Abstract
Sensitive detection of α-synuclein (α-syn) pathology is important in the diagnosis of disorders like Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy and in providing better insights into the etiology of these diseases. Several monoclonal antibodies that selectively react with aggregated α-syn in pathological inclusions and reveal extensive and underappreciated α-syn pathology in the brains of diseased patients were previously reported by Duda et al. (Ann Neurol 52:205-210, 2002). We sought to characterize the specificity of some of these antibodies (Syn 505, Syn 506 and Syn 514); using C-terminal and N-terminal truncations of α-syn, all three antibodies were determined to require N-terminal epitopes that minimally comprise amino acids 2-4, but possibly extend to amino acid 12 of α-syn. The selectivity of these antibodies was further assessed using biochemical analysis of human brains and reactivity to altered recombinant α-syn proteins with duplication variants of amino acids 1-12. In addition, by expressing wild-type or a double mutant (E46K/A53T) of α-syn in cultured cells and by comparing their immunoreactivities to another antibody (SNL-4), which has a similar primary epitope, it was determined that Syn 505, Syn 506 and Syn 514 recognize conformational variants of α-syn that is enhanced by the presence of the double mutations. These studies indicate that antibodies Syn 505, Syn 506 and Syn 514 preferentially recognize N-terminal epitopes in complex conformations, consistent with the dramatic conformational change associated with the polymerization of α-synuclein into amyloid fibrils that form pathological inclusions.
Keywords: α-Synuclein, Antibodies, Fibrillization, Lewy bodies, Parkinson's disease
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by cardinal features of resting tremor, brady-kinesia, postural instability, and muscle rigidity, as well as numerous other non-motor symptoms that can include cognitive impairment, psychosis and autonomic impairment [28, 46]. Pathological analysis of PD brains reveals intracytoplasmic, perikaryal inclusions, known as Lewy bodies (LBs), and similar inclusions in neuronal processes, termed Lewy neurites (LNs) [20, 23, 33]. Amyloidogenic 10-15 nm-wide fibrils, comprised of the protein α-synuclein (α-syn), are the major component of LBs and LNs [20, 23, 33, 52]. Several genetic mutations in α-syn, including missense mutations and short chromosomal duplications and triplications that include the α-syn gene, have been identified in familial forms of PD [2, 13, 31, 41, 47, 54] and serve as compelling evidence for the role of α-syn in the pathobiology of PD. Furthermore, α-syn is the major component of pathological inclusions in other neurodegenerative diseases, including dementia with Lewy bodies (DLB), the Lewy body variant of Alzheimer's disease (LBVAD), and multiple system atrophy (MSA), which are collectively termed α-synucleinopathies [11, 14, 23, 35, 49, 52, 53]. Therefore, the ability to detect α-syn preferentially in pathological inclusions is important to both characterization and post-mortem diagnosis of several disorders.
α-Syn is a highly-expressed 140 amino acid neuronal protein that is normally soluble and localized to neuronal synaptic terminals [20, 23, 33, 45]. The abundance of α-syn in the neuropil can overshadow the presence of smaller pathological aggregates. We previously reported on monoclonal antibodies that could preferentially detect pathological α-syn, and these antibodies revealed extensive and underappreciated α-syn inclusions in the striatum of PD and DLB patients [12]. Here, some of these antibodies are further characterized, and it is demonstrated that their abilities to preferentially detect pathological inclusions are likely a product of preferential recognition of α-syn in pathological conformations.
Materials and methods
Antibody generation
Syn 505, Syn 506 and Syn 514 are mouse monoclonal antibodies that were previously generated using recombinant human α-syn oxidized/nitrated in vitro [12]. Syn 208 is mouse monoclonal antibody that was raised against full-length recombinant human α-syn and recognizes an epitope between amino acid residues 89-110 in human α-syn [19, 53]. SNL-4 is a rabbit polyclonal antibody raised against a synthetic peptide corresponding to residues 2-12 in human α-syn [19]. Syn211 is a mouse monoclonal antibody specific for human α-syn, requiring amino acids 121-125 [19].
Tissue processing and immunohistochemistry
The harvesting, fixation and further processing of the tissue specimens were conducted as previously described [11, 43]. Immunohistochemistry was performed using monoclonal antibodies followed by the avidin-biotin complex (ABC) detection system (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine using previous published procedures [11].
Biochemical fractionation of human brain tissue
Amygdala (0.6 g) were homogenized in 10 ml/g of high-salt (HS) buffer (50 mM Tris, pH 7.4, 750 mM NaCl, 10 mM EDTA supplemented with protease inhibitors) and samples were centrifuged at 100,000g for 30 min. The HS-insoluble pellets were extracted by homogenization with HS/T buffer (HS buffer containing 1% Triton X-100) and centrifuged at 100,000g for 30 min. The pellets were re-extracted in HS buffer/1 M sucrose, layered on a 1.2/1.5/2.2 M discontinuous sucrose gradient in HS buffer and centrifuged at 200,000g for 2 h. The resulting layers and inter-phases were collected separately. Preliminary experiments demonstrated that the majority of HS/T insoluble, aggregated α-syn was present in the 1.5/2.2 M interphase. These fractions were diluted 10-fold in HS buffer and sedimented at 100,000g for 30 min. The pellets were extracted with 200 μl SDS-sample buffer (10 mM Tris, pH 6.8, 1 mM EDTA, 40 mM DTT, 1% SDS, 10% sucrose) by homogenization, sonication for 2 s and heating to 100°C for 5 min. Five μl of each extract was used for Western blot analysis.
Gel electrophoresis and Western blotting
Proteins on slab gels were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and electrophoretically transferred onto nitrocellulose membranes in buffer containing 25 mM Tris, 190 mM glycine and 10% methanol. The membranes were blocked with Tris buffered saline (50 mM Tris, pH 7.6, 150 mM NaCl) containing 5% dry milk, incubated with primary antibodies followed by a goat anti-mouse antibody (Jackson Immunoresearch Laboratories Inc., West Grove, Pennsylvania) or goat anti-rabbit antibody (Cell Signaling Technology, Danvers, MA) conjugated to horseradish peroxidase. The immunocomplexes were detected with enhanced chemiluminescence reagents (NEN, Boston, MA), followed by exposure onto X-ray film.
Expression and purification of synuclein proteins
The bacterial-expression vector pRK172 with the WT or A53T human α-syn cDNA, human β-syn cDNA, human γ-syn cDNA, murine α-syn cDNA or canary (zebra finch) α-syn cDNA cloned into the Nde I and Hind III restriction sites was previously published [16, 21, 22]. The vector expressing the double mutant E46K/A53T was generated by using complementary sets of synthetic single-stranded DNA containing the mutant sequence for E46K and the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Specific stop codons were created in the pRK172 plasmid expressing WT α-syn using the QuikChange site-directed mutagenesis kit (Stratagene) to generate plasmids expressing carboxy-truncated proteins of α-syn. These proteins were purified as previously described [6, 21, 25]. PCR was performed with human WT α-syn in expression vector pRK172 with forward primer sequences: CAT ATG GAT GTA TTC ATG AAA GGA CTT TCA AAG GCC AAG ATG GAT GTA TTC ATG AAA GGA CTT TCA AAG GCC AAG GAG GGA GTT to produce a duplication of amino acids 1-12 at the amino-terminus; or CAT ATG AAG GCC AAG TCA CTT GGA AAA ATG TTC GTA GAT ATG ATG GAT GTA TTC ATG AAA GGA CTT TCA AA to reproduce a duplication in reverse of amino acids 12-1 at the amino-terminus. Reverse primer utilized was AAG CTT TAG GCT TCA GGT TCG TAG TCT TGA T. PCR products were subcloned into pRK172 with restriction enzymes NdeI and HindIII. All cDNA modifications were confirmed by DNA sequencing as a service provided by DNA Sequencing Facility of the University of Pennsylvania.
Shorter α-syn carboxy-truncated proteins were expressed as glutathione-S-transferase (GST) fusion proteins by cloning the human α-syn cDNA into the Sma I restriction site of the bacterial expression plasmid, pGEX-2T (Amersham Pharmacia Biotech, Piscataway, NJ). Stop codons were created in the α-syn cDNA to cause the specific premature termination of translation. Following expression in BL21 (DE3) bacteria, cell pellets were lysed in phosphate-buffer saline (PBS)/1% Triton X-100 by sonication and bacterial debris were removed by centrifugation. The fusion proteins were purified on glutathione conjugated agarose beads (Sigma, St. Louis, MO) and eluted with free glutathione. Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL) and bovine serum albumin as a standard.
Epitope mapping
The epitopes recognized by each monoclonal antibody were mapped using Western blot analysis where 50 ng of recombinant carboxyl-terminal truncated α-syn proteins or 100 ng of GST/carboxy-terminal truncated α-syn fusion proteins were resolved on 15 and 12% polyacrylamide gels, respectively. Synthetic α-syn peptides (V.M. Keck Biotechnology Resource Center, New Haven, CT) were used for further epitope mapping by enzyme linked immunosorbant assay (ELISA). Each peptide (100 μl at a concentration of 67 μM) was incubated on individual wells of 96-well ELISA plates overnight. After blocking with 5% horse serum in PBS for 2 h, antibodies diluted in PBS/2% horse serum were added to the plates. Following incubation with a goat anti-mouse antibody conjugated to horseradish peroxidase (Jackson Immunoresearch Laboratories Inc., West Grove, PA) followed by washing, the complex was reacted with 3,3′,5,5′-tetramethylbenzidine. The reaction was terminated by adding 10% phosphoric acid and quantified with spectrometric plate reader at a wavelength of 450 nm.
In vitro sedimentation assays
Recombinant human α-syn (A53T and E46K/A53T mutants) was produced in BL21 E. coli and purified to homogeneity as previously described [25]. Samples were diluted to 1 mg/ml in 100 mM Na acetate, pH 7.4 and were subjected to constant agitation for 72 h at 37°C, as previously described [21, 25]. Each experiment was performed with 3-4 independent samples per condition, analyzed concurrently. For sedimentation analysis of fibril formation, samples were sedimented at 100,000g for 20 min. SDS-sample buffer was added to supernatant and pellet and samples were heated to 100°C for 5 min. Each fraction, supernatant (S) and pellet (P), was resolved by SDS-PAGE, gels were stained with Coomassie and following quantification by densitometry with by ImageJ software (NIH). The percentage of protein in pellets was calculated as [P/(P + S)] × 100.
Independent assessment of the rate of fibrillization was also determined by K114 amyloid fluorometry, as previously described [7]. Briefly, a fraction of each sample was incubated with K114 (10 μM) in 100 mM glycine, pH 8.5, and fluorescence signal was measured (λex = 380 nm, λem = 550 nm, cutoff = 530 nm) with SpectraMax Gemini fluorometer and SoftMax Pro software (Molecular Devices, Sunnyvale, CA).
Comparisons were completed by two-way, parametric t tests using GraphPad InStat software (San Diego, CA).
Cell culture and transfection
The mammalian-expressing vectors pcDNA3.1 cloned with either WT or A53T human α-syn were previously described [40]. The construct with the double E46K and A53T mutations was generated as described above for the pRK172 construct. QBI293 cells were maintained using Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. QBI293 cells were plated onto glass coverslips and transfected at approximately 60% confluency using FuGene6 transfection reagent (Roche Applied Sciences, Indianapolis, IN). Transfection procedures were completed as per manufacturer's recommendations with 0.25 μg of cDNA per 6.5-mm well (24-well plate). Cells were maintained for 48 h after transfection prior to fixation.
Immunofluorescence
Immunofluorescence of transfected QBI293 cells were completed as previously described [37]. Cells were fixed at -20°C with 100% MeOH for 20 min, followed by 50% MeOH and 50% acetone for 5 min. Following washes with phosphate buffered saline (PBS), coverslips were blocked with PBS containing 10% goat serum and 5% bovine serum albumin (BSA), and primary antibodies were diluted into blocking solution for 1 h at RT. Antibodies were utilized at the following concentrations: SNL-4 (1:500), Syn 505 (1:1,000), Syn 506 (1:2,000), Syn 514 (1:1,000). After PBS washes, coverslips were incubated with goat anti-mouse secondary conjugated to Alexa 594 and goat anti-rabbit secondary conjugated to Alexa 488. Coverslips were mounted using Vectashield mounting medium (Vector Laboratories).
To quantify immunofluorescence data, pictures were taken of 3-4 fields per experiment, chosen for similar intensity of SNL-4 immunofluorescence and in a blinded fashion for Syn 505, Syn 506, or Syn 514 reactivity. Within each experiment identical exposure times were used across conditions for each antibody. Cells were counted in a blinded fashion followed by statistical analyses by paired t tests using GraphPad software.
Results
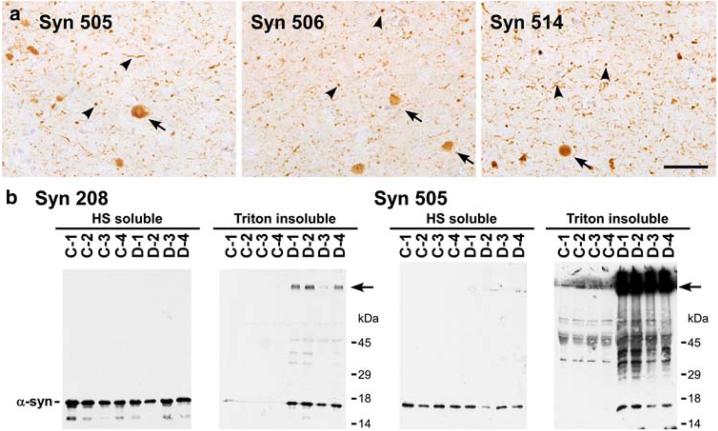
Syn 505, Syn 506, and Syn 514 are mouse monoclonal antibodies that were previously identified for their ability to preferentially immunostain pathological α-syn compared to normal α-syn [12]. The sensitivity of these antibodies uncovered an unprecedented and extensive burden of α-syn pathology in the striatum of LB disorders [12], but they also revealed extensive neuritic and “dot-like” α-syn pathology in other brain areas such as the cortex (Fig. 1a, arrowheads) [12]. These antibodies were raised against recombinant human α-syn that was oxidized/nitrated in vitro with peroxynitrite, but these antibodies were not specific to oxidized α-syn as they recognized nitrated, non-nitrated and recombinant α-syn purified with reducing agents to prevent oxidation [12].
Fig. 1.
Immunohistochemistry and biochemical analysis of DLB brains with monoclonal antibodies that selectively detect pathological α-syn. a Immunostaining with Syn 505, Syn 506 and Syn 514 in the cingulate cortex of a DLB brain detecting LBs (arrows), but far greater neuritic inclusions and smaller “dot-like” aggregates (arrowheads) and relatively weak reactivity for the normal neuropil distribution of α-syn. (Bar scale = 50 μm). b Comparative immunoblotting analysis of biochemical brain samples using antibodies Syn 208 and Syn 505. The tissue from four controls (C1-4) and four DLB patients (D1-4) were fractionated biochemically as described in “Materials and methods.” HS-soluble and Triton-insoluble extracts were resolved on SDS-polyacrylamide gels and Western blots were probed with either Syn 208 or Syn 505. α-Syn was detected in the HS-fraction from all brains using either Syn 208 or Syn 505. Syn 208 detected monomeric α-syn predominantly in the Triton-insoluble extracts from DLB patients. Higher molecular mass α-syn aggregates, some that did not enter the resolving gel (arrow), were also weakly detected. Syn 505 also revealed Tritoninsoluble monomeric α-syn in diseased brains, but, in contrast, the major species detected were higher molecular mass α-syn aggregates that did not enter the resolving gel (arrow)
To begin to understand the relative selectivity of these antibodies, they were used to probe biochemical fractions from control and diseased brains that were processed for immunoblotting, as described in “Material and methods.” Analyses using a conventional anti-α-syn antibody (Syn 208) showed that α-syn is present in the soluble (HS) fractions from control and disease brains as a monomer. α-Syn was present in the detergent insoluble (Triton-insoluble) fractions of diseased brains (Fig. 1b) primarily as a monomer, but also as higher molecular mass species (arrow), some of which did not enter the SDS-polyacrylamide resolving gel. This finding is consistent with many previous studies [1, 8, 17, 26, 53], demonstrating that the presence of detergent insoluble α-syn reflects the presence of α-syn inclusions. The same immunoblotting analyses using Syn 505 also showed the accumulation of α-syn in the detergent-insoluble fraction from diseased brain, but this antibody demonstrated a remarkable ability to detect aggregated α-syn that does not enter the resolving SDS-polyacrylamide gel (arrow), preferentially over that of the monomeric α-syn. Similar analyses with Syn 506 and Syn 514 also revealed enhanced reactivity for high molecular mass species of α-syn (data not shown), but their specificities were not as remarkable as that of Syn 505.
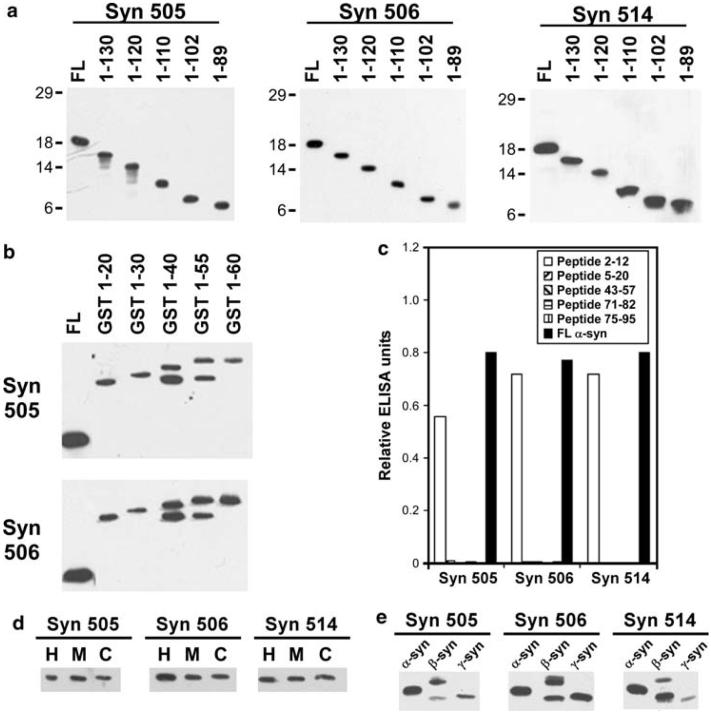
To further understand the specificity of these antibodies, the primary recognition sequence was determined. All three antibodies (Syn 505, Syn 506 and Syn 514) reacted strongly with α-syn carboxy-truncated to 89 residues (Fig. 2a). The epitope for Syn 505 and Syn 506 were further determined using shorted truncations (1-20 to 1-60) of α-syn expressed as an amino-terminally tagged GST fusion protein (Fig. 2b). The immunoreactivity of both of these antibodies for GST-α-syn 1-20 demonstrated that the required epitope is in the first 20 amino acid residue of α-syn. Syn 514 was not reactive with any GST fusion proteins of either full-length or truncated forms of α-syn (data not shown), preventing concurrent analysis of this antibody. However, this suggests that amino-terminal tagging may interfere with the Syn 514 epitope. ELISA mapping with synthetic peptides showed that all three antibodies reacted with a peptide corresponding to residues 2-12 in α-syn, but not with a panel of other peptides corresponding to amino acid segments within the amino-terminal half of the protein, including peptide 5-20 (Fig. 2c). These results indicate that these antibodies minimally require an epitope containing amino acids 2-4; however the epitope may extend up to amino acid 12.
Fig. 2.
Antibodies Syn 505, Syn 506 and Syn 514 recognize N-terminal α-syn epitopes. Western blot analysis using a recombinant carboxy-terminal truncated α-syn proteins or b GST/carboxy-truncated α-syn fusion proteins were performed as described in “Material and methods.” c Peptide mapping using ELISA analysis and synthetic peptides corresponding to various amino acids stretches within α-syn demonstrating that the minimal required epitopes for Syn 505, Syn 506 and Syn 514 comprises residues 2-4, and may extend through amino acid 12. d Immunoblotting analysis for reactivity of Syn 505, Syn 506 and Syn 514 with human (H), murine (M) or canary (C) α-syn. e Immunoblot analysis to assess the reactivity of Syn 505, Syn 506 and Syn 514 with human α-syn, β-syn or γ-syn
Since the amino-terminus of α-syn is highly conserved among various vertebrate species [15], the comparative immunoreactivity of Syn 505, Syn 506 and Syn 514 towards recombinant human, murine and canary α-syn was assessed. These antibodies reacted equally with α-syn from each of these species (Fig. 2d). β-Syn and γ-syn are two other members of the small synuclein family of protein, but unlike α-syn they are not present in pathological inclusions [1, 15, 50-52]. Nevertheless, the amino-termini of β-syn and γ-syn are very similar to that of α-syn [15], and Syn 505, Syn 506 and Syn 514 could recognize α-, β- and γ-syn with similar immunoreactivity (Fig. 2e). This ability to react with all three members of the small synuclein family proteins was consistent with the mapping of the epitopes of these antibodies to the amino-terminus.
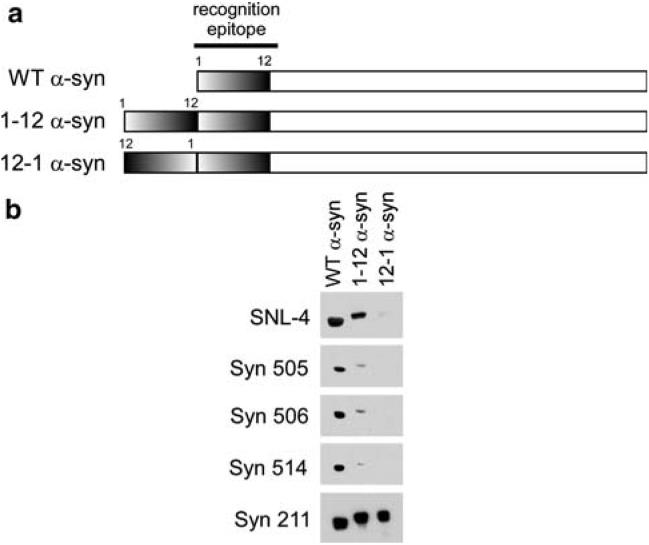
We hypothesized that perhaps the selective recognition of pathological α-syn by these antibodies may be due to unique folding of the protein that may bring two N-terminal amino acid segments into close proximity. To test this hypothesis, two different recombinant proteins were generated: (1) 1-12 α-syn was generated such that the amino acid residues 1-12 was duplicated in tandem at the N-terminus of α-syn, and (2) 12-1 α-syn was created so that the same 12 residues are present before the normal N-terminus but in the reverse orientation prior to the full α-syn sequence (Fig. 3a). The reactivity of the antibodies was analyzed by immunoblotting. Two-times the relative concentration of WT α-syn was resolved on SDS-polyacrylamide gels to account for the possible double-epitope of the 1-12 and 12-1 α-syn proteins (Fig. 3b). Analysis with antibody Syn211, which recognizes an epitope in the C-terminus of α-syn [19], was used to demonstrate similar immunoreactivity for all proteins. Unexpectedly, Syn 514, Syn 505, and Syn 506 demonstrated approximately 10-fold lower immunoreactivity for 1-12 α-syn than WT α-syn and no immunoreactivity (even upon overexposure; data not shown) was noted with 12-1 α-syn. By comparison, the SNL-4 antibody, a rabbit polyclonal antibody raised against a synthetic peptide corresponding to residues 2-12, demonstrated 2:1 immunoreactivity between WT α-syn and 1-12 α-syn and trace immunoreactivity of 12-1 suggesting that this antibody only recognizes residues 1-12 in α-syn when present at the N-terminus. These findings for Syn 505, Syn 506 and Syn 514 suggest that the epitopes recognized by these antibodies are not solely dependent on primary amino acid sequence and does not likely result from a double-epitope of amino termini in close proximity. Additionally, their recognition may be altered by complex conformational changes.
Fig. 3.
Immunoblotting characterization of Syn 505, Syn 506 and Syn 514 using recombinant α-syn protein with altered N-terminus. a Diagram of the N-terminal altered proteins. Protein 1-12 α-syn was generated as described in “Material and methods” so that the amino acid sequence of residues 1-12 was duplicated in tandem at the N-terminus. Protein 12-1 α-syn was generated so that residues 1-12 are present before the normal N-terminal, but in the reverse orientation. b Immuno-blotting analysis with antibodies SNL-4, Syn 505, Syn 506, Syn 514, and Syn 211. Two-times the relative concentration of WT α-syn was resolved on SDS-polyacrylamide gels to account for the possible double-epitope of the 1-12 and 12-1 α-syn protein. Antibody Syn 211, which reacts with an epitope at the C-termimus of α-syn and would not be affected by these N-terminal alterations, was used as a control. Surprisingly, the analysis revealed that Syn 505, 506, and 514 antibodies recognized WT α-syn with approximately 10-fold greater immunore-activity than 1-12 α-syn, and these antibodies could not detect 12-1 α-syn. SNL-4, a polyclonal antibody raised to amino acid sequence 2-12 in α-syn, was used for comparison. SNL-4 reacted similarly with WT α-syn and 1-12 α-syn, but very weakly with 12-1 α-syn
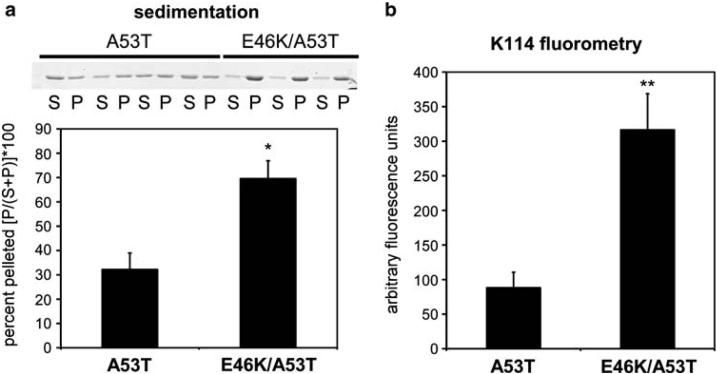
To further assess that these antibodies recognized altered conformational epitopes that may be more highly represented in pathological inclusions, we aimed to create a cellular model of α-syn where the protein should present a greater tendency to generate these conformational alterations. Previous studies have shown that the mutations A53T or E46K in α-syn increase the rate of polymerization of α-syn in vitro relative to WT α-syn [3, 4, 22, 25, 38]. We reasoned that the presence of both these mutations may increase the propensity of α-syn to polymerize even more quickly and may yield a protein that has a greater tendency to generate the conformational epitopes recognized by these antibodies. Polymerization assays were performed in vitro, comparing the double mutation (E46K/A53T) α-syn against A53T α-syn. Sedimentation assays and K114 amyloid fluorometry demonstrated that E46K/A53T α-syn had a greater propensity to polymerize relative to A53T α-syn (Fig. 4). Negative-staining electron microscopic analysis of E46K/A53T α-syn revealed that this protein assembled into 10-15 nm fibrils similar to A53T α-syn (data not shown).
Fig. 4.
Analyses of the polymerization of A53T and E46K/A53T α-syn mutations. Recombinant A53T and E46K/A53T α-syn were incubated at 1 mg/ml for 72 h, as described in “Materials and methods.” a Representative Coomassie blue stained SDS-polyacrylamide showing the proteins in the soluble fractions (S) or in the pellets (P) following sedimentation analysis, and quantitative summary (* P = 0.003, n =7). b K114 amyloid fluorometry analyses (** P = 0.001, n = 7) demonstrating the increased propensity of E46K/A53T α-syn to polymerize compared to A53T α-syn. Data represent averages ± S.E.M
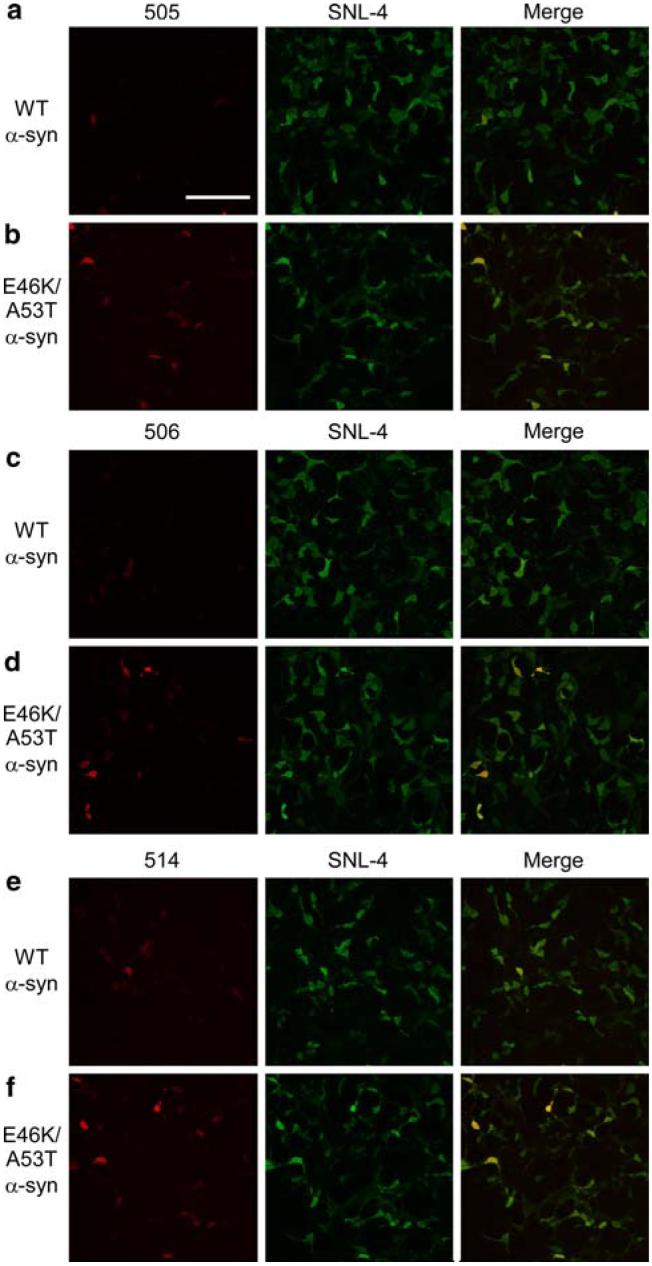
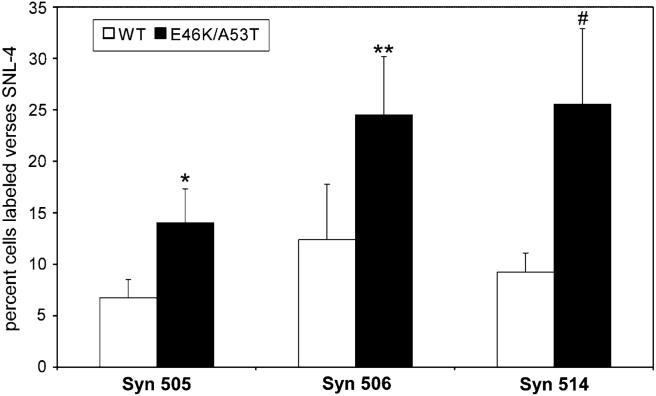
Taking advantage of the increased fibrillization rate of E46K/A53T α-syn, QBI293 cells were transiently transfected with either WT α-syn or E46K/A53T mutant α-syn. After 48 h in culture, cells expressing high levels of α-syn demonstrated cytoplasmic distribution and no inclusionlike structures were noted for any condition (see below; data not shown). Moreover, biochemical extraction revealed the absence of Triton-insoluble α-syn in either the WT or E46K/A53T transfected cells suggesting that insoluble aggregations of α-syn were not formed in these cells (data not shown). Cells were analyzed by immunostaining with SNL-4 and either Syn 505, Syn 506 or Syn 514 (Fig. 5). The ability of SNL-4 to detect all transfected cells was confirmed by double-immunofluorescence with Syn 211 (data not shown). Remarkably, only a minority of cells stained with SNL-4 was positive for Syn 505, Syn 506 or Syn 514, but the percentage of cells double-labeled was significantly increased in cells expressing E46K/A53T α-syn compared to WT α-syn (Figs. 5, 6). These data further support the notion that Syn 505, Syn 506, and Syn 514 recognize structural variants that can be present at low levels in non-aggregated soluble protein and the abundance of these conformations are enhanced by the presence of mutations in α-syn that increase the rate of fibrillization.
Fig. 5.
Double-immunofluorescence analysis of QBI293 cells transfected with WT or E46K/A53T α-syn. Representative fields of QBI293 cells transfected with WT α-syn (a, c, d) or E46K/A53T α-syn (b, d, f). Cells were immunostained with either Syn 505 (a, b), Syn 506 (c, d), or Syn 514 (e, f) (each in red, left) and with SNL-4 (green, center). Increased single-label immunofluorescence (left panels) and double-label immunofluorescence (merge, right panels) with Syn 505, Syn 506, and Syn 514 were noted in E46K/A53T α-syn over WT α-syn transfected cells. Representative images were chosen from a single experiment. (Bar scale = 200 μm)
Fig. 6.
Summary of double-immunofluorescence analyses of QBI293 cells transfected with WT or E46K/A53T α-syn. Percent of SNL-4 labeled cells that were co-labeled with Syn 505, 506, or 514. Data represent averages ± S.E.M. Comparisons demonstrate increased double-labeling in E46K/A53T α-syn transfected cells. (* P = 0.02, n = 5; ** P < 0.0001, n =4; # P = 0.05, n =6)
Discussion
In these studies, the properties of antibodies that selectively detect aggregated α-syn in pathological inclusions [12] (Fig. 1) were characterized. All three antibodies, Syn 505, Syn 506 and Syn 514, have N-terminal epitopes that minimally require amino acids residues 2-4 in α-syn; however, the sequence recognized may extend further up to residue 12 (Fig. 2). The selectivity of these antibodies to detect aggregated α-syn cannot be solely due to their reactivity to the N-terminus, since SNL-4, an antibody that also reacts with the extreme N-terminus, does not share this selectivity [19]. The epitope for Syn 514 may require a free amino-terminus as revealed by the paucity of reactivity with GST-α-syn, while Syn 505 and Syn 506 do not necessarily require a free amino-terminus. Similarly, weak immunoreactivity to the protein 1-12 α-syn demonstrates that the epitopes required for these antibodies are not solely dependent on primary amino acid sequence. Therefore, complex conformational changes may be responsible for the observed antibody recognition patterns.
The specificity of these antibodies is further supported by comparing the immunofluorescent staining of cultured cells expressing WT or E46K/A53T α-syn. Antibodies Syn 505, Syn 506 and Syn 514 selectively reacted with a subpopulation of cells that are reactive with SNL-4. Further, these antibodies detected a significantly greater percentage of cells that express E46K/A53T α-syn compared to cells expressing WT α-syn. Although E46K/A53T α-syn has a greater propensity to fibrillize in vitro, the expression of E46K/A53T α-syn did not form visible or Triton-insoluble aggregates in these cells. Therefore, the increase in the percentage of immunoreactive cells is not the result of aggregate formation in this model. The presence of the E46K and A53T mutations likely engenders subtle structural changes that promotes increased polymerization in vitro as well as significant changes in secondary structure, as previously suggested [5, 21, 44]. When expressed in cells, these pre-aggregate structural variants, which presumably are increased in abundance by the E46K/A53T double mutations, can be detected with antibodies Syn 505, Syn 506 and Syn 514.
It is interesting that these antibodies that preferentially recognize α-syn in pathological inclusions were generated to oxidized/nitrated α-syn. Although these antibodies do not recognize any specific oxidative or nitrative covalent modification [12], it is possible that oxidative cross-linking [48] that occurs during the in vitro oxidation method used may have contributed to the generation of antibodies with specific conformational requirement. This cross-linking may stabilize conformational epitopes that would otherwise not be stable in the procedure used to immunize mice.
The conformational anti-α-syn antibodies characterized here are important tools to study α-syn pathology and have provided further insights into the importance of α-syn aggregates in PD and related disorders especially since the role of LBs in neurodegeneration has been contested [20, 24]. It has been suggested that the density of LBs is likely not sufficient to account for the neurodegeneration seen in the synucleinopathies, and there has been speculation that they may represent adaptive responses to other primary insults [24]. Conversely, many reports have demonstrated a clear correlation between cortical LBs and dementia [27, 29, 34, 36, 42]. Moreover, the use of unique tools (such as the antibodies characterized here) or of specific techniques (such as pre-treatment with either formic acid or protease K) [30, 39] has demonstrated that the abundance of α-syn pathology has been grossly under-estimated by conventional α-syn immunohistochemistry. The major form of α-syn aggregates is a profusion of neuritic and pre-synaptic α-syn inclusions [12, 30, 39], the relative density of which correlates with the relative density of LBs [10]. The presynaptic accumulation of α-syn aggregates can lead to synaptic degeneration [30], which may result in more profound neurodegeneration. Collectively these intracytoplasmic protein aggregates may act as cellular “plugs” effectively interfering with normal axonal transport as demonstrated in transgenic mouse models of synucleinopathies [18, 32]. In fact, it has been hypothesized that these axonal aggregates may be the primary insult that lead to the formation of somal LBs via supersaturation of the cell soma with α-syn and that the axonal transport blockade may be the cause of eventual neuronal demise [9]. The antibodies characterized here have unique abilities to detect conformers present in pathological inclusions, are valuable tools to monitor the abundance of pathological inclusions and could be used to better understand structural changes that occur during protein aggregation.
Acknowledgments
We thank Drs. John Q. Trojanowski and Virginia M.-Y. Lee (Center for Neurodegenerative Disease Research, University of Pennsylvania) and the families of patients who made this research possible.
This work was funded by grants from the National Institute on Aging (AG09215) and the National Institute of Neurological Disorders and Stroke (NS053488). E.A.W. was supported by a training grant (T32 AG00255) from the National Institute on Aging.
References
- 1.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VMY, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 2.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 3.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 5.Conway KA, Harper JD, Lansbury PT. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 6.Crowther RA, Jakes R, Spillantini MG, Goedert M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 1998;436:309–312. doi: 10.1016/s0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 7.Crystal AS, Giasson BI, Crowe A, Kung MP, Zhuang ZP, Trojanowski JQ, Lee VM. A comparison of amyloid fibrillogenesis using the novel fluorescent compound K114. J Neurochem. 2003;86:1359–1368. doi: 10.1046/j.1471-4159.2003.01949.x. [DOI] [PubMed] [Google Scholar]
- 8.Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J, Sima A, D'Amato C, Albin R, Gilman S, Yen SH. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol. 1999;155:1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duda JE. Pathology and neurotransmitter abnormalities of dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;17(Suppl 1):3–14. doi: 10.1159/000074677. [DOI] [PubMed] [Google Scholar]
- 10.Duda JE, Giasson BI, Lee VMY, Trojanowski JQ. Is the initial insult in Parkinson's disease and dementia with Lewy bodies a neuritic dystrophy? Ann N Y Acad Sci. 2003;991:295–297. [Google Scholar]
- 11.Duda JE, Giasson BI, Gur TL, Montine TJ, Robertson D, Biaggioni I, Hurtig HI, Stern MB, Gollomp SM, Grossman M, Lee VMY, Trojanowski JQ. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol. 2000;59:830–841. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
- 12.Duda JE, Giasson BI, Mabon ME, Lee VMY, Trojanoswki JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 13.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 14.Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson's disease: looking for the way out of a quackmire. Neuron. 2005;47:479–482. doi: 10.1016/j.neuron.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 15.George JM. The synucleins. Genome Biol. 2002;3 doi: 10.1186/gb-2001-3-1-reviews3002. REVIEWS3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giasson BI, Duda JE, Forman MS, Lee VMY, Trojanoswki JQ. Prominent perikaryal expression of α- and β-synuclein in neurons of dorsal root ganglion and in medullary neurons. Exp Neurol. 2001;172:354–362. doi: 10.1006/exnr.2001.7805. [DOI] [PubMed] [Google Scholar]
- 17.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VMY. Oxidative damage linked to neurodegeneration by selective alpha- synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 18.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanoswki JQ, Lee VMY. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 19.Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VMY. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson's disease. J Neurosci Res. 2000;59:528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Giasson BI, Lee VMY. Are ubiquitination pathways central to Parkinson's disease? Cell. 2003;114:1–8. doi: 10.1016/s0092-8674(03)00509-9. [DOI] [PubMed] [Google Scholar]
- 21.Giasson BI, Murray IV, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 22.Giasson BI, Uryu K, Trojanowski JQ, Lee VMY. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 23.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg MS, Lansbury PT. Is there a cause-and-effect relationship between alpha-synuclein fibrillization and Parkinson's disease? Nat Cell Biol. 2000;2:E115–E119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 26.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuro-pathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol (Berl) 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 27.Haroutunian V, Serby M, Purohit DP, Perl DP, Marin D, Lantz M, Mohs RC, Davis KL. Contribution of Lewy body inclusions to dementia in patients with and without Alzheimer disease neuro-pathological conditions. Arch Neurol. 2000;57:1145–1150. doi: 10.1001/archneur.57.8.1145. [DOI] [PubMed] [Google Scholar]
- 28.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 29.Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, Clark CM, Glosser G, Stern MB, Gollomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 30.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 32.Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Lennox G, Lowe J, Landon M, Byrne EJ, Mayer RJ, Godwin-Austen RB. Diffuse Lewy body disease: correlative neuro-pathology using anti-ubiquitin immunocytochemistry. J Neurol Neurosurg Psychiatry. 1989;52:1236–1247. doi: 10.1136/jnnp.52.11.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Dementia with Lewy bodies. Neurology. 1999;52:893. doi: 10.1212/wnl.52.4.893. [DOI] [PubMed] [Google Scholar]
- 36.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol (Berl) 2000;100:285–290. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 37.Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26:10068–10078. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 39.Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, Muller V, Odoy S, Fujiwara H, Hasegawa M, Iwatsubo T, Trojanowski JQ, Kretzschmar HA, Haass C. Misfolded protein-ase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VMY, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 42.Samuel W, Galasko D, Masliah E, Hansen LA. Neocortical Lewy body counts correlate with dementia in the Lewy body variant of Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55:44–52. doi: 10.1097/00005072-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt ML, Murray J, Lee VMY, Hill WD, Wertkin A, Trojanowski JQ. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991;139:53–65. [PMC free article] [PubMed] [Google Scholar]
- 44.Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid- like cross-beta conformation. Proc Natl Acad Sci USA. 2000;97:4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sidhu A, Wersinger C, Vernier P. Does alpha-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J. 2004;18:637–647. doi: 10.1096/fj.03-1112rev. [DOI] [PubMed] [Google Scholar]
- 46.Simuni T, Hurtig HI. Parkinson's disease: the clinical picture. In: Clark CM, Trojanowski JQ, editors. Neurodegenerative dementias. McGraw-Hill; New York: 2000. pp. 193–203. [Google Scholar]
- 47.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 48.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 49.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 50.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 51.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 53.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VMY. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 54.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez TE, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]