Abstract
Alpha-naphthylisothiocyanate (ANIT) causes intrahepatic cholestasis by injuring biliary epithelial cells. Adaptive regulation of hepatobiliary transporter expression has been proposed to reduce liver injury during cholestasis. Recently, the oxidative stress transcription factor Nrf2 (nf-e2–related factor 2) was shown to regulate expression of hepatobiliary transporters. The purpose of this study was to determine whether ANIT-induced hepatotoxicity and regulation of hepatobiliary transporters are altered in the absence of Nrf2. For this purpose, wild-type and Nrf2-null mice were administered ANIT (75 mg/kg po). Surprisingly, ANIT-induced hepatotoxicity was similar in both genotypes at 48 h. Accumulation of bile acids in serum and liver was lower in Nrf2-null mice compared with wild-types treated with ANIT. Transporter mRNA profiles differed between wild-type and Nrf2-null mice after ANIT. Bsep (bile salt export pump), Mdr2 (multidrug resistance gene), and Mrp3 (multidrug resistance–associated protein) efflux transporters were increased by ANIT in wild-type, but not in Nrf2-null mice. In contrast, mRNA expression of two hepatic uptake transporters, Ntcp (sodium-taurocholate cotransporting polypeptide) and Oatp1b2 (organic anion transporting peptide), were decreased in both genotypes after ANIT, with larger declines in Nrf2-null mice. mRNA expression of the transcriptional repressor of Ntcp, small heterodimeric partner (SHP), was increased in Nrf2-null mice after ANIT. Furthermore, hepatocyte nuclear factor 1α (HNF1α), which regulates Oatp1b2, was downregulated in ANIT-treated Nrf2-null mice. Preferential upregulation of SHP and downregulation of HNF1α and uptake transporters likely explains why Nrf2-null mice exhibited similar injury to wild-types after ANIT. A subsequent study revealed that treatment of mice with the Nrf2 activator oltipraz protects against ANIT-induced histological injury. Despite compensatory changes in Nrf2-null mice to limit ANIT toxicity, pharmacological activation of Nrf2 may represent a therapeutic option for intrahepatic cholestasis.
Keywords: Nrf2, ANIT, Nqo1, oxidative stress, Mrps
Cholestasis is an impairment of bile flow leading to accumulation of bile acids and other chemicals in liver and blood (Trauner et al., 1998). There are two types of cholestasis. Intrahepatic cholestasis within the liver is caused by hepatitis, drug-induced liver injury, pregnancy, and primary biliary cirrhosis (Watanabe et al., 2007). Extrahepatic cholestasis is obstruction of bile flow outside the liver, that can occur in various clinical conditions, such as gallstones, cholangiocarcinoma, pancreatic cancer, and primary sclerosing cholangitis (Hofmann, 2002). In rodents, α-naphthylisothiocyanate (ANIT) is a widely used chemical to model human intrahepatic cholestasis. ANIT is detoxified in hepatocytes by conjugation with glutathione (GSH). The ANIT-GSH conjugate is transported by multidrug resistance–associated protein 2 (Mrp2) into bile, where ANIT dissociates from GSH. The released ANIT selectively damages bile-duct epithelial cells and causes cholangitis and subsequent intrahepatic cholestasis (Dietrich et al., 2001; Ogawa et al., 2000).
Hepatic uptake and biliary excretion of many endogenous and exogenous compounds are mediated by basolateral and canalicular transporters. Basolateral uptake transporters, such as sodium taurocholate cotransporting polypeptide (Ntcp) and organic anion transporting polypeptides (Oatps), transport bile acids and organic anions from sinusoidal blood into hepatocytes. Subsequent secretion of cholephilic compounds from hepatocytes into bile is largely dependent on numerous canalicular transporters, including Mrp2, bile salt export pump (Bsep), breast cancer resistance protein, and multidrug resistance 1a, 1b, and 2 isoforms (Mdr1a, 1b, 2). The basolateral export transporters, Mrp3 and Mrp4, transport bilirubin glucuronide and bile acids, respectively, from hepatocytes into blood during cholestasis (Takikawa, 2002). Basolateral uptake systems are reduced and efflux pumps are elevated in animal models of cholestasis and human cholestatic diseases, such as primary biliary cirrhosis (Lee et al., 2001; Zollner et al., 2003). Differential regulation of hepatobiliary transporters is thought to represent an adaptation to cholestasis by limiting the accumulation of toxic biliary constituents and preventing progression of liver injury.
Nuclear factor, erythroid 2–related factor 2 (Nrf2) belongs to the cap‘n'collar family of basic leucine zipper transcription factors. Under physiological conditions, Nrf2 is sequestered by the cytosolic regulatory protein Kelch-like erythroid-cell-derived protein with CNC homology-associated protein 1 (Keap1) in the cytoplasm and is rapidly degraded by the ubiquitin-proteasome pathway (Ishii et al., 2000, 2002). When electrophilic or oxidative insults target the Nrf2-Keap1 complex, Nrf2 degradation is inhibited and Nrf2 translocates to the nucleus. Nrf2 is an essential factor in the coordinate induction of some detoxifying and antioxidant enzymes, including NAD(P)H:quinone oxidoreductase 1 (Nqo1), glutathione-S-transferases, and heme oxygenase-1. Nrf2 heterodimerizes with Maf proteins and binds cis-acting antioxidant response elements (AREs) in the promoter regions of genes (Ishii et al., 2000, 2002). More recently, our laboratory and others identified AREs in the upstream regions of mouse Mrp2–4, and demonstrated induction of these transporters following chemical activation of Nrf2 (Maher et al., 2007; Vollrath et al., 2006).
Constitutive activation of Nrf2 by deletion of Keap1 protects mice against acetaminophen hepatotoxicity (Okawa et al., 2006). In contrast, Nrf2-null mice are more susceptible to liver injury produced by aflatoxin B1, pentachlorophenol, and acetaminophen (Enomoto et al., 2001; Jowsey et al., 2003; Umemura et al., 2006). A recent study showed that bile-duct ligation, an extrahepatic cholestasis model, increases numerous genes regulated by Nrf2, that may constitute a defense system against oxidative stress generated in liver during bile-duct ligation (Aleksunes et al., 2005). However, whether Nrf2 protects the liver from injury during intrahepatic cholestasis is not known. Therefore, this study was undertaken to determine the role Nrf2 plays in the adaptive response to intrahepatic cholestasis. Goals of this study are to determine whether Nrf2 is activated during ANIT-induced intrahepatic cholestasis, functional outcomes of Nrf2 deficiency on ANIT-induced toxicity and bile acid–related pathways, and whether Nrf2 can regulate adaptive changes in transporter expression during cholestasis. Furthermore, it was determined whether pharmacological activation of Nrf2 can protect against ANIT-induced intrahepatic cholestasis.
MATERIALS AND METHODS
Materials.
ANIT and all other chemicals were purchased from Sigma-Aldrich (St Louis, MO). Serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), and conjugated bilirubin were assayed using colorimetric assays from Pointe Scientific, Inc. (Canton, MI). Serum and hepatic bile acids were quantified using a colorimetric assay from Wako Chemicals (Richmond, VA).
Reagents.
Nrf2 antibody (H-300, sc-13032) was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Nqo1 antibody (ab2346) was purchased from Abcam (Cambridge, MA). Mrp2 (immunofluorescence only), Ntcp, and Bsep antibodies were provided by Dr Bruno Stieger (University Hospital, Zurich, Switzerland). Mrp2 (M2III-5) and Mrp3 antibodies (M3II-2) for Western blot analysis were provided by Dr George Scheffer (VU Medical Center, Amsterdam, The Netherlands).
Animals.
Wild-type and Nrf2-null breeding pairs of mice on a mixed C57BL/6 and AKR background were obtained from Dr Jefferson Chan (University of California, Irvine, CA) (Chan et al., 1996). Male mice (aged 8–10 weeks, n = 4–6) were used for experiments. A single dose of ANIT (75 mg/kg, 10 ml/kg in corn oil) was administered orally to mice, blood samples obtained, and livers removed 48 h after ANIT treatment. In a separate experiment, mice (n = 4–6) received ip injections of vehicle or oltipraz (50 mg/kg) once daily for 2 days. ANIT (75 mg/kg, po) or vehicle (10 ml/kg) was administered 4 h after the first dose of oltipraz. Blood samples and livers were collected 48 h after ANIT treatment. Animals received humane care as outlined in the Guide for the Care and Use of Laboratory Animals (NIH publication 86-23, revised 1985). Studies were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Serum ALT, ALP, conjugated bilirubin, and bile acid quantification.
Blood was collected 48 h after ANIT treatment. Serum samples were analyzed by standard enzymatic-colorimetric assays using ALT, ALP, conjugated bilirubin, and bile acid assay kits in accordance with the manufacturer's protocols. The absorption of each sample was assessed by spectrophotometry at wavelengths of 340, 405, 555, and 560 nm, respectively.
RNA isolation.
Total RNA was isolated using RNAzol B reagent (Tel Test, Inc., Friendswood, TX) according to the manufacturer's protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. The integrity of each RNA sample was evaluated by formaldehyde-agarose gel electrophoresis before analysis.
Branched DNA signal amplification assay.
Pooled total RNA samples from four to six mice were used to determine the effects of ANIT on bile acid metabolism related genes and transcription factors expression. Genes that were altered by ANIT, including mouse Nqo1, Ntcp, Oatp1a1, Oatp1a4, Oatp1b2, Mrp2–4, Bsep, Mdr2, Cyp7a1, Cyp8b1, Cyp27a1, HNF1α (hepatocyte nuclear factor 1α), and SHP (small heterodimeric partner), were confirmed by quantifying individual mouse total RNA samples by the branched DNA (bDNA) assay as previously described (QuantiGene, High Volume bDNA Signal Amplification Kit; Panomics, Fremont, CA) (Aleksunes et al., 2005; Maher et al., 2006). Multiple oligonucleotide probe sets (containing capture, label, and blocker probes) specific to mouse Cyp7a1, Cyp8b1, Cyp27a1, HNF1α, and SHP mRNA transcripts were designed using ProbeDesigner software v1.0 (Bayer Corp., Diagnostics Div., Emeryville, CA) (Supplementary Table 1). Specific mouse Nqo1, Ntcp, Oatp1a1, Oatp1a4, Oatp1b2, Mrp2–4, Bsep, and Mdr2 probe sets were described previously (Aleksunes et al., 2005; Maher et al., 2006).
Western blotting.
Liver cytosol and plasma membrane preparations were made as described previously (Aleksunes et al., 2006). Nuclear extracts were prepared using the NE-PER Nuclear Extraction Kit according to the manufacturer's directions (Pierce Biotechnology, Rockford, IL). Protein concentrations were determined using Pierce protein assay reagents according to the manufacturer's recommendations (Pierce Biotechnology). Liver nuclear (for Nrf2 expression), cytosol (for Nqo1 expression), and membrane preparations (for Mrp2, Mrp3, Bsep, and Ntcp expression) were loaded and separated on 8% (Mrp2, Mrp3, Bsep) or 10% (Ntcp, Nrf2, Nqo1) sodium dodecyl sulfate–polyacrylamide gels. Proteins were transferred overnight at 4°C to polyvinylidene difluoride membranes. Membranes were blocked for 2 h in blocking buffer (1% nonfat dry milk with 0.5% Tween 20). All primary and secondary antibodies were diluted in blocking buffer. Primary antibody dilutions were as follows: Nrf2 (1:1000), Nqo1 (1:1000), Mrp2 (M2III-5, 1:600), Mrp3 (M3II-2, 1:2000), Bsep (K44, 1:2000), and Ntcp (K4, 1:1000). Blots were subsequently incubated with a species-appropriate horseradish peroxidase–conjugated secondary antibody for 1 h. Protein-antibody complexes were detected using an ECL chemiluminescent kit (Pierce Biotechnology) and exposed to Fuji Medical X-ray film (Fisher Scientific, Springfield, NJ). The intensity of the protein bands was semiquantified using Quantity One Software (Bio-Rad Laboratories, Hercules, CA).
Histopathology.
Liver samples were fixed in 10% formalin prior to routine processing and paraffin embedding. Liver sections (5 μm in thickness) were stained with hematoxylin and eosin and evaluated for hepatocellular necrosis.
Indirect immunofluorescence analysis.
Immunostaining for Mrp2, Mrp3, and Ntcp was performed on frozen liver sections (5 μm) as described previously (Aleksunes et al., 2006; Maher et al., 2007). Briefly, liver cryosections were blocked with 5% serum/phosphate-buffered saline with 0.1% Triton X for 1 h, and then incubated with primary antibody diluted 1:100 in blocking buffer for 2 h at room temperature. Sections were subsequently washed, and incubated for 1 h with goat anti-rat Alexa 488 IgG for Mrp3 detection and goat anti-rabbit Alexa 488 IgG for Mrp2 and Ntcp detection (Invitrogen Corporation, Carlsbad, CA). Secondary antibodies were diluted 1:200 in blocking buffer. Images were captured on an Olympus BX41 fluorescent microscope with a DP70 camera and DP Controller software (Olympus America, Inc., Center Valley, PA). Three tissues were analyzed and found to be similar for each treatment group, and one representative image is presented. Negative controls without primary antibody were included in the analysis (data not shown). All sections were both stained and imaged under uniform conditions for each antibody. Images were cropped and brightness and contrast were adjusted the same for each antibody in Adobe Photoshop CS2 (San Jose, CA).
Statistical analysis.
All data were analyzed by analysis of variance, followed by a Duncan's multiple range post hoc test. Significance was set at p < 0.05. Bars represent means ± SEM.
RESULTS
Activation of Nrf2 in Liver of Mice and Nqo1 Protein Expression after ANIT Treatment
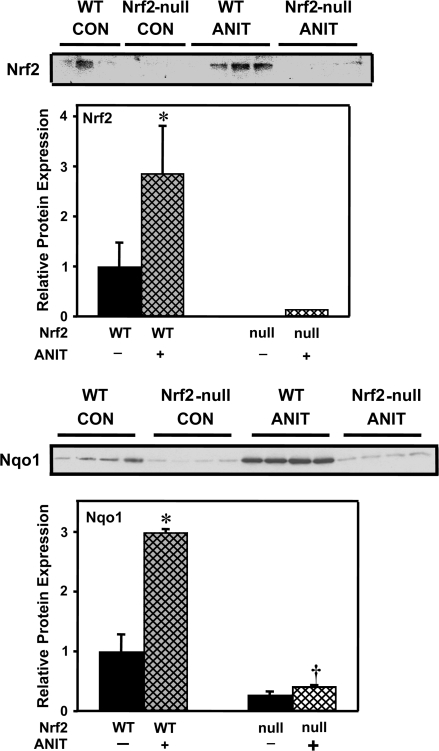
To determine whether Nrf2 is activated in mouse liver after ANIT treatment, nuclear accumulation of Nrf2 protein was determined by Western blot. Nuclear levels of Nrf2 protein increased 183% in wild-type mice at 48 h after ANIT treatment (Fig. 1). mRNA and protein expression of the prototypical Nrf2 target gene Nqo1 was quantified by the bDNA assay and Western blot, respectively (Venugopal and Jaiswal, 1996). Similar to previous reports, the basal expression of Nqo1 is reduced in Nrf2-null mice, compared with wild-type counterparts (Aleksunes et al., 2005; Maher et al., 2007). Both mRNA (data not shown) and protein expression of Nqo1 were increased 91 and 199%, respectively, in wild-type mice 48 h after ANIT treatment, but were unchanged in Nrf2-null mice (Fig. 1).
FIG. 1.
Nuclear Nrf2 protein and Nqo1 protein expression in wild-type and Nrf2-null mouse liver 48 h after ANIT treatment. Upper panel: Western blot of liver nuclear extract proteins (30 μg protein/lane) stained with an antibody that detects mouse Nrf2. Immunoreactive bands were semiquantitated by densitometric analysis. Data are expressed as mean ± SEM (each group, n = 3 or 4 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Lower panel: Western blot of liver cytosolic proteins stained with an antibody that detects mouse Nqo1 (50 μg protein/lane). Immunoreactive bands were semiquantitated by densitometric analysis. Data are expressed as mean ± SEM (each group, n = 4 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Daggers (†) represent a statistically significant difference (p < 0.05) compared with ANIT-treated wild-type mice.
Serum ALT and ALP in Wild-Type and Nrf2-Null Mice after ANIT Treatment
Hepatobiliary toxicity was determined 48 h after ANIT treatment of wild-type and Nrf2-null mice. Serum ALT (marker for hepatocyte toxicity) and ALP (marker for biliary toxicity) were increased in both genotypes after ANIT. However, there were no significant differences in serum ALT or ALP between ANIT-treated WT and Nrf2-null mice (data not shown).
Biomarkers for Cholestasis after ANIT Treatment
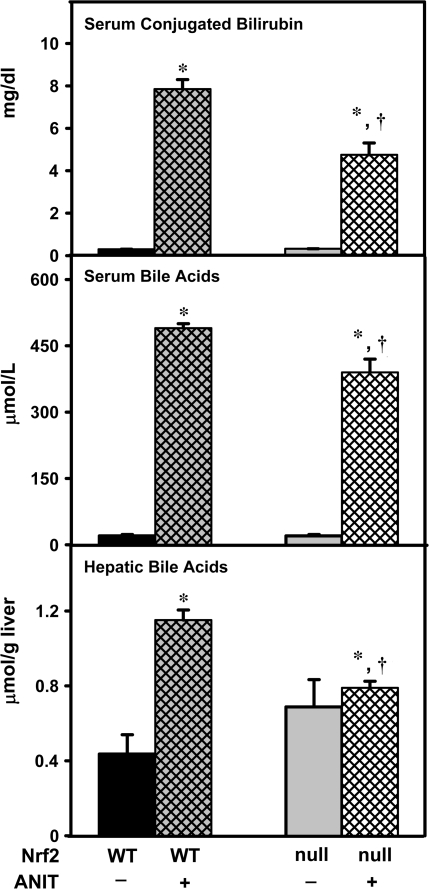
Serum bile acids and conjugated bilirubin, as well as hepatic bile acids were quantified in wild-type and Nrf2-null mice 48 h after ANIT treatment as biomarkers of cholestasis. Serum conjugated bilirubin was increased in ANIT-treated wild-type and Nrf2-null mice, with a higher increase in wild-type mice (Fig. 2). Serum and hepatic bile acid concentrations were elevated in wild-type and Nrf2-null mice after ANIT treatment, with higher concentrations observed in wild-type mice (Fig. 2).
FIG. 2.
Serum conjugated bilirubin and bile acids and hepatic bile acids in wild-type and Nrf2-null mice after ANIT treatment. At 48 h after ANIT treatment, conjugated bilirubin and bile acids were quantified in serum. Hepatic bile acids were also quantified. Data are expressed as mean ± SEM (each group, n = 4–6 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Daggers (†) represent a statistically significant difference (p < 0.05) compared with ANIT-treated wild-type mice.
mRNA Expression of Basolateral Uptake or Efflux and Canalicular Efflux Transporters after ANIT Treatment
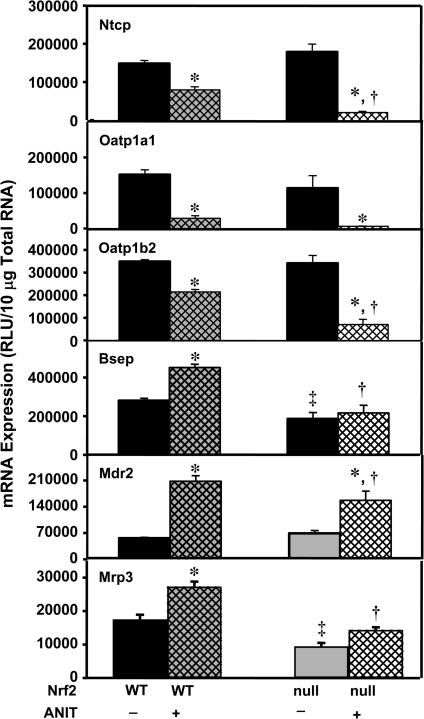
Because adaptive regulation has been proposed as a mechanism to limit hepatotoxicity, mRNA profiles of uptake and efflux transporters were quantified in wild-type and Nrf2-null mice after ANIT. Basal levels of Ntcp, Oatp1a1, 1a4 (data not shown), and 1b2 were similar between wild-type and Nrf2-null mice. Basal hepatic Bsep mRNA was 34% lower in Nrf2-null mice than in wild-type mice (Fig. 3). Forty-eight hours after ANIT treatment, mRNA levels of Ntcp, Oatp1a1, and Oatp1b2 mRNA were decreased in wild-type mice 47, 81, and 39%, respectively. Interestingly, Ntcp and Oatp1b2 mRNA were further reduced in Nrf2-null mice (Fig. 3). mRNA levels of Oatp1a4 were not changed in either group after ANIT treatment (data not shown). mRNA expression of Bsep and Mdr2 was increased in ANIT-treated wild-type mice by 61 and 270%, respectively, but was lower in Nrf2-null mice (Fig. 3). The basal expression of Mrp3 mRNA was lower in Nrf2-null mice compared with wild-type mice. After ANIT treatment, the mRNA expression of Mrp3 was increased in wild-type mice by 56%, with no change in Nrf2-null mice (Fig. 3). Mrp2, Mrp4, and Bcrp mRNA were not altered in either group after ANIT treatment (data not shown). In pooled RNA samples, no differences in other transporters, including Abcg5 and Abcg8, were observed in either group after ANIT treatment (data not shown).
FIG. 3.
Basolateral uptake (Ntcp, Oatp1a1, Oatp1b2), efflux (Mrp3), and apical efflux (Bsep, Mdr2) transporter mRNA expression in wild-type and Nrf2-null mouse liver after ANIT treatment. Total RNA was isolated from both control and ANIT-treated mouse liver and analyzed by the bDNA signal amplification assay as described in “Materials and Methods.” Data are presented as mean relative light units (RLU) ± SEM (each group, n = 4–6 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Daggers (†) represent a statistically significant difference (p < 0.05) compared with ANIT-treated wild-type mice. Double daggers (‡) represent a statistically significant difference (p < 0.05) compared with control wild-type mice.
Protein Expression and Immunofluorescence of Mrp2, Mrp3, and Ntcp after ANIT Treatment
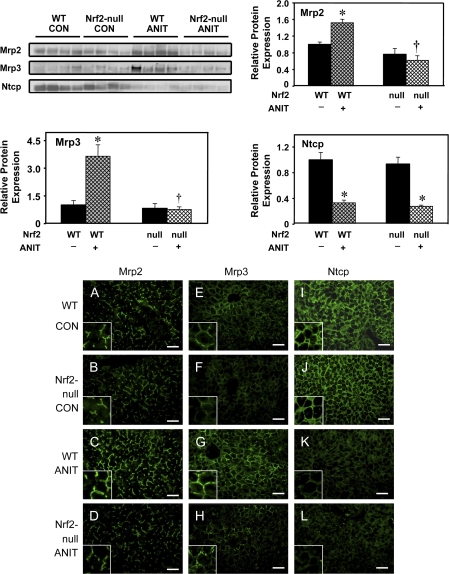
To determine whether changes in mRNA expression of hepatobiliary transporters after ANIT treatment corresponded with protein levels, Western blotting was performed with liver membrane fractions. Although the mRNA expression of Mrp2 was not changed after ANIT treatment in any group (Fig. 3), protein expression of Mrp2 was increased in livers from wild-type mice by 34%, but was not changed in livers from Nrf2-null mice (Fig. 4). ANIT increased protein expression of Mrp3 in wild-type mice by 265%, but not in Nrf2-null mice, corresponding to the mRNA results (Fig. 4). ANIT treatment similarly decreased Ntcp protein 67 and 72% in the two genotypes (Fig. 4). No difference in Bsep protein expression was seen after ANIT treatment (data not shown). Mrp1 and Mrp4 proteins were not detected by Western blot, suggesting that expression of both transporters is low in mouse liver (data not shown). Of note, there are no commercially available or published antibodies that specifically detect mouse Mdr2.
FIG. 4.
Protein expression and immunofluorescent analysis of Mrp2, Mrp3, and Ntcp in livers from wild-type and Nrf2-null mice after ANIT treatment. Representative Western blot of liver membrane proteins stained with antibodies that detect mouse Mrp2, Mrp3, and Ntcp protein (50 μg protein/lane). Immunoreactive bands were semiquantitated by densitometric analysis. Data are expressed as mean ± SEM (each group, n = 4 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Daggers (†) represent a statistically significant difference (p < 0.05) compared with ANIT-treated wild-type mice. Indirect immunofluorescence against canalicular Mrp2 and sinusoidal Mrp3 and Ntcp (green) was conducted on liver cryosections (5 μm) obtained at 48 h from control wild-type (A, E, I), control Nrf2-null (B, F, J), ANIT-treated wild-type (C, G, K) and ANIT-treated Nrf2-null (D, H, L) mice. Portions of images were enlarged and provided as inserts. Representative images are shown. Bar, 50 μm.
Mrp2, Mrp3, and Ntcp immunofluorescence was performed on frozen liver sections obtained from wild-type and Nrf2-null mice treated with either vehicle or ANIT in order to determine patterns of transporter expression. Compared with vehicle-treated counterparts (Figs. 4A and 4B), ANIT treatment enhanced canalicular Mrp2 staining throughout the liver lobule in wild-type mice (Fig. 4C), with no increase in Mrp2 in livers from Nrf2-null mice, similar to Western blot results (Fig. 4D). Mrp3 protein is typically localized to the basolateral membrane of hepatocytes, as evidenced by a distinct honeycomb pattern. Similar to Western blot analysis, limited immunofluorescent staining of Mrp3 was observed in the liver lobule from vehicle-treated mice (Figs. 4E and 4F). Enhanced staining of Mrp3 was prominent on the basolateral membranes of hepatocytes throughout the liver (greatest in centrilobular hepatocytes) of ANIT-treated wild-type (Fig. 4G), but not in Nrf2-null livers (Fig. 4H). Ntcp is uniformly expressed at high levels in vehicle-treated mouse livers and exhibits a honeycomb appearance that is characteristic of its sinusoidal localization (Figs. 4I and 4J). Ntcp immunofluorescence was similarly reduced in livers from ANIT-treated wild-type and Nrf2-null mice (Figs. 4K and 4L), corresponding with Western blot analysis (Fig. 4).
mRNA Expression of Bile Acid Synthetic Enzymes after ANIT Treatment
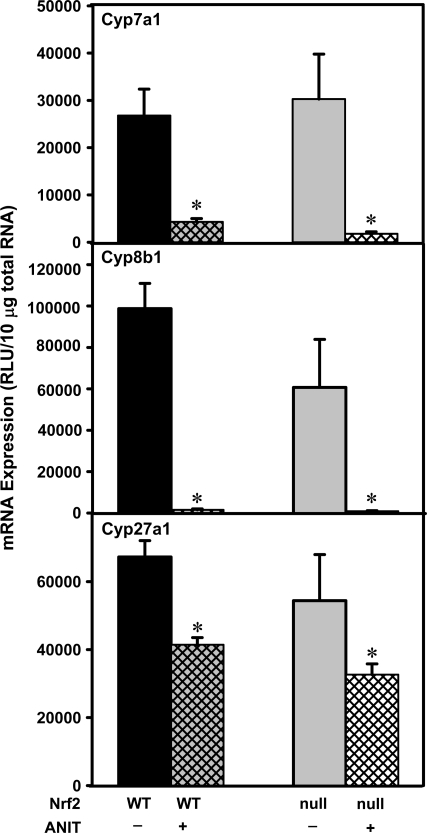
Hepatic bile acid concentrations are dependent on a balance between uptake, synthesis, and elimination. The mRNA expression of bile acid synthetic enzymes was quantified to determine their contribution in dictating serum and hepatic bile acid concentrations in these mice (Fig. 5). The mRNA expression of cholesterol 7α-hydroxylase (Cyp7a1), the rate-limiting enzyme of bile acid synthesis, was decreased similarly in both genotypes after ANIT treatment (Fig. 5). The mRNA expression of sterol-12α-hydroxylase (Cyp8b1) was also markedly decreased in both genotypes after ANIT treatment (Fig. 5). The mRNA expression of sterol-27α-hydroxylase (Cyp27a1), a key enzyme in the alternative acidic pathway of bile acid synthesis, was also reduced in both genotypes after ANIT treatment, but to a lesser degree (Fig. 5). The mRNA expression of these bile acid synthetic enzymes tended to be further decreased in Nrf2-null mice than in wild-type mice after ANIT treatment, although these changes were not statistically significant (Fig. 5).
FIG. 5.
Bile acid synthesis mRNA expression in wild-type and Nrf2-null mouse liver after ANIT treatment. Total RNA was isolated from both control and ANIT-treated mouse liver and analyzed by the bDNA signal amplification assay as described in “Materials and Methods.” Data are presented as mean relative light units (RLU) ± SEM (each group, n = 4–6 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice.
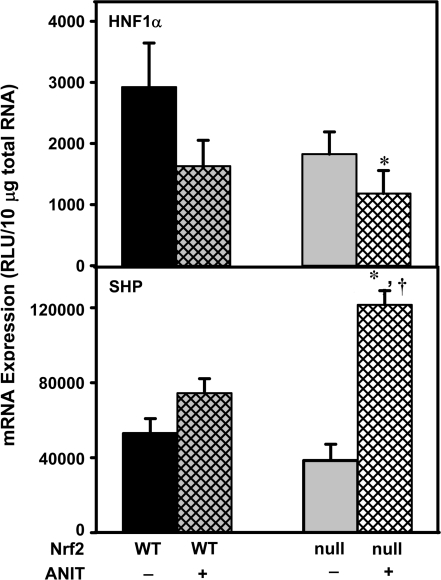
mRNA Expression of HNF1α and SHP after ANIT Treatment
Although Nrf2-null mice are unable to upregulate efflux transporter pathways after ANIT treatment, reduced expression of uptake transporters and bile acid synthetic enzymes might contribute to lower levels of bile acids in their livers. To identify alternate regulatory pathway(s) that could explain reduced bile acid uptake and no difference in hepatic injury in the absence of the Keap1-Nrf2 pathway, the hepatic mRNA levels of a number of transcription factors were screened in pooled samples and HNF1α and SHP demonstrated differential expression between ANIT-treated wild-type and Nrf2-null mice. Therefore, mRNA of HNF1α and SHP were quantified in individual, replicate samples. The mRNA of HNF1α in Nrf2-null mice and wild-type mice given ANIT tended to be lower than in wild-type mice; the decrease in HNF1α was statistically lower only when ANIT was given to Nrf2-null mice (Fig. 6). The mRNA expression of SHP after ANIT tended to be increased in wild-type mice, however, SHP mRNA was increased 116% in Nrf2-null mice (Fig. 6). In addition, the mRNA expression of other transcription factors was quantified in pooled RNA samples and it was determined that HNF4α, PPAR-α (peroxisome proliferator–activated receptor), PPAR-γ, LXR, and FXR mRNA levels were not changed in either genotype after ANIT treatment.
FIG. 6.
HNF1α and SHP mRNA expression in wild-type and Nrf2-null mouse liver after ANIT treatment. Total RNA was isolated from both control and ANIT-treated mouse liver and analyzed by the bDNA signal amplification assay as described in “Materials and Methods.” Data are presented as mean relative light units (RLU) ± SEM (each group, n = 4–6 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Daggers (†) represent a statistically significant difference (p < 0.05) compared with ANIT-treated wild-type mice.
Pharmacological Activation of Nrf2 during ANIT-Induced Liver Injury
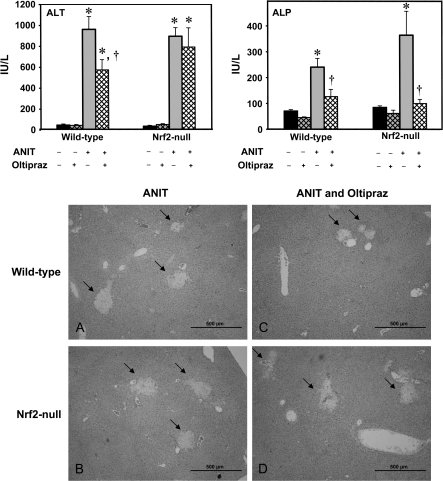
In the genetic model of Nrf2 deficiency, Nrf2-null mice exhibited alternate compensatory responses leading to no overall difference in hepatocyte and biliary toxicity. Therefore, a pharmacological approach was conducted to determine whether Nrf2 can protect against intrahepatic cholestasis. Oltipraz is a Nrf2 activator (Petzer et al., 2003). The protection study was evaluated by serum markers for hepatocyte necrosis (ALT), cholangiocyte damage (ALP), as well as liver histopathology. In these studies, ANIT (75 mg/kg) increased serum ALT and ALP 48 h after treatment, as observed in the prior study (Fig. 7). Pre- and post-treatment with oltipraz attenuated serum ALT in wild-type, but did not change serum ALT in Nrf2-null mice, indicating that oltipraz protected livers from ANIT-induced hepatotoxicity, via the Nrf2 pathway (Fig. 7). Although pre- and post-treatment with oltipraz reduced serum ALP in wild-type, interestingly, it also decreased serum ALP in Nrf2-null mice, suggesting that oltipraz protected cholangiocytes from ANIT-induced biliary toxicity, independent of Nrf2 (Fig. 7). To confirm differences in hepatocyte injury, histopathological analysis was conducted. Examination demonstrated that control- and oltipraz-treated liver histology did not differ between genotypes (data not shown). A similar degree of large foci of hepatocyte necrosis in the periportal regions were observed in both genotypes 48 h after ANIT treatment, whereas the adjacent remaining parenchyma was largely unaffected (Figs. 7A and 7B). However, pre- and post-treatment with oltipraz suppressed the degree of foci of periportal necrosis in wild-type mice, but not in Nrf2-null mice (Figs. 7C and 7D). These histopathological findings corresponded to the serum ALT data.
FIG. 7.
Effect of pretreatment with oltipraz on ANIT-induced hepatobiliary toxicity and histological examination in wild-type and Nrf2-null mice. Oltipraz (50 mg/kg) was administered before and after ANIT treatment (75 mg/kg, orally), and blood and livers were collected 48 h after ANIT treatment. Serum ALT and ALP were quantified. Data are presented as mean ± SEM (each group, n = 4–6 animals). Asterisks (*) represent a statistically significant difference (p < 0.05) compared with vehicle-treated mice. Daggers (†) represent a statistically significant difference (p < 0.05) compared with ANIT-treated wild-type mice. Liver hematoxylin and eosin staining is presented as follows: ANIT-treated wild-type (A), ANIT-treated Nrf2-null (B), ANIT and oltipraz-treated wild-type (C), ANIT and oltipraz-treated Nrf2-null (D) mice. Representative images are shown. Bar, 500 μm.
DISCUSSION
Nrf2 activation protects the liver from xenobiotic toxicity by regulating expression of several detoxifying and antioxidant enzymes as well as transporters (Enomoto et al., 2001; Jowsey et al., 2003; Okawa et al., 2006; Umemura et al., 2006). The purpose of the present study was to determine whether Nrf2 plays a protective and/or adaptive role in intrahepatic cholestatic liver injury. For this purpose, ANIT was administered to wild-type and Nrf2-null mice to induce intrahepatic cholestasis and hepatobiliary toxicity (Dietrich et al., 2001; Ogawa et al., 2000). ANIT activated Nrf2 as evidenced by Nrf2 translocation and Nqo1 mRNA and protein induction (Fig. 1). Despite activation of Nrf2, hepatobiliary toxicity was similar between wild-type and Nrf2-null mice after ANIT (data not shown). A lack of difference in ANIT susceptibility suggests that Nrf2-null mice compensate via alternate, Nrf2-independent mechanisms. In order to delineate the signaling mechanisms responsible for no difference in injury between wild-type and Nrf2-null mice, mRNA expression of hepatobiliary transporters, bile acid synthetic, and transcription factor genes was quantified (Figs. 3, 5, 6). Induction of efflux transporters Mdr2, Mrp3, and Bsep was dependent on Nrf2 expression (Fig. 3). Uptake transporter mRNA levels were downregulated in both genotypes after ANIT and decreased even further in Nrf2-null mice (Fig. 3). Preferential upregulation of SHP (Fig. 6) and downregulation of HNF1α and their downstream uptake transporter target genes likely explains why Nrf2-null mice exhibited similar injury to wild-types after ANIT. Therefore, in the absence of Nrf2, mice compensate by altering SHP and HNF1α signaling in order to limit bile acid burden and injury to the liver.
A preliminary time-course of ANIT injury and transporter regulation was conducted. Markers for liver injury and cholestasis, such as serum ALT, bile acids, and bilirubin were increased 24 h after ANIT treatment, and peaked at 48 h (data not shown), in agreement with a previous report (Kim and Kim, 2005). Furthermore, mRNA expression of Nqo1 as well as most hepatobiliary transporters was not altered at 24 h, but was increased by 48 h after ANIT treatment (data not shown) (Venugopal and Jaiswal, 1996). Therefore, 48 h after ANIT was selected for the present study.
In the present study, the basal and inducible expression of Mrp3 after ANIT treatment was dependent upon Nrf2 (Fig. 3). Induction of Mrp3 has also been observed after treatment with the Nrf2 activator, oltipraz, and in response to acetaminophen hepatotoxicity (Aleksunes et al., 2008; Maher et al., 2007). Using chromatin immunoprecipitation, Nrf2 was shown to bind to an ARE at −9919 bp in the mouse Mrp3 gene (Maher et al., 2007). Mrp3 protein levels were increased only in livers of wild-type mice after ANIT treatment, corresponding with the mRNA expression data (Figs. 3, 4). It is known that conjugated bilirubin is transported by Mrp3 (Belinsky et al., 2005). In the present study, serum conjugated bilirubin was increased in wild-type mice given ANIT, but this elevation was attenuated in Nrf2-null mice after ANIT treatment (Fig. 2). This likely reflects impaired induction of Mrp3 in Nrf2-null mice.
Farnesoid X receptor (FXR) is a bile acid sensor and is activated during cholestasis. Mdr2 and Bsep are targets of FXR signaling (Gerloff et al., 2002; Lew et al., 2004; Liu et al., 2003). Serum and hepatic bile acid concentrations were increased by ANIT in both genotypes; although, to a lesser degree in Nrf2-null mice (Fig. 2). Similarly, the induction of Mdr2 was reduced in Nrf2-null mice (Fig. 3). Thus, the upregulation of Mdr2 in both genotypes appears to be regulated by FXR activated by the accumulation of bile acids after ANIT treatment. In contrast, the basal expression and induction of Bsep after ANIT was completely blocked in Nrf2-null mice (Fig. 3). Therefore, the mRNA expression of Bsep could be directly regulated by Nrf2 as well as FXR, although there is no report designating Bsep as a Nrf2 target gene.
Although the mRNA expression of Mrp2 was unchanged 48 h after ANIT treatment (Fig. 3), Mrp2 protein levels were increased in wild-type mice after ANIT treatment, but were attenuated in Nrf2-null mice. These findings indicate that Mrp2 is potentially regulated by post-transcriptional/post-translational mechanisms via Nrf2 after ANIT (Fig. 4) (Maher et al., 2007). Furthermore, a previous study demonstrated that proteasome inhibitors inhibited the degradation of the MRP2 mutant protein (Keitel et al., 2000). Also, it has recently been shown that Nrf2 regulates genes involved in the ubiquitin-proteasome pathway (Hu et al., 2006). Taken together, protesomal degradation may be involved in regulation of Mrp2 protein expression by Nrf2. Induction of Mrp2 during liver injury is generally thought to be a protective response. However, previous research showed that ANIT conjugated to GSH is excreted into bile by Mrp2, and that transport-deficient (TR−) rats, which lack Mrp2, are protected from ANIT-induced hepatotoxicity (Dietrich et al., 2001). A pharmacokinetic study of ANIT showed that the plasma half-life of ANIT in rats is about 6 h after an oral dose (Hu and Morris, 2005). Thus, it is anticipated that most ANIT is cleared from the rat within 48 h. Taken together, the upregulation of Mrp2 at 48 h probably does not confer subsequent damage to cholangiocytes by transporting more ANIT into bile. Instead, higher levels of Mrp2 protein may serve to eliminate Mrp2 substrates, such as glutathione disulfide and/or glutathione conjugates of 4-hydroxynonenal, a final product of lipid peroxidation, to aid in the recovery of the liver following ANIT toxicity (Ji et al., 2002; Suzuki and Sugiyama, 1998).
Bile acid homeostasis is dictated not only by efflux, but also by the synthesis of hepatic uptake transporters. The mRNA expression of uptake transporters, such as Ntcp and Oatps, were decreased after ANIT treatment in wild-type, but were further reduced in Nrf2-null mice (Fig. 3). Bile acid synthetic genes were similarly decreased in ANIT-treated wild-type and Nrf2-null mice (Chisholm et al., 1999; Liu et al., 2003). It was hypothesized that in the absence of Nrf2, the expression of alternate signaling pathways may be altered after bile acid accumulation leading to an apparent lack of difference in ANIT injury. Using pooled RNA samples, we quantified mRNA expression of several transcription factors, and two candidate bile acid–related transcription factors, HNF1α and SHP, were differentially altered between genotypes. The liver-enriched transcription factor HNF1α is an important regulator of uptake transporters, including Ntcp, Oatp1a1, and Oatp1b2, and was reduced in ANIT-treated Nrf2-null mice (Geier et al., 2002; Tanaka et al., 2006). SHP is a repressor that is activated by bile acids via FXR binding to the SHP promoter. SHP dimerizes with LRH-1 and inhibits transcription of Ntcp, Cyp7a1, and Cyp8b1 (del Castillo-Olivares and Gil, 2001; Denson et al., 2001; Goodwin et al., 2000). ANIT treatment tended to increase SHP mRNA in wild-type mice, and was further increased when given to Nrf2-null mice (Fig. 6). The data suggest that the HNF1α and FXR-SHP-LRH-1 pathways may compensate for loss of the antioxidant defense system in Nrf2-null mice. Enhanced SHP signaling in Nrf2-null mice likely decreases Ntcp and Cyp7a1 as well as hepatic bile acid concentrations (Figs. 2, 3, 5), and in turn, leads to no difference in hepatobiliary toxicity, as evaluated by serum ALT and ALP (data not shown). A second approach to determining the role of Nrf2 in intrahepatic cholestasis employed the Nrf2 activator oltipraz. Previous studies have shown that oltipraz activates Nrf2 and increases expression of a number of antioxidant and detoxification genes as well as Mrp transporters through ARE activation (Petzer et al., 2003). Pre- and post-treatment with oltipraz protects mice from ANIT-induced liver injury via Nrf2 (Fig. 7). To gain some insights into this mechanism, the gene expression of hepatic transporters was quantified using pooled samples. Unexpectedly, oltipraz did not further enhance upregulation of Mrp3, Bsep, Mdr2, and Nqo1 or the downregulation of Ntcp, Oatp1a1, and Oatp1a4 mRNA (Supplementary Fig. 1). Therefore, oltipraz may protect liver from ANIT-induced liver injury by other mechanisms. Further studies should be conducted to identify the mechanism(s) involved in the protection of oltipraz against ANIT-induced liver injury. Interestingly, oltipraz also conferred protection against cholangiocyte injury in wild-type and Nrf2-null mice through an unknown signaling mechanism (Fig. 7).
In the present study, bile acids and likely lipid peroxides accumulated in livers leading to oxidative stress. To counteract these events, hepatic expression of bile acid synthetic and uptake transporter genes was reduced. In wild-type mice, Nrf2 was activated leading to increased expression of detoxification/drug metabolizing enzymes (Nqo1) and efflux transporters. In contrast, Nrf2-null mice were unable to activate the antioxidant defense, and instead activated the SHP signaling pathway. Pronounced SHP activation in Nrf2-null mice is likely responsible for lower bile acids after ANIT. Altered SHP and HNF1α levels are likely responsible for further downregulation of uptake transport genes in the absence of Nrf2, preventing bile acid accumulation, and resulting in no differences in injury between genotypes. Because of compensatory changes in transcriptional signaling in Nrf2-null mice, caution should be exercised when designing and interpreting future cholestatic liver injury experiments. Lastly, a role for Nrf2 in mitigating ANIT-induced histological injury was demonstrated using oltipraz as a pharmacological activator.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health grants (ES-09649, ES-09716, ES-07079, and RR021940).
Supplementary Material
Acknowledgments
We thank Drs Bruno Stieger and George Scheffer for generously providing antibodies and Dr Jefferson Chan for providing Nrf2-null breeding pairs.
References
- Aleksunes LM, Slitt AL, Maher JM, Augustine LM, Goedken MJ, Chan JY, Cherrington NJ, Klaassen CD, Manautou JE. Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol. Appl. Pharmacol. 2008;226:74–83. doi: 10.1016/j.taap.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell. Stress Chaperones. 2006;11:356–363. doi: 10.1379/CSC-217.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol. Sci. 2005;83:44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Dawson PA, Shchaveleva I, Bain LJ, Wang R, Ling V, Chen ZS, Grinberg A, Westphal H, Klein-Szanto A, et al. Analysis of the in vivo functions of Mrp3. Mol. Pharmacol. 2005;68:160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm JW, Nation P, Dolphin PJ, Agellon LB. High plasma cholesterol in drug-induced cholestasis is associated with enhanced hepatic cholesterol synthesis. Am. J. Physiol. 1999;276:G1165–1173. doi: 10.1152/ajpgi.1999.276.5.G1165. [DOI] [PubMed] [Google Scholar]
- del Castillo-Olivares A, Gil G. Suppression of sterol 12alpha-hydroxylase transcription by the short heterodimer partner: Insights into the repression mechanism. Nucleic Acids Res. 2001;29:4035–4042. doi: 10.1093/nar/29.19.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–147. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- Dietrich CG, Ottenhoff R, de Waart DR, Oude Elferink RP. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology. 2001;167:73–81. doi: 10.1016/s0300-483x(01)00459-0. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- Geier A, Kim SK, Gerloff T, Dietrich CG, Lammert F, Karpen SJ, Stieger B, Meier PJ, Matern S, Gartung C. Hepatobiliary organic anion transporters are differentially regulated in acute toxic liver injury induced by carbon tetrachloride. J. Hepatol. 2002;37:198–205. doi: 10.1016/s0168-8278(02)00108-3. [DOI] [PubMed] [Google Scholar]
- Gerloff T, Geier A, Roots I, Meier PJ, Gartung C. Functional analysis of the rat bile salt export pump gene promoter. Eur. J. Biochem. 2002;269:3495–3503. doi: 10.1046/j.1432-1033.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. Cholestatic liver disease: Pathophysiology and therapeutic options. Liver. 2002;22(Suppl. 2):14–19. doi: 10.1034/j.1600-0676.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- Hu K, Morris ME. Pharmacokinetics of alpha-naphthyl isothiocyanate in rats. J. Pharm. Sci. 2005;94:2441–2451. doi: 10.1002/jps.20460. [DOI] [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006;79:1944–1955. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5. [DOI] [PubMed] [Google Scholar]
- Ji B, Ito K, Suzuki H, Sugiyama Y, Horie T. Multidrug resistance-associated protein2 (MRP2) plays an important role in the biliary excretion of glutathione conjugates of 4-hydroxynonenal. Free Radic. Biol. Med. 2002;33:370–378. doi: 10.1016/s0891-5849(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Jowsey IR, Jiang Q, Itoh K, Yamamoto M, Hayes JD. Expression of the aflatoxin B1-8,9-epoxide-metabolizing murine glutathione S-transferase A3 subunit is regulated by the Nrf2 transcription factor through an antioxidant response element. Mol. Pharmacol. 2003;64:1018–1028. doi: 10.1124/mol.64.5.1018. [DOI] [PubMed] [Google Scholar]
- Keitel V, Kartenbeck J, Nies AT, Spring H, Brom M, Keppler D. Impaired protein maturation of the conjugate export pump multidrug resistance protein 2 as a consequence of a deletion mutation in Dubin-Johnson syndrome. Hepatology. 2000;32:1317–1328. doi: 10.1053/jhep.2000.19791. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim YC. Effects of betaine supplementation on hepatic metabolism of sulfur-containing amino acids in mice. J. Hepatol. 2005;42:907–913. doi: 10.1016/j.jhep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473–1484. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- Lew JL, Zhao A, Yu J, Huang L, De Pedro N, Pelaez F, Wright SD, Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem. 2004;279:8856–8861. doi: 10.1074/jbc.M306422200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Callaghan TN, Cheng X, Cheung C, Gonzalez FJ, Klaassen CD. Alterations in transporter expression in liver, kidney, and duodenum after targeted disruption of the transcription factor HNF1alpha. Biochem. Pharmacol. 2006;72:512–522. doi: 10.1016/j.bcp.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Suzuki H, Hirohashi T, Ishikawa T, Meier PJ, Hirose K, Akizawa T, Yoshioka M, Sugiyama Y. Characterization of inducible nature of MRP3 in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G438–446. doi: 10.1152/ajpgi.2000.278.3.G438. [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- Petzer JP, Navamal M, Johnson JK, Kwak MK, Kensler TW, Fishbein JC. Phase 2 enzyme induction by the major metabolite of oltipraz. Chem. Res. Toxicol. 2003;16:1463–1469. doi: 10.1021/tx034154e. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cMOAT/MRP2. Semin. Liver Dis. 1998;18:359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- Takikawa H. Hepatobiliary transport of bile acids and organic anions. J. Hepatobiliary Pancreat. Surg. 2002;9:443–447. doi: 10.1007/s005340200055. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Chen C, Maher JM, Klaassen CD. Kupffer cell-mediated downregulation of hepatic transporter expression in rat hepatic ischemia-reperfusion. Transplantation. 2006;82:258–266. doi: 10.1097/01.tp.0000226243.69023.54. [DOI] [PubMed] [Google Scholar]
- Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N. Engl. J. Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- Umemura T, Kuroiwa Y, Kitamura Y, Ishii Y, Kanki K, Kodama Y, Itoh K, Yamamoto M, Nishikawa A, Hirose M. A crucial role of Nrf2 in in vivo defense against oxidative damage by an environmental pollutant, pentachlorophenol. Toxicol. Sci. 2006;90:111–119. doi: 10.1093/toxsci/kfj076. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath V, Wielandt AM, Iruretagoyena M, Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem. J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Takashimizu S, Kojima S, Kagawa T, Nishizaki Y, Mine T, Matsuzaki S. Clinical and pathological features of a prolonged type of acute intrahepatic cholestasis. Hepatol. Res. 2007;37:598–607. doi: 10.1111/j.1872-034X.2007.00115.x. [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J. Hepatol. 2003;38:717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.