Abstract
A variety of plant species emit volatile compounds in response to mechanical stresses such as herbivore attack. Although these volatile compounds promote gene expression leading to anti-herbivore responses, the underlying transduction mechanisms are largely unknown. While indirect evidence suggests that the cytoplasmic free Ca2+ concentration ([Ca2+]c) plays a crucial role in the volatile-sensing mechanisms in plants, these roles have not been directly demonstrated. In the present study, we used Arabidopsis leaves expressing apoaequorin, a Ca2+-sensitive luminescent protein, in combination with a luminometer, to monitor [Ca2+]c transients that occur in response to a variety of volatile compounds and to characterized the pharmacological properties of the increase in [Ca2+]c. When leaves were exposed to volatiles, [Ca2+]c was transiently raised. The [Ca2+]c increases induced by acyclic compounds were disrupted by Ruthenium Red, a potential plasma-membrane and endo-membrane Ca2+-permeable channel inhibitor, but not by 1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA), an extracellular Ca2+-chelator, suggesting that acyclic compounds promote Ca2+-release from intracellular stores. On the other hand, the electrophilic compound (E)-2-hexenal promoted Ca2+-influx via ROS production by natural oxidation at the aquarius phase. In a gpa1-2 mutant lacking a canonical Gα subunit, the [Ca2+]c transients induced by all tested volatiles were not attenuated, suggesting that G-protein coupled receptors are not involved in the volatile-induced [Ca2+]c transients in Arabidopsis leaves.
Key words: Arabidopsis thaliana, Ca2+, electrophile, green leaf volatiles, reactive oxygen species
Introduction
Land plants are exposed to a variety of environmental stresses and exhibit physiological responses in order to adapt to and resist these stresses. After mechanical stimuli such as wounding or pathogen/herbivore attack, plants produce and emit a variety of volatile compounds such as jasmonates (JAs), salicylates (SAs) and green leaf volatiles.1 Volatiles from herbivore-damaged leaves (HIPVs: herbivore induced plant volatiles) play multiple roles in protection against attacking herbivores, including induction of the pathogen-related (PR) proteins and attraction of the natural predators of the herbivore.2 Interestingly, such anti-herbivore responses were observed not only in the attacked plant but also in neighbouring plants. In lima bean leaves infested with herbivorous mite (Tetranychus urticae), terpenoid compounds were specifically emitted in response to herbivore damage, promoting the expression of defensive genes in neighbouring plant leaves.3
The major chemical components that released from T. urticae-infested leaf are (E)-β-ocimene, (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) and (E,E)-4,8,12-trimethyl-1,3,7-tridecatetraene (TMTT). Although wounded leaves also specifically emit the C6-alcohol, (Z)-3-hexenol, immediately after wounding, this compound does not promote defence gene expression in neighbouring plant leaves.3 Fumigation with the above three HIPVs also promoted the expression of PR genes, indicating that HIPVs directly promote a series of defence responses. Pharmacological analyses revealed that Ca2+-influx, Ser/Thr-protein kinases and type 1/2A protein phosphatases are involved in HIPV-responses in lima bean leaves.3 However, possible candidates for volatile compound receptors and the underlying signal transduction pathways in which they participate are still obscure.
In humans and animals, G-protein coupled receptors (GPCRs) act as olfactory receptors.4 In general, heterotrimeric G-proteins are activated by the specific binding of odorant molecules (including volatiles) to GPCRs. The heterotrimeric G-protein then binds to and activates adenylate cyclase, resulting in production of cAMP and activation of plasma membrane cyclic nucleotide gated channels (CNGCs), which allow entry of cations including Ca2+. GPCRs also act as sensors for hormones and growth factors due to their specific binding affinity for a variety of chemical compounds.4
In plant science, a line of A. thaliana mutated for the GPCR-like protein, gcr1 is found to be less sensitive toward the cytokinin, gibberellin and brassinosteroid of plant hormones.5,6 Recently, Liu et al.7 identified an orthologue of GPCRs in Arabidopsis, GCR2, and demonstrated that GCR2 associates with the Gα subunit, GPA1. GCR2 localizes to the plasma membrane and binds ABA with high affinity at a physiological concentration (Kd = 20.1 nM). The binding of ABA promotes the dissociation of the GCR2-GPA1 complex, and GCR2 overexpression results in hypersensitivity to ABA, indicating that GCR2 is a plasma membrane ABA receptor. Although there are 25 recognized GPCR orthologues, there is only one canonical Gα (GPA1) subunit, one Gβ subunit, and two Gγ subunits in the Arabidopsis genome.8–13
GCR1 also associates with GPA1 and the gcr1 mutant exhibits hypersensitivity to ABA.14 Considered together, it appears that GPA1 may associate with a broad spectrum of GPCRs and serve as a common component of GPCR-related signal transduction.
Although many reports have indicated the involvement of GPCRs and Ca2+ in the plant volatile-sensing pathway, their role has not been well demonstrated through a genetic approach and monitoring of cytosolic Ca2+ concentration ([Ca2+]c) in plant cells. Here we monitored [Ca2+]c in Arabidopsis leaves expressing the luminous Ca2+-reporting protein apoaequorin and obtained direct evidence that volatiles promote transient increases in [Ca2+]c. Furthermore, we found that GPCRs may not be involved in the volatile-induced [Ca2+]c transients. Finally, the properties of the volatile-induced [Ca2+]c increases were analyzed by pharmacological approaches.
Results
Volatiles promote the transient elevation of [Ca2+]c in Arabidopsis leaves.
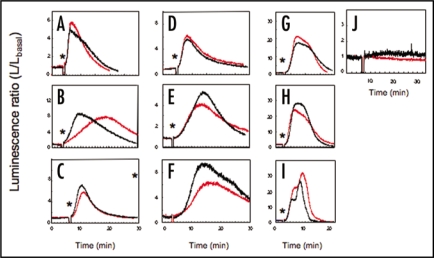
Aequorin-reconstituted Arabidopsis leaves were exposed to the vapour of a variety of volatile-compounds (their structures are illustrated in Fig. 1) at the indicated concentrations. The aequorin luminescence was monitored in order to examine the time-course of the volatile-induced increase in [Ca2+]c (Fig. 2A–J; traces in black). When a piece of cotton impregnated with volatiles was enclosed in the dish with Arabidopsis leaves to produce a vapour of the volatile compound, the intensity of aequorin luminescence was immediately and/or gradually raised, and then exponentially decayed. All of the listed volatiles, including HIPVs (terpenoids; acyclic compounds and bicyclic compounds) and GLVs (green leaf volatiles; C6-aldehydes and C6-alcohols and their acetates) promoted transient increases in [Ca2+]c with different kinetic patterns and intensities. Among the listed volatiles used in the present study, C6-aldehyde, (E)-2-hexenal (Fig. 2A) rapidly promoted the [Ca2+]c transient, which peaked within 3 min. Alcohols with similar structures such as (E)-2-hexen-1-ol and cyclic organic compounds including bicyclic (Fig. 2G–I), and acyclic compounds (Fig. 2C–E) promoted a relatively slow increase in [Ca2+]c, which peaked at around 5–10 min from the start of exposure. These results suggest that the volatile-induced [Ca2+]c increase is a common event in the plant response to volatile-exposure, but each molecule may promote this response through a different mechanism.
Figure 1.
Volatile compounds used in this study.
Figure 2.
Volatile-induced [Ca2+]c transients in Arabidopsis leaves. Typical time-courses of aequorin luminescence in the leaves from AQ plants (black) and gpa1-2-AQ plants (red) are shown. Four leaves were packed in a Petri dish. The pieces of cotton with HIPVs were packed into the dish with Arabidopsis leaves at the time indicated by asterisks. Volatiles; (A) (E)-2-hexenal (B) (E)-2-hexen-1-ol (C) β-ocimene (D) β-myrcene (E) (E)-DMNT (F) bornyl acetate (G) (+)-α-pinene (H) (-)-α-pinene (I) (-)-β-pinene, were fumigated at the indicated time (asterisks in each columns). As control experiments, a piece of cotton, impregnated with dichloromethane was packed into a Petri dish after the evaporation of the solvent (J). Typical traces from each 3–5 representative data were shown.
The GPCR pathway is not involved in the volatile-induced [Ca2+]c transients.
The impact of volatiles on [Ca2+]c in an apoae-quorin-expressing GPA1 mutant line (gpa1-2 AQ) was also tested to clarify the role of heterotrimeric G-proteins in volatile-induced [Ca2+]c transients (Fig. 2A–J; traces in red). Although GPCRs act as volatile-receptors in olfactory sensory nerves in animals and GPA1 is a multifunctional, solitary canonical Gα protein in Arabidopsis,7 we observed no significant attenuation of the peak height of the [Ca2+]c transients in response to each volatile tested in the present study (p = 0.3–0.9; the two-tailed Student's t test). These results suggest that GPA1 and GPCRs are not involved in the volatile-induced [Ca2+]c increase in plant cells. We further investigated the effects of an adenylyl cyclase inhibitor (2′,5′-dideoxyadenosine, DA) or phospholipase C inhibitors (U-73122 and neomycin) on the [Ca2+]c transient in response to β-ocimene, a major HIPV in Arabidopsis (Fig. 3). Pre-conditioning of these three compounds had little or no effect on the β-ocimene-induced [Ca2+]c transient (the two-tailed Student's t test; p = 0.236, 0.602, and 0.599 respectively), implying that adenylyl cyclase and phospholipase C, major components of the olfactory system in animal cells, may not be involved in the upstream events of activation of Ca2+-permeable channels or in the resulting [Ca2+]c increase that occurs during plant volatile-sensing.
Figure 3.
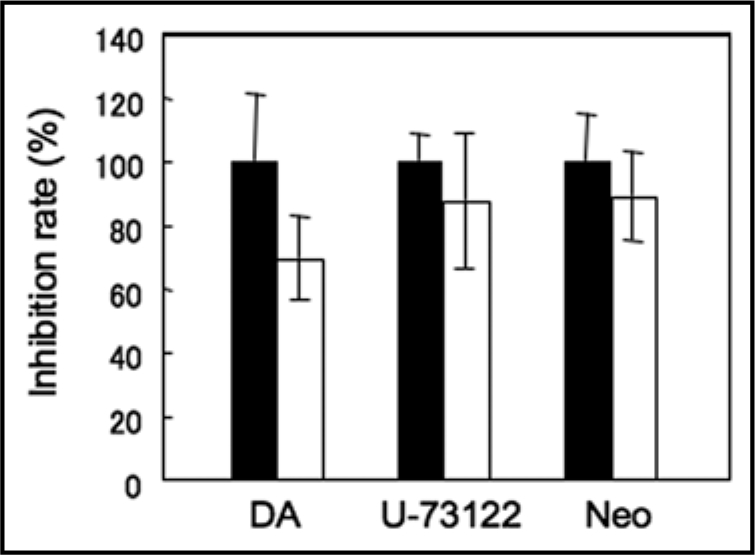
Effects of the inhibitors of the olfactory signalling cascade. The inhibition rates against the peak amplitudes of luminescence in response to the fumigation with β-ocimene are shown. 2′,5′-dideoxyadenosine (DA; 1 mM), U-73122 (0.1 mM) and neomycin (Neo; 0.5 mM) were extracellularly applied to Arabidopsis leaves for 2–3 hrs prior to the fumigation. Data represent means ± SEs (n = 4–8).
Because our previous pharmacological analyses revealed that Ser/Thr-protein kinases and type 1/2A protein phosphatases are also involved in HIPV-induced expression of defence-related genes in lima bean leaves,3 the impact of protein kinase inhibitors and a Ser/Thr protein phosphatase inhibitor on β-ocimene-induced [Ca2+]c increase was further examined (Fig. 4). Pre-conditioning with K252a, a Ser/Thr protein kinase inhibitor, and calyculine A, a protein phosphatase inhibitor had no effect, suggesting that protein phosphorylation and dephosphorylation might be not involved in the up-stream events of β-ocimene-induced [Ca2+]c increase. In contrast, staurosporine, another Ser/Thr protein kinase inhibitor, resulted in an approximately two-fold enhancement of the amplitude of the β-ocimene induced [Ca2+]c transient. In Arabidopsis leaves, treatment with staurosporine also promoted the expression of defence related genes, and this was further promoted by the simultaneous treatment with volatiles,15 suggesting that a staurosporine-sensitive Ser/Thr protein kinase may negatively regulate the [Ca2+]c increase and the subsequent defence responses.
Figure 4.
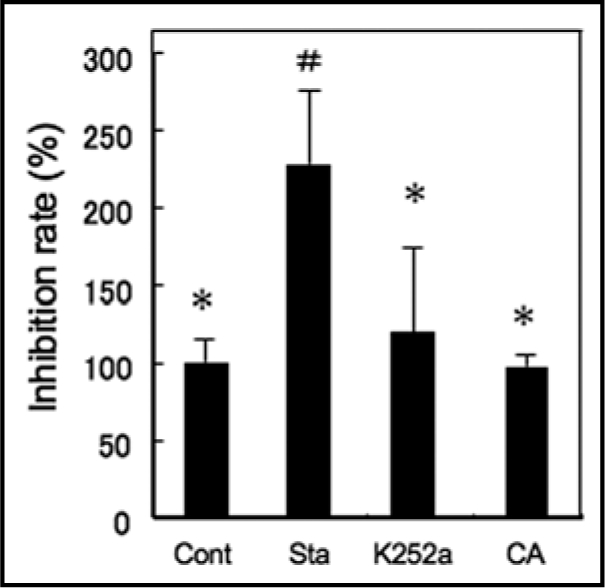
Effects of the inhibitors for protein kinase and phosphatase against β-ocimene induced [Ca2+]c transient. The inhibition rates against the peak amplitudes of luminescence in response to fumigation with β-ocimene are shown. Staurosporine (Sta; 10 µM), K252a (10 µM) and Calyculine A (CA; 1 µM) were extracellularly applied to Arabidopsis leaves for 2–3 hrs prior to the fumigation. Data represent means ± SEs (n = 3–4). # and *p < 0.05, the two-tailed Student's t test.
Volatiles promote a cytosolic calcium transient by two distinct pathways.
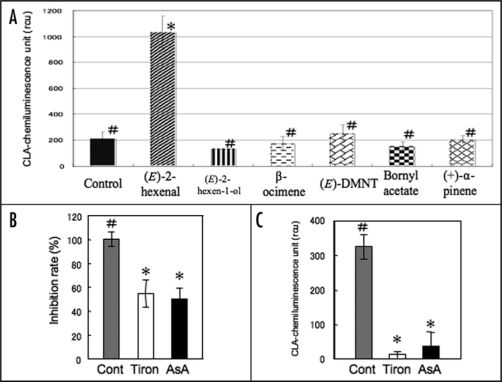
Compounds containing the highly electrophilic α,β-unsaturated carbonyl group strongly activate the expression of defence related genes in Arabidopsis.16 The electrophile 4-hydroxy-2-nonenal dramatically induced the production of reactive oxygen species (ROS) in rat liver epithelial cells and reduced cell viability.17 Among the typical volatiles used in the present study, only (E)-2-hexenal, a C6-aldehyde containing an α,β-unsaturated carbonyl group, promoted the generation of O2− (Fig. 5A). ROS activate the plasma membrane Ca2+-permeable channel and promote Ca2+-influx in Arabidopsis guard cells, root cells and tobacco BY-2 suspension cell culture.18–20 Preconditioning with the ROS-scavengers ascorbic acid and Tiron attenuated the amplitude of the [Ca2+]c transient in response to (E)-2-hexenal (Fig. 5B). Interestingly, (E)-2-hexenal naturally generated O2− when the vapor was dissolved in the CLA-containing solution (Fig. 5C). The other volatile compounds tested in this study did not promote ROS production and the time-courses of the [Ca2+]c transients were also distinct from the (E)-2-hexenal-induced [Ca2+]c transient.
Figure 5.
Volatile-dependent superoxide generation and the participation in [Ca2+]c transients in Arabidopsis leaves. (A) Volatiles-dependent superoxide generation in the leaves from AQ plants. Data represent means ± SEs (n = 3–7). (B) The effect of ROS scavengers, Tiron (40 mM) or ascorbic acid (AsA, 10 mM) on the (E)-2-hexenal-promoted [Ca2+]c transient. Inhibition rates against the peak amplitudes of luminescence in control experiments were shown. Data represent means ± SEs (n = 4). (C) Superoxide production in cell free system. CLA-containing medium were exposed to (E)-2-hexenal. CLA luminescence of the medium (control), with Tiron or ascorbic acid. Data represent means ± SEs (n = 3). # and *p < 0.05, the two-tailed Student's t test. rcu; relative chemiluminescence units.
To date, a large body of electrophysiological data and recent molecular biological studies have revealed that plants possess distinct types of Ca2+ channels with different gating mechanisms in both plasma- and endo-membranes. To further examine the mechanisms of the volatiles-induced [Ca2+]c increase, we assessed the effects of BAPTA, an extracellular Ca2+-chelator, and Ruthenium Red (RuR), a potential inhibitor of both plasma membrane and endomembrane Ca2+-permeable channels,21 on the [Ca2+]c transients induced by three major volatiles; β-ocimene, (E)-DMNT and (E)-2-hexenal (Fig. 6). The [Ca2+]c transients induced by β-ocimene was inhibited by RuR but not by BAPTA, suggesting the involvement of Ca2+-release from intracellular stores in β-ocimene-induced [Ca2+]c transients. The [Ca2+]c transient induced by (E)-DMNT was partially inhibited by BAPTA and almost inhibited by RuR. On the other hand, the [Ca2+]c transient induced by (E)-2-hexenal was largely inhibited by BAPTA. This result further suggests that electrophiles of volatiles that contain an α,β-unsaturated carbonyl group promote rapid ROS production and the activation of ROS-sensitive Ca2+-permeable channels in plasma membrane as a consequence.
Figure 6.
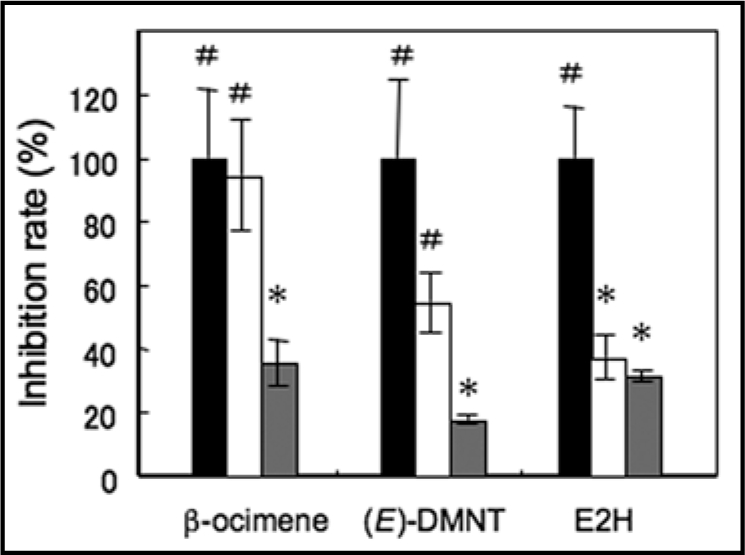
Ca2+ stores for the volatiles-promoted [Ca2+]c transients in Arabidopsis leaves. Effects of 10 mM BAPTA (white bars), a Ca2+ chelator, and 0.5 mM ruthenium red (gray bars), a plasma membrane and endomembrane Ca2+-permeable channel inhibitor on the volatile-promoted [Ca2+]c transients were tested. Inhibition rates against the peak amplitudes of luminescence in control experiments were shown. Black bars indicate the relative peak amplitudes of the luminescence in control experiments for each treatment. Data represent means ± SEs (n = 3–6). # and *p < 0.05, the two-tailed Student's t test.
Discussion
Plant volatiles including terpenoids, C6-volatiles and electrophiles are synthesized and emitted in response to herbivore damage to plant leaves, and promote a variety of defence responses in both damaged and neighbouring plants.3 In Arabidopsis mutants whose synthesis of volatiles had been modified, the attractiveness of the plants to parasitoids and their susceptibility to pathogens were affected.22 In addition, exposure to volatile C6-aldehydes and alloocimene promoted the expression of defence-related genes and the resistance to pathogens in Arabidopsis leaves.15 In the present study, we first demonstrated that a variety of plant volatiles could promote the [Ca2+]c transient in Arabidopsis leaves, which is one of the key components required for defence gene expression in the lima bean.3 Based on our pharmacological analyses, it was revealed that the [Ca2+]c transients induced by acyclic compounds, major components of HIPVs, were mainly induced by Ca2+-release from intracellular Ca2+-stores. In contrast, (E)-2-hexenal, an electrophilic compound, mainly promoted Ca2+-influx from the extracellular space. Fumigation of (E)-2-hexenal dynamically promoted the generation of O2− in Arabidopsis leaves. Interestingly, generation of O2− was also observed to some extent in cell-free conditions, and ROS scavengers attenuated the (E)-2-hexenal-induced [Ca2+]c transient, suggesting that ROS-dependent activation of plasma membrane Ca2+-permeable channels participates in the electrophile-dependent [Ca2+]c transient. Among the C6-volatiles, (E)-2-hexenal specifically inhibits root elongation in Arabidopsis seedlings.23 In plant lines that are mutant for γ-amino butylic acid (GABA) transaminase, (E)-2-hexenal-dependent growth inhibition was impaired and GABA accumulation was observed.22 Besides these biological and physiological effects, natural plant aldehydes including (E)-2-hexenal were identified as natural insecticides against stored-product beetles in fumigation tests with wheat grains.24
ROS generation is also closely related to disease resistance and hypersensitivity. Exposure to salicylic acid, a plant hormone that promotes disease tolerance, results in rapid and transient generation of O2−, and in turn O2− likely triggers the influx of Ca2+ into the cells.25,26 Considered together, these findings indicate that natural plant aldehydes have multiple functions through generation of ROS, and through the specific binding to the potential receptor and the natural generation of O2−.
The underlying signalling pathways for the volatile responses are still obscure. In animal olfactory sensors, GPCRs specifically bind to volatiles and activate heterotrimeric G-proteins, which consequently activate their effectors. In contrast, we found that the only canonical G-protein α subunit, GPA1 was not involved in volatiles-promoted [Ca2+]c transients in Arabidopsis leaves (Fig. 2), suggesting that GPCRs are not involved in the volatile-induced [Ca2+]c transients.
Although a broad spectrum of volatiles can promote [Ca2+]c transients in both plant and animal cells, the mechanisms are distinct. In the case of olfactory nerves, G-proteins stimulate adenylate cyclase resulting in the production of cyclic AMP, which activates cyclic nucleotide-gated channels in the plasma membrane. These then allow Ca2+-influx from extracellular spaces. In contrast, our pharmacological assays indicated that the volatiles-promoted [Ca2+]c transients in Arabidopsis leaves were mainly the result of Ca2+-release from intracellular Ca2+-stores. Recently, Bonaventure et al.27 reported that a gain of function allele of AtTPC1, which encodes a slow-activating vacuolar (SV) channel in Arabidopsis thaliana, activated oxylipin biogenesis after leaf wounding. In fou2 (fatty acid oxygenation upregulated 2) mutants that carry a point mutation in the extracellular loop of AtTPC1, the current-voltage relationship in SV channel activity was negatively shifted and the time for its activation was faster in comparison to wild type AtTPC1. These results strongly suggest that Ca2+-release from the vacuole might be involved in wound-induced volatile synthesis. Expression of defence genes in response to fungal infection was enhanced in fou2 mutants and attenuated in tpc1-2, a knockout line of AtTPC1.28 These results suggest that TPC1 channels are involved in the defence responses as described previously.29,30
In tpc1-2 mutants, the expression levels of LOX1 (lipoxygenase 1) in both infected and not infected leaves were comparable to those in wild type plants, whereas fou2 mutants showed elevated expression of LOX1 in both situations. This suggests that uncharacterized Ca2+-permeable channel(s), rather than TPC1, may coordinate pathogen-induced induction of LOX1 expression, and that the slight increase in the [Ca2+]c level near the vacuolar membrane in fou2 mutants at the steady state might non-specifically activate the signalling components involved in regulation of LOX1 activity and oxylipin biogenesis.
The [Ca2+]c transient that occurred in response to β-ocimene was mainly mediated by Ca2+-release from intracellular Ca2+-stores. Preconditioning with staurosporine, an inhibitor of Ser/Thr protein kinases, increased the amplitude of the transient. These results may indicate that the activities of TPC1 channels and/or other Ca2+-permeable channels that mediate volatile-induced Ca2+-release are negatively regulated by phosphorylation. Identification of the primary targets for volatiles, including Ca2+-permeable channel(s) that coordinate intracellular Ca2+-release, and the underlying signalling components will be required to elucidate the mechanisms of interplant communication involving plant volatiles. A further understanding of these mechanisms may contribute to the development of ecological approaches to improvement of plant resistance against herbivore and/or pathogen attack.
Materials and Methods
Plant materials.
Seedlings of Arabidopsis thaliana L. ecotype Wassilewskija that express apoaequorin specifically in cytosol31 (AQ) were used to monitor the cytosolic free Ca2+ concentration. A line of gpa1-2 (T-DNA insertion mutant of the α subunit of the heterotrimetric G protein of A. thaliana),32 expressing apoae-quorin (gpa1-2 AQ) was made by performing a cross between gpa1-2 plants and AQ plants. These seedlings were grown on plates containing Murashige-Skoog salt, 0.5% gellan gum, 1% sucrose, 0.01% myo-inositol and 4.6 mM Mes-KOH (pH 5.7) at about 25°C, under a long day conditions (16 hours of daylight and 8 hours of darkness) with 100 µmol m−2s from white light fluorescent tubes. Leaves from two-week-old seedlings were used for experiments.
Chemicals.
Chemically synthesized coelenterazine was a generous gift from Prof. M. Isobe and M. Kuse (Nagoya University). β-ocimene was a generous gift from Mr. K. Suzuki (International flavors & fragrances Inc., Japan). The other chemicals were obtained from Sigma (St. Louis, MO, USA) unless specified otherwise.
Aequorin reconstitution, [Ca2+]c monitoring and volatile exposure.
Excised leaves were immersed in liquid MS medium including 2.5 µM of chemically synthesized coelenterazine,33 and incubated overnight in the dark in order to reconstitute the aequorin. Four leaves from each plant were placed in a Petri dish (φ35 mm dish, and a volume of 9 mL; AGC Techno Glass, Funabashi, Japan), and settled in the chamber of a luminometer (Lumi-counter 2500, Microtech Nichion Co., Funabashi, Japan) in order to measure the luminescence emitted from the leaves. The data were stored on a compatible computer by using luminescence curve-analyzing software (Microtech Nichion). Volatile compounds were dissolved in pure dichloromethane and 10 µl of cold solutions were impregnated into a piece of cotton wool. After evaporation of the solvent, we enclosed a piece of cotton in the Petri dish with Arabidopsis leaves for [Ca2+]c monitoring. (E)-2-hexenal, (E)-2-hexen-1-ol, (+)-α-pinene, (-)-α-pinene and (-)-β-pinene were packed into a Petri dish at 2.5 µmole. β-ocimene, β-myrcene, (E)-DMNT (synthesized in the laboratory) and bornyl acetate were packed into a Petri dish at 0.5 µmole. (Described concentrations of volatiles are estimated values when all of the compounds are evaporated in a petri dish.) The leaves were transferred from MS medium to an experimental Petri dish 15–20 min prior to the experiments. All of the recordings were made at room temperature (25–28°C). The obtained data were processed and analyzed by Excel 2004 for Mac (Microsoft Co.). For the statistical analysis of the data, the luminescence ratio (L/Lbasal) was calculated by dividing the luminescence intensities of aequorin luminescence (L) with the steady luminescence intensity before fumigating volatiles (Lbasal), as described previously.34,35
Monitoring of superoxide radical production.
A superoxide (O2−)-specific chemi-luminescent reagent, 2-methyl-6-phenyl-3,7-dihydrolimidazo[1,2-a]pyrazin-3-one (CLA) was purchased from Tokyo Kasei (Tokyo, Japan). To monitor the production of superoxide radical in the medium, CLA was dissolved in MS medium at 0.1 mM. CLA-containing medium (0.1 ml) was spotted in the Petri dish to immerse the leaves, and then the cotton containing volatiles was placed in the dish. In cell free experiments, CLA-containing medium alone (0.1 ml × 4 times) was spotted into the Petri dish. The luminescence of CLA was measured and analyzed as described above.
Pharmacological assay.
The adenylyl cyclase inhibitor [2′,5′-dideoxyadenosine], the phospholipase C inhibitor [U-73122], the Ser/Thr protein kinase inhibitors [staurosporine (Wako Pure Chemical, Osaka, Japan), K252a (Wako)] and the Ser/Thr protein phosphatase inhibitor [calyculine A (Wako)] were prepared in DMSO to give 0.1 M, 2.5 mM, 1mM, 1mM and 0.1mM stock solutions, respectively. The inhibitor of phospholipase C [neomycin; Sigma], the Ca2+ chelator [1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA; Dojindo, Kumamoto, Japan)], the scavenger of reactive oxygen [ascorbic acid], the superoxide radical scavenger [1,2-Dihydroxy-3,5-benzenedisulfonic acid, disodium salt, monohydrate(Tiron; Dojindo)], and a potential endomembrane Ca2+-permeable channel inhibitor [Ruthenium Red (RuR; Sigma)], were prepared in distilled water at adequate concentrations. Each drug was added to the liquid MS medium containing coelenterazine in a Petri dish and leaves were incubated in the medium for 2–3 hours. If the corresponding stock solutions were prepared with DMSO, the control leaves were also immersed in the MS medium containing DMSO at the same concentration and used as controls.
Acknowledgements
We are grateful to the late Prof. S. Muto (Nagoya University, Japan) for general discussion regarding the present study and for sharing the Lumicounter2500, Mr. K. Suzuki (International flavors & fragrances Inc., Japan) for the generous gift of β-ocimene and Prof. M. Isobe and Dr. Kuse (Nagoya University, Japan) for the generous gift of chemically synthesized coelenterazine. We are also grateful to Prof. A. Trewavas FRS, FRSE (University of Edinburgh, UK) for the permission to use the pMAQ2 binary vector. This work was supported in part by a grant for Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency to T.A. and J.T.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/8275
References
- 1.Pichersky E, Gershenzon J. The formation and function of plant volatiles: Perfumes for pollinator attraction and difense. Curr Opin Plant Biol. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 2.Takabayashi J, Dicke M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996;1:109–113. [Google Scholar]
- 3.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 2000;406:512–515. doi: 10.1038/35020072. [DOI] [PubMed] [Google Scholar]
- 4.Luttrell LM. Reviews in molecular biology and biotechnology: transmembrane signaling by G protein-coupled receptors. Mol Biotechnol. 2008;39:239–264. doi: 10.1007/s12033-008-9031-1. [DOI] [PubMed] [Google Scholar]
- 5.Plakidou-Dymock S, Dymock D, Hooly R. A higher plant seven-transmembrane receptor that influences sensitivity to cytokinins. Curr Biol. 1998;8:315–324. doi: 10.1016/s0960-9822(98)70131-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen JG, Pandey S, Huang J, Alonso JM, Ecker JR, Assmann SM, Jones AM. GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol. 2004;135:1–9. doi: 10.1104/pp.104.038992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, et al. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Yanofsky MF, Meyerowitz EM. Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA. 1990;87:3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H. GTP-binding proteins in plants: new members of an old family. Plant Mol Biol. 1994;26:1611–1636. doi: 10.1007/BF00016493. [DOI] [PubMed] [Google Scholar]
- 10.Weiss CA, Garnaat CW, Mukai K, Hu Y, Ma H. Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1) Proc Natl Acad Sci USA. 1994;91:9554–9558. doi: 10.1073/pnas.91.20.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason MG, Botella JR. Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein gamma-subunit cDNA. Proc Natl Acad Sci USA. 2000;97:14784–14788. doi: 10.1073/pnas.97.26.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason MG, Botella JR. Isolation of a novel G-protein gamma-subunit from Arabidopsis thaliana and its interaction with Gbeta. Biochim Biophys Acta. 2001;1520:147–153. doi: 10.1016/s0167-4781(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 13.Jones AM, Assmann SM. Plants: the latest model system for G-protein research. EMBO Rep. 2004;5:572–578. doi: 10.1038/sj.embor.7400174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey S, Assmann SM. The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell. 2004;16:1616–1632. doi: 10.1105/tpc.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana. Plant Cell Physiol. 2005;46:1093–1102. doi: 10.1093/pcp/pci122. [DOI] [PubMed] [Google Scholar]
- 16.Alméras E, Stolz S, Vollenweider S, Reymond P, Méne-Saffrané L, Farmer EE. Reactive electrophile species active defence gene expression in Arabidopsis. Plant J. 2003;34:205–216. doi: 10.1046/j.1365-313x.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 17.Feng Q, Kumagai T, Torii Y, Nakamura Y, Osawa T, Uchida K. Anticarcinogenic antioxidants as inhibitors against intracellular oxidative stress. Free Radic Res. 2001;35:779–788. doi: 10.1080/10715760100301281. [DOI] [PubMed] [Google Scholar]
- 18.Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 19.Foreman J, Demidchik V, Bothwell JHF, Mylona H, Torres MA, Linstead P, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 20.Kawano T, Sahashi N, Uozumi N, Muto S. Involvement of apoplastic peroxidases in the chitosaccharide-induced immediate oxidative burst and cytosolic Ca2+ increase in tobacco suspension culture. Plant Peroxid Newslett. 2000;14:117–124. [Google Scholar]
- 21.Pottosin II, Oxana RD, Muñiz J. Cooperative block of the plant endomembrane ion channel by ruthenium red. Biophys J. 1999;77:1973–1979. doi: 10.1016/S0006-3495(99)77038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, et al. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance aganst both herbivores and pathogens. Proc Natl Acad Sci USA. 2006;103:16672–16676. doi: 10.1073/pnas.0607780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirabella R, Rauwerda H, Struys EA, Jakobs C, Triantaphylidès C, Haring MA, et al. The Arabidopsis her1 mutant implicates GABA in E-2-hexenal responsiveness. Plant J. 2008;53:197–213. doi: 10.1111/j.1365-313X.2007.03323.x. [DOI] [PubMed] [Google Scholar]
- 24.Hubert J, Münzbergová Z, Santino A. Plant volatile aldehydes as natural insecticides against stored-product beetles. Pest Manag Sci. 2008;64:57–64. doi: 10.1002/ps.1471. [DOI] [PubMed] [Google Scholar]
- 25.Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular generation of superoxide followed by an increase in cytosolic calcium ion in tobacco suspension culture: The earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- 26.Kawano T, Muto S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco suspension culture. J Exp Bot. 2000;51:685–693. [PubMed] [Google Scholar]
- 27.Bonaventure G, Gfeller A, Proebsting WM, Hoerstensteiner S, Chételat A, Martinoia E, et al. A gain-of-function allele of TPC1 activates oxylipin biogenesis after leaf wounding in Arabidopsis. Plant J. 2007;49:889–898. doi: 10.1111/j.1365-313X.2006.03002.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonaventure G, Gfeller A, Rodríguez VM, Armand F, Farmer EE. The fou-2 gain-of-function allele and the wild-type allele of the Two Pore Channel 1 contribute to different extents or by different mechanism to defense gene expression in Arabidopsis. Plant Cell Physiol. 2007b;48:1775–1789. doi: 10.1093/pcp/pcm151. [DOI] [PubMed] [Google Scholar]
- 29.Kadota Y, Furuichi T, Ogasawara Y, Goh T, Higashi K, Muto S, et al. Identification of putative voltage dependent Ca2+ permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 Cells. Biochem Biophy Res Commun. 2004;317:823–830. doi: 10.1016/j.bbrc.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 30.Kurusu T, Yagala T, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005;42:798–809. doi: 10.1111/j.1365-313X.2005.02415.x. [DOI] [PubMed] [Google Scholar]
- 31.Furuichi T, Mori IC, Takahashi K, Muto S. Sugar-induced increase in cytosolic Ca2+ in Arabidopsis thaliana whole plants. Plant Cell Physiol. 2001;42:1149–1155. doi: 10.1093/pcp/pce150. [DOI] [PubMed] [Google Scholar]
- 32.Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM. Modulation of Cell Proliferation by Heterotrimetric G protein in Arabidopsis. Science. 2001;292:2066–2069. doi: 10.1126/science.1059040. [DOI] [PubMed] [Google Scholar]
- 33.Isobe M, Takahashi H, Usami K, Hattori M, Nishigohri Y. Bioluminescence mechanism on new system. Pure Appl Chem. 1994;66:765–772. [Google Scholar]
- 34.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2007;146:505–514. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Critical consideration on the relationship between auxin transport and calcium transients in gravity perception of Arabidopsis seedlings. Plant Signal Behav. 2008;3:521–524. doi: 10.4161/psb.3.8.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]