Abstract
Adipose tissue secretes factors linked to colon cancer risk including leptin. A hallmark of cancer is sustained angiogenesis. While leptin promotes angiogenesis in adipose tissue, it is unknown whether leptin can induce epithelial cells to produce factors that may drive angiogenesis, vascular development and therefore cancer progression. The purpose of this study was to compare the effects of leptin-stimulated colon epithelial cells differing in adenomatous polyposis coli (Apc) genotype (gatekeeper tumor suppressor gene for colon cancer) on angiogenesis. We employed novel colonic epithelial cell lines derived from the Immorto mouse [young adult mouse colon (YAMC)] and the Immorto-Min mouse [Immorto-Min colonic epithelial cell (IMCE)], which carries the Apc Min mutation, to study the effects of leptin-stimulated colon epithelial cells on angiogenesis. We utilized ex vivo rat mesenteric capillary bioassay and human umbilical vein endothelial cell (HUVEC) models to study angiogenesis. IMCE cells stimulated with leptin produced significantly more vascular endothelial growth factor (VEGF) than YAMC (268 ± 18 versus 124 ± 8 pg/ml; P < 0.01) cells. Leptin treatment induced dose-dependent increases in VEGF only in IMCE cells. Conditioned media from leptin (50 ng/ml)-treated IMCE cells induced significant capillary formation compared with control, which was blocked by the addition of a neutralizing antibody against VEGF. Conditioned media from leptin-treated IMCE cells also induced HUVEC cell proliferation, chemotaxis, upregulation of adhesion proteins and cell-signaling activation resulting in nuclear factor kappa B nuclear translocation and DNA binding due to VEGF. This is the first study demonstrating that leptin can induce preneoplastic colon epithelial cells to orchestrate VEGF-driven angiogenesis and vascular development, thus providing a specific mechanism and potential target for obesity-associated cancer.
Introduction
Increasing epidemiological evidence has demonstrated that obesity is associated with an increased risk of cancer, especially colon cancer (1–3). Obese individuals have a large amount of adipose tissue that secretes various growth factors and cytokines that play a role in the low-grade, chronic inflammatory state that is linked to their obesity and subsequent cancer risk. Leptin, an adipocyte-derived hormone that classically plays a crucial role in regulating energy balance, is elevated in obese individuals (4). It is unclear whether elevated leptin levels may have effects on colon epithelial cells and the development of colon cancer or if it may indirectly mediate the process via a wide variety of other cell types located in the gut.
Elevated serum leptin levels are associated with several cancers including colon (5), prostate (6) and breast (7). In addition, elevated leptin levels are positively correlated with the likelihood of developing larger and more advanced tumors (7,8). Conversely, elevated leptin levels have not been associated with late stage/large tumors, most probably due to the weight loss, which is typical in advanced stage cancer patients. In vitro data are more consistent regarding the effects of leptin on cell fate. In tumor cell lines, leptin treatment induces cell proliferation in colon (9–11), breast (12,13), gastric (13), prostate (13,14) and ovarian cancer (15). Based on these data, it is probably that leptin has cancer cell stage-specific and tissue-specific actions that ultimately result in a growth-promoting effect on neoplastic cells.
In vitro models, animal studies and clinical evidence lend support to the hypothesis that cancer development largely depends on the ability of survival-advantaged mutant cells (such as ApcMin/+ colonic epithelial cells) to hijack and exploit the normal physiological processes of the host (16). Truncating mutations in the adenomatous polyposis coli (APC) gene are initiating events in colorectal carcinogenesis; a majority of adenomas in inherited and sporadic forms of colorectal cancers have mutations in Apc (17). The normal cellular functions of APC, including proliferation, migration, differentiation and apoptosis are disrupted by these truncating mutations (18).
Epithelial cells, when stimulated, can produce immunomodulatory mediators that can modulate neoplastic phenotypes such as angiogenesis and cell growth and survival (19). Hanahan et al. (20) suggest that there is a set of six traits that are shared by virtually all types of human cancer, titled the ‘hallmarks of cancer’. One of these traits is sustained angiogenesis. Angiogenesis, the growth of new blood vessels, is critical for the growth and spread of tumors. This event supplies the growing tumor with many things including oxygen, nutrients, growth factors and hormones (21). The proliferative index of tumor cells decrease with the increasing distance from the nearest vessel. Further, the growth of these tumors does not become rapid until this vascularization occurs (22). Driving these vascularization events is the expression of angiogenic growth factors. A clear correlation was observed between the expression of angiogenic growth factors and progression and prognosis of tumors (21,23).
In a homeostatic situation, proangiogenic factors are counterbalanced with antiangiogenic factors. Tumors seem to be able to alter the ‘angiogenic switch’ by swaying the ratio of angiogenesis inducers to angiogenesis inhibitors in favor of angiogenesis (20). One of the main proangiogenic factors is vascular endothelial growth factor (VEGF). VEGF expression is associated with advanced tumor progression and a poor prognosis in colon cancer (21,22). ‘Activation of the VEGF/VEGF receptor axis triggers multiple signaling networks that result in endothelial cell survival, mitogenesis, migration and differentiation. VEGF also mediates vessel permeability and has been associated with malignant effusions’ (21).
In addition to VEGF, certain chemokines have proangiogenic capabilities (24). Various CXC and CC chemokines have different angiostatic properties, ranging from induction of endothelial cell migration and/or proliferation in vitro, neovascularization in vivo or to act as angiostatic molecules themselves. Among the CC chemokines, CCL1 (I-309), CCL2 (MCP-1), CCL1 (eotaxin), CCL15 (Leukotactin-1) and CCL16 (HCC-4) have direct roles in angiogenesis. These chemokines have corresponding receptors that are expressed on endothelial cells (24). Macrophage inflammatory protein 3 (MIP3), a member of the CC chemokine family, has been recently reported to induce endothelial cell migration and tube formation via CCR1, a hallmark of angiogenesis (24,25).
Previously, our laboratory demonstrated that leptin preferentially promotes the survival and proliferation of a preneoplastic colon epithelial cell line [Immorto-Min colonic epithelial cell (IMCE) (ApcMin/+)] compared with the normal colon epithelial cell line [young adult mouse colon (YAMC) (Apc+/+)] (26). These cell lines (YAMC and IMCE) are excellent models of normal and preneoplastic cells. The phenotypic changes seen in the IMCE (ApcMin/+) cells, including growth factor-induced migration, cell–cell communication and inducible nitric oxide synthase/cyclooxygenase expression, are consistent with known early phenotypes in human colorectal cancer (27–29). While leptin itself promotes angiogenesis in adipose tissue (30,31), it is not known whether leptin can induce epithelial cells to produce factors that may drive an angiogenic response such as endothelial chemoattraction and growth factor production. Therefore, the purpose of this study was to investigate the role of leptin-exposed colon epithelial cells differing in Apc genotype on angiogenesis.
Materials and methods
YAMC (Apc+/+) and IMCE (ApcMin/+) cells and cell culture conditions
The YAMC (Apc+/+) and IMCEs (ApcMin/+) were kindly provided by Dr Robert Whitehead (Vanderbilt University; Nashville, TN). Cells were cultured as described previously (29). Briefly, cells were cultured in RPMI (Life Technologies, Rockville, MD) growth media containing newborn calf serum (Gemini Bio-Products, Woodland, CA), murine interferon-γ (Life Technologies), insulin–transferrin–selenium (Life Technologies) and penicillin–streptomycin (Invitrogen, Grand Island, NY) at 33°C on collagen I (Life Technologies) -coated flasks until reaching ∼70% confluence. Cells were then transferred to 39°C in serum- and interferon-γ-free medium overnight to allow for a brief stabilization period. These cell lines behave like normal cells in that they are contact inhibited and undergo apoptosis if they achieve maximal confluence. Conditions have been optimized for cells to proliferate slowly for 24 h at 39°C and then undergo cell death over 5–8 days, similar to the life cycle of a normal colon epithelial cell (32).
Leptin treatment of IMCE and YAMC cells
After the stabilization period of 24 h at 39°C, the IMCE (ApcMin/+) and YAMC (Apc+/+) cells were treated with either serum-free RPMI medium alone (CON-control), 1 ng/ml leptin (L1) or 50 ng/ml leptin (L50) (R&D Systems, Minneapolis, MN). After 48 h of treatment, media was collected from the flasks of treated cells and stored at −80°C until use. This media collected from these treated IMCE and YAMC cells is hereafter referred to as conditioned media. Total protein was measured using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL) in the conditioned media samples to adjust for cell number/confluency if necessary. However, total protein was not different between the conditioned media samples (data not shown). The dose of leptin and collection period was based on our previous studies using leptin, IMCE (ApcMin/+) and YAMC (Apc+/+) cells (26) as well as physiological leptin levels found in obese mice (30 ng/ml) (33) and obese human subjects (31.3 ng/ml) (34). Leptin levels in the conditioned media were measured by commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems) to verify that leptin was no longer present and hence could not have direct effects.
VEGF ELISA
The release of VEGF into the culture medium was quantified by sandwich ELISA according to the manufacturer's instructions (R&D Systems). Briefly, 50 μl of undiluted culture medium was added to each well and incubated according to instructions. Upon completion of the assay procedure, the plate was read at 450 nm wavelength using a Synergy HT plate reader (Bio-Tek, Winooski, VT). The role of p38 and nuclear factor kappa B (NFκB) in leptin driven VEGF production from IMCE (ApcMin/+) cells was tested by cotreating IMCE (ApcMin/+) cells with leptin (50 ng/ml) and a specific p38 inhibitor, SB202190 (A.G. Scientific, San Diego, CA) at a concentration of 50 nM or a specific NFκB inhibitor (35), pyrrolidine dithiocarbamate (PDTC) (Tocris, Ellisville, MO), at a concentration of 100 nM.
Angiogenesis assay (rat mesentery)
All animals were cared for according to Michigan State University animal care and use protocols. A total of 40 male Sprague-Dawley rats were used for this study (12–20 weeks of age). The rats were euthanized with CO2, and the small intestine and its mesenteric attachments were removed. The first and last loop of the intestine and mesentery were removed from the jejunum and ileum by cutting along the adipose tissue adjacent to the external wall of the gut. These segments of the mesentery were divided into individual wedge-shaped windows, as described previously (36). Each rat yielded 15–18 windows. Windows were then randomized to various study treatments, three tissue control windows (immediately fixed), three windows for media control (treatment with serum-free media alone) and three windows per conditioned media treatment group. Windows were exposed to 1 ml conditioned media in 24-well culture plates and placed in a 37°C incubator for 24 h. VEGF and MIP3 antibody neutralization experiments were carried out using a 30 min pretreatment with anti-VEGF antibody or anti-MIP3 antibody at 1 μg/ml (Santa Cruz Biotechnology, Santa Cruz, CA) and then cotreatment with serum-free or conditioned media.
After the treatment period, tissue windows were fixed for 30 min in 4% formalin. The tissue was then rinsed in phosphate-buffered saline and incubated in tetramethylrhodamine derivative of the Griffonia simplicifolia I lectin (Vector laboratories, Burlingame, CA) as described previously (37). After rinsing, the mesenteric windows were mounted on microscope slides in a water-soluble mountant Gelvatol (Monsanto, St Louis, MO) for subsequent analysis by epifluorescence microscopy.
Angiogenic response to conditioned media was quantified by visual assessment of percent coverage of the microscope field by angiogenesis. Differences among the groups were tested using the one-way analysis of variance in combination with Bonferroni's multiple comparison test with statistical significance discerned at P < 0.05. Experiments were repeated at least three times and data shown are representative from one experiment. Data were assessed statistically using Prism® software (Graph Pad, San Diego, CA).
HUVEC cells and culture conditions
Human umbilical vein endothelial cells (HUVECs) were obtained from Cascade Biologics (Portland, OR) and cultured in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Invitrogen), 1% penicillin–streptomycin and Low Serum Growth Supplement (Cascade Biologics). Cells were cultured on collagen I-coated flasks or 96-well plates at 37°C in 5% CO2. At confluence, media was changed to Dulbecco's modified Eagle's medium with 0.25% fetal bovine serum and 1% penicillin–streptomycin for 12 h before treatment.
Conditioned media and leptin treatment of HUVECs
After the 12 h low serum incubation period, the HUVECs were treated with conditioned media collected from the leptin-treated IMCE (ApcMin/+) and YAMC (Apc+/+) cells (as described above) for 0, 5, 15, 30 or 60 min and 0, 0.5, 1.5, 3 or 6 h. HUVECs were also treated with leptin directly (1 or 50 ng/ml). Flasks of cells were scraped in sample buffer for western blot. Cells cultured in 96-well plates were used for proliferation assays.
Leptin-treated HUVEC cell proliferation assay
HUVECs were grown as described above. Briefly, cells were seeded in 96-well plates. After the 12 h low serum incubation period, the cells were treated with conditioned media from control and leptin (1 and 50 ng/ml)-treated IMCE (ApcMin/+) and YAMC (Apc+/+) cells or leptin alone (1 and 50 ng/ml). The antibody neutralization experiments were carried out using an anti-VEGF or anti-VEGFR-2 antibody (Santa Cruz Biotechnology) at a concentration of 1 μg/ml alone or with cotreatment of conditioned media from IMCE (ApcMin/+) treated with leptin (50 ng/ml). The NFκB inhibitor experiment was carried out using a specific NFκB inhibitor, PDTC (Tocris), at a concentration of 1.0, 0.1 and 0.001 μM with a cotreatment of conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells.
Cell proliferation was measured after 24 h of treatment using the commercial CelTiter96 Aqueous kit according to manufacturer's instructions (Promega Madison, WI). Briefly, 20 μl per well of CellTiter96 Aqueous One solution reagent was added to the 96-well plate containing the cells in 100 μl of culture media and incubated for 1 h at 37°C in 5% CO2. Upon completion of the assay procedure the plate was read at 490 nm using the Synergy HT plate reader (Bio-Tek).
HUVEC chemotaxis
HUVEC cells were cultured as described above. HUVEC cell number was assessed by trypan blue dye exclusion using a hemocytometer. Cells were then collected and prepared per manufacturer's instructions for the QCM™ chemotaxis ECM510 cell migration assay (Chemicon, Temecula, CA). Briefly, 40 000 HUVEC cells were seeded in the upper chamber of the provided 96-well plates. The lower chambers were filled with conditioned medium from control or leptin-treated IMCE (ApcMin/+) or YAMC (Apc+/+) cells. The plates were incubated overnight to allow for HUVEC cell migration through the pores and into the lower chamber or to the outside bottom of the chamber. Any cells attached to the outside of the chamber were detached using the provided detachment buffer and collected according to manufacturer's instructions. Cells were detected using a compound that fluoresces when exposed to non-specific enzymes in live cells (provided with the kit). The plate was read at an excitation wavelength of 485 nm and emission wavelength of 530 nm using a Cytofluor fluorescent plate reader (Millipore Corporation, Bedford, MA) and data were analyzed.
Western blotting
Cells were grown and treated as described above. At collection, cells were washed twice with cold phosphate-buffered saline, scraped into 1 ml sample buffer (sodium dodecyl sulfate Reducing Buffer; 0.5 M Tris–HCl, glycerol, 10% sodium dodecyl sulfate and 0.5% bromophenol blue in ddH20), sonicated and boiled. Samples were then loaded on an equal protein basis of 40 μg per lane, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to an Immobilon-FL polyvinylidene difluoride membrane (Millipore Corporation). Membranes were probed with primary antibodies against inter-cellular adhesion molecule (ICAM)-1 (1:100), vascular cell adhesion molecule (VCAM)-1 (1:100), E-selectin (1:1000), pSAPK (1:500), stress-activated protein kinase (SAPK) (1:500), p42/44 (1:1000), pp42/44 (1:1000) and NFκB (1:1000) (Santa Cruz Biotechnology) with shaking overnight at 4°C. Actin (1:1000; Santa Cruz Biotechnology) was also run for normalization. Incubation with the primary antibody was followed by appropriate infrared-labeled second antibodies and detected using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Nuclear and cytoplasmic protein extraction
HUVECs were cultured and treated with conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells for 0, 0.5, 1.5, 3.0 and 6.0 h. Nuclear and cytoplasmic protein fractions were then extracted. Cells were scraped in Meng-Mo buffer [25 mM 3-(N-morpholino)propanesulfonic acid, 2 mM ethylenediaminetetraacetic acid, 0.02% sodium azide (NaN3), 10% glycerol, 20 mM sodium molybdate (Na2MoO4·2H2O)]-containing protease inhibitor and lysed using a Dounce homogenizer (38). Cytoplasmic fraction was separated and nuclear pellet isolated via centrifugation. Cytoplasmic fraction was removed and nuclear pellet resuspended in Meng-Mo buffer. Pellet was washed and high-salt nuclear extracts were prepared by suspending the nuclear pellet in MENG-Mo containing 500 mM NaCl.
Nuclear activation assay
NFκB activation was measured in HUVEC nuclear extracts using the TransAM™ Transcription Factor Assay Kit according to the manufacturer's instructions (Active Motif, Carlsbad, CA) as described previously (39). Briefly, 20 μg of nuclear extract was applied to the plates and incubated according to manufacturer's instructions. Substrate-activated horseradish peroxidase-conjugated secondary antibody provided a sensitive colorimetric readout that was quantified by spectrophotometry at 450 nm wavelength using a Synergy HT plate reader (Bio-Tek).
Statistical analysis
Data were assessed statistically using Prism® software (Graph Pad) and statistical significance was set at P < 0.05. Differences among the groups were tested using the one-way analysis of variance in combination with Bonferroni's multiple comparison test, with statistical significance discerned at P < 0.05. Experiments were repeated at least three times and data shown are from a representative experiment.
Results
Leptin analysis of conditioned media from IMCE (ApcMin/+) and YAMC (Apc+/+) cells
Leptin was measured by commercial ELISA in the conditioned media of the leptin-treated IMCE (ApcMin/+) and YAMC (Apc+/+) cells after the 48 h treatment period [data not shown, (39)]. This verified that leptin was no longer present in the media and ruled out a direct effect of any residual leptin.
VEGF production is induced by leptin treatment in IMCE (ApcMin/+) cells
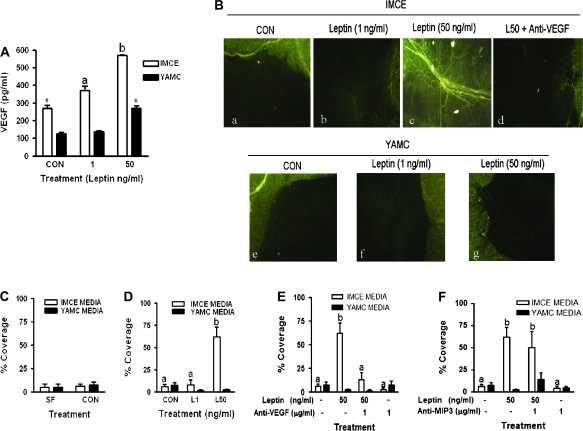
IMCE (ApcMin/+) control cells made significantly more VEGF than YAMC (Apc+/+) (268 ± 18 versus 124 ± 8 pg/ml, P < 0.01) control cells (Figure 1A). Leptin treatment induced a dose-dependent increase in VEGF production in IMCE (ApcMin/+) cells significant compared with control at all doses tested. In YAMC (Apc+/+) cells leptin significantly induced VEGF only at the highest leptin concentration (50 ng/ml) (Figure 1A).
Fig. 1.
VEGF production from IMCE and YAMC cells treated with leptin and induction of angiogenesis. (A) The effect of leptin on IMCE (ApcMin/+) and YAMC (Apc+/+) cell production of VEGF. Cells were treated with leptin (1 or 50 ng/ml) for 48 h; media was collected and analyzed for VEGF using ELISA. Results are representative of at least three separate experiments. *P < 0.01 (compared with YAMC CON); a = P < 0.05 (compared with IMCE CON). (B) Epifluorescence visualization of microvessels in rat mesenteric windows using the Griffonia simplicifolia I lectin. Rat mesenteric windows were treated with conditioned media collected at 48 h from IMCE (ApcMin/+) or YAMC (Apc+/+) control or leptin (1 or 50 ng/ml) treated cells. Representative stimulus-specific capillary coverage is illustrated by G.simplicifolia I labeling for: (a) media from IMCE control cells, (b) media from IMCE leptin (1 ng/ml)-treated cells, (c) media from IMCE leptin (50 ng/ml)-treated cells, (d) media from IMCE leptin (50 ng/ml)-treated cells cotreated with anti-VEGF antibody (1 ug/ml), (e) media from control YAMC cells, (f) media from YAMC leptin (1 ng/ml)-treated cells and (g) media from YAMC leptin (50 ng/ml)-treated cells. (C) Graphical illustration of percent capillary coverage of rat mesenteric windows comparing control media from IMCE and YAMC cells, which was not different. (D) Graphical illustration of percent capillary coverage of rat mesenteric windows after treatment with conditioned media from leptin (1 and 50 ng/ml)-treated IMCE and YAMC cells. Only the L50 media induced a significant increase in capillary coverage of the rat mesentery window. (E) Graphical illustration of percent capillary coverage of rat mesenteric windows after a pretreatment with an anti-VEGF neutralization antibody followed by treatment with conditioned media from leptin (0 and 50 ng/ml) treated IMCE and YAMC cells. The capillary formation induced by the leptin (50 ng/ml)-treated IMCE media was blocked by cotreatment with anti-VEGF antibody. (F) Graphical illustration of percent capillary coverage of rat mesenteric windows after a pretreatment with an anti-MIP3 neutralization antibody followed by treatment with conditioned media from leptin (0 and 50 ng/ml)-treated IMCE and YAMC cells. Cotreatment of leptin (50 ng/ml)-treated IMCE media with an antibody against MIP3 did not block capillary formation. Data shown are the mean ± standard deviation of 10 mesenteric windows per treatment from one representative experiment. The experiments were repeated three times. a = no statistical difference; b = P < 0.001 (compared with all treatments). CON = conditioned media from control IMCE or YAMC cells, L1 = conditioned media from leptin (1 ng/ml) treated IMCE or YAMC cells, L50 = conditioned media from leptin (50 ng/ml) treated IMCE or YAMC cells.
Leptin treatment of IMCE cells induces angiogenesis in rat mesenteric windows
Digital images of rat mesenteric windows visualized the angiogenesis. A significant difference was observed in the mesentery windows incubated with conditioned media from IMCE (ApcMin/+) cells. Control-treated windows are shown in Figure 1B-a. With the addition of leptin at a dose of 1 ng/ml, formation of capillaries started to occur and a small amount of the mesenteric window showed angiogenesis (Figure 1B-b). Interestingly, when a larger dose of leptin was used (50 ng/ml), the window was filled with a large vessel as well as many small capillaries growing out from the layer of adipose tissue as well as sprouting from the center of the large vessel that runs through the mesentery window (Figure 1B-c). Bar graphs further illustrate the angiogenesis by showing the percent coverage achieved based on the photographs described above. Conditioned media from leptin-treated IMCE (ApcMin/+) cells significantly induced angiogenesis at the 50 ng/ml dose (P < 0.001) compared with both control and a 1 ng/ml leptin dose (Figure 1D).
As demonstrated in Figure 1B, mesenteric windows incubated with conditioned media from control and 1 ng/ml leptin-treated YAMC (Apc+/+) cells showed no vessel growth coming from the outer ring of adipose tissue adjacent to the external wall of the gut (Figure 1B-e,f and C). However, the formation of a few small capillaries was induced when the mesenteric window was incubated with media from YAMC (Apc+/+) cells treated with a 50 ng/ml dose of leptin that was not significantly different from control or 1 ng/ml leptin (Figure 1B-g and D).
Figure 1B also demonstrated visually the antibody neutralization experiments. Pretreatment with anti-VEGF antibody significantly decreased the amount of capillary formation induced by the 50 ng/ml dose of leptin (P < 0.001) (Figure 1B-d and E). Pretreatment with anti-MIP3 antibody failed to block the capillary formation induced with the addition of the high dose of leptin alone (image not shown; Figure 1F).
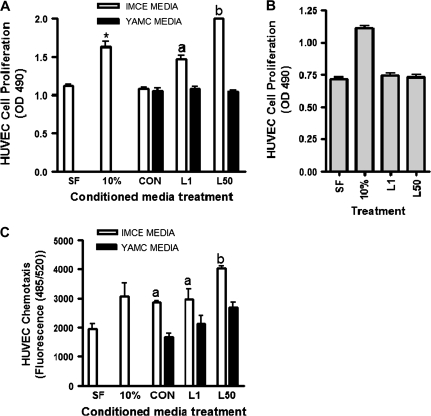
Effect of leptin-treated IMCE cells on HUVEC cell proliferation and chemotaxis
Treatment with media that contained 10% serum (as a positive control) significantly induced HUVEC cell proliferation as expected. However, conditioned media from control IMCE (ApcMin/+) or YAMC (Apc+/+) cells did not induce cell proliferation compared with cells treated with serum-free media alone. Conditioned media from leptin-treated IMCE (ApcMin/+) cells significantly increases HUVEC cell proliferation in a dose-dependent manner compared with treatment with serum-free media alone and conditioned media from control IMCE (ApcMin/+) cells (Figure 2A). Treating the HUVEC cells directly with leptin did not induce cell proliferation (Figure 2B). Conditioned media from leptin-treated YAMC (Apc+/+) cells showed no effect on HUVEC cell proliferation at either dose (Figure 2A). Conditioned media from leptin-treated IMCE (ApcMin/+) cells also significantly increased HUVEC cell migration compared with treatment with serum-free media alone, whereas the conditioned media from the YAMC (Apc+/+) cells showed no significant effect (Figure 2C).
Fig. 2.
HUVEC proliferation and migration in response to conditioned media. (A) The effect of conditioned media from IMCE (ApcMin/+) or YAMC (Apc+/+) control or leptin (1 or 50 ng/ml)-treated cells on HUVEC cell proliferation as measured using the Aqueous One kit. *P < 0.05 (compared with SF control); a = P < 0.05 (compared with CON); b = P < 0.001 (compared with CON). (B) The effect of direct leptin treatment alone on HUVEC cell proliferation. (C) The effect of conditioned media from IMCE (ApcMin/+) or YAMC (Apc+/+) control or leptin (1 or 50 ng/ml)-treated cells on HUVEC cell migration using the QCM chemotaxis assay. a = P < 0.05 (compared with SF control); b = P < 0.001 (compared with CON and L1). Results are representative of three separate experiments. SF = treatment with serum free medium alone, 10% = treatment with media that contained 10% serum (as a positive control), CON = conditioned media from control IMCE or YAMC cells, L1 = conditioned media from leptin (1 ng/ml) treated IMCE or YAMC cells, L50 = conditioned media from leptin (50 ng/ml) treated IMCE or YAMC cells.
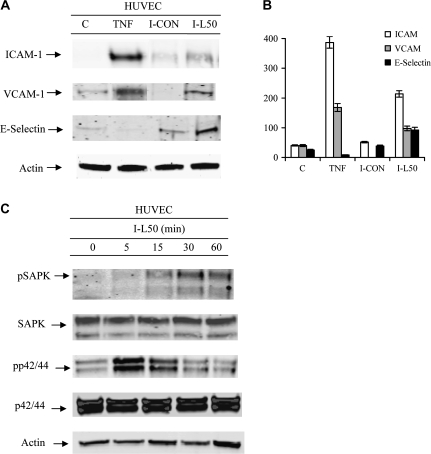
Leptin-treated IMCE cell media increased adhesion proteins in HUVEC cells
HUVECs were incubated with conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells. Total cell lysates from HUVEC cells were collected and probed using western blot. This treatment induced increased ICAM-1, VCAM-1 and E-selectin at 6 h post-treatment (Figure 3A and B) in HUVECs. As a positive control, tumor necrosis factor-α treatment induced adhesion molecules in HUVECs.
Fig. 3.
Adhesion molecule production by and activation of HUVECs as demonstrated by western blot. (A) The effect of conditioned media from IMCE (ApcMin/+) control or leptin (50 ng/ml)-treated cells on ICAM-1, VCAM-1 or E-Selectin upregulation at 6 h post-treatment of HUVEC cells. (B) Densitometric representation of the western blots in A for ICAM, VCAM and E-Selectin. (C) The effect of conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells at various time points on HUVEC cell activation (phosphorylation) of SAPK or p42/44. Total protein controls pair matched for SAPK and p42/44 are shown. Actin shown as a protein loading control. Results are representative of two separate experiments. C = media from HUVEC control cells, TNF = 5 ng/ml tumor necrosis factor-α as positive control, I-CON = conditioned media from IMCE (ApcMin/+) control cells, I-L50 = conditioned media from leptin (50 ng/ml) treated IMCE (ApcMin/+) cells.
Leptin-treated IMCE cell media increased cell-signaling molecules in HUVEC cells
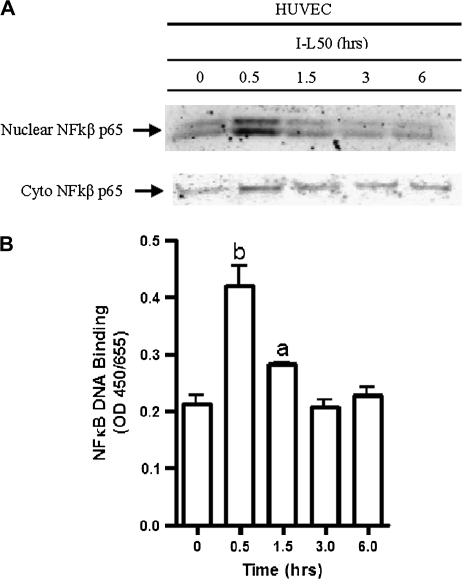
Media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells increased HUVEC cell activation of SAPK in a time-dependent fashion (Figure 3C) reaching highest levels at 30 and 60 min. Media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells increased HUVEC cell activation of p42/44 after 5 min, decreased slightly after 15 and down by 30 min (Figure 3C). Nuclear NFκB protein was increased at 0.5 h in HUVECs treated with conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) (I-L50) cells (Figure 4A). Further, NFκB p65 DNA binding was increased significantly at 0.5 h and decreased in a time-dependent manner; however, was still significantly elevated at 1.5 h (Figure 4B).
Fig. 4.
NFκB nuclear translocation and DNA binding in HUVECs following conditioned media treatment. (A) The effect of conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells on HUVEC cytoplasmic and nuclear NFκB translocation. Cells were treated overnight in serum free medium prior to exposure to treatment. Nuclear extracts were collected at time points shown. (B) The effect of conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells on nuclear NFκB DNA binding at various time points using the TransAM NFκB kit. a = P < 0.05 (compared with 0 h); b = P < 0.001 (compared with 0 h). Results are representative of two separate experiments.
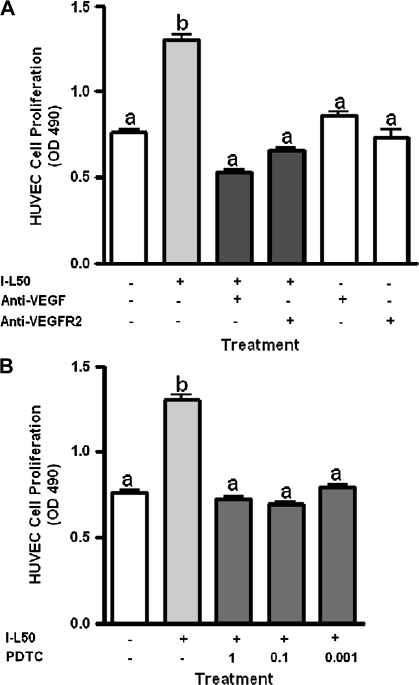
Effect of VEGF, VEGFR-2 and NFκB inhibitors on I-L50 stimulated HUVEC cell proliferation
Leptin (50 ng/ml)-treated IMCE (ApcMin/+) (I-L50) -conditioned media significantly increased HUVEC cell proliferation (Figure 5A). This effect was significantly decreased by cotreatment with anti-VEGF antibody and anti-VEGFR-2 antibody compared with I-L50 alone (Figure 5A). Figure 5B depicts a significant increase in I-L50-induced HUVEC cell proliferation that was significantly decreased by treatment with PDTC, an NFκB inhibitor.
Fig. 5.
Inhibition of conditioned media driven HUVEC cell proliferation. (A) The effect of conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells and coincubation with anti-VEGF or anti-VEGFR-2 antibody (1 μg/ml) on HUVEC cell proliferation as measured using the Aqueous One proliferation assay. (B) The effect of conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells and cotreatment with pyrrolidine dithiocarbamate (PDTC; NFκB inhibitor) at 1.0, 0.1 or 0.001 μM on HUVEC cell proliferation. Results are representative of three separate experiments. a = no significant difference; b = P < 0.01 (compared with all treatments). I-L50 = conditioned media from leptin (50 ng/ml) treated IMCE (ApcMin/+) cells.
Discussion
The impact of obesity and cancer are a priority for the health of the nation. Leptin, an adipocyte-derived hormone crucial in regulating energy balance, is elevated in obese individuals. We show for the first time that leptin induces only colon epithelial cells carrying a mutation in Apc, a gatekeeper mutation in colon cancer, to produce increased concentrations of VEGF. We go on to show that the VEGF in the conditioned media of the epithelial cells is responsible for increased capillary formation in a functional angiogenesis bioassay. Based on this observation of increased VEGF and the importance of the phenotype of angiogenesis in carcinogenesis (20), we wanted to understand if this increase in VEGF was bioactive and thus, resulted in increased angiogenic potential. We found that this increase in VEGF was only translated into increased angiogenic potential in the IMCE cells treated with leptin. This is evidenced by data indicating that pretreatment with a neutralizing anti-VEGF antibody significantly decreased the amount of capillary formation induced by the 50 ng/ml dose of leptin (P < 0.001) suggesting a possible mechanism (Figure 1B-d and E). Numerous studies have demonstrated leptin's ability to induce angiogenesis by stimulating increased production of VEGF (14,30). However, it is novel that leptin differentially induced VEGF production in colon epithelial cells. Similar to our findings, leptin significantly induced expression of VEGF as well as induced cell migration and increased the expression of growth factors controlling proliferation, metastasis and angiogenesis in prostate cancer cells (14). Our data indicate that VEGF production is increased in IMCE (ApcMin/+) cells probably via signaling through NFκB. Pretreatment with anti-MIP3 antibody failed to block the capillary formation induced by treatment with conditioned media from high dose leptin-treated IMCE cells (Figure 1F), demonstrating that the mechanism of angiogenesis is not through MIP3.
Endothelial cells must undergo a series of events to support capillary formation. These events include endothelial cell chemoattraction, increased endothelial cell expression of adhesion proteins for cell–to-cell adhesion and endothelial cell proliferation (40,41). We sought to understand which of these events were regulated by the colon epithelial cell treated with leptin. We utilized HUVEC cells, a model of human endothelial cells, to identify the potential mechanism through which the increased capillary formation occurs. Conditioned media from leptin-treated IMCE (ApcMin/+) cells significantly increased HUVEC cell migration in a dose-dependent manner. Conditioned media from leptin (50 ng/ml)-treated IMCE (ApcMin/+) cells also significantly increased HUVEC cell proliferation (Figure 2A).
The proliferative effect of conditioned media from leptin-treated IMCE (ApcMin/+) cells on HUVEC cells was significantly decreased by cotreatment with either a neutralizing anti-VEGF antibody or a neutralizing anti-VEGFR-2 antibody compared with I-L50 alone (Figure 5A). These data demonstrate that when signaling through the VEGF pathway is blocked, either through ligand or receptor inhibition, proliferation is inhibited. This further supports the role of VEGF in I-L50-stimulated HUVEC cell proliferation. I-L50 media also induced the phosphorylation of mitogen-activated protein kinase p42/44 and SAPK (Figure 3C). Binding of VEGF ligands to its receptor activates p42/44 and SAPK pathways and leads to functional changes that include increased cell migration and invasion as well as increased NFκB p65 translocation into the nucleus (42). Further, VEGF promotes growth of cancer cells and cell proliferation through activating VEGFR-2 signaling (43,44).
We identified that conditioned media from the leptin-treated epithelial cells caused endothelial cell proliferation, migration and upregulation of adhesion molecules probably resulting from NFκB activation and nuclear translocation. The increase in NFκB nuclear translocation and DNA binding, shown in figure 4, supports a role for HUVEC cell NFκB activation by conditioned media from leptin-treated IMCE (ApcMin/+) cells. Figure 5B shows a significant increase in I-L50-induced HUVEC cell proliferation that is decreased significantly by cotreatment with PDTC, a NFκB inhibitor, at all doses tested. Further, neutralizing antibodies against VEGF and VEGFR-2 block the HUVEC cell proliferation, demonstrating that inhibition of NFκB signaling decreases proliferation and that it acts through the VEGFR-2 receptor. This is further confirmation that in the VEGF pathway, VEGFR-2 signals through NFκB to increase adhesion molecules, thereby suggesting a mechanism by which leptin-treated IMCE (ApcMin/+) cells induce proliferation in HUVECs.
VEGFR-2 regulates endothelial cell migration, proliferation and differentiation and plays a key role in mitogenic, chemotactic and prosurvival signals (45,46). VEGFR-2 is the chief mediator of the angiogenic and permeability-increasing effect of VEGF. Conversely, VEGFR-1 has been shown to have no effect on cell proliferation (42). The major function of VEGFR-1 signaling in endothelial cells is the release of tissue-specific growth factors (45). Activation of VEGFR-2, not VEGFR-1, is required for the antiapoptotic effects of VEGF in HUVECs (45). A study by Von Marschall et al. (2000) found specific immunostaining for both VEGFR-1 and VEGFR-2 in the endothelial cells of vascular structures surrounding tumor cells of pancreatic cancer samples; however, it was higher for VEGFR-2 than VEGFR-1 (43% compared with 29%). In contrast, no receptor expression was observed in endothelial cells of normal pancreas or chronic pancreatitis, indicating that upregulation of the VEGF receptors is specific to cancer cell progression in the pancreas and not associated with chronic inflammation (44).
Conditioned media from leptin-treated IMCE (ApcMin/+) cells stimulated expression of adhesion molecules ICAM, VCAM and E-selectin. We believe that this is due to VEGF, as studies indicate that VEGF induces adhesion molecules in HUVECs and is mediated largely through NFκB activation (23). Cell adhesion molecules play a key role in the formation of the vasculature and have been detected in the blood vessels during angiogenesis (40). Adhesion molecules play a large part in tumor development by mediating both interactions between cancer cells and interactions between cancer and other cells, including endothelial cells (47).
In general, our data are consistent with data from the Min mouse model. The Min mouse carries a mutation in the Apc gene (identical to our colon epithelial cells) resulting in small intestinal polyp formation. Korsisaari et al. (2007) treated Min mice with a monoclonal antibody-targeting VEGF-A and genetic deletion of VEGF-A selectively in intestinal epithelial cells (48). They established that blocking VEGF-A signaling resulted in tumor growth cessation and long-term survival in an intestinal adenoma model. The authors go on to suggest that ‘VEGF-A inhibition may be a previously uncharacterized strategy for the prevention of the angiogenic switch and growth in intestinal adenomas’ (48). Goodland et al. (2006) treated Min mice with an oral-signaling inhibitor of VEGF to investigate the role of VEGF-2 in adenoma development and growth. They blocked angiogenesis and limited the growth of adenomas to ≤1 mm (49). Together, these results indicate that VEGFR-2 signaling may play key roles in the development and progression of intestinal adenomas. While our cells are colon epithelial cells from the Min mouse and these tumors are small intestinal, together these data suggest that angiogenesis signals are critical at much earlier stages of cancer progression than previously suggested. In addition, obesity, and in particular elevated leptin, may act to enhance or speed the process of angiogenesis in colon cancer progression by inducing ApcMin/+ epithelial cells to produce more VEGF.
In regards to the observation that leptin administration to ApcMin/+ mice did not enhance polyp formation (11), we proposed at least two important differences, aside from the in vivo versus in vitro experimental systems including dose of leptin and cell type, which may explain the apparent contradictory findings. First, the serum levels of leptin achieved by administration to ApcMin/+ mice (7.4 versus control 2.3 ng/ml) did not achieve concentrations consistent with ApcMin/+ mice with high-fat diet-induced obesity (14.39 versus control 5.93 ng/ml) (50). The concentrations of leptin used in the present in vitro study are more reflective of leptin levels in C57BL/6J mice with diet-induced obesity (30 ng/ml) (33). As such, the concentrations of leptin used in this study are relevant to obese mice but not in murine leptin administration studies, which achieve only modest increases in serum leptin concentrations. Finally, the cell lines used in this study are derived from the colon of the min mice, whereas most polyps form in the small intestines of ApcMin/+ mice. It may be that small intestinal epithelial cells respond differently to leptin than colon epithelial cells.
Our data begin to explain the mechanism behind leptin as a risk for colon cancer. Taken together, these findings represent an important key observation that strengthens the potential mechanism behind the obesity–colon cancer link. Obesity is a risk factor for several cancers including colon cancer; however, the mechanism is poorly understood. We show that a hallmark of cancer, sustained angiogenesis, is regulated very early in the process of colon carcinogenesis by the epithelial cells carrying an Apc mutation. Our in vitro data show that leptin acts in a mechanistically distinct manner to induce and orchestrate angiogenesis cross talk in models of normal and preneoplastic colon epithelial cells and endothelial cells. These findings, if confirmed in relevant animal and human model systems, enhance the biologic plausibility that leptin may act at an early stage of carcinogenesis to regulate epithelial–endothelial cell cross talk. This may represent a target for prevention of obesity-associated cancer.
Funding
Michigan Agriculture Experiment Station; National Cancer Institute (R03CA130033).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- APC
adenomatous polyposis coli
- ELISA
enzyme-linked immunosorbent assay
- HUVEC
human umbilical vein endothelial cell
- ICAM
inter-cellular adhesion molecule
- IMCE
immorto-min colonic epithelial cell
- MIP3
macrophage inflammatory protein 3
- NFκB
nuclear factor kappa B
- PDTC
pyrrolidine dithiocarbamate
- SAPK
stress-activated protein kinase
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- YAMC
young adult mouse colon
References
- 1.Bianchini F, et al. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 2.Calle EE, et al. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 3.Gunter MJ, et al. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J. Nutr. Biochem. 2006;17:145–156. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Vendrell J, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes. Res. 2004;12:962–971. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 5.Stattin P, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol. Rep. 2003;10:2015–2021. [PubMed] [Google Scholar]
- 6.Saglam K, et al. Leptin influences cellular differentiation and progression in prostate cancer. J. Urol. 2003;169:1308–1311. doi: 10.1097/01.ju.0000055903.18400.25. [DOI] [PubMed] [Google Scholar]
- 7.Chen DC, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Goktas S, et al. Prostate cancer and adiponectin. Urology. 2005;65:1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 9.Attoub S, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. FASEB J. 2000;14:2329–2338. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 10.Hardwick JC, et al. Leptin is a growth factor for colonic epithelial cells. Gastroenterology. 2001;121:79–90. doi: 10.1053/gast.2001.25490. [DOI] [PubMed] [Google Scholar]
- 11.Aparicio T, et al. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut. 2005;54:1136–1145. doi: 10.1136/gut.2004.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okumura M, et al. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim. Biophys. Acta. 2002;1592:107–116. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 13.Somasundar P, et al. Differential effects of leptin on cancer in vitro. J. Surg. Res. 2003;113:50–55. doi: 10.1016/s0022-4804(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 14.Frankenberry KA, et al. Leptin induces cell migration and the expression of growth factors in human prostate cancer cells. Am. J. Surg. 2004;188:560–565. doi: 10.1016/j.amjsurg.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Choi JH, et al. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J. Clin. Endocrinol. Metab. 2005;90:207–210. doi: 10.1210/jc.2004-0297. [DOI] [PubMed] [Google Scholar]
- 16.de Visser KE, et al. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 17.Haramis AP, et al. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoud NN, et al. Genotype-phenotype correlation in murine Apc mutation: differences in enterocyte migration and response to sulindac. Cancer Res. 1999;59:353–359. [PubMed] [Google Scholar]
- 19.Kagnoff MF, et al. Epithelial cells as sensors for microbial infection. J. Clin. Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D, et al. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 21.Hicklin DJ, et al. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 22.Reinmuth N, et al. Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc. Res. Tech. 2003;60:199–207. doi: 10.1002/jemt.10258. [DOI] [PubMed] [Google Scholar]
- 23.O'Byrne KJ, et al. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur. J. Cancer. 2000;36:151–169. doi: 10.1016/s0959-8049(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 24.Hwang J, et al. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine. 2005;30:254–263. doi: 10.1016/j.cyto.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Son KN, et al. Human CC chemokine CCL23 enhances expression of matrix metalloproteinase-2 and invasion of vascular endothelial cells. Biochem. Biophys. Res. Commun. 2006;340:498–504. doi: 10.1016/j.bbrc.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Fenton JI, et al. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol. Biomarkers Prev. 2005;14:1646–1652. doi: 10.1158/1055-9965.EPI-04-0916. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead RH, et al. Derivation of conditionally immortalized cell lines containing the Min mutation from the normal colonic mucosa and other tissues of an “Immortomouse”/Min hybrid. Epithelial Cell Biol. 1994;3:119–125. [PubMed] [Google Scholar]
- 28.Mei JM, et al. Expression of prostaglandin endoperoxide H synthase-2 induced by nitric oxide in conditionally immortalized murine colonic epithelial cells. FASEB J. 2000;14:1188–1201. doi: 10.1096/fasebj.14.9.1188. [DOI] [PubMed] [Google Scholar]
- 29.Fenton JI, et al. Membrane-type matrix metalloproteinases mediate curcumin-induced cell migration in non-tumorigenic colon epithelial cells differing in Apc genotype. Carcinogenesis. 2002;23:1065–1070. doi: 10.1093/carcin/23.6.1065. [DOI] [PubMed] [Google Scholar]
- 30.Sierra-Honigmann MR, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 31.Ronti T, et al. The endocrine function of adipose tissue: an update. Clin. Endocrinol. (Oxf) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 32.Fenton JI, et al. Stage matters: choosing relevant model systems to address hypotheses in diet and cancer chemoprevention research. Carcinogenesis. 2006;27:893–902. doi: 10.1093/carcin/bgi355. [DOI] [PubMed] [Google Scholar]
- 33.Ahren B. Plasma leptin and insulin in C57BI/6J mice on a high-fat diet: relation to subsequent changes in body weight. Acta Physiol. Scand. 1999;165:233–240. doi: 10.1046/j.1365-201x.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 34.Considine RV, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 35.Bessho R, et al. Pyrrolidine dithiocarbamate, a potent inhibitor of nuclear factor kappa B (NF-kappa B) activation, prevents apoptosis in human promyelocytic leukemia HL-60 cells and thymocytes. Biochem. Pharmacol. 1994;48:1883–1889. doi: 10.1016/0006-2952(94)90586-x. [DOI] [PubMed] [Google Scholar]
- 36.Hansen-Smith FM, et al. Regional differences in spontaneously occurring angiogenesis in the adult rat mesentery. Microvasc. Res. 1994;47:369–376. doi: 10.1006/mvre.1994.1029. [DOI] [PubMed] [Google Scholar]
- 37.Hansen-Smith FM. Capillary network patterning during angiogenesis. Clin. Exp. Pharmacol. Physiol. 2000;27:830–835. doi: 10.1046/j.1440-1681.2000.03341.x. [DOI] [PubMed] [Google Scholar]
- 38.Hord NG, et al. Physicochemical and immunocytochemical analysis of the aryl hydrocarbon receptor nuclear translocator: characterization of two monoclonal antibodies to the aryl hydrocarbon receptor nuclear translocator. Mol. Pharmacol. 1994;46:618–626. [PubMed] [Google Scholar]
- 39.Fenton JI, et al. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507–1515. doi: 10.1093/carcin/bgl018. [DOI] [PubMed] [Google Scholar]
- 40.Kim I, et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J. Biol. Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay D, et al. Complexity in the vascular permeability factor/vascular endothelial growth factor (VPF/VEGF)-receptors signaling. Mol. Cell. Biochem. 2004;264:51–61. doi: 10.1023/b:mcbi.0000044374.85095.df. [DOI] [PubMed] [Google Scholar]
- 42.Fan F, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24:2647–2653. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 43.Su JL, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9:209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 44.von Marschall Z, et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–1372. doi: 10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, et al. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 46.Cebe-Suarez S, et al. The role of VEGF receptors in angiogenesis; complex partnerships. Cell. Mol. Life Sci. 2006;63:601–615. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallicchio M, et al. Celecoxib decreases expression of the adhesion molecules ICAM-1 and VCAM-1 in a colon cancer cell line (HT29) Br. J. Pharmacol. 2008;153:870–878. doi: 10.1038/sj.bjp.0707634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korsisaari N, et al. Inhibition of VEGF-A prevents the angiogenic switch and results in increased survival of Apc+/min mice. Proc. Natl Acad. Sci. USA. 2007;104:10625–10630. doi: 10.1073/pnas.0704213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodlad RA, et al. Inhibiting vascular endothelial growth factor receptor-2 signaling reduces tumor burden in the ApcMin/+ mouse model of early intestinal cancer. Carcinogenesis. 2006;27:2133–2139. doi: 10.1093/carcin/bgl113. [DOI] [PubMed] [Google Scholar]
- 50.Mai V, et al. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res. 2003;63:1752–1755. [PubMed] [Google Scholar]