Abstract
The second messenger, 3′,5′-cyclic monophosphate (cGMP), is a critical component of many different processes in plants while guanylyl cyclases that catalyse the formation of cGMP from GTP have remained somewhat elusive in higher plants. Consequently, two major aims are the discovery of novel GCs and the identification of cGMP mediated processes. Recently, we have reported temporal signatures of ozone (O3)-induced hydrogen peroxide (H2O2) and nitric oxide (NO) generation, their effect on cGMP generation, and consequent transcriptional changes of genes diagnostic for stress responses in tobacco. We demonstrated that O3 and NO induced early transcriptional activation of the scavenger encoding proteins, alternative oxidase (AOX1a), glutathione peroxidase (GPX) and the induction of ethylene production through aminocyclopropancarboxylic acid synthase (ACS2) are cGMP-independent. By contrast, the early response of the phenylalanine ammonia lyase gene (PALa) and the late response of the gene encoding the pathogenesis-related protein (PR1a) show critical dependence on cGMP. Here we show differential cGMP responses to virulent and avirulent Pseudomonas syringae strains and propose that host-pathogen recognition and/or down-stream processes are transduced by complex cGMP signatures. This is in accordance with the identification of a growing number of multi-domain molecules in Arabidopsis that are reported to contain putative functional GC catalytic centers.
Key words: plant stress; ozone; nitric oxide; salicylic acid; ethylene; 3′,5′-cyclic monophosphate (cGMP)
Guanylyl Cyclases and cGMP in Higher Plants
It is emerging that cyclic nucleotides and hence the cyclic nucleotide generating enzymes such as adenylyl and guanylyl cyclases (GCs)1 have key roles in many and diverse biological processes.2–5 Here we shall mainly focus on GCs and cGMP. The latter is critically implicated in responses to both abiotic and biotic stress responses,6–8 the gating of channels,9,10 plant hormone signal transduction,11,12 nitric oxide (NO)-dependent signaling13–16 as well as the regulation of transcription.17 While there are currently only two experimentally confirmed GCs in higher plants,18,19 this number has been predicted to significantly increase based on the presence of putative GC catalytic centers in many Arabidopsis thaliana proteins20 and the number (>100) of annotated nucleotide cyclases in Chlamydomonas reinhardtii.4,20 We hypothesize that many more processes that are also critically dependent on the second messenger cGMP remain to be discovered and that catalytic domains capable of generating cGMP from GTP are part of a growing family of highly diverse multi-domain enzymes.
Cyclic GMP in Plant Stress Responses
It has been previously been demonstrated that cGMP levels in Arabidopsis thaliana seedlings increase very rapidly (onset: ≤5 seconds), time dependently and, importantly, to different degrees in response to salt and osmotic stress, and that salt stress activates two distinct cGMP signalling pathways.7 The osmotic pathway is independent of the second messenger [Ca2+]c while the ionic response pathway, triggered by high NaCl, is [Ca2+]c-dependent. These findings are an indication that cGMP plays a complex role in stress responses that cannot be accounted for by simple “on/off” mechanisms. Contrary to the rapid changes in response to osmoticum dependent and ionic stress, responses to NO6 and gravitropic stimulus appear to be much slower21 and to the best of our knowledge, to-date there is no indication to suggest a link between the causing stimulus and the amplitude of resulting cGMP increases in biotic interactions between pathogens and their plant hosts.
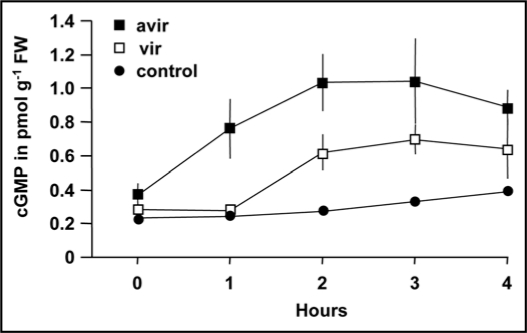
We have measured cGMP accumulation in Arabidopsis thaliana leaves following inoculation with virulent (DC3000) and avirulent (AvirB) Pseudomonas syringae strains (Fig. 1) and observed that one hour post inoculation, significant increases are registered in response to the avirulent strain only. The induced elevation persisted over the entire duration of the experiment. The virulent strain also caused an increase in cGMP level, but the onset was delayed and the increase remained smaller than that caused by the avirulent strain.
Figure 1.
Time-course of cGMP generation in Arabidopsis thaliana leaves in response to virulent and avirulent Pseudomonas syringae strains. Both strains induced distinct time dependent increase in cGMP levels that peaked at ≥2 hours with the avirulent strain causing a more rapid response. Leaves of four week old plants were pressure inoculated with 106 cfu/cm2 bacterial cells suspended in 10 mM MgCl2 solution. Whole leaf tissue was removed at the indicated time points and frozen immediately in liquid nitrogen. Cyclic GMP was extracted from N2 snap-frozen ground leaves using the trichloroacetic acid extraction procedure as detailed in the manual to the Amersham cGMP assay kit. Cyclic GMP concentrations were subsequently measured with the Amersham Biosciences cGMP[125I] assay system kit (code RPA 525, Amersham Biosciences, Little Chalfont, UK) using the acetylation protocol. Error bar values represent the mean (+/- SEM; n = 3) and the data are representative of two independent experiments.
The earlier induction of cGMP accumulation in response to inoculation with the avirulent strain is consistent with the immediate recognition of specific pathogen avirulent (avr) gene encoded molecules by resistance (R) genes in plants. The specific interaction of pathogen derived avr genes and corresponding R genes in plants triggers activation of plant defense responses.22 This process involves production of a range of signalling molecules such as reactive oxygen species, NO, jasmonic acid (JA), ethylene and salicylic acid (SA), and transcriptional activation of defense-related genes.23 The response is often accompanied by a form of programmed host cell death referred to as hypersensitive response (HR)24 that in turn is characterised by the formation of necrotic lesions at the infection site that can inhibit the spread of biotrophic pathogens.25
In the absence of specific pathogen recognition by plant R gene products (as is the case for the virulent DC3000 strain), pathogens are able to grow and spread. However, virulent pathogens can still induce activation of the plant defense system to a certain level (basal defense) that is not dissimilar to that activated by avirulent pathogens.26 To explain this overlap it has been hypothesised that R proteins may not recognize pathogen virulence (avirulence) molecules directly, but rather detect the cellular consequence of pathogen infection.22 Nevertheless, the earlier induction of cGMP levels by avirulent infection is consistent with the earlier detection of the pathogen and activation of defence responses. The early induction is also consistent with a study in Arabidopsis suspension culture cells that showed that an avirulent race of Pseudomonas syringae (race m6) can cause a significant increase in NO concentrations after 30 minutes while the virulent race m4 strain failed to increase cellular NO levels even after six hours.27 These results are thus consistent with avirulent pathogens inducing cGMP synthesis via NO-dependent pathways and virulent cGMP induction occurring through NO-independent pathways.
In tobacco, cGMP has been implicated in NO-dependent defence responses and being required for induction of expression of defense-related genes, pathogenesis-related 1 gene (PR-1) and the phenylalanine ammonia lyase gene (PAL).6,28 Additionally, in Arabidopsis cell cultures cGMP has been shown to be required for NO induced cell death in response to challenge by avirulent bacterial pathogens.27 While these studies used GC inhibitors and a cell-permeable cGMP analogue (8Br-cGMP) to help elucidate the role of cGMP in defence responses, to the best of our knowledge, this is the first report to show a direct pathogen dependent increases in tissue cGMP levels in planta.
Outlook
Research on animal GCs suggests that a number of different GC domain combinations and architectures exist20 and that GCs can be divided in two groups, the soluble GCs and the particulate GCs. The former typically have a highly conserved NO binding site29–31 and consequently play a key role in NO sensing and signal transduction; the latter typically serve as transmembrane receptors where the cytosolic GC domain is located next to a kinase domain. One of the best-studied examples of the second type are the Atrial Natriuretic Peptide (ANP) receptors. To-date there are two experimentally confirmed GCs reported in higher plants, the first, while soluble, is not NO sensitive.18 In this molecule the GC domain combines with a novel cysteine protease-like domain,32 a combination that is also found in Chlamydomonas reinhardtii. The second19 is the particulate brassinosteroid receptor (AtBRI1) and contains a leucine rich ligand binding domain, a transmembrane domain and intracellular GC and kinase domains reminiscent of the ANP receptor.33 Incidentally, the wall-associated receptor kinase-like 10 precursor (At1g79680) that we have identified as a candidate GC18 has a domain architecture not dissimilar to AtBRI1. But in this case, in place of the leucine rich ligand binding domain is the extracellular wall-binding anchor. It is noteworthy that At1g79680 is also differentially expressed in response to avirulent and virulent Pseudomonas syringae strains (data extracted from “Genevestigator”34) and it is conceivable that the molecule has a critical role in cGMP-dependent signalling in response to biotic challenges.
Finally, the link between the reported NO increases and the consequent increase in cellular cGMP levels in plants remain to be discovered. In animals, soluble GCs can function as heme sensors that selectively bind NO and do so by the highly conserved H-NOX family (heme nitric oxide/oxygen-binding domain).30,31 The core H-NOX signature is: Hx{12}Px{14,16} YxSxR, where “x” stands for any amino acid and the number in the curly brackets determine the length of the gap. Interestingly, this motif is present in four Arabidopsis thaliana proteins that are potentially capable of binding NO and might have GC activity.
In summary, in the near future we are likely to see the identification and characterisation of an increasing number of GCs in higher plants as well as reports of biological responses, processes and pathways35 that depend critically on highly specific temporal, spatial and stimulus specific cGMP signatures.
Acknowledgements
This project was supported by at South African National Research Foundation grant to C.G.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8066
References
- 1.Wood DW, Setubal JC, Kaul R, Monks DE, Kitajima JP, Okura VK, et al. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science. 2001;294:2317–2323. doi: 10.1126/science.1066804. [DOI] [PubMed] [Google Scholar]
- 2.Moutinho A, Hussey PJ, Trewavas AJ, Malho R. cAMP acts as a second messenger in pollen tube growth and reorientation. Proc Natl Acad Sci USA. 2001;98:10481–10486. doi: 10.1073/pnas.171104598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newton RP, Smith CJ. Cyclic nucleotides. Phytochemistry. 2004;65:2423–2437. doi: 10.1016/j.phytochem.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Schaap P. Guanylyl cyclases across the tree of life. Front Biosci. 2005;10:1485–1498. doi: 10.2741/1633. [DOI] [PubMed] [Google Scholar]
- 5.Meier S, Gehring C. Emerging roles in plant biotechnology for the second messenger cGMP—guanosine 3',5'-cyclic monophosphate. Afr J Biot. 2006;5:1687–1692. [Google Scholar]
- 6.Durner J, Wendehenne D, Klessig D. Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donaldson L, Ludidi N, Knight MR, Gehring C, Denby K. Salt and osmotic stress cause rapid increases in Arabidopsis thaliana cGMP levels. FEBS Lett. 2004;569:317–320. doi: 10.1016/j.febslet.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Gottig N, Garavaglia BS, Daurelio LD, Valentine A, Gehring C, Orellano EG, et al. Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proc Natl Acad Sci USA. 2008;105:18631–18636. doi: 10.1073/pnas.0810107105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshi T. Regulation of voltage dependence of the KAT1 channel by intracellular factors. J Gen Physiol. 1995;105:309–328. doi: 10.1085/jgp.105.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng Q, Mercier RW, Yao W, Berkowitz GA. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen JV, Jones RL. cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell. 1996;8:2325–2333. doi: 10.1105/tpc.8.12.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pharmawati M, Maryani MM, Nikolakopoulos T, Gehring CA, Irving HR. Cyclic GMP modulates stomatal opening induced by natriuretic peptides and immunoreactive analogues. Plant Physiol Biochem. 2001;39:385–394. [Google Scholar]
- 13.Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- 14.Prado AM, Porterfield DM, Feijo JA. Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development. 2004;131:2707–2714. doi: 10.1242/dev.01153. [DOI] [PubMed] [Google Scholar]
- 15.Delledonne M. NO news is good news for plants. Curr Opin Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, et al. Nitric oxide, stomatal closure and abiotic stress. J Exp Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 17.Maathuis FJM. cGMP modulates gene transcription and cation transport in Arabidopsis roots. Plant J. 2006;45:700–711. doi: 10.1111/j.1365-313X.2005.02616.x. [DOI] [PubMed] [Google Scholar]
- 18.Ludidi N, Gehring C. Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem. 2003;278:6490–6494. doi: 10.1074/jbc.M210983200. [DOI] [PubMed] [Google Scholar]
- 19.Kwezi L, Meier S, Mungur L, Ruzvidzo O, Irving H, Gehring C. The Arabidopsis thaliana brassinosteroid receptor (AtBRI1) contains a domain that functions as a guanylyl cyclase in vitro. PLoS ONE. 2007;2:449. doi: 10.1371/journal.pone.0000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier S, Seoighe C, Kwezi L, Irving H, Gehring C. Plant nucleotide cyclases: An increasingly complex and growing family. Plant Sig Behav. 2007;2:536–539. doi: 10.4161/psb.2.6.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, Neill SJ, Tang Z, Cai W. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nimchuk Z, Eulgem T, Holt BF, 3rd, Dangl JL. Recognition and response in the plant immune system. Annu Rev Genet. 2003;37:579–609. doi: 10.1146/annurev.genet.37.110801.142628. [DOI] [PubMed] [Google Scholar]
- 23.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 24.Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 25.Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glazebrook J. Genes controlling expression of defense responses in Arabidopsis—2001 status. Curr Opin Plant Biol. 2001;4:301–308. doi: 10.1016/s1369-5266(00)00177-1. [DOI] [PubMed] [Google Scholar]
- 27.Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 2000;24:667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 28.Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, et al. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA. 2000;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Brandish P, Ballou D, Marletta M. A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc Natl Acad Sci USA. 1999;96:14753–14758. doi: 10.1073/pnas.96.26.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boon E, Huang S, Marletta M. A molecular basis for NO selectivity in soluble guanylate cyclase. Nat Chem Biol 2005. 2005;1:53–59. doi: 10.1038/nchembio704. [DOI] [PubMed] [Google Scholar]
- 31.Boon EM, Marletta MA. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr Opin Chem Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Ginalski K, Zemojtel T. ECEPE proteins: a novel family of eukaryotic cysteine proteinases. Trends Biochem Sci. 2004;29:524–526. doi: 10.1016/j.tibs.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin H, Goeddel DV, et al. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;388:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann P, Hennig L, Gruissem W. Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci. 2005;10:407–409. doi: 10.1016/j.tplants.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Suita K, Kiryu T, Sawada M, Mitsui M, Nakagawa M, Kanamaru K, et al. Cyclic GMP acts as a common regulator for the transcriptional activation of the flavonoid biosynthetic pathway in soybean. Planta. 2009;229:403–413. doi: 10.1007/s00425-008-0839-5. [DOI] [PubMed] [Google Scholar]