memory, but not spatial working memory, in a win-stay radial arm maze task is also improved by forty days of E2 treatment via silastic capsules given to 24 month-old female mice 18 months after ovariectomy (Heikkinen et al., 2004). Improvement in other tasks has also been observed; silastic E2 implants improved spontaneous alternation in a T-maze (Miller et al., 1999) and novel object recognition (Vaucher et al., 2002) in 24 month-old female mice. In contrast to these positive effects of E2 treatment, negative or null effects of treatment have been reported in other aged females treated with E2 post-training. In one study, a single E2 injection given immediately after training to 22 month-old female mice had no effect on novel object recognition (Gresack et al., 2007b), and in another study, chronic E2 treatment administered via injections of E2 daily or every 4 days (vehicle injected all other days) to female mice from 18–21 months of age had no effect on novel object recognition and working memory errors in the radial arm maze and a detrimental effect on reference memory errors in the radial arm maze (Gresack and Frick, 2006a). Although these data could indicate that E2 must be in the circulation during training to improve memory in aged females, the fact that post-training E2 enhances spatial reference memory in the water maze (Harburger et al., 2007) argues against this as an explanation for all types of memory. Rather, it may be that E2 must be in the circulation during testing to improve certain types of memory, like object recognition or spatial memory tested in the radial arm maze, in aged females. This possibility is supported for object recognition by the fact that performance in this task is improved in 22–24 month-old mice by 21 days of E2 silastics prior to training and testing (Vaucher et al., 2002).
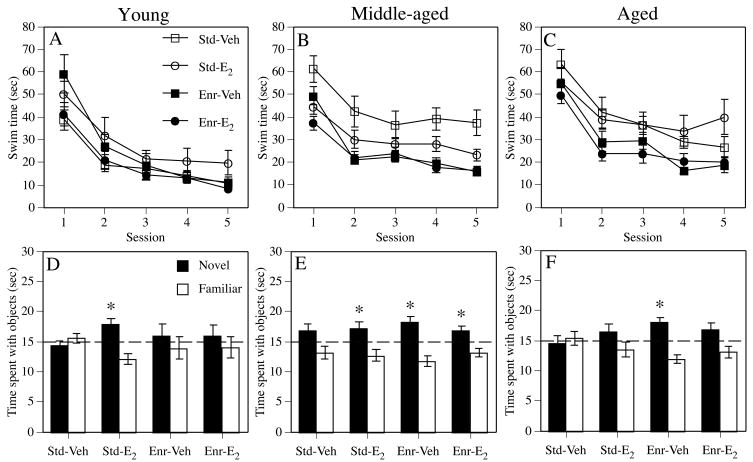
Although the aforementioned studies provide valuable information about the effects of E2 on memory in aging females, with many findings suggesting that treatment is beneficial, they do not allow the effectiveness of E2 to be directly compared between middle-aged and aged females. Such comparisons can only be made if females of different ages are given the same E2 treatment and tested on the same behavioral tasks. Although only a handful of such studies have been conducted, studies of this kind can reveal key insights about how age influences the mnemonic response to E2. Studies of avoidance learning in female rats indicate that memory at any age is not improved by E2; treatment either impaired or has no effect on such learning in ovariectomized female rats at 12–13 months, 17–18 months, or 20 months of age (Foster et al., 2003). However, E2 did protect against the detrimental effects of the cholinergic antagonist scopolamine on T-maze active avoidance in ovariectomized 12–13 month-old, but not 20 month-old, female rats, suggesting protective effects of E2 in middle-aged, but not aged, females (Savonenko and Markowska, 2003). Benefits limited to middle-aged females have also been observed for other types of memory. For example, E2 enhanced spatial water maze acquisition in 4 and 16 month-old ovariectomized rats, but not in 24 month-old rats (although minor benefits were observed at this age in a spatial probe trial) (Talboom et al., 2008). More strikingly, our lab recently reported widely discrepant effects of post-training E2 treatment on spatial reference memory in the Morris water maze and novel object recognition tasks in ovariectomized young, middle-aged, and aged female mice; in 5 month-old females, E2 enhanced object recognition, but impaired spatial memory, in 17 month-old females, E2 enhanced both types of memory, and in 22 month-old females, E2 had no beneficial effect on either type of memory (see Std-Veh and Std-E2 groups in Fig. 3) (Gresack et al., 2007a).
Figure 3.
Female mice were housed from the age of 3 weeks in standard (Std) conditions (5 mice/shoebox cage) or enriched (Enr) conditions (up to 10 mice in a large cage with many objects) up to and through behavioral testing at 5, 17, or 22 months of age. Mice were ovariectomized approximately 2 weeks before behavioral testing and were injected i.p. with vehicle (Veh) or 0.2 mg/kg E2 immediately after training each day. (A–C) Mice were tested for 5 days in a spatial Morris water maze task. Among young females, spatial memory was impaired by E2 alone, but improved by the combination of E2 and enrichment. Among middle-aged females, E2 improved spatial memory in standard-housed mice only, whereas enrichment improved spatial memory regardless of hormone treatment. Among aged females, only enrichment improved spatial memory. Each point represents the mean (± SEM) for one session. (D–F) Only E2 alone enhanced 48-hour object recognition relative to chance (dashed line at 15 seconds; *p < 0.05) in young females, whereas only enrichment alone enhanced object recognition in aged females. Object recognition in middle-aged females was enhanced relative to chance by E2 alone, enrichment alone, and the combination of both treatments. Each bar represents the group mean (± SEM) for the retention trial. Adapted from (Gresack et al., 2007a).
Collectively, the studies by Savonenko and Markowska, 2003, Talboom et al., 2008 and Gresack et al., 2007a suggest beneficial effects of E2 in middle-aged, but not aged, females. As such, these findings support the increasingly popular notion that there is a critical period during early menopause in which hormone replacement may effectively improve memory (Maki, 2006; Sherwin, 2006; Zandi et al., 2002b). This so-called “critical period hypothesis” suggests that hormone therapy will only benefit cognitive function if initiated when menopausal symptoms are present during early menopause (i.e., during middle-age) (Maki, 2006). Indeed, meta-analyses of clinical studies in menopausal women report that hormone therapy is more effective in recently menopausal women experiencing physiological symptoms of menopause at the time of treatment than in those who are many years beyond the onset of menopause (Yaffe et al., 1998). The fact that the subjects in the Women’s Health Initiative (WHIMS and WHISCA) studies were all age 65 or older, and were asymptomatic, helps to support the contention that estrogen treatment after the critical period is not an effective means of preventing age-related cognitive decline.
Influence of the ovaries and duration of hormone loss prior to treatment
In addition to age, duration of hormone deprivation prior to E2 treatment is also relevant to consideration of the critical period hypothesis. In women, age and duration of hormone deprivation are typically linked (except in the case of surgical menopause for younger women), so differentiating between effects of age and length of hormone deprivation in women can be challenging. In rodents, bilateral ovariectomy can be more easily used to distinguish between age and hormone deprivation. Regardless of age, ovariectomy is standard practice in rodent hormone replacement studies, allowing investigators more control over E2 levels in circulation. Most investigators ovariectomize their females in close proximity (a month or less) to the start of E2 treatment, with the assumption that allowing the ovaries to age naturally provides the most useful model of natural brain aging. However, some investigators report interesting differences in the mnemonic response to E2 between short- and long-term ovariectomy. For example, Daniel, Hulst, and Berberling (2006) tested three groups of ovariectomized rats in the radial arm maze at 17 months of age; one group was ovariectomized at 12 months and treated with E2-secreting silastic capsules for 5 months prior to testing, and other groups were ovariectomized at 12 or 17 months and treated with E2 silastics for 1 week prior to testing. Rats in which silastics were implanted at the time of ovariectomy, regardless of whether treatment started at 12 or 17 months, exhibited improved spatial working memory in the radial arm maze when tested at 17 months. In contrast, spatial working memory in rats ovariectomized at 12 months and treated at 17 months was not affected by E2, suggesting that E2 treatment is not effective when initiated after 5 months of hormone deprivation. In support of this notion, two other studies reported no effect of E2 on spatial working memory among rats ovariectomized for 7 or 18 months. In one study, rats ovariectomized at 13 months of age and treated with E2 silastics for 5 days at 21 months of age showed no improvement in a T-maze delayed non-matching to position task (although spatial working memory was improved in a water maze task) (Markowska and Savonenko, 2002), and in another, rats ovariectomized at 5 months of age and treated for 40 days with E2 pellets at 23 months of age showed no improvement in radial arm maze and T-maze tasks (Heikkinen et al., 2004). Further, Gibbs, 2000b showed that 10 months of hormone deprivation followed by 6–8 weeks of weekly E2 injections had no effect on spatial working memory in a delayed non-match to position task among 23 month-old rats. However, a shorter period of hormone deprivation (3 months) could be offset by 5–9 months of E2 silastic treatment, as spatial working memory was improved at 22–25 months of age (Gibbs, 2000b). Together, these studies indicate that long-term (> 3 months) hormone deprivation reduces the beneficial effect of E2, and fit well with the critical period hypothesis suggesting that hormone therapy may be most effective when given soon after hormone loss.
Knowing about this critical period may help women decide when to initiate hormone therapy, but this information does not address the question of whether hormone therapy is necessary in the first place. That is, does long-term ovarian hormone loss itself impair memory to the extent that treatment would be required? A handful of studies have examined the effects of long-term ovariectomy on memory, and the results do not provide any clear answers. Bimonte-Nelson and colleagues have demonstrated that 1.5–6 months of ovariectomy starting after 14 months of age improves spatial working and reference memory in aged rats tested in a water escape-motivated radial arm maze, whereas 21 days of ovariectomy impairs both spatial working and reference memory in the same task (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004). This seemingly unlikely benefit of long-term ovariectomy has been linked with elevated progesterone in aged intact rats, a hypothesis supported by detrimental effects of progesterone treatment in ovariectomized aged female rats on spatial working memory in this task (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004). The improvements induced by long-term ovariectomy are also consistent with the beneficial effects of long-term ovariectomy on delayed recognition learning in monkeys (Lacreuse et al., 2000). However, the beneficial effects of long-term ovariectomy in rats observed by Bimonte-Nelson and colleagues have not been found by other investigators. For example, Sato et al., 2003 reported that 15 month-old rats tested in a dry-land radial arm maze 3 months after ovariectomy were impaired in numerous measures of spatial working memory (Sato et al., 2003). Further, a recent longitudinal study of rats ovariectomized at 13 months found no effect on spatial working memory tested in a T-maze delayed non-match to position task until 7 months after surgery or until 4 months after surgery when delays of 5, 15, or 30 minutes were inserted between trials (Markowska and Savonenko, 2002). This study also found that rats tested 6 months after ovariectomy surgery were more sensitive to the disruptive effects of scopolamine than sham-operated controls (Markowska and Savonenko, 2002).
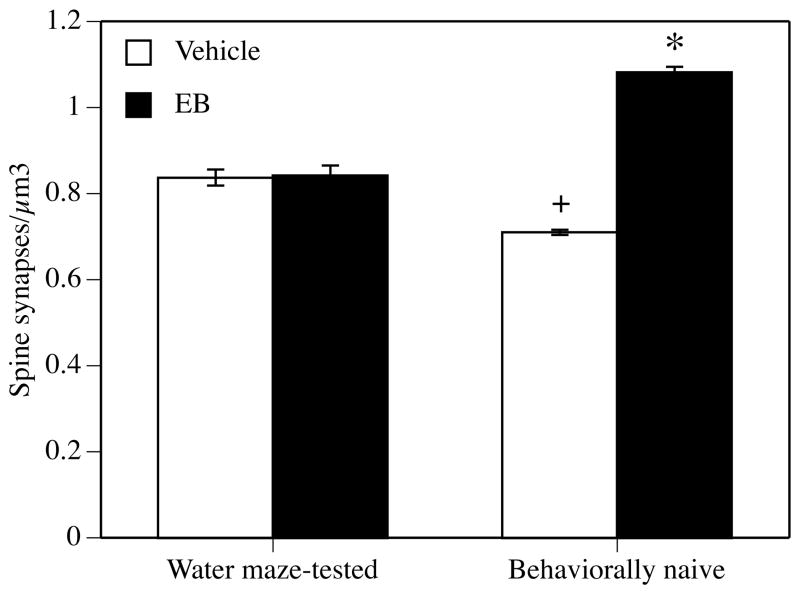
In the hippocampus and prefrontal cortex, long-term ovariectomy alters cholinergic function, although these changes have not been directly linked to behavior. In one recent study, ChAT protein levels were increased in the hippocampus, but not prefrontal cortex, of 2 month-old and 15 month-old rats treated for 10 days with E2 silastics implanted immediately after ovariectomy (Bohacek et al., in press). However, among rats ovariectomized at 10 months of age and treated at 15 months of age, ChAT protein levels were increased in the prefrontal cortex, but not hippocampus (Bohacek et al., in press). Another study of cholinergic function in rats found that ChAT and trkA mRNA in specific basal forebrain nuclei were significantly decreased 6 months, but not 3 months after ovariectomy (conducted at 13 months of age) relative to age-matched gonadally intact controls (Gibbs, 1998). The results of these two studies may indicate that long-term ovarian hormone loss alters the functioning of the septohippocampal and basalocortical cholinergic systems, which could lead to impaired spatial working memory in tasks, like the radial arm maze, in which hippocampal and cortical cholinergic involvement has been demonstrated (Olton et al., 1992; Sengstock et al., 1992). However, the work by Bimonte-Nelson and colleagues is inconsistent with the notion that long-term ovariectomy is detrimental to neural and mnemonic functioning. Given that Bimonte-Nelson and colleagues have replicated the beneficial effects of long-term ovariectomy on spatial working and reference memory (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004), the discrepancy between their data and those of others may be related to the stress of water maze testing, which has been shown to interfere with the hippocampal response to E2 treatment (Frick et al., 2004) (Fig. 4). This, and other, possibilities must be addressed before conclusions can be made in rodents about the effects of long-term ovariectomy on memory. Additional data concerning effects of long-term ovariectomy on other types of memory and in mice should be collected to determine the extent to which any of these findings generalize to other cognitive domains and other rodent species.
Figure 4.
CA1 spine synapse density in vehicle- and estradiol-treated rats that were behaviorally naïve or tested in the Morris water maze. Young ovariectomized rats were given two injections, 24 hours apart, of sesame oil or 10 μg estradiol benzoate (EB). Forty-eight hours after the second injection, rats were tested in a 1-day spatial Morris water maze task and then immediately perfused for analysis of CA1 spine synapse density. Spine synapse density did not differ between vehicle and EB-treated rats tested in the water maze. In contrast, behaviorally naïve EB-treated rats exhibited a significantly higher density of spine synapses than behaviorally naïve vehicle controls and than both groups tested in the water maze (*p < 0.05 relative to all other groups). Behaviorally naïve controls also had fewer spines than both water maze-tested groups (+p < 0.05 relative to all other groups). Each bar represents the mean (± SEM). Reprinted from (Frick et al., 2004).
Another critical issue related to the ovaries is whether they are present during treatment and testing. Because most women retain their ovaries at menopause, this issue is clearly relevant to clinical use of hormone therapies, and it is surprising that more studies have not been conducted using gonadally intact female rodents. To date, only three studies have tested the effects of E2 on gonadally intact aging females, so it is too premature to judge whether the presence of ovarian tissue affects the mnemonic response to E2. However, the results, at least for spatial reference memory, suggest a positive effect of E2 in intact aging females. One study from my lab reported that 5 μg estradiol benzoate given to gonadally intact 27–28 month-old female mice for 5 days prior to Morris water maze testing, and then each day 4 hours prior to testing, significantly improved spatial task acquisition (Fig. 5A) and increased hippocampal levels of the presynaptic protein synaptophysin (Fig. 5B) (Frick et al., 2002b). This effect was dose-dependent, as a 1 μg dose had no effect on spatial memory or synaptophysin levels (Frick et al., 2002b). Interestingly, the 5 μg dose had no effect on spatial water maze acquisition in ovariectomized 17 month-old mice (unpublished observations), although it is unclear whether this difference was due to age or ovariectomy surgery. Another study of gonadally intact 20 month-old female mice found that a single post-training injection improved spatial water maze retention and inhibitory avoidance (Frye et al., 2005). However, neither the Frick et al., 2002b or Frye et al., 2005 studies included comparisons to ovariectomized rats, so neither could address whether the presence of ovaries afforded an advantage over the absence of ovaries in response to E2.
Figure 5.
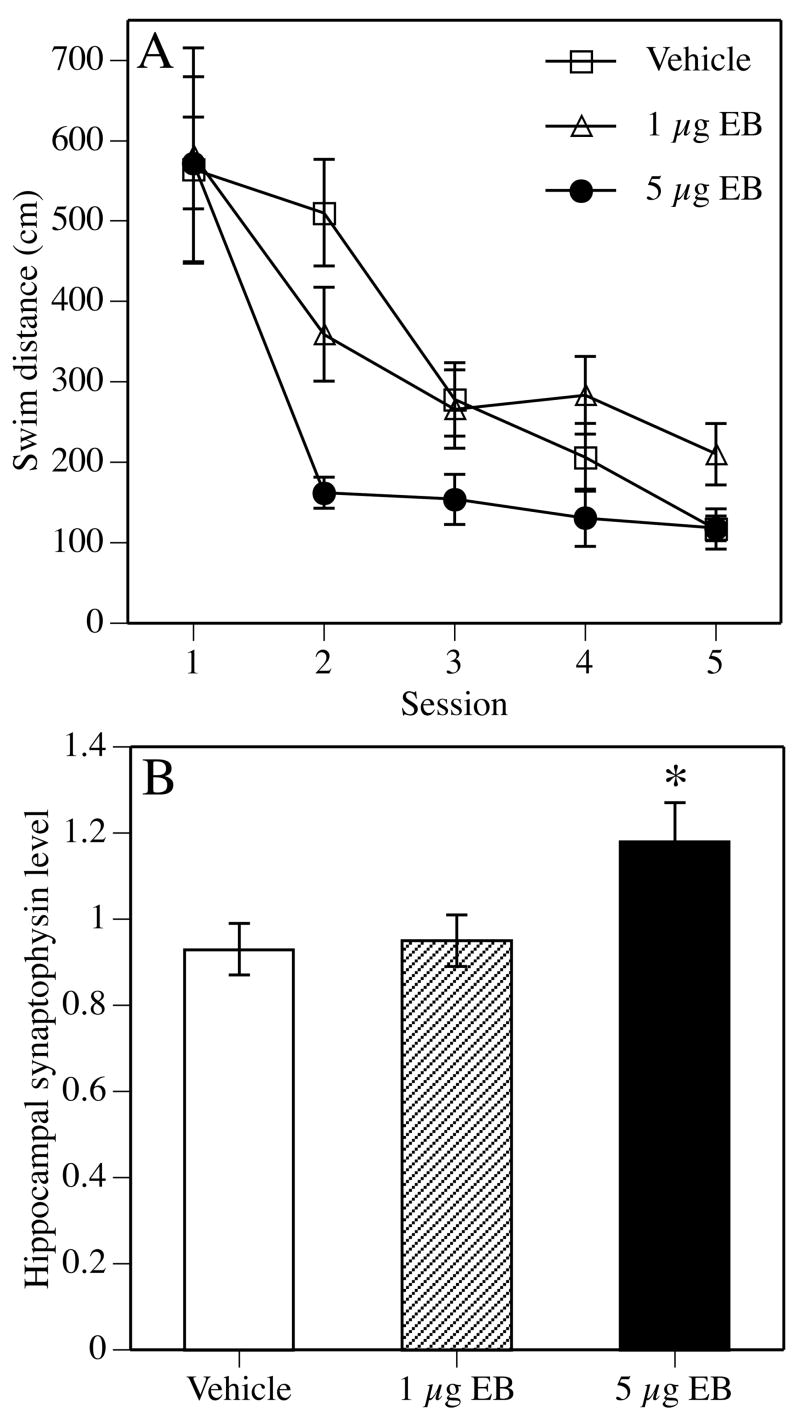
Gonadally intact 27–28 month-old female mice were injected subcutaneously with sesame oil vehicle or 5 μg estradiol benzoate (EB) for 5 days prior to spatial Morris water maze testing, and then each day 4 hours prior to testing. (A) Although all mice learned to find the platform, mice receiving 5 μg EB learned significantly faster than vehicle controls or mice receiving 1 μg EB. Each point represents the mean (± SEM) for an entire session. (B) At the conclusion of testing, synaptophysin protein levels were measured in whole hippocampus. Synaptophysin levels are expressed as the amount of synaptophysin in each sample relative to the amount of synaptophysin in a homogenate of whole mouse brain. Mice receiving 5 μg EB exhibited significantly higher hippocampal synaptophysin levels than vehicle controls. Adapted from (Frick et al., 2002b).
A comparison between intact and ovariectomized females was provided by a study of 20 month-old female rats that were untreated or were treated with E2 silastics for 6 days after sham or ovariectomy surgery. In this study, total number of errors in a T-maze active avoidance task was not affected by ovariectomy or E2 treatment, however among untreated rats, ovariectomized rats were more sensitive than intact rats to the disruptive effects of the cholinergic antagonist scopolamine (Savonenko and Markowska, 2003). In another measure of performance, E2 treatment increased the number of trials to criterion performance in intact rats relative to ovariectomized rats, indicating a detrimental effect of E2 on the performance of intact rats (Savonenko and Markowska, 2003). These results seem to contrast with the beneficial effects of E2 in intact aged female mice described above, and resolving this discrepancy will require considerably more data on this subject. Task, dose, and type of memory tested could all contribute to the differences between studies, as could species. In intact middle-aged mice, levels of E2 and progesterone are reduced relative to intact young mice (Nelson et al., 1992), whereas in intact aged rats, E2 levels are similar to and progesterone levels are more than 4-fold higher than intact young rats (Bimonte-Nelson et al., 2003). Given that the hormonal milieu of the intact aging rat and mouse differs considerably, baseline E2 and progesterone levels may influence the extent to which ovariectomy influences memory on its own and in combination with E2. As mentioned previously, elevated endogenous progesterone levels in aged rats have been hypothesized to underlie observed impairments in spatial working and reference memory in a water escape-motivated radial arm maze (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004). Because most women taking hormone therapy retain their ovaries, addressing the discrepancies between the mouse and rat data, as well as understanding whether the ovaries influence the mnemonic response to E2, is imperative to the application of rodent data to menopausal women.
Timing of treatment relative to training
Although the aging studies discussed thus far suggest that E2 can influence certain types of memory, the vast majority are confounded by the fact that E2 administration prior to training can influence numerous non-mnemonic performance factors such as motivation, attention, and sensorimotor function (McGaughy and Sarter, 1999; Morgan and Pfaff, 2001; Pfaff et al., 2002), in addition to memory. Therefore, this work does not address the issue of whether E2 specifically modulates memory in tasks designed to test memory. This shortcoming can be addressed if E2 is given immediately after training (post-training) in tasks where rodents are trained in a single day and then treated with E2 immediately after training. Most studies conducted in both young and aging rodents utilize a form of E2 encapsulated in 2-hydroxypropyl-β-cyclodextrin which can successfully cross the blood-brain barrier and is metabolized within 24 hours (Pitha et al., 1986; Taylor et al., 1989). Retention is then tested 24 or more hours later. Because E2 is not in the circulation during training or testing, its specific effects on memory consolidation can be examined in the absence of non-mnemonic confounds, which may confound interpretation of the data (McGaugh, 1989).
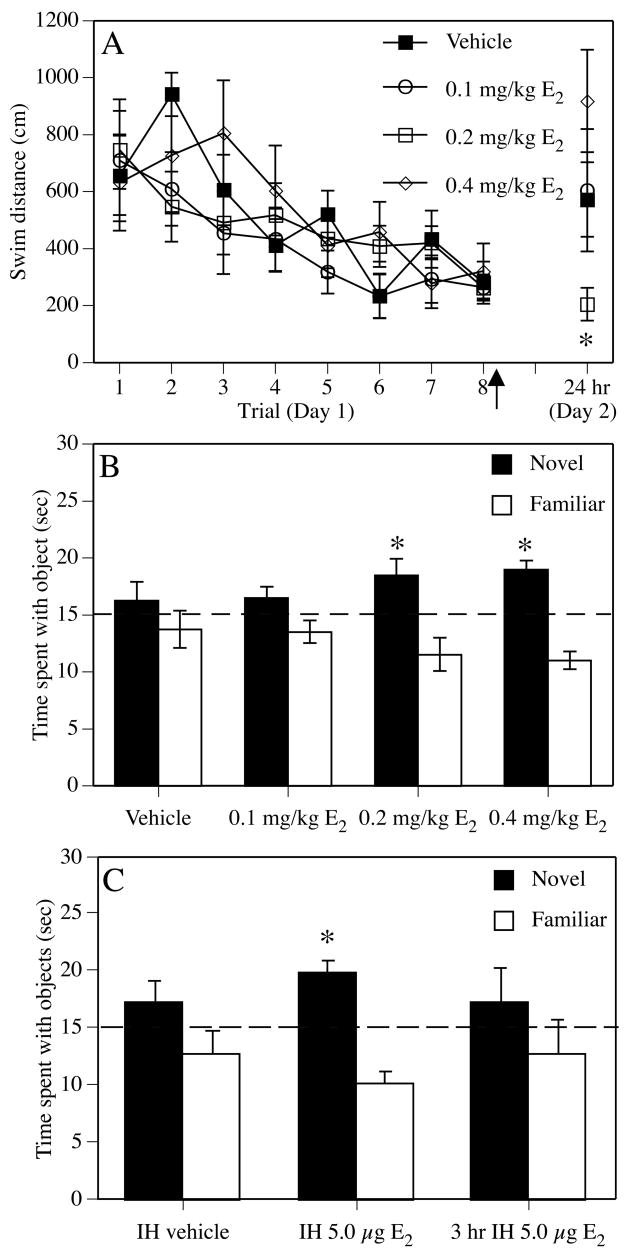
All post-training studies conducted to date report beneficial effects of E2 on memory consolidation. In the Morris water maze, young ovariectomized rats (Packard and Teather, 1997b) and mice (Gresack and Frick, 2006b) receiving a single intraperitoneal (i.p.) injection of 0.2 mg/kg cyclodextrin-encapsulated E2 immediately after eight consecutive spatial training trials remembered the location of the hidden escape platform 24 hours later significantly better than those receiving vehicle, 0.1 mg/kg E2, or 0.4 mg/kg E2 (Fig. 6A). The memory facilitation produced by E2 is time-dependent, as administration 2 hours post-training does not enhance memory in this task (Packard and Teather, 1997b). Using this same protocol, post-training injection of 0.2 mg/kg E2 also significantly enhanced spatial reference memory consolidation in ovariectomized 22 month-old mice (Harburger et al., 2007) (Fig. 7). This beneficial effect in aged females is supported by another study in intact 24 month-old female and male mice in which post-training injections of 10 μg E2 dissolved in oil enhanced spatial memory in the water maze using a different 2-day testing protocol (Frye et al., 2005). However, when given immediately after training each day in a 5-day Morris water maze protocol, post-training i.p. injections of 0.2 mg/kg E2 improved spatial reference memory consolidation in 17 month-old, but not 22 month-old, ovariectomized mice (Gresack et al., 2007a) (Fig. 3). These findings raise two interesting points. The first is the discrepancy between the effects of post-training E2 on spatial memory in aged females in the 2-day and 5-day Morris water maze protocols. Although the reasons behind this discrepancy are currently unclear, we have previously hypothesized that the stress due to repeated daily injections may contribute to the lack of effect of E2 in the 5-day protocol (Gresack et al., 2007a). The second point, which is consistent with the critical period hypothesis, is the effectiveness of the same E2 treatment in middle-aged, but not aged, females, again illustrating the fact that age can play a pivotal role in the ability of E2 to enhance memory.
Figure 6.
Effects of post-training estradiol on memory consolidation in young ovariectomized mice. (A) 0.2 mg/kg E2 significantly improved spatial memory retention in the Morris water maze. All groups learned to find the platform similarly on Day 1 (training trials 1–8). Mice were injected intraperitoneally (i.p.) with vehicle or E2 immediately following trial 8 (arrow). Twenty-four hours later, only mice receiving 0.2 mg/kg E2 remembered the platform location, as indicated by shorter swim distances on Day 2 relative to vehicle controls and mice receiving 0.4 mg/kg E2 (*p < 0.05). Each point represents the mean (± SEM) for a single trial. (B) During object recognition testing, groups receiving 0.2 mg/kg or 0.4 mg/kg E2 spent significantly more time than chance (dashed line at 15 seconds; *p < 0.05) with the novel object 48 hours after injection), suggesting intact memory for the familiar object. (A) and (B) reprinted from (Gresack and Frick, 2006b). (C) Intrahippocampal infusion of E2 immediately, but not 3 hours later, also enhances novel object memory consolidation. Mice receiving immediate bilateral dorsal hippocampal infusions of 5 μg E2, but not vehicle, spent significantly more time than chance with the novel object 48 hours after infusion (dashed line at 15 seconds; *p < 0.05). For both panels, each bar represents the group mean (± SEM) for the retention trial. Adapted from (Fernandez et al., 2008).
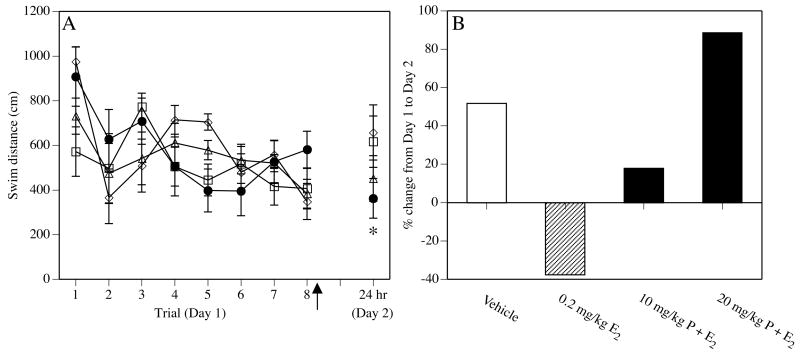
Figure 7.
Aged ovariectomized females were trained in a spatial Morris water maze task and then immediately injected i.p. (arrow) with vehicle, 0.2 mg/kg E2, 10 mg/kg progesterone + 0.2 mg/kg E2, or 20 mg/kg progesterone + 0.2 mg/kg E2 (n = 11 for vehicle, n = 10 for all other groups). (A) All mice learned to find the platform during training on Day 1. On Day 2, the performance of all mice but those receiving 0.2 mg/kg E2 alone deteriorated relative to that during the last trial of Day 1 (*p < 0.05 for the 0.2 mg/kg E2 group Day 1 vs Day 2), suggesting that only 0.2 mg/kg E2 alone enhanced memory for the platform location in aged females. The 20 mg/kg dose of progesterone completely blocked this effect. (B) Percent change from trial 8 of Day 1 to trial 1 of Day 2. Positive numbers indicate worse performance on Day 2 relative to Day 1. Only 0.2 mg/kg E2 alone improved performance from Day 1 to Day 2. Adapted from (Harburger et al., 2007).
In addition to spatial reference memory, other types of memory are also improved by post-training E2. In young rodents, a single post-training systemic injection of cyclodextrin-encapsulated or oil-dissolved E2 also enhances non-spatial reference memory consolidation (Farr et al., 2000), spatial working memory consolidation (Gresack and Frick, 2004), and spatial and non-spatial object memory consolidation (Frye et al., 2007; Gresack and Frick, 2004; Gresack and Frick, 2006b; Gresack et al., 2007a; Gresack et al., 2007b; Luine et al., 2003; Walf et al., 2006) (Fig. 6B). Like spatial reference memory consolidation, the beneficial effects of E2 on object memory consolidation occur rapidly, as injections given 2–3 hours post-training to young ovariectomized females do not object recognition memory (Fernandez et al., 2008; Luine et al., 2003; Walf et al., 2006). Further, post-training infusions of 5 μg cyclodextrin-encapsulated E2 directly into the dorsal hippocampus of young female rats and mice enhance both spatial reference memory (Packard and Teather, 1997a) and object recognition memory (Fernandez et al., 2008) consolidation (Fig. 6C). Also like spatial reference memory, the effects of acute post-training E2 depend on age, such that an improvement is seen during a 48-hour retention test in ovariectomized 17 month-old females treated with a single 0.2 mg/kg E2 injection, but not during either 24- or 48-hour retention tests in 21–22 month-old females (Gresack et al., 2007a; Gresack et al., 2007b) (see Std-Veh and Std-E2 groups in Fig. 3). Again, these data fit well with the critical hypothesis. Moreover, comparisons with other studies invite some speculative conclusions. For example, the fact that spatial reference memory consolidation was enhanced in aged females in the 2-day Morris water maze task by a single E2 injection (Harburger et al., 2007) (Fig. 7), but was not enhanced in the radial arm maze by chronic E2 injections (Gresack and Frick, 2006a), may suggest that post-training E2 in aged females can improve memories that are simple, like the location of a single platform overnight, but not those that are significantly more complex, like the location of multiple platforms over the course of a two-week testing period. Further, the fact that a single post-training injection of E2 in aged females could enhance 24-hour retention in the 2-day spatial water maze task, but not 24- or 48-hour object memory consolidation in the object recognition task, may imply that spatial reference memory is more sensitive than object recognition memory to the effects of acute E2 treatment in aged females. Finally, the fact that object recognition was improved in 22–24 month-old ovariectomized mice by pre-training E2 administered via silastic capsules (Vaucher et al., 2002), but not by post-training E2 injections (Gresack and Frick, 2006a; Gresack et al., 2007a; Gresack et al., 2007b), may indicate that E2 must be in the circulation for object memory consolidation to be enhanced in aged females.
No study has directly compared the effects of pre- and post-training E2 treatment on memory in young or aging rodents, so the importance of circulating E2 to enhancing memory in aging females is unknown. Nevertheless, the data thus far indicate that E2 can specifically enhance certain types of memory in middle-aged and aged female rodents in the absence of performance-related confounds, which is important for the development of future hormone-based treatments for reducing age-related memory decline in humans. Although effects of a single post-training E2 injection may not seem relevant to issues of age-related memory decline, the distinction between E2 effects on memory and other psychological processes is important for the use of hormone therapy in menopausal women; if motivational or affective changes alone are responsible for hormone therapy-induced improvements in memory tasks, then treatments that directly target these processes, rather than memory, could be used instead of hormones. Although it is unlikely that the effects of E2 in memory tasks are due entirely to non-mnemonic factors, understanding exactly how E2 modulates memory may lead to the development of novel treatments that produce the beneficial effects of E2 without having to administer the hormone itself. Such development may provide a useful and effective strategy for reducing age-related memory decline, and would require the elucidation of the molecular mechanisms underlying estrogenic modulation of memory. As will be discussed later this review, the rapid time frame in which post-training E2 enhances memory consolidation may provide a unique opportunity to understand the molecular mechanisms through which E2 modulates memory.
Type of treatment
The nature of the E2 treatment itself also requires careful consideration. The duration of treatment (e.g., acute or chronic) may not substantially affect the ability of E2 to improve memory in aging females, given that acute post-training and longer-term pre-training treatments can both improve the same type of memory at a given age. For example, spatial memory in the water maze is improved in aged female mice by a single post-training injection of E2 (Frye et al., 2005; Harburger et al., 2007) and by estradiol benzoate given 5 days prior to and then throughout testing (Frick et al., 2002b). One study of middle-aged females directly compared the effects of acute (2 days of injections) and chronic (28 days) E2 treatments on spatial water maze acquisition and found that both treatments improved performance (Markham et al., 2002). Thus, other factors, such as age at treatment, duration of hormone deprivation, and inclusion of a progestin, may be more important determinants of how a given E2 treatment will affect a specific type of memory. Nevertheless, too few studies comparing the effects of acute and chronic treatments have been conducted in aging females to conclude that duration of treatment is not an important factor influencing the mnemonic response to E2.
Perhaps more important than duration of treatment may be whether the treatment reproduces hormone fluctuations similar to the natural cycle. Hormone therapies prescribed for women, including those used by the WHI studies, do not simulate the cyclic nature of estrogen and progestin fluctuations characteristic of the menstrual cycle. Women in the WHI received daily doses of 0.625 mg conjugated equine estrogens (CEE; Premarin®) or 0.625 mg CEE plus 0.25 mg medroxyprogesterone acetate (MPA; PremPro®). The “continuous” nature of these treatments, where the same dose of hormone is administered each day, may contribute to their failure to improve memory, given that female brains are exposed to cycling levels of estrogens and progestins for much of their lives. At this time, however, it is unclear if cyclic hormone therapy is any more effective in enhancing cognitive function than continuous treatment. Because truly replicating the cycle is very difficult, the studies published thus far have attempted to simulate only certain aspects of the cycle (typically, the preovulatory estrogen surge). As such, these treatments may be considered “intermittent” rather than “cyclic”.
Only three studies thus far have directly compared the effects of continuous and intermittent E2 treatments in aging female rodents, so very little data is available on the subject. One study, in which rats were ovariectomized at 13 months of age, found that continuous and intermittent E2 treatments were similarly beneficial. Starting at 14 months of age, rats were treated with E2-secreting silastic pellets (in low and high doses) or with one injection of 10 μg E2 every other week for four weeks prior to spatial Morris water maze testing (Bimonte-Nelson et al., 2006). Although the high dose E2 pellet had no effect on performance, the low dose E2 pellet and intermittent E2 injection treatments significantly improved task acquisition (Bimonte-Nelson et al., 2006). Another study in rats ovariectomized at 13 months at treated at 18–20 months found that the effects of continuous E2 treatment (via pellets) on spatial working memory were greatly enhanced by priming with E2 injections (given 3 of 4 days for 4 cycles) (Markowska and Savonenko, 2002). The results of these studies contrast with other data collected in mice ovariectomized at 18 months of age and treated with vehicle every day, 0.2 mg/kg cyclodextrin-encapsulated E2 every day, or 0.2 mg/kg E2 every 4 days from 18 to 21 months of age (Gresack and Frick, 2006a). In this study, continuous E2 treatment had no effect on spatial working or reference memory tested in the radial arm maze or on object recognition, whereas intermittent E2 treatment impaired spatial reference memory and tended to impair spatial working memory and object recognition (Gresack and Frick, 2006a). A primary factor in the discrepancy between the Bimonte-Nelson et al., 2006 and Gresack and Frick, 2006a studies could be age at treatment, as subjects in the two studies differed greatly in age (benefits were seen by Bimonte-Nelson and colleagues at 15 months of age, but not by Gresack and Frick at 21 months of age). Further, differences in route of administration (pellets vs. injection) and type of E2 (cyclodextrin-encapsulated or not) may also contribute to the discrepant results. However, although the Bimonte-Nelson et al., 2006 study found a beneficial effect of intermittent treatment, whereas Gresack and Frick, 2006a found a detrimental effect of such treatment, neither study found that cyclic E2 treatment was more effective than intermittent E2 treatment, which is very useful information. Nevertheless, too little data have been published comparing continuous and intermittent E2 treatment regimens to judge the relative efficacy of these treatments on memory in females of any age. This critically important issue deserves much more future study aimed at determining: 1) the cognitive domains in which continuous and intermittent E2 treatment differ in effectiveness, 2) whether age at treatment or duration of hormone loss influences the effectiveness of comparable continuous and intermittent E2 treatments, and 3) whether treatments that more accurately mimic the natural cycle (including progesterone fluctuations) are any more effective in modulating memory in aging females than continuous or intermittent treatments with E2 alone.
Co-administration of a progestin
The effects of progestins, such as progesterone, on memory at any age are not well understood. Of particular relevance to this review are effects of progesterone on memory when combined with estrogens. Data from the WHI indicate that treatment with CEE plus the synthetic progestin MPA increased the risk of global cognitive decline and dementia (Rapp et al., 2003b; Shumaker et al., 2003), and impaired verbal memory (Resnick et al., 2006). It is easy to suggest that MPA was responsible for the detrimental effects of the WHI treatment, since MPA has been shown to reduce CEE’s neuroprotective effects on hippocampal neurons (Nilsen and Brinton, 2002) and to act similar to glucocorticoids (Poulin et al., 1989), which promote neurodegeneration and cognitive impairment during aging (McEwen et al., 1999). However, the arm of the WHI in which only CEE was administered also found an increased risk of global cognitive decline and dementia (Espeland et al., 2004; Shumaker et al., 2004), which may suggest little effect of progestins, like MPA, on cognitive function in older women.
The current rodent literature adds little to help resolve this issue. Two studies report beneficial effects of treatment with chronic E2 plus progesterone on memory in ovariectomized rats, one testing spatial working memory and the other testing spatial reference memory. In one study, rats ovariectomized at 13 months of age were implanted with E2-secreting silastic capsules either immediately or 3 months after ovariectomy, or received weekly injections of 10 μg E2 followed 48 hours later by 500 μg progesterone starting 3 months after ovariectomy (Gibbs, 2000b). Spatial working memory in a delayed non-match to position task was tested in all rats at 22–25 months of age. Although rats receiving injections of E2 plus progesterone, but not E2 alone, reached criterion performance faster than controls, all treatments reduced the mean number of errors per testing block (Gibbs, 2000b), suggesting a benefit to both types of treatment. In another study, rats were ovariectomized at 14 months of age and treated at 15–16 months of age with 2 days of E2 (16.67 μg/day) or 28 days of E2 or E2 plus progesterone (via silastic capsules); all treatments improved spatial Morris water maze task acquisition (Markham et al., 2002). Although these studies might suggest that E2 plus progesterone treatment is as effective, if not slightly more effective, at improving spatial memory in aging females, other studies report diametrically contrasting results. For example, in another study using the spatial Morris water maze task, progesterone given continuously (via pellets) completely reversed the beneficial effects of continuous or intermittent E2 on spatial task acquisition in 15 month-old ovariectomized rats (Bimonte-Nelson et al., 2006). Similarly, immediate post-training i.p. injection of 20 mg/kg cyclodextrin-encapsulated progesterone completely reversed the memory enhancing effects of 0.2 mg/kg cyclodextrin-encapsulated E2 in 22 month-old ovariectomized mice tested in a 2-day spatial Morris water maze task (Harburger et al., 2007) (Fig. 7). The reasons for the striking inconsistencies between these reversals and the beneficial effects of E2 and progesterone (Gibbs, 2000b; Markham et al., 2002) are not obvious. As such, the data regarding the influence of combined hormone therapy on memory in aging females are inconclusive. However, understanding how both estrogens and progestins affect cognitive function is of tremendous clinical importance, given that co-administration is recommended for women with a uterus because of the protection from uterine cancer afforded by a progestin (Persson et al., 1996). Future work will need to assess how combined treatment influences different types of memory at different ages, and to determine, if applicable, the most effective methods of administering combined treatment for reducing age-related memory decline.
Environmental factors
Finally, environmental factors, such as education, appear to alter the mnemonic response to E2. Clinical trials of hormone therapies are susceptible to a “healthy user bias” due to the fact that women who initiate hormone therapy are generally more educated (Keating et al., 1999; Launer et al., 1999; Tang et al., 1996) and healthier (Matthews et al., 1996) than women who do not elect treatment. This selection bias may skew perceptions about the effectiveness of hormone therapy because women who are well educated (> 11 yrs) are less likely to develop dementia than poorly educated (< 8 yrs) women (Launer et al., 1999). One recent longitudinal study reports that high childhood cognition levels are associated with an older age of menopause onset and better cognitive performance at menopause (Kok et al., 2006). Furthermore, some evidence suggests that estrogens may more effectively improve cognition in women with less education than in those with greater education (Matthews et al., 1999), a situation that could result if differences in baseline neural and cognitive function allow more room for improvement in women with less education. The over-representation of well-educated women in studies of hormone therapy may lead to a false perception that such treatment is ineffective for all women, when, in fact, certain populations (e.g., those with less education) may benefit from treatment.
We sought to examine this issue in an aging mouse model using a paradigm called environmental enrichment. Environmental enrichment treatments involve housing rodents together in cages with cognitively and physically stimulating objects. Controls are group (social) or singly (isolated) housed and not exposed to enriching objects. Enrichment improves hippocampal-dependent memory and enhances hippocampal morphology and plasticity in young-adult (Davis et al., 2004; Duffy et al., 2001; Kempermann et al., 1997; Rampon et al., 2000), middle-aged (Frick et al., 2003; Kempermann et al., 1998), and aged (Frick et al., 2002b; Nakamura et al., 1999; Soffié et al., 1999; Winocur, 1998) rats and mice. A link between enrichment and endogenous ovarian hormone levels was provided by a 1999 study in which spatial reference memory in the Morris water maze was impaired in intact female rats relative to ovariectomized rats; this impairment was eliminated by exposure to environmental enrichment (Daniel et al., 1999). Our laboratory subsequently showed that exposing female mice to an enriched environment from weaning to adulthood influences their mnemonic response to exogenous E2 (Gresack and Frick, 2004). Mice were exposed for 3 hrs/day to enriched or control environments from 3 weeks to 7 months of age, at which point they were given i.p. injections of 0.2 mg/kg E2 immediately after training in the radial arm maze and novel object recognition tasks. E2 enhanced spatial working memory and object recognition in mice raised in standard environments, but had no effect on those raised in enriched environments (Gresack and Frick, 2004), suggesting that enrichment prevents E2 from improving memory. We subsequently replicated the effect on object memory consolidation in young mice exposed to 24 hour/day enrichment from 3 weeks to 5 months of age (Gresack et al., 2007a) (Fig. 3D), and in those exposed to 4 weeks of enrichment starting at 5 months of age (Gresack et al., 2007b). Interestingly, different relationships between enrichment and post-training E2 treatment were observed in middle-aged and aged female mice raised in standard or enriched housing conditions. Among middle-aged females, object memory was enhanced by E2 regardless of housing condition (Fig. 3E), whereas spatial reference memory in the Morris water maze was enhanced by E2 in standard housed, but not enriched, mice (Fig. 3B) (Gresack et al., 2007a). In aged females, E2 alone had no effect on object or spatial memory (Fig. 3C and 3F), and interfered with the beneficial effects of enrichment on object memory (Fig. 3F) (Gresack et al., 2007a; Gresack et al., 2007b). As mentioned earlier, these data support the critical period hypothesis, and are consistent with clinical data suggesting that estrogen treatment may be most effective in women with less education (Matthews et al., 1999). As such, the rodent data suggest that estrogen treatment may be most beneficial for cognition in women who have experienced recent ovarian hormone loss and less cognitive and/or physical stimulation. Although it is uncertain how well these findings will generalize to humans, they clearly indicate that environmental factors should be considered in assessing the ability of E2 to reduce age-related memory decline.
What’s next for hormone therapy?
The studies conducted thus far have provided invaluable insights into how hormones such as E2 and progesterone modulate memory in aging females. Among the most firm conclusions to be drawn from the available data are that middle-aged female rodents seem to benefit more than aged rodents from several types of E2 treatments, and that environmental factors influence the mnemonic response to E2. Further, post-training studies have revealed that E2 can specifically modulate memory consolidation in middle-aged and aged females. However, many issues remain unresolved as described above. Specifically, questions of how duration of ovarian hormone deprivation, presence of the ovaries, addition of a progestin, and cyclicity of treatment influence the mnemonic response to E2 need to be more fully addressed. Of greatest importance to women considering hormone therapy may be understanding the influence of ovarian hormone deprivation on the mnemonic response to E2, given that such information could weigh heavily in their decision to initiate hormone therapy. Further, determining the dose and duration of treatment necessary to benefit memory, the length of time any benefits may last post-treatment, and the relative effectiveness of estrogen and estrogen plus progestin treatments for modulating various types of memory should help individual women decide on a treatment that will work best for them. Ideally, these issues should be addressed systematically using common methods and hormone formulations, with results replicated within and between labs to establish external validity and generalizability among rodent species and to menopausal women.
Because many women will take hormone therapy to alleviate menopausal symptoms, it is incumbent upon the research community to determine how treatment may affect cognitive function in these women, both during and after treatment. However, estrogens and progestins are complicated compounds that affect tissues throughout the body. Thus, it is reasonable to question the desirability of working towards the goal of optimizing ovarian hormone treatments to modulate memory, as it may not be possible to develop treatments that have no effects in peripheral tissues such as the breast, uterus, or heart.
SERMs
One way in which to reap the benefits of ovarian hormone treatment, while mitigating its side effects, is through the use of selective estrogen receptor modulators (SERMs). Historically, SERMs have been non-steroidal compounds that act as estrogen agonists in some tissues and antagonists in others. A comprehensive review of the SERM literature is beyond the scope of this review (see (Zhao et al., 2005) for a recent review), but some of the most common of these mixed agonist/antagonist SERMs are discussed below, including tamoxifen, raloxifene, phytoestrogens, and ICI 182,780. In general, these SERMs have been disappointing in terms of their ability to improve memory in female rodents and postmenopausal women. Tamoxifen, a SERM used for the prevention and treatment of breast cancer in postmenopausal women, acts as an ER antagonist in tissues such as breast and an ER agonist in several tissues including bone, liver, and uterus (Mitlak and Cohen, 1997; Shang and Brown, 2002). In cultured hippocampal neurons, tamoxifen has modest effects as an ER agonist, but acts as a competitive antagonist in the presence of E2 (Zhao et al., 2005). In postmenopausal women, tamoxifen use has been associated with impaired verbal memory (Jenkins et al., 2004) and an increase in reported memory problems (Paganini-Hill and Clark, 2000). These data are consistent with studies in mice showing that tamoxifen impairs spatial memory consolidation and retrieval (Chen et al., 2002a; Chen et al., 2002b). Raloxifene, which is currently approved for the treatment of osteoporosis, also acts a both an ER agonist and antagonist depending on the tissue (Wijayarante et al., 1999). In hippocampal cultures, raloxifene’s neuroprotective effects suggest action as a partial to full ER agonist (Zhao et al., 2005). However, treatment of postmenopausal women with raloxifene for 3 years had no effect on verbal memory or attention (Yaffe et al., 2001). Similarly, raloxifene did not mimic the beneficial effects of E2 on spatial working memory in young female rats (Gibbs et al., 2004) or aged rhesus monkeys (Lacreuse et al., 2002). Phytoestrogens are plant-derived estrogens that are structurally similar to endogenous estrogens, and include such compounds as genistein, genistin, diadzein, and formononetin (Zhao et al., 2005). Phytoestrogens exhibit neuroprotective abilities in hippocampal cultures, but do not promote neurite outgrowth (Zhao et al., 2005). One study in rats found that soy phytoestrogens significantly improved working memory in the radial arm maze (Pan et al., 2000). However, studies of women eating soy diets suggest no beneficial effect of these compounds; in women already using estrogen therapy, a high soy diet actually blocked the beneficial effects of the therapy (Kreijkamp-Kaspers et al., 2004; Rice et al., 1995). Perhaps the most promising of this group of SERMs may be ICI 182,780, which is typically considered a pure anti-estrogen. Recent work suggests that this compound may actually act as an estrogen agonist in the hippocampus, as it increases kinase activation, attenuates glutamate excitotoxicity, and protects against β-amyloid induced neurotoxicity similar to E2 (Zhao et al., 2005). One recent study in young female rats found that ICI 182,780 alone enhanced spatial learning, despite the fact that it also reversed the beneficial effects of E2 on spatial learning when the two compounds were given in combination (Zurkovsky et al., 2006). However, ICI 182,780 may only have agonist-like properties under certain circumstances. For example, my lab recently found that, unlike E2 alone, ICI 182,780 alone did not enhance novel object recognition in young female mice, although it blocked the beneficial effects of E2 when the compounds were given in combination (Fernandez et al., 2008). Thus, in order for ICI 182,780 to be considered a potential SERM for reducing age-related memory decline, the conditions under which it may act as an agonist must be delineated, and its effectiveness in aging females must be established. An additional complication with the potential clinical use of ICI 182,780 and similar compounds is that they cannot cross the blood brain barrier. However, studies are currently underway to design ICI-like compounds that can do so (Zhao et al., 2005).
One of the primary hurdles to the development of compounds that selectively bind to one of the cloned ERs is that the ligand binding domains of ERα and ERβ share over 50% homology and differ in only two amino acids within the binding site (Mosselman et al., 1996). Despite this challenge, some relatively selective SERMs that act only as ER agonists (not antagonists) have recently been developed and the results, thus far, seem promising. The most selective of these compounds are the ER-specific agonist propyl pyrazole triol (PPT), which has 410-fold selectivity for ERα over ERβ (Stauffer et al., 2000), and the ER-specific agonist diarylpropionitrile (DPN), which has 70-fold selectivity for ERβ over ERα (Meyers et al., 2001). Thus far, these compounds appear to improve certain types of memory in young ovariectomized rodents. In young female rats, DPN modestly improved spatial Morris water maze acquisition (Rhodes and Frye, 2006), whereas PPT enhanced spatial learning in an object placement task (Frye et al., 2007). Although both compounds have been reported to enhance novel object recognition in rats (Walf et al., 2006), my laboratory has found a beneficial effect of only DPN on novel object recognition in young female mice (Fernandez et al., 2006). Together, these data seem to suggest an important role of ERα in mediating memory for object locations and for ERβ in spatial reference memory and object recognition. However, it is important to note that both DPN and PPT can still bind to both ERs, which complicates the interpretation of their effects. Furthermore, these drugs also presumably bind putative membrane estrogen receptors, so even if they were completely selective for one of the cloned receptors, it would still be difficult to elucidate the specific receptor mechanisms through which they work. Regardless of receptor specificity, it remains possible that these compounds could be sufficiently selective to benefit cognitive function without causing clinically significant side effects in other tissues.
Molecular-based therapies
An alternative to further refining SERMs would be to elucidate the molecular mechanisms underlying memory-enhancing effects of hormones and develop drugs that target those mechanisms. As mentioned earlier in this review, E2 can rapidly affect cellular function in a way that cannot be attributed to traditional genomic action of ERα and ERβ. For example, E2 potentiates kainate-induced currents in cultured hippocampal CA1 neurons within 3 minutes of application, and this effect is independent of ERα and ERβ activation (Gu et al., 1999). In CA1, E2 also rapidly enhances field excitatory postsynaptic potentials (EPSPs) (Bi et al., 2000; Foy et al., 1999; Teyler et al., 1980), increases the amplitude of intracellular EPSPs (Wong and Moss, 1992), and potentiates excitatory postsynaptic currents (Rudick and Woolley, 2003). Accordingly, long-term potentiation in CA1 is enhanced by E2 (Bi et al., 2000; Foy et al., 1999), an effect that is blocked by the tyrosine kinase inhibitor, PP2 (Bi et al., 2000). Numerous other studies have demonstrated that exogenous E2 activates several signaling cascades hippocampal neurons, including the ERK/MAPK (Fitzpatrick et al., 2002; Kuroki et al., 2000; Wade and Dorsa, 2003), PI3K/Akt (Mannella and Brinton, 2006; Yokomaku et al., 2003), tyrosine kinase (Bi et al., 2000), and protein kinase A (PKA) (Shingo and Kito, 2005) pathways.
The ERK/MAPK pathway is of particular interest for hormonal modulation of memory given recent data showing that this pathway is critical for many kinds of learning and memory, including hippocampal-dependent learning and memory (see (Adams and Sweatt, 2002; Sweatt, 2004) for reviews). ERK is one of a family of MAP kinases that is phosphorylated (i.e., activated) as part of a G-protein initiated signal transduction cascade. Ligand (e.g., a growth factor or hormone) binding to a G-protein activates the molecules (in order) Ras, Raf, MAPK/ERK Kinase (MEK), and ERK (Adams and Sweatt, 2002). Phosphorylated ERK (pERK) can then translocate into the cell nucleus where it leads to phosphorylation of cAMP response element binding protein (CREB). Phosphorylated CREB (pCREB) then binds to the DNA response element CRE, which mediates transcription of numerous genes and leads to the translation of proteins including synaptic proteins like synaptophysin (Adams and Sweatt, 2002). Although other signal transduction cascades (e.g., cAMP/PKA, PKC) are implicated in memory consolidation, particular attention has been focused on ERK because ERK activation is necessary for PKA and PKC to activate CREB (Adams and Sweatt, 2002; Impey et al., 1998a; Murphy and Segal, 1997). Both pERK and pCREB are increased in the hippocampus within 1 hour after training in hippocampal-dependent tasks such as the spatial water maze (Blum et al., 1999), object recognition (Kelly et al., 2003), contextual fear conditioning (Atkins et al., 1998; Impey et al., 1998b), and inhibitory avoidance (Taubenfeld et al., 1999; Taubenfeld et al., 2001). Further, treatment with MEK inhibitors such as PD098059 or an anti-sense CREB oligonucleotide completely blocked training induced increases in pERK and pCREB (Atkins et al., 1998; Guzowski and McGaugh, 1997; Selcher et al., 1999). Importantly, these compounds also block long-term memory consolidation. In the water maze, infusion of PD098059 or an anti-sense CREB oligo into dorsal hippocampus prior to training impaired 48-hour retention (Blum et al., 1999; Guzowski and McGaugh, 1997). PD098059 also impaired 48-hour retention when injected immediately, but not 1 hour, after training, suggesting a 1 hour time window in which ERK regulates memory consolidation (Blum et al., 1999; Guzowski and McGaugh, 1997).
Recent work suggests that E2 alters hippocampal physiology by activating ERK and CREB. In hippocampal cell lines, E2 increased pERK, pCREB, and CRE-mediated gene transcription within 10–20 minutes of application (Fitzpatrick et al., 2002; Wade and Dorsa, 2003). MEK inhibitors completely blocked both these effects and also blocked E2-mediated neuroprotection from β-amyloid and excitotoxicity (Bi et al., 2000; Fitzpatrick et al., 2002; Wade and Dorsa, 2003; Wade et al., 2001). In cultured hippocampal neurons, a MEK inhibitor blocked the E2-induced increase in synaptic protein levels (Yokomaku et al., 2003) and an anti-sense CREB oligo blocked E2-induced increases in pCREB and spine density (Murphy and Segal, 1997). In the intact rat, a single intracerebroventricular infusion of E2 increased pERK in CA1 and CA3 within 5 minutes (Kuroki et al., 2000). In addition, the phosphorylation of the p42 isoform in the hippocampus is significantly reduced by ovariectomy in rats and restored by E2 replacement (Bi et al., 2001). Although some of these effects may be mediated by classical estrogen receptors, other studies suggest non-genomic mechanisms (Wade et al., 2001; Watters et al., 1997). These studies include data indicating that a bovine serum albumin-conjugated form of E2 (BSA-E2) that cannot pass through the cell membrane can induce similar changes in hippocampal ERK activation as free E2 (Fernandez et al., 2008; Kuroki et al., 2000). Regardless of the specific receptor mechanism, the data clearly indicate an important role for ERK and/or CREB in mediating E2-induced changes in hippocampal plasticity. The question remains as to whether these molecules are also involved in E2-induced alterations in memory.
Evidence from my laboratory suggests that ERK activation is necessary for E2 to modulate memory. Using post-training E2 treatments, we have found that the 0.2 mg/kg dose of E2 that enhanced spatial and object memory consolidation (Fig. 3, Fig. 6) also significantly increased activation of the p42 isoform of ERK in the dorsal hippocampus within 60 minutes of a single i.p. injection or 5 minutes of an intracranial infusion (Fernandez et al., 2008; Lewis et al., 2008) (Figs. 8 and 9). Increases in both ERK activation and in object memory were significantly attenuated by dorsal hippocampal infusion of the cAMP inhibitor Rp-cAMPs or the NMDA antagonist APV (Fig. 8), suggesting that the E2-induced enhancement of object memory involves NMDA receptors and protein kinase A (PKA) activation in the dorsal hippocampus (Lewis et al., 2008). However, neither treatment completely blocked the E2-induced increase in dorsal hippocampal ERK activation, indicating the involvement of other kinases upstream of ERK (e.g., receptor tyrosine kinases, protein kinase C). Regardless of the upstream activators, the involvement of ERK appears to be necessary for post-training 0.2 mg/kg E2 to enhance object memory consolidation, as demonstrated by the fact that systemic MEK inhibition by SL327 completely blocked dorsal hippocampal ERK activation (Fig. 9A and 9B) and that dorsal hippocampal infusion of the MEK inhibitor U0126 completely blocked the E2-induced enhancement of object memory consolidation (Fig. 9C) (Fernandez et al., 2008). Further, this study also demonstrated that effects of E2 on object memory and dorsal hippocampal ERK activation could be mediated entirely by membrane-associated estrogen receptors; a membrane-impermeable form of E2, bovine serum albumin-conjugated E2 (BSA-E2), had the same memory-and ERK-enhancing effects as E2 (Fig. 9D and 9E), and effects on object memory were blocked by intrahippocampal infusion of U0126 (Fig. 9E) (Fernandez et al., 2008).
Figure 8.
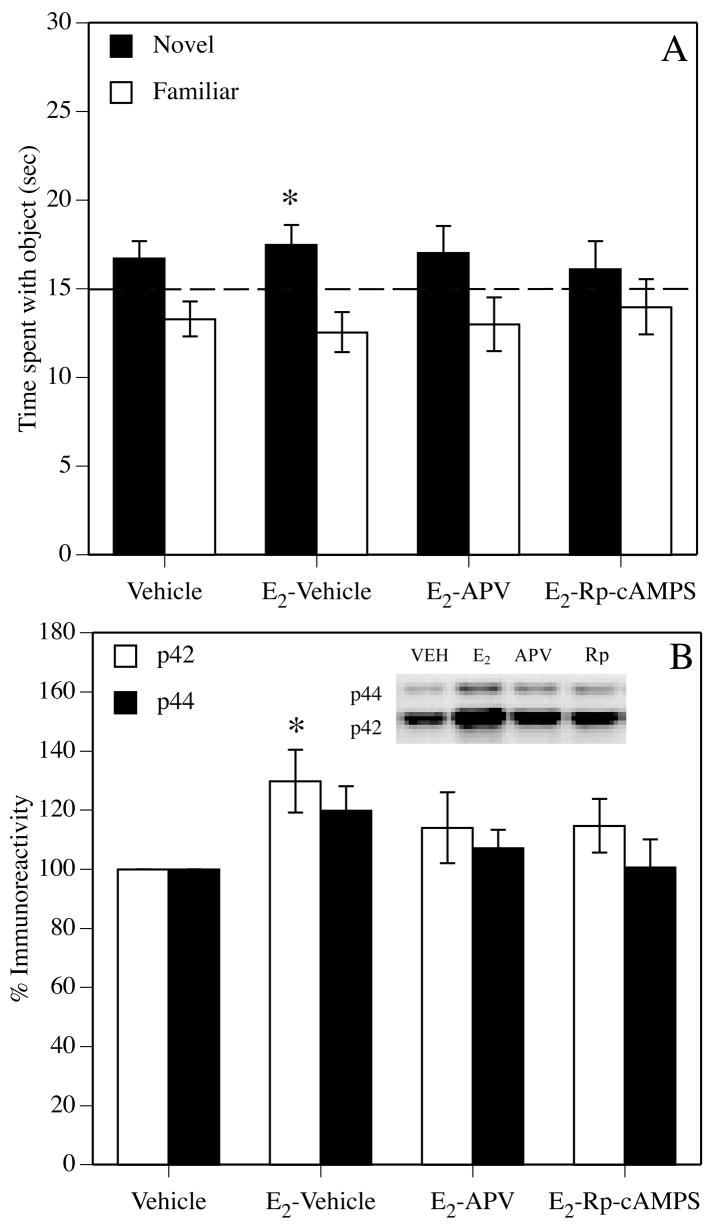
(A) Mice were trained in the novel object recognition task and then immediately injected i.p. with vehicle or 0.2 mg/kg E2 and intrahippocampally infused with vehicle, the NMDA receptor antagonist APV (D-2-Amino-5-phosphonovaleric acid; 5.0 mg/ml, 2.5 μg/side), or the cAMP inhibitor Rp-cAMPS (Rp-Cyclic 3′,5′-hydrogen phosphorothioate adenosine triethylammonium salt; 36 mg/ml, 18.0 μg/side). Mice treated with i.p. E2 and intrahippocampal vehicle (E2-Vehicle) spent significantly more time with the novel object than mice treated with i.p. and intrahippocampal vehicle (Vehicle; *p < 0.05 relative to Vehicle). Neither group treated with i.p. E2 and intrahippocampal APV or Rp-cAMPS (E2-APV and E2-Rp-cAMPS) spent significantly more time with the novel object than vehicle controls, suggesting that dorsal hippocampal infusions of either drug reduced the E2-induced enhancement of object recognition. The dashed line at 15 seconds represents chance performance. Each bar represents the group mean (± SEM) for the retention trial. (B) Effects of E2 on dorsal hippocampal activation of the two isoforms of ERK, p42 and p44. Western blotting was used to measure phospho-ERK levels, which were normalized to total p42/p44 ERK levels. Data are presented as percent increase in immunoreactivity relative to vehicle controls. E2 alone significantly increased levels of phosphorylated p42 30% above vehicle controls (*p < 0.05). Intrahippocampal infusion of APV or Rp-cAMPS attenuated this increase (14% over control). Neither APV nor Rp-cAMP completely blocked the activation of p42. Each bar represents mean (± SEM) immunoreactivity. Inset: Representative Western blots for phosphorylated/total p42 and p44. Reprinted from (Lewis et al., 2008).
Figure 9.
(A) Representative Western blots showing phosphorylated p42 and p44 ERK protein levels in dorsal hippocampus 1 hour after i.p. 0.2 mg/kg E2 injection. (B) E2 significantly increased phospho-p42, but not phospho-p44, ERK levels, and 30 mg/kg SL327 completely blocked this increase. Each bar represents mean (± S.E.M.) percent change from Vehicle (Veh) controls (*p < 0.05 relative to vehicle). (C) Mice receiving 0.2 mg/kg E2 plus intrahippocampal (IH) infusions of vehicle spent significantly (*p < 0.05) more time with the novel object than chance (dashed line at 15 seconds) 48 hours after training, thus, demonstrating memory for the familiar object. This beneficial effect of E2 was blocked by IH infusion of U0126 (0.5 μg/side). (D) Mice receiving intracerebroventricular (ICV) infusions of bovine serum albumin-conjugated E2 (BSA-E2, 5 μM) spent significantly more time with the novel object than chance. This effect was blocked by IH infusions of U0126 (0.5 μg/side). Controls receiving ICV infusions of vehicle or 850 pg/μl regular E2 (free E2) did not prefer the novel object. Each bar represents the mean (± S.E.M.) time spent with each object (*p < 0.05 relative to chance). (E) ICV BSA-E2 infusions significantly increased phospho-p42, but not phospho-p44, ERK levels in the dorsal hippocampus 5 minutes after infusion. Each bar represents mean (± S.E.M.) percent change from vehicle controls (*p < 0.05 relative to vehicle). Inset: Representative Western blots showing phosphorylated p42 and p44 ERK protein levels.
Downstream from ERK, a recent microarray analysis from my laboratory identified several genes of interest in the dorsal hippocampus altered by 0.2 mg/kg E2 1 hr after injection, including reduced expression of insulin-like growth factor (IGF) binding protein 2 (IGFBP2) (Pechenino and Frick, 2007). Numerous interactions between IGF and E2 have been previously documented (Mendez et al., 2006). IGFBP2 typically acts to sequester IGF-1 and prevent this protein from activating elements of the IGF cascade such as PI3K and Akt (Chesik et al., 2007). Thus, reduced expression of IGFBP2 would lead to greater availability of IGF-1, which should lead to increased activation of PI3K and Akt. Because PI3K can lead to ERK activation, an E2-induced reduction in IGFBP2 should lead to increased activation of ERK, thereby providing another route by which E2 can modulate ERK. Together, the data from this molecular line of experimentation demonstrate that dorsal hippocampal ERK activation is critical to the beneficial effects of acute E2 treatment on object memory consolidation, and suggest that the development of treatments that increase ERK activation in the dorsal hippocampus may be one way in which to obtain the beneficial effects of E2 without incurring the side effects of hormone treatment.
However, as with behavior, it is important to consider how age-related alterations in the hippocampus may affect the molecular response to E2. Because hippocampal ERK phosphorylation and ERK mRNA levels are reduced in aged rats (Bi et al., 2003; Gooney et al., 2004; Simonyi et al., 2003; Zhen et al., 1999), E2 may activate ERK to a lesser extent in aged animals. Such a reduction in ERK signaling could diminish the mnemonic response to E2 in aged females. Indeed, recent work has shown that age-related reductions in the response of basal forebrain neurons to intrahippocampal infusion of another important modulator, nerve growth factor, are due to disruptions in basal forebrain ERK activation (Williams et al., 2007; Williams et al., 2006). Nevertheless, because the aged hippocampus is able to mount robust ERK activation in response to other growth factors (Mo et al., 2005), E2 may still be able to activate ERK in the aged hippocampus. Because this effect has yet to be tested, it remains to be seen whether any ERK activation produced in the aged hippocampus by E2 is necessary for, or is even associated with, E2-induced improvements in learning and memory among middle-aged and aged females. Such work will be pivotal to establishing the validity of this approach for the design of treatments for reducing age-related memory decline.
Although this line of research is in its infancy, an approach focusing on molecules through which E2 acts to modulate memory may lead to specific targets to which non-steroidal drugs that mimic the beneficial effects of E2 can be designed. Because these drugs would modulate the downstream effectors of estrogen receptors, rather than the receptors themselves, resulting drugs should not produce the detrimental side effects inherent to hormone- and SERM-therapy. Critical to this approach is the validation of molecular targets in aging females, by not only establishing that E2 activates these targets in the aging hippocampus, but also that such activation is necessary for E2 to enhance memory. If such targets can be identified and validated in aging females, then this molecular approach to hormone therapy may ultimately prove useful in providing treatments that can safely and effectively reduce age-related memory decline.
Conclusions
Studies conducted in aging female rodents have begun to shed light on the importance of estrogens and progestins as critical modulators of age-related memory decline. There are numerous practical and scientific advantages of using rodents as models of age-related cognitive decline; of these, the short rodent life span should allow for relatively rapid advances in understanding how factors like age, hormone deprivation, type of treatment, and the environment influence the neural and mnemonic response to E2. The fact that fewer than two-dozen empirical studies have been published examining effects of E2 treatment on memory in aging female highlights the need for much more research on this topic. Continuing this work is of great importance to both gerontology and women’s health, given how little is currently known about ovarian hormone therapy in aging females. Further, the development of non-steroidal treatments that mimic the beneficial effects of E2 on memory but lack side effects in peripheral tissue is also paramount to reaping the potential benefits of hormone therapy. As such, interdisciplinary studies that aim to identify the molecular mechanisms through which E2 modulates memory in young and aging females should set the stage for hormone-based therapies of the future.
Acknowledgments
The experimental work from my lab discussed in this review was supported by Yale University, an American Federation for Aging Research/Pfizer grant in Hormones and Aging, an Investigator Initiated Research Grant (IIRG-03-6051) from the Alzheimer’s Association, NIMH MH065460, and NIH AG022525. I wish to thank Drs. Stephanie Fernandez, Lauren Harburger, and Michael Lewis for their helpful comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Ann Rev Pharmcol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Janssen WG, Shah RA, Morrison JH. Estrogen modulates synaptic N-methyl-D-aspartate receptor subunit distribution in the aged hippocampus. J Comp Neurol. 2004;474:419–426. doi: 10.1002/cne.20148. [DOI] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-α in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Nat Acad Sci USA. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E. The oestrous cycle in the mouse. Am J Anat. 1922;30:297–371. [Google Scholar]
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: Results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: Results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: A large and reliable sex difference. Behav Brain Res. 1998;93:185–90. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: Relations to β-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol. 2002;68:209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- Bach ME, Bara M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Nat Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellantoni MF, Blackman MR. Menopause and its consequences. In: Schneider EL, Rowe JW, editors. Handbook of the Biology of Aging. Academic Press; New York: 1996. pp. 415–430. [Google Scholar]
- Berry B, McMahan R, Gallagher M. Spatial learning and memory at defined points of the estrous cycle: Effects of performance on a hippocampal-dependent task. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Nat Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Foy MR, Thompson RF, Baudry M. Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiol Aging. 2003;24:977–983. doi: 10.1016/s0197-4580(03)00012-5. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Nat Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: Relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm ACE. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm ACE. Ovarian hormones and cognition in the aged female rat: II. Progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. doi: 10.1111/j.1365-2826.2008.01752.x. in press. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav. 2007;52:237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:410–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: Recent insights and remaining challenges. Learn Mem. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Neurosci Rev. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burkitt J, Widman D, Saucier DM. Evidence for the influence of testosterone in the performance of spatial navigation in a virtual water maze in women but not men. Horm Behav. 2007;51:649–654. doi: 10.1016/j.yhbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Watson RI. An evaluation of psychologic effects of sex hormone administration in aged women. I. Results of therapy after six months. J Gerontol. 1952;7:228–244. doi: 10.1093/geronj/7.2.228. [DOI] [PubMed] [Google Scholar]
- Chen D, Wu CF, Shi B, Xu YM. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol Biochem Behav. 2002a;71:277–284. doi: 10.1016/s0091-3057(01)00656-6. [DOI] [PubMed] [Google Scholar]
- Chen D, Wu CF, Shi B, Xu YM. Tamoxifen and toremifene impair retrieval, but not acquisition, of spatial information processing in mice. Pharmacol Biochem Behav. 2002b;72:417–421. doi: 10.1016/s0091-3057(01)00782-1. [DOI] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor binding protein-2 as a regulator of IGF actions in CNS: implications in multiple sclerosis. Cytokine Growth Factor Rev. 2007;18:267–278. doi: 10.1016/j.cytogfr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Columbo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Nat Acad Sci USA. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DGM. The Women’s Health Initiative Memory Study: Findings and implications for treatment. Lancet Neurol. 2005;4:190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Crowley WF, Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotrophin-releasing hormone (GnRH) secretion in men and women. Rec Prog Horm Res. 1985;41:473–531. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiol Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Davis CD, Jones FL, Derrick BE. Novel environments enhance the induction and maintenance of long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:6497–6506. doi: 10.1523/JNEUROSCI.4970-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW. The effects of aging on hippocampal and cortical projections of the forebrain cholinergic system. Brain Res Rev. 1987;12:423–438. doi: 10.1016/0165-0173(87)90007-5. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer’s disease. Prog Brain Res. 2007;163:741–753. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. doi: 10.1016/j.mce.2008.11.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: The impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Sutherland RJ. The aging hippocampus: Navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology. 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Dunbar GL, Rylett RJ, Schmidt BM, Sinclair RC, Williams LR. Hippocampal choline acetyltransferase activity correlates with spatial learning in aged rats. Brain Res. 1993;604:266–272. doi: 10.1016/0006-8993(93)90378-z. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Declarative memory: Insights from cognitive neurobiology. Annual Review of Psychology. 1997;48:547–572. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The Cognitive Neuroscience of Memory. Oxford University Press; New York, NY: 2002. [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Estradiol potentiates acetylcholine and glutamate-mediated post-trial memory processing in the hippocampus. Brain Res. 2000;864:263–269. doi: 10.1016/s0006-8993(00)02184-3. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Frick KM. Differential estrogen receptor mechanisms involved in memory consolidation. Soc Neurosci Abstr, Program. 2006:#462.5. [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Felicio LS, Mobbs CV, Nelson JF. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev. 1984;5:467–497. doi: 10.1210/edrv-5-4-467. [DOI] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Björklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Fischer W, Gage FH, Björklund A. Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur J Neurosci. 1989;1:34–45. doi: 10.1111/j.1460-9568.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against β-amyloid toxicity requires expression of estrogen receptor α or β and activation of the MAPK pathway. J Neurochem. 2002;82:674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Interaction of rapid signal transduction cascades and gene expression in mediating estrogen effects on memory over the lifespan. Front Neuroendo. 2005;26:51–64. doi: 10.1016/j.yfrne.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety, and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Delaney SS, Berger-Sweeney J. Sex differences in neurochemical markers that correlate with behavior in aging mice. Neurobiol Aging. 2002a;23:145–158. doi: 10.1016/s0197-4580(01)00237-8. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002b;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell RD, Kodsi MH, McKinney M, Southerland S, Vella-Rountree L, Lewis MH. Markers for biogenic amines in the aged rat brain: Relationshp to decline in spatial learning ability. Neurobiol Aging. 1990;11:507–514. doi: 10.1016/0197-4580(90)90111-c. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: Effects of estrogen and progesterone. J Neurosci. 1996;16:1049–1055. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Exp Neurol. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000a;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000b;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Aggarwal P. Estrogen and basal forebrain cholinergic neurons: Implications for brain aging and Alzheimer’s disease-related cognitive decline. Horm Behav. 1998;34:98–111. doi: 10.1006/hbeh.1998.1451. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997;749:143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gooney M, Messaoudi E, Maher FO, Bramham CR, Lynch MA. BDNF-induced LTP in dentate gyrus is impaired with age: Analysis of changes in cell signaling events. Neurobiol Aging. 2004;25:1323–1331. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006a;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006b;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007a;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Kerr KM, Frick KM. Short-term environmental enrichment decreases the mnemonic response to estrogen in young, but not aged, female mice. Brain Res. 2007b;1160:91–101. doi: 10.1016/j.brainres.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Gu Q, Korach KS, Moss RL. Rapid action of 17β-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Nat Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hart SA, Patton JD, Woolley CS. Quantitative analysis of ER alpha and GAD colocalization in the hippocampus of the adult female rat. J Comp Neurol. 2001;440:144–155. doi: 10.1002/cne.1376. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puoliväli J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Exp Gerontol. 2004;39:1277–1283. doi: 10.1016/j.exger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Estrogen-containing hormone therapy and Alzheimer’s disease risk: Understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: Morphomolecular senescence of cortical circuits. TINS. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: A meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Nat Acad Sci USA. 2004a;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998a;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998b;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho-Oncology. 2004;13:61–66. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- Keating NL, Cleary PD, Rossi AS, Zaslavsky AM, Ayanian JZ. Use of hormone replacement therapy by postmenopausal women in the United States. Ann Int Med. 1999;130:545–553. doi: 10.7326/0003-4819-130-7-199904060-00002. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;12:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok HS, Kuh D, Cooper R, van der Schouw YT, Grobbee DE, Wadsworth ME, Richards M. Cognitive function across the life course and the menopausal transition in a British birth cohort. Menopause. 2006;13:19–27. doi: 10.1097/01.gme.0000196592.36711.a0. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EH, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand and binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Moss MB. Cognitive function in aged ovariectomized female rhesus monkeys. Behav Neurosci. 2000;114:506–513. doi: 10.1037//0735-7044.114.3.506. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JRM, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Rates and risk factors for dementia and Alzheimer’s disease. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- LeFevre J, McClintock MK. Reproductive senescence in female rats: A longitudinal study of individual differences in estrous cycles and behavior. Biol Reprod. 1988;38:780–789. doi: 10.1095/biolreprod38.4.780. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Evans HM. The oestrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;6:1–148. [Google Scholar]
- Lu KH. Changes in ovarian function and gonadotrophin and prolactin secretion in aging female rats. In: Meites J, editor. Neuroendocrinology of aging. Plenum Press; New York: 1983. pp. 103–122. [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SSC. Chronological changes in sex steroid, gonadotrophin, and prolactin secretion in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–490. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, MacLusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 1975;86:293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and risk for dementia: Where do we go from here? Gynecol Endocrinol. 2004;19:354–359. doi: 10.1080/09513590400018207. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: Is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiat. 2001;158:227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phophatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Social recognition memory: Influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92:881–888. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: Relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Becker JB. Neuroendocrinology of sexual behavior in the female. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press; Cambridge, MA: 2002. pp. 117–151. [Google Scholar]
- McEwen BS, de Leon M, Lupien S, Meaney M. Corticosteroids, the aging brain and cognition. Trends Endocrinol Metab. 1999;10:92–96. doi: 10.1016/s1043-2760(98)00122-2. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Dissociating learning and performance: Drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Effects of ovariectomy, 192 IgG-saporin-induced cortical cholinergic deafferentation, and administration of estradiol on sustained attention performance in rats. Behav Neurosci. 1999;113:1216–1232. doi: 10.1037//0735-7044.113.6.1216. [DOI] [PubMed] [Google Scholar]
- Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor α and β immunoreactive neurons in normal adult and aged female rat hippocampus: A qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: Cellular and molecular mechanisms. Front Neuroendo. 2006;27:391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Miller MM, Hyder SM, Assayag R, Panarella SR, Tousignant P, Franklin KBJ. Estrogen modulates spontaneous alternation and the cholinergic phenotype in the basal forebrain. Neuroscience. 1999;91:1143–1153. doi: 10.1016/s0306-4522(98)00690-3. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J Neurosci. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitlak BH, Cohen FJ. In search of optimal long-term female hormone replacement: The potential of selective estrogen receptor modulators. Horm Res. 1997;48:155–163. doi: 10.1159/000185507. [DOI] [PubMed] [Google Scholar]
- Mo L, Ren Q, Duchemin AM, Neff NH, Hadjiconstantinou M. GM1 and ERK signaling in the aged brain. Brain Res. 2005;1054:125–134. doi: 10.1016/j.brainres.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: How basic neuroscience can inform hormone therapy. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Letters. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: A randomized controlled trial. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Nat Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Kobayashi S, Ohashi Y, Ando S. Age-changes of brain synapses and synaptic plasticity in response to an enriched environment. J Neurosci Res. 1999;56:307–315. doi: 10.1002/(SICI)1097-4547(19990501)56:3<307::AID-JNR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH, Finch CE. Differential contributions of ovarian and extraovarian factors to age-related reduction in plasma estradiol and progesterone during the estrous cycle of C57BL/6J mice. Endocrinology. 1992;130:805–810. doi: 10.1210/endo.130.2.1733727. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: Evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: Synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Nat Acad Sci USA. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley CA, Hautamaki RD, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh, synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987;403:389–392. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto Y, Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Olton DS, Givens BS, Markowska AL, Shapiro M, Golski S. Mnemonic functions of the cholinergic septohippocampal system. In: Squire LR, Weinberger NM, Lynch G, McGaugh JL, editors. Memory: Organization and locus of change. Oxford University Press; Oxford, New York: 1992. [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95:333–42. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997a;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 2000;64:165–176. doi: 10.1023/a:1006426132338. [DOI] [PubMed] [Google Scholar]
- Pan Y, Anthony M, Watson S, Clarkson TB. Soy phytoestrogens improve radial arm maze performance in ovariectomized retired breeder rats and do not attenuate benefits of 17beta-estradiol treatment. Menopause. 2000;7:230–235. doi: 10.1097/00042192-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Bayley PJ, Bui BK, Hansen LA, Thal LJ. Choline acetyltransferase activity and cognitive domain scores of Alzheimer’s patients. Neurobiol Aging. 2000;21:11–17. doi: 10.1016/s0197-4580(00)00090-7. [DOI] [PubMed] [Google Scholar]
- Pechenino AS, Frick KM. Effects of estradiol treatment on gene expression in the hippocampus of young female mice. Soc Neurosci Abstr, Program. 2007:#195.21. [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Brit Med Journal. 1978;25:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy--long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67:327–332. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Frohlich J, Morgan M. Hormonal and genetic influences on arousal--sexual and otherwise. TINS. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- Pitha J, Harman SM, Michel ME. Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J Pharm Sci. 1986;75:165–167. doi: 10.1002/jps.2600750213. [DOI] [PubMed] [Google Scholar]
- Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124:809–816. doi: 10.1016/j.neuroscience.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Poulin R, Baker D, Poirer D, Labrie F. Androgen and glucocorticoid receptor-mediated inhibition of cell proliferation by medroxyprogesterone acetate in ZR-75-1 human breast cancer cells. Breast Cancer Res Treat. 1989;13:161–172. doi: 10.1007/BF01806528. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Renner K, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Res. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: A critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rice M, Graves A, Larson E. Estrogen replacement therapy and cognition: Role of phytoestrogens. Gerontologist. 1995;35(Suppl 1):169. [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Robel P, Young J, Corpechot C, Mayo W, Perche F, Haug M, Simon H, Baulieu EE. Biosynthesis and assay of neurosteroids in rats and mice: functional correlates. J Steroid Biochem Mol Biol. 1995;53:355–360. doi: 10.1016/0960-0760(95)00074-a. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: Plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperbert C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Selective estrogen receptor modulators regulate phasic activation of hippocampal CA1 pyramidal cells by estrogen. Endocrinology. 2003;144:179–187. doi: 10.1210/en.2002-220581. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. J Reprod Dev. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sato T, Teramoto T, Tanaka K, Ohnishi Y, Irifune M, Nishikawa T. Effects of ovariectomy and calcium deficiency on learning and memory of eight-arm radial maze in middle-aged female rats. Behav Brain Res. 2003;142:207–216. doi: 10.1016/s0166-4328(03)00010-x. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengstock GJ, Johnson KB, Jantzen PT, Meyer EM, Dunn AJ, Arendash GW. Nucleus basalis lesions in neonate rats induce a selective cortical cholinergic hypofunction and cognitive deficits during adulthood. Exp Brain Res. 1992;90:163–174. doi: 10.1007/BF00229268. [DOI] [PubMed] [Google Scholar]
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Can estrogen keep you smart? Evidence from clinical studies. J Psychiat Neurosci. 1999;24:315–321. [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Front Neuroendo. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Estradiol induces PKA activation through the putative membrane receptor in the living hippocampal neuron. J Neural Trans. 2005;112:1469–1473. doi: 10.1007/s00702-005-0371-8. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology. 1997a;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-αand -β mRNA in the rat central nervous system. J Comp Neurol. 1997b;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Estrogen binding and estrogen receptor characterization (ERα and ERβ) in the cholinergic neurons of the rat basal forebrain. Neuroscience. 2000;96:41–49. doi: 10.1016/s0306-4522(99)00520-5. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Murch K, Sun GY. Extracellular signal-regulated kinase 2 mRNA expression in the rat brain during aging. Neurochem Res. 2003;28:1375–1378. doi: 10.1023/a:1024948532633. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O’Neill R, Zubieta JK. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil Steril. 2001;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- Soffié M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res. 1999;101:37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendo. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. The Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14(2):215–23. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Bear MF, Alberini CM. A molecular correlate of memory and amnesia in the hippocampus. Nat Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein β and δco-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. J Neurosci. 2001;21:84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: Behavioral and physiological effects of episode-like pulses in rats. Pharm Res. 1989;6:641–646. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. Gonadal steroids: Effects on excitability of hippocampal pyramidal cells. Science. 1980;209:1017–1019. doi: 10.1126/science.7190730. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. A plethora of estrogen receptors in the brain: Where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KBJ. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Foy MR, Foy JG, Thompson RF. 17β-estradiol suppresses expression of long-term depression in aged rats. Brain Res Bull. 2000;53:783–787. doi: 10.1016/s0361-9230(00)00377-4. [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER) α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V. Impaired recognition memory and decreased prefrontal cortex spine density in aged female rats. Ann NY Acad Sci. 2007;1097:54–57. doi: 10.1196/annals.1379.026. [DOI] [PubMed] [Google Scholar]
- Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC. Effects of estrogen on cognition, mood, and cerebral blood flow in AD. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and non-spatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women. The Women’s Health Initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ. Tamoxifen activation of the estrogen receptor/AP-1 pathway: Potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endo. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Strumble RG, Clark AW, Coyle JT, DeLong MR. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155(1):45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wijayarante AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP. Comparative analyses of mechanistic differences among antiestrogens. Endocrinology. 1999;140:5828–5840. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- Williams B, Granholm AC, Sambamurti K. Age-dependent loss of NGF signaling in the rat basal forebrain is due to disrupted MAPK activation. Neurosci Let. 2007;413:110–114. doi: 10.1016/j.neulet.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology. 2006;188:605–618. doi: 10.1007/s00213-006-0477-1. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Winocur G. Environmental influences on cognitive decline in aged rats. Neurobiol Aging. 1998;19:598–597. doi: 10.1016/s0197-4580(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Wise PM. New understanding of the complexity of the menopause and challenges for the future. In: Bellino FL, editor. Proceedings of the International Symposium on the Biology of Menopause; Norwell, MA: Springer; 2000. pp. 1–8. [Google Scholar]
- Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharmcol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: Correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Glinn MA, Ostrowski NL, Su Y, Ni B, Cole HW, Bryant HU, Paul SM. Raloxifene and estradiol benzoate both fully restore hippocampal choline acetyltransferase activity in ovariectomized rats. Brain Res. 1999;847:98–104. doi: 10.1016/s0006-8993(99)02062-4. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline. Neurology. 2000a;54:1949–1953. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Krueger K, Sarkar S, Grady D, Barrett-Connor E, Cox DA, Nickelsen T. Cognitive function in postmenopausal women treated with raloxifene. NEJM. 2001;344:1207–1213. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. The Lancet. 2000b;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: Effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-β mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Yen SSC. The human menstrual cycle: Neuroendocrine regulation. In: Yen SSC, Jaffe RB, Barbieri RL, editors. Reproductive endocrinology: Physiology, pathophysiology, and clinical management. W.B. Saunders; Philadelphia: 1999. pp. 191–217. [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endo. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002a;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Brietner JCS. Hormone replacement therapy and incidence of Alzheimer’s disease in older women: The Cache County Study. JAMA. 2002b;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhao L, O’Neill K, Brinton RD. Selective estrogen receptor modulators (SERMs) for the brain: Current status and remaining challenges for developing NeuroSERMs. Brain Res Rev. 2005;49:472–493. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zhen X, Uryu K, Cai G, Johnson GP, Friedman E. Age-associated impairment in brain MAPK signal pathways and the effect of caloric restriction in Fisher 344 rats. J Gerontol A Biol Sci Med Sci. 1999;54A:B539–B548. doi: 10.1093/gerona/54.12.b539. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144:26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiol Learn Mem. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]