Abstract
Most molecular and cellular studies of cognitive function have focused on either normal or pathological states, but recent research with transgenic mice has started to address the mechanisms of enhanced cognition. These results point to key synaptic and nuclear signalling events that can be manipulated to facilitate the induction or increase the stability of synaptic plasticity, and therefore enhance the acquisition or retention of information. Here, we review these surprising findings and explore their implications to both mechanisms of learning and memory and to ongoing efforts to develop treatments for cognitive disorders. These findings represent the beginning of a fundamental new approach in the study of enhanced cognition.
A number of psychiatric and neurological disorders, such as Alzheimer’s disease1, schizophrenia2, depression3, Parkinson’s disease4, learning disabilities5, age-related cognitive decline6 and mental retardation7 are associated with learning and memory (L&M) impairments. The dominant paradigm for the development of treatments for these disorders is based on the idea that insights into the specific mechanisms that underlie each of these conditions will lead to the development of targeted interventions. The problem with this approach is that there are a large number of different causes for cognitive deficits. Genetic analyses of schizophrenia and depression, two disorders that are associated with significant cognitive impairments, have shown that they are caused by numerous mutations and many developmental and environmental factors. Similar genetic heterogeneity has been demonstrated in every other major cause for cognitive deficits, including Alzheimer’s disease, learning disabilities, mental retardation and age-related cognitive decline. Thus, developing targeted therapies for each of the multiple causes of these conditions will be a formidable task. Even if targeted therapies for one or more of these specific genetic conditions are forthcoming, it is unlikely that they will have a significant impact on the cognitive impairments thought to afflict more than one in 20 people worldwide. Therefore, in addition to the prevalent targeted therapy approach, there is a need to develop alternative strategies that could have a more general impact on cognition. One possibility would be to develop strategies to ameliorate cognitive deficits irrespective of their specific genetic or environmental cause.
Although there is considerable evidence for animals and people with dramatic cognitive enhancements8,9, mechanistic studies of exceptional cognition are relatively rare. Remarkably, transgenic and KO studies in mice have revealed a surprisingly large number of mutations that seem to enhance cognitive function (TABLE 1). These mutations target a number of different signalling pathways and affect a plethora of behaviours. Surprisingly, nearly all of them seem to enhance a form of synaptic plasticity referred to as long-term potentiation (LTP).
Table 1.
Mouse models showing enhancement of learning and memory
| Gene | Tg/KO | Strain | Behavioural phenotypes | Plasticity phenotypes | Comments | Refs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| wm | cxt | cue | ext | obj | LTP | LTD | |||||
| NMDA receptor-related signalling | |||||||||||
| NR2B | Tg | B6/CBF1 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | — | NA | 9 |
| Cdk5 | C-KO | NA | ↑ | ↑ | — | ↑ | ND | ↑ | ND | Only reverse water maze enhanced | 38 |
| p25 | C-Tg | C57Bl/6J | ↑ | ↑ | — | ND | ND | ↑ | ND | Only transient expression enhances memory | 41 |
| KIF17 | Tg | BDF | ↑ | ND | ND | ND | ND | ND | ND | Working memory is also enhanced | 29 |
| Cavβ3 | KO | B6/129 | ND | ↑ | — | ND | ↑ | ↑ | — | NA | 26 |
| Calcium homeostasis-related signalling | |||||||||||
| RyR3 | KO | C57Bl/6J | ↑ | ND | ND | ND | ND | ↑ | ↓ | But also see 50 | 189 |
| Ncx2 | KO | B6/129 | ↑ | ↑ | — | ND | ↑ | ↑ | ↓ | NA | 53 |
| Kinase and phosphatase | |||||||||||
| Calcineurin | C-I | C57Bl/6J | ↑ | ND | ↑ | ↓ | ↑ | ↑ | — | But also see 75 | 72, 73 |
| PP1 | C-I | C57Bl/6J | ↑ | ND | ND | ND | ↑ | ↑ | ↓ | NA | 64, 190 |
| AC1 | Tg | C57Bl/6 | ND | — | — | ↓ | ↑ | ↑ | ND | NA | 80 |
| Ap oa1 | Tg | C57Bl/6J | ND | ↑ | ND | ND | ↑ | ↑ | ND | NA | 82 |
| CaMKIV | Tg | C57Bl/6N | ND | ↑ | ND | ND | ND | ↑ | ND | Also see 93 | 94 |
| RNA and protein synthesis | |||||||||||
| eIF2α | Tg | C57Bl/6J | ↑ | ↑ | ↑ | ND | ND | ↑ | ND | NA | 104 |
| GCN2 | KO | 129SvEv | ↑ | ↓ | — | ND | ND | ↑ | — | Learning and LTP are impaired with strong training | 103 |
| ATF4, C/EBP | C-I | C57Bl/6 | ↑ | ND | ND | ND | ND | ↑ | ↓ | Learning is enhanced after only weak training | 99 |
| Proto-oncogenes | |||||||||||
| H-ras | Tg | B6/129 | ↑ | ↑ | ND | ND | ND | ↑ | ND | NA | 135 |
| Cbl-b | KO | C57Bl/6 | ↑ | ND | ND | ND | ND | — | ND | Only remote memory is enhanced | 140 |
| Structural genes | |||||||||||
| tPA | Tg | NA | ↑ | ND | ND | ND | ND | ↑ | — | NA | 117 |
| HB-GAM | Tg | FVB/NHsd | ↑ | — | ↓ | ND | ND | ↑, — | ND | Gene controls inhibition and this complicates the LTP studies | 131, 132 |
| TLCN | KO | C57 × CBA | — | — | ND | ND | ND | ↑ | ND | Learning and memory for non-aversive tasks are enhanced | 126 |
| GAP43 | Tg | C57Bl/6 | ↑ | ND | ND | ND | ND | ↑ | ND | But also see 192 | 191 |
| GABA-related signalling | |||||||||||
| GABAAα5 | KO | B6/129 | ↑ | ND | ND | ND | ND | — | ND | NA | 163 |
| GRPR | KO | C57Bl/6J | — | ↑ | ↑ | ND | ND | ↑ | ND | LTP in amygdala is enhanced | 193 |
| Glial signalling | |||||||||||
| S100b | KO | C57Bl/6J | ↑ | ↑ | ND | ND | ND | ↑ | ND | NA | 148 |
| DAAO | KO | NA | ↑ | ND | ND | ND | ND | ↑ | ND | NA | 151 |
| Miscellaneous | |||||||||||
| ORL1 | KO | B6/129 | ↑ | ↑ | — | ND | ND | ↑ | ND | Enhanced fear memory only 7 days after training | 21, 42 |
| 5-HT3R | Tg | B6SJL/F2 | ND | ↑ | — | — | ND | ND | ND | NA | 154 |
| MAOA | KO | C3H/HeJ | ND | ↑ | ↑ | ND | ND | ND | ND | NA | 155 |
| HDC | KO | C57Bl/6 (129Sv) | ↑ | ↑ | ↑ | ND | ↓ | ↑ | — | Water maze phenotype was found in 129Sv background | 157, 158 |
| HCN1 | KO | B6/129 | ↑ | — | — | ND | ND | ↑ | ND | LTP at perforant path is enhanced. Also see 195 | 194 |
| DEF45 | KO | B6/129 | ↑ | ND | ND | ND | ↑ | ND | ND | Better recognition memory, only up to 3 hours after training | 196, 197 |
| Kvβ1.1 | KO | C57Bl/6 | ↑ | ND | ND | ND | ND | ↑ | ND | 18 month-olds mice were used. Also 198 | 164 |
| EC-SOD | Tg | C57Bl/6 × C3H | ↑ | ↓ | — | ND | ND | ↑ | ND | 20 month-olds mice were used. Also see 200 | 199 |
GABA, γ-aminobutyric acid; C-I, conditional inhibition; C-KO, conditional knockout; C-Tg, conditional transgenic; cue, cued fear conditioning; cxt, context fear conditioning; ext, fear extinction; KO, knockout; LTD, long-term depression; LTP, long-term potentiation; NA, not applicable; ND, not determined; NMDA, N-methyl-D-aspartate; obj, object recognition task; Tg, transgenic; wm, water maze; —, no change.
This Review focuses on a subset of representative mutant mice with enhanced performance in well-characterized L&M behavioural tasks such as the Morris water maze and fear conditioning (BOX 1). We classify these ‘smart’ mutant mice into several groups according to the signal transduction pathways affected, and briefly summarize how each of the mutations is thought to affect synaptic plasticity and L&M. We will also highlight commonalities and propose mechanisms that could be targeted for the development of drugs for L&M disorders.
Box 1. Behavioural tests for learning and memory
Morris water maze
This is one of the most used spatial learning and memory (L&M) tasks known to depend on the hippocampus. Animals swim in a murky pool of water to find the location of a submerged platform just beneath the surface of the water. To escape the water, mice use a variety of cues and strategies, including spatial cues around the pool in the room. Animals are trained for several days and the time/path length they take to find the platform is usually measured as a learning index. A more sensitive measure of spatial learning is performance in probe trials in which the platform is removed from the pool and the mice are allowed to search for it for a short period of time (for example, 60 seconds). A common learning index for this test is the percentage of time that the mice spend looking for the platform in the quadrant where the platform was during training.
Novel-object recognition task
This is a non-aversive, non-spatial test that requires hippocampal function. In this test, animals are allowed to freely explore two objects in an open field during training sessions. In the test sessions, one of the objects is replaced by a novel object. Short (for example, 1 hour) or long-term memory (for example, 24 hours) is measured as a ratio of time spent exploring the novel object versus the familiar object. Variants of this task use other stimuli, such as smells and even conspecifics (social recognition).
Radial arm maze
This is a spatial learning task with various versions. The apparatus has several arms (most commonly eight) that can be baited with food pellets at the end. Food-deprived animals are allowed to enter the arms and search for the hidden food. In a common version of this task, which is sensitive to both hippocampus and prefrontal cortex lesions, food-deprived animals are first (phase A) allowed to retrieve food pellets from 4 accessible arms of an 8-arm maze (the remaining 4 arms are blocked). After a retention interval of (for example, 2 minutes) animals are brought back to the maze (phase B) and are given access to all 8 arms, but only the 4 previously blocked arms are now baited. Within-phase errors are committed when mice enter an arm previously visited in the same trial; across-phase errors are committed when mice enter an arm in phase B that they had already visited in phase A.
Fear conditioning
This is a Pavlovian aversive learning task in which animals associate a non-aversive conditioned stimuli (CS), such as a tone or context, with an aversive unconditioned stimulus (US; for example, footshock). Conditioned responses, usually freezing (cessation of all but respiratory movement) are used as measures of memory. There are two common versions of this test: in tone conditioning the CS is a tone that precedes and co-terminates with the US; in context conditioning the CS is the context in which the animals are conditioned (that is, a chamber). Fear memories can last a lifetime but they can also be extinguished by repeated exposures to the CS without the US.
Conditioned taste aversion
This is an aversive learning task in which animals associate a food source (for example, saccharine flavoured water; CS) with malaise usually induced by LiCl injection (US). Avoidance of the food previously associated with malaise is used as a memory index.
Inhibitory avoidance
In this task the training apparatus has metal grids on the floor which can deliver a footshock. One part of the grid is covered to provide a safe platform for animals. During training, animals are placed on the safe platform and once they voluntarily step down to the grids they automatically receive a shock. Memory is assessed by measuring the time the animals spend on the platform before stepping down.
Passive avoidance
Here the animal learns to inhibit a natural tendency, namely to step into an apparently safer, dark compartment that has previously been associated with footshock.
Latent inhibition
In this group of tasks, extended pre-exposure to a stimulus prevents its association with an US, such as footshock. For example, extensive exposure to a conditioning chamber before conditioning weakens or even prevents association between the chamber and the footshock.
NMDA receptors and enhanced cognitive function
The critical role of N-methyl-D-aspartate receptors (NMDARs) in synaptic plasticity and memory has been extensively researched with both genetic and pharmacological manipulations10–18. NMDARs are composed of an obligatory subunit NR1 and other modulatory subunits including NR2 (with A, B, C and D subtypes) and NR3 (with A and B subtypes). The receptor subunit compositions change during development19,20.
Upregulation of NR2B
There have been previous reports of mice with enhanced L&M21, but the doogie mouse, which overexpressed the NR2B subunit in the adult forebrain, was the first widely-publicized ‘smart’ mouse9. Normally, the expression of NR2B is decreased during post-natal development19. The prolonged NMDAR currents resulting from overexpression of NR2B led to the enhancement of hippocampal CA1 LTP, a finding that is consistent with more robust levels of LTP during the developmental stages expressing higher levels of this receptor subunit22. Doogie mice were shown to have enhanced performance in several different hippocampal L&M tasks (BOX 1), including novel-object recognition9 and spatial memory tested with the Morris water maze23. In addition, both contextual and tone (cued) fear memory and fear extinction were enhanced in doogie mice. Fear extinction is thought to reflect learning that the tone no longer is associated with shock, and is known to be NMDAR-dependent24. A recent follow-up study showed that even aged doogie mice out-perform age-matched controls, indicating that the NR2B-dependent L&M enhancement lasts into old age25.
Mutant mouse in which the β3 subunit of the voltage-dependent Ca2+ channels (Cavβ3) was knocked out also showed enhanced LTP and memory due to increased NMDAR function. The NMDA-mediated current and NMDAR-dependent LTP were increased in the hippocampus of Cavβ3-knockout mice. Although the underlying molecular mechanism is unclear, NR2B expression was slightly, but significantly increased in the knockout mice. These mice performed better than controls in a series of hippocampus-dependent L&M tasks, including contextual fear conditioning, novel-object recognition and social transmission of food preferences26. These studies suggest that NR2B or related downstream signalling molecules could be promising targets for the development of cognitive enhancement strategies for aged subjects.
Transport of NMDA receptors
The trafficking of glutamate receptors from the cytoplasm to synaptic sites is known to have an important role in synaptic plasticity and in L&M27,28. Transgenic mice overexpressing KIF17, a protein that transports NR2B along microtubules, outperform controls in spatial learning and working memory tasks29. Interestingly, both NR2B mRNA and protein levels were higher in the KIF17 transgenic mice, but it is unknown whether the levels of NR2B are increased specifically in synaptic sites. Functional inhibition of KIF17 in cultured neurons, driven by the overexpression of a dominant negative form of KIF17, decreased the number of synaptic NR2B clusters. It is therefore conceivable that enhanced transport of NR2B and thus, higher levels of this subunit in synaptic NMDARs could explain better learning in mice overexpressing KIF1730. The transcription factor cyclic-AMP response-element-binding protein (CREB) in these mice showed higher levels of phosphorylation at serine 133 (S133), which is associated with higher levels of CREB-dependent transcription31. As discussed below, this transcription factor is required for the stability of LTP and memory32,33 and it is activated downstream of NMDAR stimulation34. Therefore, it is possible that overexpression of KIF17 results in a train of events leading to higher levels of NR2B at synaptic sites, larger NMDAR currents, stronger CREB activation, higher and more stable LTP, faster learning and better memory.
Degradation of NMDA receptors
The Ca2+-dependent protease calpain regulates the degradation of NMDA receptors35,36. Calpain is activated by Ca2+ entry through NMDARs and rapidly cleaves NMDAR subunits resulting in a decrease in the number of functional NMDA receptors in the postsynaptic density36,37. There is evidence that cyclin-dependent kinase 5 (Cdk5) may regulate calpain-dependent proteolysis of NR2B; deletion of Cdk5 in adult mouse forebrain reduced NR2B degradation and consequently augmented NMDA-mediated current, resulting in stronger LTP and enhanced contextual fear conditioning, faster fear extinction and more flexible learning in the reversal water maze task38. This indicates that Cdk5 plays a part in modulating the proteolysis of NR2B and that this is important for synaptic plasticity and learning.
The involvement of Cdk5 in L&M is complex, perhaps because this molecule has a variety of substrates and binds many different cofactors39,40. Chronic activation of p25, a strong activator of Cdk5, caused neuronal loss in the cortex and hippocampus, and severely impaired synaptic plasticity and learning41. Surprisingly, transient expression of p25 in mice forebrain enhanced synaptic plasticity and hippocampus-dependent memory, including contextual fear conditioning and the Morris water maze41. NR2A phosphorylation and NMDAR-mediated currents were both increased after transient overexpression of p25 (REF. 41). It would be interesting to investigate whether calpain-mediated NR2B cleavage and/or the NR2B-mediated current is changed in this mouse model.
Activation of CaMKII
One of the first genetic manipulations reported to enhance learning and synaptic plasticity in mice involved the nociceptin receptor (ORL1)21. Mice lacking ORL1 showed normal pain sensitivity but enhanced L&M in the Morris water maze and passive avoidance tasks (BOX1). Moreover, LTP was significantly enhanced in this mutant. Follow-up studies indicated that the ORL1 mutation resulted in enhanced NMDA receptor function and more rapid activation of its key downstream effector, a calcium calmodulin kinase II (αCaMKII)42. Interestingly, application of the nociceptin peptide should increase the function of the nociceptin receptor and, not surprisingly, this had opposite molecular, electrophysiological and behavioural effects to nociceptin receptor knockout43. These results suggest that nociceptin-mediated signalling regulates NMDA receptor-dependent activation of αCaMKII and functions as a key constraint of plasticity and L&M.
Besides genetic manipulations, pharmacological drugs targeting glutamatergic systems have been developed. For example, ampakines, which are known to enhance attention span and L&M, positively modulate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid-type glutamate receptors (AMPARs) and subsequently facilitate NMDAR-dependent LTP induction44. Interestingly, the appetite hormone leptin is reported to enhance synaptic plasticity and L&M by upregulating NMDAR function and αCaMKII activity45,46. These findings demonstrate that upregulation of NMDAR function can result in enhancements of L&M (FIG. 1). One of the consequences of upregulating NMDAR function is an increase in synaptic Ca2+. Next, we describe how manipulating other regulators of intracellular calcium can also result in enhanced plasticity and L&M.
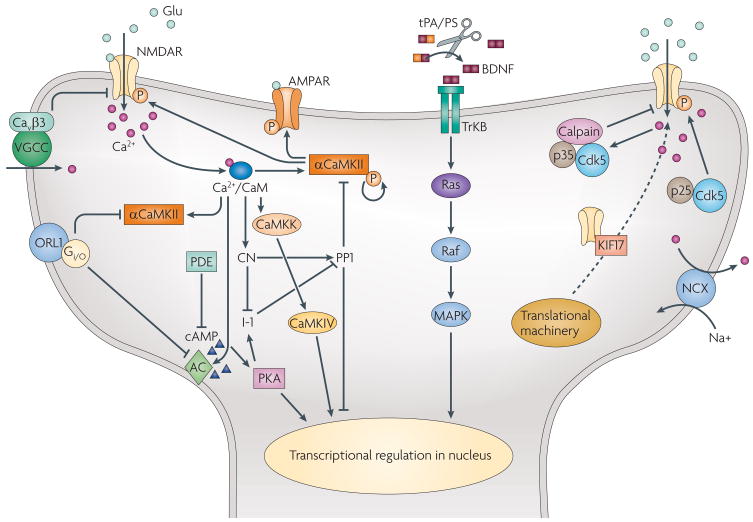
Figure 1. NMDAR-dependent signalling and downstream kinases and phosphatases implicated in learning and memory enhancement.
N-methyl-D-aspartate receptor (NMDAR) function can be positively regulated by α calcium calmodulin kinase (αCaMKII) phosphorylation and by the transient activation of cyclin-dependent kinase 5 (Cdk5) through the positive regulator p25. Transport of the NR2B subunit to synaptic sites can be increased by overexpressing the motor protein KIF17. Calpain, possibly modulated by Cdk5, downregulates NR2B by proteolysis. The β3 subunit of voltage-gated calcium channel (VGCC) and the nociceptin receptor ORL1 also negatively regulate NMDAR expression or function by unknown mechanisms. Calcium influx through NMDARs activates αCaMKII, which in turn positively regulates NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) function, contributing to the induction and expression of long-term potentiation (LTP), respectively. In addition, neuronal calcium concentration can be regulated by Na+/Ca2+ exchangers (NCXs), which extrude Ca2+ from neurons. Calcium/calmodulin (CaM) activates downstream kinases and phosphatases: it activates adenylyl cyclase (AC) to produce cAMP, which activates protein kinase A (PKA) and eventually regulates cyclic-AMP response-element-binding protein (CREB) activity in the nucleus. By phosphorylating inhibitor-1 (I-1), PKA can antagonize the action of protein phosphatase 1 (PP1), which is activated by the calcium/CaM-activated phosphatase calcineurin (CN). CaM also activates calcium CaM kinase kinase (CaMKK), which in turn activates calcium/CaM kinase IV (CaMKIV), another positive regulator of transcription. Activation of TrkB by brain-derived neurotrophic factor (BDNF) triggers the mitogen-activated protein kinase (MAPK) signalling pathway and ultimately regulates transcription. Sharp and blunted arrows represent positive and negative regulation, respectively. tPA/PS, tissue-type plasminogen activator/plasmin; PDE, phosphodiesterase.
Tipping Ca2+ homeostasis
Postsynaptic rises in Ca2+ and subsequent activation of downstream signalling molecules are crucial for long-term forms of synaptic plasticity, including LTP and long-term depression (LTD)47,48. In addition to influx through membrane channels, Ca2+ can be released from internal stores through inositol trisphosphate (IP3) or ryanodine-sensitive receptors (RyRs). RyR3-deficient mice showed better L&M in the spatial version of the Morris water maze. Unlike controls, in which the induction of NMDA-independent LTP can only be triggered after very strong stimulation protocols (four stimulus trains, 200 Hz, 500 ms), which are thought to recruit internal Ca2+ sources49, in these mutants NMDA-independent LTP could be induced with a relatively weak stimulation protocol (four stimulus trains, 100 Hz, 100 ms), suggesting that this mutation altered the dynamic interaction between extracellular and intracellular calcium release involved in LTP. By contrast, another mutation of this receptor failed to enhance LTP or improve learning in mutant mice50. Differences in the genetic background of the mouse strains51 could explain the difference between the results of these two studies. A great deal of caution is required when interpreting memory-enhancing phenotypes in mutants: does the mutation enhance memory by upregulating memory mechanisms or by merely compensating for unknown mutations in the genetic background of a specific inbred mouse line? Using hybrid strains, in which most of recessive alleles are likely to be suppressed, would help to defray this concern. In this Review we emphasize memory-enhancing mechanisms, such as enhanced NMDAR signalling, with convergent evidence from different mutants, different strains and from different learning tasks. This convergence is crucial for demonstrating the specificity and reliability of the findings.
Clearance of intracellular Ca2+ is another important mechanism for regulating intracellular Ca2+ concentrations. The Na+/Ca2+ exchanger (NCX) is known to have a role in Ca2+ homeostasis by extruding Ca2+ from neurons at high turnover rates in the brain52. Mutant mice lacking NCX2, the predominant isoform in adult brain, exhibited enhanced performance in a number of hippocampal tasks, including the Morris water maze, contextual conditioning and object recognition53. Previous studies indicated that the magnitude and temporal pattern of internal Ca2+ triggers different downstream signalling pathways and determines whether LTP or LTD are induced54,55. Remarkably, not only did the NCX2 mutants show enhanced LTP, physiological protocols that normally induce LTD, triggered LTP instead. This result indicates that it is possible to enhance L&M even when the fundamental rules of potentiation and depression are seemingly altered.
These studies demonstrate that it is possible to enhance plasticity and L&M by manipulating mechanisms that regulate intracellular calcium concentrations. However the relation between these processes and L&M is poorly understood.
Balance between kinases and phosphatases
There is growing evidence that opposing kinases and phosphatases that are downstream of NMDARs determine whether incoming signals enhance or suppress synaptic plasticity and therefore facilitate or dampen L&M processes55,56. CaMKII, as discussed above, protein kinase A (PKA), mitogen-activated protein kinase (MAPK) and protein kinase Mζ (PKMζ) are well-known positive regulators57–61, whereas the phosphatase calcineurin (also known as PP2B) and protein phosphatase 1 (PP1) serve as negative regulators of both synaptic plasticity and L&M62–65.
Calcineurin
Calcineurin can affect synaptic plasticity in many different ways, including modulation of NMDAR-mediated currents, PP1 and the GTPase activity of dynamin I66–68. In addition, the existence of calcineurin-activated adenylyl cyclase (AC) in hippocampal neurons was also reported69.
Mice expressing a truncated, constitutively active form of calcineurin exhibited deficits in hippocampal LTP and memory62,70, supporting the idea that this phosphatase inhibits LTP and learning71. When calcineurin was inhibited in adult mice forebrain in a spatially and temporally regulated fashion, namely by expressing the reverse tetracycline-responsive transcription factor (rtTA) under the control of a forebrain-specific αCaMKII promoter (see BOX 2 for details)72, the inducible expression of a calcineurin inhibitor significantly suppressed calcineurin activity in the hippocampus and cortex of mutant mice, leading to enhancements in LTP and improvements in both short- and long-term object recognition memory. Enhanced L&M was also observed in the spatial version of the Morris water maze, conditioned taste aversion and cued fear conditioning72,73. These and other studies have indicated that enhancing calcineurin function disrupts LTP and learning, whereas decreasing its function facilitates these two processes74.
Box 2. Evolution of transgenic technology
The first generation of mutant mice used in molecular and cellular cognition studies included germ-line targeted disruption or overexpression of genes of interest. Following studies with α calcium calmodulin kinase II (αCaMKII) homozygous-null mutants, showing that disruption of this gene led to impairments in long-term potentiation (LTP) and learning and memory (L&M)59,60, similar findings were reported upon deletion of the tyrosine kinase Fyn172. These early studies demonstrated the power of mammalian transgenic and knockout approaches in integrative studies of physiological mechanisms of behaviour, and they pointed to LTP as a key synaptic mechanism involved in L&M.
The second generation of mouse transgenic technologies introduced spatial and temporal control17,33,173–175. For example, transgenic mice with the P1 bacteriophage Cre recombinase under the regulation of the αCaMKII promoter175 allowed the deletion of loxP-flanked genes in principal neurons of the postnatal forebrain. Temporal control of transgenic expression in the brain was first achieved by employing the tetracycline system176. Doxycycline binds to the tetracycline-responsive transcription factor tTA (or rtTA) and is used in this system to temporally turn on or off transgene expression17,173,176. This transcription factor can be expressed under the control of cell-type and region-specific promoters, such as the αCaMKII promoter, to add spatial control to this inducible system. Introducing heterologous genes from another organism can be combined with this technique. For example, overexpression of anAplysiaoctopamine receptor (Ap oa1), a Gs-protein-coupled receptor, which selectively stimulates PKA signalling, provided an ideal system to study the role of acute manipulation of cAMP signalling in synaptic plasticity and L&M82,177. Despite its power, the doxycycline system is limited by the requirement for the derivation and crossbreeding of several mouse mutant lines.
Another powerful inducible and region restricted transgenic system takes advantage of gene fusions with the mutant version of the ligand-binding domain of the oestrogen receptor (LBD) under the regulation of tissue- and cell-specific promoters such as the αCaMKII promoter33. Binding of tamoxifen to the LBD changes its conformation, excluding large heat-shock proteins that prevent the fusion protein of interest to bind, therefore modulating the function of its partners33. The significant advantage of this system is that it requires only a single transgene and manipulations can be turned on and off much faster than the tetracycline system (hours versus days)33. The limitation is that not all proteins can be regulated with LBD fusions.
Recently, chemical-regulation of engineered proteins, such as kinases, has been used to quickly activate and inactivate them in plasticity and L&M studies178. Genetic manipulations with even higher spatial and temporal resolution, targeting specific neurons in networks of interest, are currently under development and will undoubtedly have a key role in the next generation of molecular and cellular cognition studies179. Even though mutant mouse studies of L&M have certain limitations180, it is obvious that they have had an enormous impact on our understanding of the molecular mechanisms of cognitive function, including those that can be recruited to enhance L&M.
A genetic approach to inhibit calcineurin, by deletion of its regulatory subunit CNB1 in excitatory forebrain neurons of adult mice, led to suppression of hippocampal LTD and only to slight enhancements of LTP75. This mutant did not show enhanced performance in hippocampus-dependent memory tasks including the Morris water maze and contextual fear conditioning72,75. Furthermore, hippocampus-dependent working memory tasks, such as the delayed matching-to-place task and radial arm maze were impaired. Another study in rats showed that blocking calcineurin expression by infusing antisense oligonucleotides into brain ventricles enhanced hippocampal LTP induction and contextual fear conditioning, but not spatial learning in the Morris water maze76,77.
These studies show that the memory enhancements seem to be sensitive to the conditions in which the experiments were carried out. Considering the complexity of calcineurin signalling in neurons, it is not surprising that two different manipulations with different degrees of calcineurin inhibition resulted in inconsistent cellular and behavioural phenotypes.
Serine/threonine phosphatase PP1
Calcineurin regulates the activity of another serine/threonine phosphatase, PP1, by dephosphorylating inhibitor-1 (I-1)68 (FIG. 1). PP1 has a key role in inducing LTD and, like calcineurin, this phosphatase is also thought to be a negative regulator of memory78. To suppress PP1, a constitutively active form of I-1 (I-1*) was inducibly and reversibly expressed in adult brain with the rtTA system64 (BOX 2). The induction of I-1* suppressed PP1 activity by ~70% in the hippocampus. Mice expressing I-1* showed improved object recognition memory when they were trained with 5-minute intervals (massed training), whereas there was no difference between genotypes when they were trained with 10-minute intervals (spaced training), as if these longer intervals occluded the advantage conferred by the expression of the I-1* transgene. Interestingly, endogenous PP1 activity was found to be significantly larger in control mice trained with the shorter intervals (massed training), suggesting that one of the physiological consequences of using longer intervals between trainings is the repression of PP1 activity. The mutants also showed enhanced L&M in the spatial version of the Morris water maze. Strikingly, inhibition of PP1 after training strengthened memory, suggesting that PP1 expression normally weakens memory. Whether this is due to active erasure mechanisms or to failure of memory retrieval remains to be resolved. CaMKII, the AMPA receptor subunit GluR1 and CREB-dependent gene expression are modulated by PP1 and changes in any of one of these components could modulate the stability of memory.
Adenylyl cyclases
ACs have a role in synaptic plasticity and memory by coupling NMDAR Ca2+ signalling to downstream cAMP-dependent pathways79, including the PKA-dependent phosphorylation of I-1 and therefore, the inactivation of PP1. Of all the ACs identified, AC1 and AC8 are neuron-specific79. AC1 overexpression in the forebrain facilitated LTP in a PKA-dependent manner, enhanced memory in an object recognition task80 and remote memory for contextual conditioning81. To achieve both temporal and spatial specificity of cAMP-PKA activation, a novel transgenic system using an Aplysia octopamine receptor (Ap oa1) in mouse forebrain was introduced82 (BOX 2). Activation of Ap oa1 in the forebrain of mice enhanced LTP and memory in contextual fear conditioning and object recognition tasks82. PKA signalling can also be pharmacologically enhanced by inhibiting phosphodiesterases (PDEs) and degradation of cAMP. PDE4 inhibitors were shown to enhance synaptic plasticity and L&M in wild-type mice and in a mouse model of Rubinstein-Taybe syndrome83–85. These mice have a heterozygous mutation in CREB-binding protein (CBP), a transcriptional co-activator that binds active (phosphorylated) CREB. Just as with the I-1*, AC1 and Ap oa1 transgenics, PDE treatments resulted in enhanced CREB activity, a result that we will explore in the next section.
Together, these studies demonstrate that the balance between kinases and phosphatases is crucial for plasticity and learning, and that shifting this balance towards kinases could be used to enhance L&M (FIG. 1).
Relieving transcriptional repression
CREB and C/EBPs
In addition to gating plasticity and memory, cAMP-PKA/phosphatase regulated signalling is crucial for the activation of transcription factors, including CREB17,32,86–89 (FIG. 2). Genetic manipulations that result in decreased CREB transcription in mice lead to deficits in both LTP and long-term memory in a wide range of tasks32,33, whereas overexpression of CREB in flies, mice and rats results in memory enhancements17,90–92. CREB is thought to regulate the transcription of proteins needed to stabilize the synaptic changes that are triggered during learning. Increasing the levels of CREB transcription is thought to boost the levels of rate-limiting proteins required for the stability of plasticity and memory103. Increased CREB activity, due to overexpression of CaMKIV in mouse forebrain, also enhanced contextual fear and social recognition memory, as well as LTP, in both hippocampus and anterior cingulate cortex93,94.
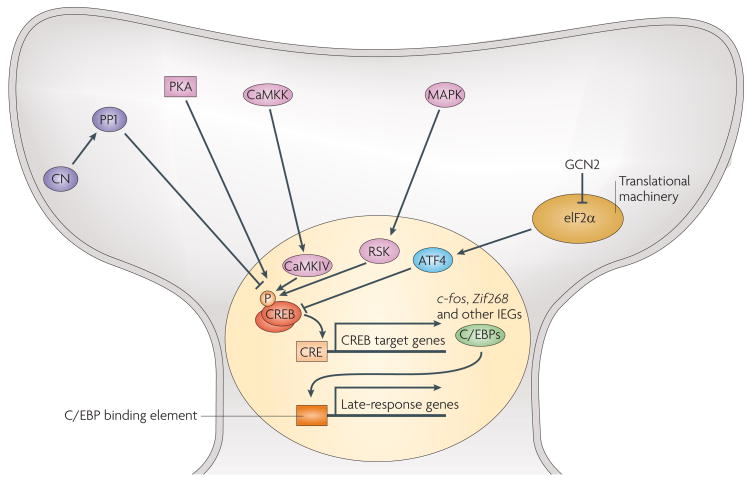
Figure 2. Regulation of CReB-dependent gene expression involved in memory formation.
The activity of cyclic-AMP response-element-binding protein (CREB) is regulated by phosphorylation or by molecular interactions. Protein kinase A (PKA), calcium calmodulin kinase IV (CaMKIV) and ribosomal S6 kinase (RSK) (activated by mitogen-activated protein (MAPK)) phosphorylate CREB at serine 133, whereas protein phosphatase-1 (PP1) dephosphorylates CREB. Another phosphatase, calcineurin (CN, also called PP2B) indirectly inhibits CREB function. Phosphorylated CREB recruits the CREB-binding protein (CBP) and activates the transcription of immediate early genes (IEGs) such as c-fos, Zif268 and C/EBPs (CCAAT/enhancer-binding protein). C/EBPs themselves function as transcription factors activating or inhibiting the expression of another group of genes (late-response genes). Transcriptional activity of CREB can be repressed by activating transcription factor 4 (ATF4), which is translationally regulated by the αsubunit of elongation factor 2 (eIF2α). Inhibition of eIF2α phosphorylation by GCN2 (general control non-depressible 2) reduces the translation of ATF4 mRNA and subsequently enhances CREB-dependent gene expression and learning and memory. ATF4 is thought to compete with CREB to bind to CRE and other transcriptional components including CBP. Sharp and blunted arrows represent positive and negative regulation, respectively. CaMKK, CaMK kinase.
Another family of transcription factors that has been implicated in memory is the CCAAT/enhancer-binding protein (C/EBP) family. The expression of these transcription factors is upregulated in Aplysia and in rodents in response to CREB activation109–111. In Aplysia, C/EBP was identified as an immediate early gene that is rapidly induced after repeated treatment with serotonin. This treatment induces long-term synaptic facilitation (LTF), the cellular mechanism of behavioural sensitization, in sensory to motor synapses in Aplysia. Importantly, inhibition of C/EBP in presynaptic sensory neurons blocked LTF95,96. In mice, the inhibition of C/EBPβ in the hippocampus blocked the consolidation of inhibitory avoidance memory97. Surprisingly, the deletion of C/EBPδ enhanced contextual fear memory98. Although it is a transcriptional activator, C/EBPδ might be less efficient than other activators with similar target sequences and therefore, work in opposition to these other transcription factors. Alternatively, C/EBPδ could mediate the activation of target genes whose products inhibit memory formation98.
Expression of a broad dominant negative inhibitor of the C/EBP family of transcription factors (EGFP-AZIP) in mouse forebrain99 suppresses the repressor isoform of C/EBPβ and decreases the expression of the activating transcription factor 4 (ATF4). ATF4, is a mammalian homologue of Aplysia CREB2 that interacts with the C/EBP family of transcription factors and is a negative regulator of CREB in vertebrates100. Thus, EGFP-AZIP, by suppressing two different transcriptional repressors, shifts the balance towards CREB and C/EBP transcriptional activator isoforms. Importantly, this lowered the threshold for LTP and memory formation: a tetanus that induces only early stages of LTP (E-LTP) in controls can induce transcription-dependent later forms of LTP (L-LTP) in EGFP-AZIP mice. Interestingly, LTD induction was reduced in hippocampal slices from mutant mice. Additionally, mutant mice showed enhanced learning when trained with a weak protocol in the Morris water maze99. These and other results101,102 show that relief of transcriptional repression can be used to enhance L&M across a wide range of species. It is important to note that C/EBP is thought to control the activation of several transcriptional cascades, therefore it is difficult to pin-point the exact molecular mechanisms underlying the memory enhancements that are observed in the EGFP-AZIP transgenics99. To identify genetic changes that might be associated with memory enhancement, gene expression profile studies were carried out with the EGFP-AZIP transgenics. Microarray analyses revealed eight genes that were differentially expressed99. Functional studies of these downstream molecules could shed light on the mechanisms responsible for the plasticity and memory enhancements of EGFP-AZIP transgenics.
eIF2/ATF4
Recently, other genetic manipulations thought to suppress ATF4 have also been reported to enhance L&M103,104. Phosphorylation of the α subunit of elongation factor 2 (eIF2α) can stimulate translation of ATF4, while inhibiting general translation105,106. Mice that are heterozygous for a point mutation which prevented phosphorylation of the eIF2α at serine 51 (eIF2α+/S51A) showed decreased levels of ATF4 (REF. 104). Stimulation protocols that induced E-LTP in controls were able to induce L-LTP in the eIF2α+/S51A mutants, therefore lowering the threshold for L-LTP induction. Importantly, these mice showed improved L&M in various behavioural tasks including contextual and cued-fear conditioning, conditioned taste aversion and latent inhibition (BOX 1).
Deletion of GCN2, a conserved eIF2α kinase, reduced phosphorylation of eIF2α, suppressed the translation of ATF4 mRNA103 and enhanced L&M. These studies support the hypothesis that the transcriptional repressor ATF4 is an important negative regulator of synaptic plasticity and memory and thus, a potential target for drugs designed to enhance these phenomena.
Epigenetic mechanisms
Neuronal gene expression can be regulated by epigenetic mechanisms such as histone modification and chromatin remodeling107. Recent evidence suggests that the repression of gene transcription by epigenetic mechanisms such as histone deacetylation in mice and rats regulates synaptic plasticity and L&M85,108–111. Moreover, enhancing histone acetylation with histone deacetylase (HDAC) inhibitors has been shown to enhance L&M through CREB and CBP-dependent transcriptional activation85,109,110,112. These results suggest that synaptic plasticity and memory can be enhanced by chromatin changes that favour transcriptional activation.
Extracellular factors
Growth factors
Molecules that orchestrate or mediate synaptic structural changes often play a role in synaptic plasticity and memory. For example, tissue-type plasminogen activator (tPA), an extracellular serine protease that converts plasminogen into plasmin, is an activity-induced gene in the hippocampus113 that modulates plasticity and memory. Genetic deletion of tPA in mice resulted in deficits in L-LTP as well as impairments in several forms of memory114–116. Importantly, transgenic neuronal overexpression of tPA enhanced both LTP and hippocampus-dependent spatial memory117. Recent findings indicated that plasmin converts brain-derived neurotrophic factor precursor (proBDNF) to mature BDNF (mBDNF) and that this conversion is critical for L-LTP in mouse hippocampus118. Various studies indicate that BDNF has a key role in synaptic plasticity and learning119,120. Interestingly, some ampakines, are also reported to increase BDNF expression121. Therefore, it is possible that the increased conversion of proBDNF to mBDNF in the tPA mutants contributes to enhanced LTP and memory.
Cell adhesion molecules
Studies in Aplysia and Drosophila melanogaster suggest that synaptic growth and plasticity involves the downregulation of cell adhesion molecules (CAMs)122–124. For example, the Aplysia cell adhesion molecule (apCAM) is internalized in response to LTF-induction in sensory neurons and blocking this internalization impairs LTF and synaptic growth124,125. This internalization is thought to relieve the CAM-dependent structural constraints during synaptic remodeling that are required for long-term plasticity and memory.
Ablation of the CAM telencephalin (TLCN, also known as intercellular adhesion molecule 5) enhanced LTP and performance in some learning tasks, especially when a positive reward was involved, such as radial maze and water-finding tasks126, but not others such as the Morris water maze or fear conditioning tasks, suggesting that the effect of this mutation is task-specific. Pre-pulse inhibition, a measure of sensorimotor gating which involves many brain regions including prefrontal cortex, hippocampus, amygdala and nucleus accumbens, was found to be enhanced in TLCN-knockout mice126. Determining how this mutation affects different brain systems might provide clues for the specific modulation of appetitive learning. The relation between CAMs and learning is complex as other CAM deletion studies resulted in behavioural abnormalities127,128.
Extracellular matrix
Heparin-binding growth-associated molecule (HB-GAM, also known as pleiotrophin) is an extracellular matrix-associated protein that is implicated in the regulation of neurite outgrowth, axon guidance and synaptogenesis129,130. Transgenic overexpression of HB-GAM in the brain improved spatial learning in the Morris water maze. Although initially these mice were thought to have deficits in LTP131, a more thorough analysis132 showed that this deficit was due to enhanced γ-aminobutyric acid A (GABAA) inhibition and that when this is accounted for, LTP mechanisms show a slight, but not statistically significant enhancement. These results are an example of the power and limitations of studying LTP in tissue slices versus the far more complex in vivo recordings. Although slice preparations facilitate the study of synaptic plasticity, results are occasionally misleading because they do not always reflect in vivo conditions. It is possible that the enhanced L&M in HB-GAM transgenic mice is caused by their higher LTP which is not masked by enhanced inhibition in vivo during learning. Interestingly, HB-GAM is also a proto-oncogene. Next we will describe other studies that involve this class of molecules in memory enhancement.
Proto-oncogenes and the regulation of memory
H-ras
Although the memory enhancement studies reviewed so far were focused on postsynaptic signalling mechanisms, there is emerging evidence for a role of presynaptic signalling133,134 in mammalian plasticity and learning. Studies of mice expressing a constitutively active form of the proto-oncogene H-ras (H-rasG12V) in axons of pyramidal neurons of the postnatal hippocampus, revealed a role for presynaptic Ras/MAPK signalling in LTP and L&M135. Confocal and electron microscopy analysis demonstrated predominant expression of H-ras in presynaptic axon terminals, suggesting that the Ras family of signalling molecules might have a role in presynaptic function. Although a postsynaptic role had also been reported previously, it remains to be demonstrated whether this has functional implications for L&M136. H-rasG12V presynaptic expression resulted in increases in the activation of MAPK and in the phosphorylation of its substrate, synapsin I. In agreement with a role for synapsin I phosphorylation in vesicle docking and neurotransmitter release, LTP in the hippocampal CA1 region was enhanced in these mutants, and behavioural studies demonstrated dramatic hippocampal-dependent learning enhancements. Importantly, a synapsin I mutation, which alone had no measurable effect in LTP and learning, reversed the physiological and behavioural enhancements of the H-rasG12V mice, indicating that H-rasG12V-dependent phosphorylation of synapsin I has a key role in the learning enhancements of these mutants. These results provided strong evidence that the learning enhancements described were caused by presynaptic mechanisms involving Ras/MAPK upregulation and subsequent phosphorylation of synapsin I at its MAPK site. Extensive studies in Aplysia137,138 have provided compelling evidence for a role for presynaptic signalling mechanisms in synaptic plasticity and learning, and the H-rasG12V studies indicated that presynaptic signalling also has a crucial role in plasticity and learning in mammals.
Cbl
Cbl belongs to another family of proto-oncogenes that are ubiquitin ligases and function as negative regulators of activated tyrosine-kinase-coupled receptors in the immune system139. Cbl-b is highly expressed in the brain including hippocampus140 and mice lacking cbl-b140 showed specific enhancements in remote memory: spatial learning and 1-day memory were normal, but memory tested at 45 days was considerably more robust in the mutants. Although little is known about the role of cbl-b in the brain, it is possible that this proto-oncogene controls plasticity processes in the neocortex that are required for remote memory141,142.
Glial regulation of memory enhancements
Studies of L&M have largely focused on neuronal cells. However, there is increasing physiological and behavioural evidence that glial cells have an active role in information processing143,144 and might also have a role in L&M145. Overexpression of S100b, a Ca2+ binding protein secreted from astrocytes146, impaired LTP and spatial learning in mice147. Targeted disruption of S100b enhanced LTP and exogenous S100b treatment reversed this enhancement148; Behavioural analysis with the Morris water maze and contextual fear conditioning revealed enhanced L&M in the S100b mutants. Future studies will reveal the underlying mechanism, as altered neuronal signalling through the neuronal S100b receptor RAGE (receptor for advanced glycation end products) might be responsible for the LTP and L&M enhancement in this mutant.
Simultaneous binding of glutamate and glycine is known to be required for efficient activation of NMDARs149. D-serine, which is mainly produced in glial cells, is a potent agonist of the glycine binding site in NMDARs150. The concentration of D-serine in the forebrain of mice lacking D-amino-acid oxidase (DAAO) was found to be higher than in control mice. Consequently, the mutant mice showed larger NMDA currents151, enhanced LTP and improved performance in the Morris water maze151. These results highlight the involvement of glial processes in L&M and suggest that manipulations of these processes could be used to enhance L&M.
Modulatory neurotransmission and memory
Although many of the studies mentioned above focused on NMDAR function, manipulations of other neuro-transmitter systems also result in plasticity and learning enhancements. For example, donepezil (an acetylcho-linesterase inhibitor)152 and modafinil, two US Food and Drug Administration approved drugs to treat cognitive deficits, are thought to affect catecholamines, serotonin, glutamate, GABA and histamine systems153. However, the exact mechanisms of action for many cognitive drugs remain unclear. Genetic manipulations can be a powerful approach to dissect the role of each neurotransmitter system in plasticity and L&M. For example, mice overexpressing the ionotropic 5-HT3 receptor displayed enhanced contextual fear conditioning, whereas cued conditioning and fear extinction were not affected154. In accordance, deletion of monoamine oxidase A (MAOA), an enzyme which metabolizes serotonin, also resulted in enhancement of classical fear conditioning155. These studies show that modulation of serotonin-mediated signalling can modulate certain forms of memory.
Similarly, manipulations of the histamine system156 have also been shown to enhance some forms of learning. Mice lacking histidine decarboxylase (HDC), an enzyme that synthesizes histidine157,158, showed enhanced L&M in the fear conditioning and the Morris water maze tasks. Unexpectedly, this mutation impaired object discrimination157. The histamine deficiency in these mutants altered dopamine metabolism in the striatum, a structure with a key role in reward and reinforcement157. Thus, it is possible that changes in motivation might have played a central role in the behavioural phenotype of these mutants. Tissue- or cell type-specific genetic lesions, may help to further elucidate the role of HDC in motivation and memory.
Integrating different signalling pathways
The studies reviewed here suggest that there might be a core of molecular mechanisms associated with enhanced cognition (FIG. 3). We noted that many of the 33 mutant mice reviewed (TABLE 1) included enhancements in NMDAR signalling. Accordingly, D-cycloserine, a partial agonist at the strychnine-insensitive glycine-binding site on the NMDAR, potentiates NMDA currents and enhances learning in rodents and humans159–161, suggesting that pharmacological upregulation of NMDAR signalling might be a general strategy for enhancing memory. Nevertheless, it is important to note that the studies reviewed here show that manipulations which do not have an obvious connection with NMDAR signalling can also enhance L&M. In general, genes associated with enhanced L&M might regulate rate-limiting steps or bottle necks in the molecular and cellular events underlying L&M. Powerful molecular tools, such as microarray analysis, could be used to unravel molecular mechanisms that are common among mutants with L&M enhancements. The relevance of these molecular genetic mechanisms to L&M could then be studied by both disrupting and overexpressing the relevant genes in mice, and then studying the resulting molecular, cellular, systems and behavioural changes.
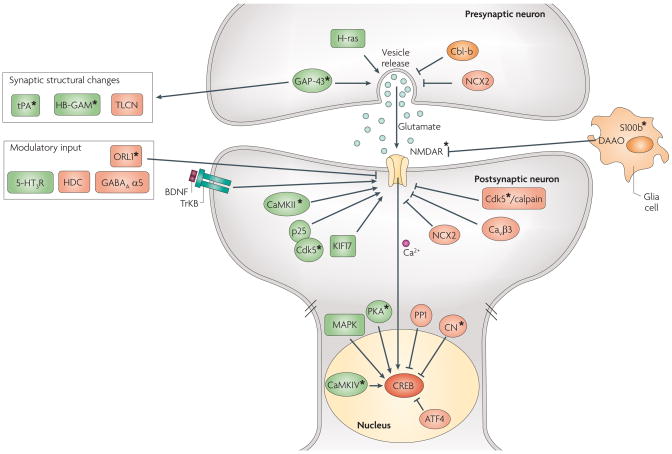
Figure 3. integrating pathways for learning and memory enhancement.
Memory enhancements have been achieved by manipulating signalling largely in four different domains of the synapse. First, in the presynaptic axonal terminal; molecular manipulations that increased glutamate release have been shown to enhance learning and memory (L&M). Second, at the postsynaptic site; manipulations that upregulate the levels or enhance the function of N-methyl-D-aspartate receptors (NMDAR) either directly (through KIF17 for example, or by phosphorylation through calcium calmodulin kinase II (CaMKII) or p25/Cdk5) or indirectly (for example through deletion of Cavβ3 or D-amino-acid oxidase (DAAO)) have been shown to enhance L&M. Third, in the nucleus through the postsynaptic transcription factor cyclic-AMP response-element-binding protein (CREB); actions of several kinases/phosphatases, that are regulated by calcium influx mainly through NMDAR, converge on CREB. De novo gene expression contributes to the stabilization and consolidation of synaptic plasticity and memory. Fourth, by structural changes at the synapse; molecules that participate in key structural changes involved in memory, such as the formation of new synapses, can be manipulated to enhance memory. Manipulations of structural molecules such as heparin-binding growth-associated molecule (HB-GAM) and telencephalin (TLCN) result in L&M enhancement. The molecules marked with an asterisk (*) are examples of genes for which bidirectional manipulations lead to deficits and enhancements in L&M. For example, inhibition of calcineurin (CN) enhances memory whereas overexpression of CN impaired L&M (see text for details). In addition to NMDAR, manipulations of other modulatory neurotransmitter systems such as serotonin, γ-aminobutyric acid (GABA) and histamine can enhance memory. Glial proteins (S100 calcium-binding protein5(S100b) and DAAO) also play active roles in L&M. Sharp and blunted arrows represent positive and negative regulation, respectively. Either the overexpression or activation of molecules in green, or the deletion or inhibition of molecules in red enhance L&M. HDC, histidine decarboxylase; NCX2, Na+/Ca2+ exchanger type 2; ORL1, nociceptin receptor; PKA, protein kinase A; PP1, protein phosphatase 1; tPA, tissue-type plasminogen activator; 5-HT3R, 5-HT3 receptor.
Conclusions
Molecular and cellular cognition studies in mice have examined more than 200 mutant genes. Although the majority of those mutations resulted in behavioural deficits (see Further Information), our review of the literature revealed that at least 33 of the mutant mice generated showed L&M enhancements (TABLE 1). Remarkably, in 26 out of 29 studied mouse lines LTP was found to be enhanced (TABLE 1). Nevertheless, it is noteworthy that not all enhancements in LTP result in L&M enhancements, probably because of disruptions in other mechanisms involved in learning (BOX 3).
Box 3. LTP enhancements without learning and memory improvements
Long-term potentiation (LTP) enhancements do not always result in better learning and memory (L&M), either because the tests used might not be sensitive enough or because the enhancements in LTP are accompanied by significant physiological disruptions that interfere with learning. Here are some examples:
Deletion of Psd95181 enhances LTP in the CA1 region of the hippocampus, but disrupts the postsynaptic density and dramatically changes the synaptic properties in the mutant mice. Not surprisingly, these mice show profound L&M deficits.
Deletion of inositol 1,4,5-triphosphate 3-kinase A also enhances LTP in the CA1 region of the hippocampus, but does not affect spatial L&M assessed by the Morris water maze182.
Deletion of Limk1 resulted in synaptic structural abnormalities183. CA1 LTP with high-frequency stimulation (50–100 Hz) was enhanced, but low frequency (10Hz)-induced LTP was impaired. Although cued fear conditioning was enhanced in these mutants, both contextual fear conditioning and initial learning in the Morris water maze were unaffected; reversal learning in the Morris water maze was impaired183.
Deletion of Fmr2 increases mortality, results in abnormalities in sensory perception, impaired contextual fear conditioning, and enhanced LTP in the CA1 region of the hippocampus 184.
Deletion of Ptpδ increases mortality rates, causes growth retardation and learning impairments in the Morris water maze and radial arm maze. These animals also show enhanced LTP in the CA1 and CA3 region of the hippocampus 185.
Dystrophin-deficient mice have altered γ-aminobutyric acid (GABA) inhibition186 and altered metabolism187. These mice show enhanced LTP but impaired L&M in object recognition tasks and in the Morris water maze188.
It is important to note that despite enhanced LTP, each of these mutations resulted in other abnormalities that could account for the L&M impairments. Many of the mutants described above were generated to model human diseases associated with cognitive deficits such as Williams syndrome (LIMK1), fragile X syndrome (FMR2) and Duchenne muscular dystrophy (dystrophin), and thus, it is not surprising to find deficits in L&M in these mutant mice.
The results summarized here demonstrate that it is possible to engineer mice with cognitive enhancements by manipulating either the acquisition or consolidation of memory. For example, NR2B, calcineurin and H-ras are thought to be involved in the acquisition of memory, whereas ORL1, CaMKIV, eIF2α and cbl-b seem to modulate memory consolidation. Calcineurin and Zif268 are involved in the establishment, but not in the extinction, of memory73. These are examples of how genetic manipulations can be used to analyse the role of specific genes in different phases of memory.
The remarkable consistency of the reviewed findings provides considerable evidence for a role of LTP mechanisms in L&M. There is compelling evidence that during learning synaptic transmission is strengthened in a manner consistent with LTP, and the vast majority of the pharmacological and genetic manipulations that disrupt LTP also impair L&M. Together with the extensive theoretical work that outlines how LTP-like mechanisms in the brain could be used in information processing and storage, this body of data provide conclusive evidence that stable changes in synaptic plasticity have a role in L&M (but see REF. 162). These data however, have not elucidated how LTP-like mechanisms are involved in processing, storing or retrieving information. It will be crucial to identify other physiological mechanisms that modulate and interact with LTP during the acquisition, consolidation and retrieval of information. Indeed, changes in inhibition (GABAAα5-knockout)163, excitability (Kvβ1.1-knockout)164 and short-term plasticity (H-rasG12V-transgenic)135 can facilitate LTP and result in L&M enhancements.
Studying enhanced cognition represents an exciting opportunity as there are a number of fascinating questions that can be addressed with mutant mice that could have an impact well beyond the molecular and cellular mechanisms explored so far. For example, how do mutations that enhance L&M affect circuit properties such as place representations in the hippocampus or emotional information processing in the amygdala? Do these representations form faster, are they more refined, flexible or specific than in normal mice? Do these mutations affect the interaction between brain regions during L&M? For example, does the cortical expression of memory-enhancing mutations accelerate the rate of cortical information transfer that is originally processed in the hippocampus?141,142 Does expressing these mutations in the hippocampus affect either the interaction between the hippocampus and striatum during spatial learning165 or the interaction between the hippocampus and the amygdala during contextual conditioning?166
There are a number of caveats that hamper the interpretation of the findings reviewed. Most of the studies described here carried out a limited behavioural analysis and did not explore in detail the behavioural nature of the L&M enhancements reported. For example, although enhancements in the Morris water maze were common among the mutants reviewed, it is not clear whether spatial components that cannot be measured in the version of the task used, would also be enhanced, such as pattern completion, pattern separation or some other specific aspect of spatial learning11,13.
Another possibility worth considering is that the enhancements of L&M described might trigger unintended physiological changes that complicate the behavioural analyses. For example, NMDAR modulates pain perception167 and it is unclear how this affected the fear conditioning studies reported. Similarly, AC1 transgenic mice showed better recognition memory, but lost memory flexibility80. Is one the result of the other? Could some of the L&M enhancements documented reflect changes in other behavioural mechanisms, such as anxiety, motivation or attention? Could some of these L&M enhancements have unknown behavioural costs that escaped the incomplete behavioural characterization of the mutants? Could more extensive tests reveal that all of these mutant mice have undetected but significant behavioural deficits? The mice studied were kept in traditional impoverished housing conditions; would the same enhancements be observed in environmentally enriched housing conditions? The answers to these questions are unknown, but the extent and consistency of the findings reviewed suggest that the key conclusions, namely that LTP-like mechanisms have a role in L&M and that there are a core of rate-limiting mechanisms that can be used to enhance L&M, may well stand the test of time.
The severity of cognitive disorders and the number of people affected by them (>5% of the population) adds urgency to the effort to develop treatments168,169. Targeting the synaptic and nuclear mechanisms associated with L&M enhancements might lead to the development of general therapies for cognitive disorders that circumvent the need to develop targeted therapies for the myriad of genetic and environmental insults underlying these disorders. Manipulations of signalling pathways that can enhance L&M in mice regardless of genetic background might be able to overcome individual genetic heterogeneity in patients and enhance cognitive function. Nevertheless, a word of caution is in order: could the manipulation of some of the systems associated with enhanced cognition lead to an increase or exacerbation of neurological and psychiatric symptoms? For example, as D-serine is suspected to be involved in brain pathologies such as schizophrenia150, DAAO-mediated signalling may not be an appropriate target for memory enhancing drugs. It is also important to stress that memory enhancing manipulations raise a number of ethical issues that are outside of the scope of this Review, but that merit careful consideration and discussion170,171.
The findings reviewed here represent the beginning of a fundamental new approach in the study of cognition, one that focuses on the molecular and cellular mechanisms that gate and limit cognitive function. We are only beginning this journey, but the results obtained so far demonstrate the tremendous potential of this approach for basic science and for clinical applications.
Acknowledgments
We would like to thank members of the Silva laboratory for discussions that shaped this Review. We would like to give B. Dobkin a special thanks for his enthusiasm for this project, the Adelson Foundation, Korea Research Foundation (KRF-2007-357-C00101), NIA (AG13622), NINDS (NS38480) and NIMH (MH077972) for funding that made this work possible.
- Rubinstein-Taybe syndrome
Rubinstein-Taybe syndrome is a genetic disorder that occurs in 1/125,000 births and is characterized by mental retardation, broad thumbs and toes, and facial abnormalities. It can be caused by heterozygous mutations in CREB binding protein (CBP).
Footnotes
DATABASES
OMIM: http://www.ncbi.nlm.nih.gov/entrez/query,fcgi?db=OMIM
Alzheimer’s disease | Parkinson’s disease
UniProtKB: http://ca.expasy.org/sprot
αCaMKII | ATF4 | Cbl-b | Cdk5 | HDC | intercellular adhesion molecule 5 | KIF17 | MAOA | ORL1 | pleiotrophin | p25 | RAGE | S100Bb
FURTHER INFORMATION
Genes to Cognition programme: http://www.genes2cognition.org
Alcino J. Silva’s homepage: http://silvalab.org
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Li W, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci USA. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harwood AJ, Agam G. Search for a common mechanism of mood stabilizers. Biochem Pharmacol. 2003;66:179–189. doi: 10.1016/s0006-2952(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 4.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16(Suppl 2):183–194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 5.Costa RM, Silva AJ. Molecular and cellular mechanisms underlying the cognitive deficits associated with neurofibromatosis 1. J Child Neurol. 2002;17:622–626. doi: 10.1177/088307380201700813. discussion 627–629, 646–651. [DOI] [PubMed] [Google Scholar]
- 6.Rosenzweig E. S. & Barnes, C. A. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 7.Ehninger D, et al. Reversal of learning deficits in a Tsc2(±) mouse model of tuberous sclerosis. Nature Med. 2008 doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luria AR. The mind of a mnemonist; a little book about a vast memory. Basic Books; New York: 1968. [Google Scholar]
- 9.Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. This paper describes the ‘doogie’ mice, which overexpress NR2B in the adult forebrain. Although this was not the first published paper reporting mice with LTP and learning and memory enhancements, the study triggered a great deal of interest in the media. [DOI] [PubMed] [Google Scholar]
- 10.Kiyama Y, et al. Increased thresholds for long-term 17. potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHugh TJ, et al. Dentate gyrus NMDA receptors 18. mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 12.Morris RG, Anderson E, Lynch GS, Baudry M. 19. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. A classic paper that used a pharmacological manipulation to demonstrate the crucial role of NMDA receptors in spatial learning. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa K, et al. Requirement for hippocampal CA3 21 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakazawa K, et al. Hippocampal CA3 NMDA 22 receptors are crucial for memory acquisition of onetime experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 15.Sakimura K, et al. Reduced hippocampal LTP and 23 spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 16.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 17.Matynia A, Kushner SA, Silva AJ. Genetic approaches to molecular and cellular cognition: a focus on LTP and learning and memory. Annu Rev Genet. 2002;36:687–720. doi: 10.1146/annurev.genet.36.062802.091007. [DOI] [PubMed] [Google Scholar]
- 18.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 19.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 20.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 21.Manabe T, et al. Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature. 1998;394:577–581. doi: 10.1038/29073. [DOI] [PubMed] [Google Scholar]
- 22.Harris KM, Teyler TJ. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. J Physiol. 1984;346:27–48. doi: 10.1113/jphysiol.1984.sp015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 24.Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 25.Cao X, et al. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- 26.Jeon D, et al. Ablation of Ca2+ channel beta3 subunit leads to enhanced N-methyl-D-aspartate receptor-dependent long term potentiation and improved long term memory. J Biol Chem. 2008;283:12093–12101. doi: 10.1074/jbc.M800816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 28.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 29.Wong RW, Setou M, Teng J, Takei Y, Hirokawa N. Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci USA. 2002;99:14500–14505. doi: 10.1073/pnas.222371099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23:131–140. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 32.Bourtchuladze R, et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. This study showed that both the stability of synaptic plasticity (LTP) and long-term memory are impaired in CREB-knockout mice, highlighting the key role of CREB in memory in the mammalian brain. [DOI] [PubMed] [Google Scholar]
- 33.Kida S, et al. CREB required for the stability of new and reactivated fear memories. Nature Neurosci. 2002;5:348–355. doi: 10.1038/nn819. This study used an inducible transgenic technique, involving the ligand-binding domain of the oestrogen receptor (LBD)-fusion protein, to study the in vivo role of CREB in memory consolidation and reconsolidation. [DOI] [PubMed] [Google Scholar]
- 34.West AE, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong YN, Wu HY, Hsu FC, Coulter DA, Lynch DR. Developmental and cell-selective variations in N-methyl-D-aspartate receptor degradation by calpain. J Neurochem. 2006;99:206–217. doi: 10.1111/j.1471-4159.2006.04096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpkins KL, et al. Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci. 2003;23:11322–11331. doi: 10.1523/JNEUROSCI.23-36-11322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nature Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 38.Hawasli AH, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nature Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–370. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- 40.Hawasli AH, Bibb JA. Alternative roles for Cdk5 in learning and synaptic plasticity. Biotechnol J. 2007;2:941–948. doi: 10.1002/biot.200700093. [DOI] [PubMed] [Google Scholar]
- 41.Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 42.Mamiya T, et al. Neuronal mechanism of nociceptin-induced modulation of learning and memory: involvement of N-methyl-D-aspartate receptors. Mol Psychiatry. 2003;8:752–765. doi: 10.1038/sj.mp.4001313. [DOI] [PubMed] [Google Scholar]
- 43.Noda Y, et al. Role of nociceptin systems in learning and memory. Peptides. 2000;21:1063–1069. doi: 10.1016/s0196-9781(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 44.Lynch G. Memory enhancement: the search for mechanism-based drugs. Nature Neurosci. 2002;5:S1035–S1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- 45.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oomura Y, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. An excellent review of the role of αCaMKII in synaptic plasticity and learning and memory. [DOI] [PubMed] [Google Scholar]
- 48.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 49.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 50.Balschun D, et al. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. Embo J. 1999;18:5264–5273. doi: 10.1093/emboj/18.19.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frankel WN. Mouse strain backgrounds: more than black and white. Neuron. 1998;20:183. doi: 10.1016/s0896-6273(00)80447-x. [DOI] [PubMed] [Google Scholar]
- 52.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 53.Jeon D, et al. Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron. 2003;38:965–976. doi: 10.1016/s0896-6273(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 54.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. An introductory review to the mechanism of two key forms of synaptic plasticity: LTP and LTD. [DOI] [PubMed] [Google Scholar]
- 55.Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- 56.Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 57.Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. This article reviews the role of negative regulators such as the transcriptional repressor CREB2 in synaptic plasticity and memory, with an emphasis on the removal of these constraints in memory storage. [DOI] [PubMed] [Google Scholar]
- 58.Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 59.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. The first paper demonstrating the use of genetic manipulations in the study of mechanisms of cognitive function in mammals. [DOI] [PubMed] [Google Scholar]
- 60.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 61.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 63.Mansuy IM, et al. Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron. 1998;21:257–265. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 64.Genoux D, et al. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- 65.Blitzer RD, et al. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- 66.Shi J, Townsend M, Constantine-Paton M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron. 2000;28:103–114. doi: 10.1016/s0896-6273(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 67.Liu JP, Sim AT, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- 68.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan GC, Tonegawa S, Storm DR. Hippocampal neurons express a calcineurin-activated adenylyl cyclase. J Neurosci. 2005;25:9913–9918. doi: 10.1523/JNEUROSCI.2376-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 71.Mansuy IM, Shenolikar S. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci. 2006;29:679–686. doi: 10.1016/j.tins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Malleret G, et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- 73.Baumgartel K, et al. Control of the establishment of aversive memory by calcineurin and Zif268. Nature Neurosci. 2008;11:572–578. doi: 10.1038/nn.2113. [DOI] [PubMed] [Google Scholar]
- 74.Mansuy IM. Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun. 2003;311:1195–1208. doi: 10.1016/j.bbrc.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Zeng H, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 76.Ikegami S, Inokuchi K. Antisense DNA against calcineurin facilitates memory in contextual fear conditioning by lowering the threshold for hippocampal long-term potentiation induction. Neuroscience. 2000;98:637–646. doi: 10.1016/s0306-4522(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 77.Ikegami S, et al. A facilitatory effect on the induction of long-term potentiation in vivo by chronic administration of antisense oligodeoxynucleotides against catalytic subunits of calcineurin. Brain Res Mol Brain Res. 1996;41:183–191. doi: 10.1016/0169-328x(96)00094-0. [DOI] [PubMed] [Google Scholar]
- 78.Morishita W, et al. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- 79.Wong ST, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nature Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- 81.Shan Q, Chan GC, Storm DR. Type 1 adenylyl cyclase is essential for maintenance of remote contextual fear memory. J Neurosci. 2008;28:12864–12867. doi: 10.1523/JNEUROSCI.2413-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Isiegas C, et al. A novel conditional genetic system reveals that increasing neuronal cAMP enhances memory and retrieval. J Neurosci. 2008;28:6220–6230. doi: 10.1523/JNEUROSCI.2935-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bourtchouladze R, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alarcon JM, et al. Chromatin acetylation, memory, and LTP are impaired in CBP ± mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 86.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 87.Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 88.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 89.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 90.Bozon B, et al. MAPK, CREB and zif268 are all required for the consolidation of recognition memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- 92.Josselyn SA, Kida S, Silva AJ. Inducible repression of CREB function disrupts amygdala-dependent memory. Neurobiol Learn Mem. 2004;82:159–163. doi: 10.1016/j.nlm.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Wu LJ, et al. Genetic enhancement of trace fear memory and cingulate potentiation in mice overexpressing Ca2+/calmodulin-dependent protein kinase IV. Eur J Neurosci. 2008;27:1923–1932. doi: 10.1111/j.1460-9568.2008.06183.x. [DOI] [PubMed] [Google Scholar]
- 94.Fukushima H, et al. Upregulation of calcium/calmodulin-dependent protein kinase IV improves memory formation and rescues memory loss with aging. J Neurosci. 2008;28:9910–9919. doi: 10.1523/JNEUROSCI.2625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 96.Lee JA, et al. Overexpression of and RNA interference with the CCAAT enhancer-binding protein on long-term facilitation of Aplysia sensory to motor synapses. Learn Mem. 2001;8:220–226. doi: 10.1101/lm.40201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nature Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- 98.Sterneck E, et al. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc Natl Acad Sci USA. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 100.Karpinski BA, Morle GD, Huggenvik J, Uhler MD, Leiden JM. Molecular cloning of human CREB-2: an ATF/CREB transcription factor that can negatively regulate transcription from the cAMP response element. Proc Natl Acad Sci USA. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 102.Bartsch D, et al. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 103.Costa-Mattioli M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Costa-Mattioli M, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 106.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 107.Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Guan Z, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. This is one of the first papers to demonstrate the role of chromatin modifications in synaptic plasticity. This study showed that the expression of an immediate early gene, C/EBP, is bidirectionally regulated by a mechanism involving histone acetylation in Aplysia. [DOI] [PubMed] [Google Scholar]
- 109.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. One of the first reports showing the key role of epigenetic mechanisms in behavioural memory in the mammalian nervous system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levenson JM, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 111.Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- 112.Vecsey CG, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 114.Huang YY, et al. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calabresi P, et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur J Neurosci. 2000;12:1002–1012. doi: 10.1046/j.1460-9568.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 116.Carmeliet P, et al. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 117.Madani R, et al. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. Embo J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- 119.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 120.Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 121.Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13. doi: 10.1016/j.ejphar.2007.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II Fasciclin II controls presynaptic structural plasticity. Neuron. 1996;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 123.Bailey CH, et al. Mutation in the phosphorylation sites of MAP kinase blocks learning-related internalization of apCAM in Aplysia sensory neurons. Neuron. 1997;18:913–924. doi: 10.1016/s0896-6273(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 124.Han JH, Lim CS, Lee YS, Kandel ER, Kaang BK. Role of Aplysia cell adhesion molecules during 5-HT-induced long-term functional and structural changes. Learn Mem. 2004;11:421–435. doi: 10.1101/lm.61104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992;256:645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- 126.Nakamura K, et al. Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur J Neurosci. 2001;13:179–189. doi: 10.1046/j.0953-816x.2000.01366.x. [DOI] [PubMed] [Google Scholar]
- 127.Cremer H, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- 128.Law JW, et al. Decreased anxiety, altered place learning, and increased CA1 basal excitatory synaptic transmission in mice with conditional ablation of the neural cell adhesion molecule L1. J Neurosci. 2003;23:10419–10432. doi: 10.1523/JNEUROSCI.23-32-10419.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys. 2002;397:162–171. doi: 10.1006/abbi.2001.2705. [DOI] [PubMed] [Google Scholar]
- 130.Rauvala H, Peng HB. HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Prog Neurobiol. 1997;52:127–144. doi: 10.1016/s0301-0082(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 131.Pavlov I, et al. Role of heparin-binding growth-associated molecule (HB-GAM) in hippocampal LTP and spatial learning revealed by studies on overexpressing and knockout mice. Mol Cell Neurosci. 2002;20:330–342. doi: 10.1006/mcne.2002.1104. [DOI] [PubMed] [Google Scholar]
- 132.Pavlov I, Rauvala H, Taira T. Enhanced hippocampal GABAergic inhibition in mice overexpressing heparin-binding growth-associated molecule. Neuroscience. 2006;139:505–511. doi: 10.1016/j.neuroscience.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 133.Humeau Y, Shaban H, Bissiere S, Luthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 134.Powell CM, et al. The presynaptic active zone protein RIM 1 alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kushner SA, et al. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manabe T, et al. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin KC, et al. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 138.Angers A, et al. Serotonin stimulates phosphorylation of Aplysia synapsin and alters its subcellular distribution in sensory neurons. J Neurosci. 2002;22:5412–5422. doi: 10.1523/JNEUROSCI.22-13-05412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rao N, Dodge I, Band H. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J Leukoc Biol. 2002;71:753–763. [PubMed] [Google Scholar]
- 140.Tan DP, Liu QY, Koshiya N, Gu H, Alkon D. Enhancement of long-term memory retention and short-term synaptic plasticity in cbl-b null mice. Proc Natl Acad Sci USA. 2006;103:5125–5130. doi: 10.1073/pnas.0601043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Frankland PW, Bontempi B. The organization of recent and remote memories. Nature Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 142.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. A comprehensive review of the molecular and cellular mechanisms underlying cortical memory consolidation. [DOI] [PubMed] [Google Scholar]
- 143.Haydon PG. GLIA: listening and talking to the synapse. Nature Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 144.McCall MA, et al. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci USA. 1996;93:6361–6366. doi: 10.1073/pnas.93.13.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Todd KJ, Serrano A, Lacaille JC, Robitaille R. Glial cells in synaptic plasticity. J Physiol Paris. 2006;99:75–83. doi: 10.1016/j.jphysparis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 146.Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995;37:417–429. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 147.Gerlai R, Wojtowicz JM, Marks A, Roder J. Overexpression of a calcium-binding protein, S100 beta, in astrocytes alters synaptic plasticity and impairs spatial learning in transgenic mice. Learn Mem. 1995;2:26–39. doi: 10.1101/lm.2.1.26. [DOI] [PubMed] [Google Scholar]
- 148.Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci USA. 2002;99:4037–4042. doi: 10.1073/pnas.052020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 150.Martineau M, Baux G, Mothet JP. D-serine signalling in the brain: friend and foe. Trends Neurosci. 2006;29:481–491. doi: 10.1016/j.tins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 151.Maekawa M, Watanabe M, Yamaguchi S, Konno R, Hori Y. Spatial learning and long-term potentiation of mutant mice lacking D-amino-acid oxidase. Neurosci Res. 2005;53:34–38. doi: 10.1016/j.neures.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 152.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 154.Harrell AV, Allan AM. Improvements in hippocampal-dependent learning and decremental attention in 5-HT(3) receptor overexpressing mice. Learn Mem. 2003;10:410–419. doi: 10.1101/lm.56103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim JJ, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci USA. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nature Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 157.Dere E, et al. Histidine-decarboxylase knockout mice show deficient nonreinforced episodic object memory, improved negatively reinforced water-maze performance, and increased neo- and ventro-striatal dopamine turnover. Learn Mem. 2003;10:510–519. doi: 10.1101/lm.67603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu L, et al. Improved learning and memory of contextual fear conditioning and hippocampal CA1 long-term potentiation in histidine decarboxylase knock-out mice. Hippocampus. 2007;17:634–641. doi: 10.1002/hipo.20305. [DOI] [PubMed] [Google Scholar]
- 159.Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- 160.Flood JF, Morley JE, Lanthorn TH. Effect on memory processing by D-cycloserine, an agonist of the NMDA/glycine receptor. Eur J Pharmacol. 1992;221:249–254. doi: 10.1016/0014-2999(92)90709-d. [DOI] [PubMed] [Google Scholar]
- 161.Jones RW, Wesnes KA, Kirby J. Effects of NMDA modulation in scopolamine dementia. Ann NY Acad Sci. 1991;640:241–244. doi: 10.1111/j.1749-6632.1991.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 162.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nature Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. An up-to-date review discussing the relationship between synaptic plasticity and memory in the hippocampus. [DOI] [PubMed] [Google Scholar]
- 163.Collinson N, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABA(A) receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Murphy GG, et al. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol. 2004;14:1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 165.Gold PE. Coordination of multiple memory systems. Neurobiol Learn Mem. 2004;82:230–242. doi: 10.1016/j.nlm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 166.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 167.Wei F, et al. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nature Neurosci. 2001;4:164–169. doi: 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- 168.Mehlman MJ. Cognition-enhancing drugs. Milbank Q. 2004;82:483–506. doi: 10.1111/j.0887-378X.2004.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nature Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 170.Rose SP. ‘Smart drugs’: do they work? Are they ethical? Will they be legal? Nature Rev Neurosci. 2002;3:975–979. doi: 10.1038/nrn984. [DOI] [PubMed] [Google Scholar]
- 171.Farah MJ, et al. Neurocognitive enhancement: what can we do and what should we do? Nature Rev Neurosci. 2004;5:421–425. doi: 10.1038/nrn1390. This review discusses the social and ethical aspects of neurocognitive enhancers. [DOI] [PubMed] [Google Scholar]
- 172.Grant SG, et al. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 173.Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- 174.Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ. Inducible, pharmacogenetic approaches to the study of learning and memory. Nature Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- 175.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 176.Mayford M, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. This study introduced the inducible transgenic doxycycline system to the study of cognitive mechanisms and addressed the role of CaMKII in memory formation. [DOI] [PubMed] [Google Scholar]
- 177.Chang DJ, et al. Activation of a heterologously expressed octopamine receptor coupled only to adenylyl cyclase produces all the features of presynaptic facilitation in aplysia sensory neurons. Proc Natl Acad Sci USA. 2000;97:1829–1834. doi: 10.1073/pnas.97.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang H, et al. Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc Natl Acad Sci USA. 2003;100:4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nature Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 180.Glaser S, Anastassiadis K, Stewart AF. Current issues in mouse genome engineering. Nature Genet. 2005;37:1187–1193. doi: 10.1038/ng1668. [DOI] [PubMed] [Google Scholar]
- 181.Migaud M, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 182.Jun K, et al. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1, 4, 5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem. 1998;5:317–330. [PMC free article] [PubMed] [Google Scholar]
- 183.Meng Y, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 184.Gu Y, et al. Impaired conditioned fear and enhanced long-term potentiation in Fmr2 knock-out mice. J Neurosci. 2002;22:2753–2763. doi: 10.1523/JNEUROSCI.22-07-02753.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uetani N, et al. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. Embo J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Knuesel I, et al. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice) Eur J Neurosci. 1999;11:4457–4462. doi: 10.1046/j.1460-9568.1999.00887.x. [DOI] [PubMed] [Google Scholar]
- 187.Rae C, et al. Abnormalities in brain biochemistry associated with lack of dystrophin: studies of the mdx mouse. Neuromuscul Disord. 2002;12:121–129. doi: 10.1016/s0960-8966(01)00253-x. [DOI] [PubMed] [Google Scholar]
- 188.Vaillend C, Billard JM, Laroche S. Impaired long-term spatial and recognition memory and enhanced CA1 hippocampal LTP in the dystrophin-deficient Dmd(mdx) mouse. Neurobiol Dis. 2004;17:10–20. doi: 10.1016/j.nbd.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 189.Futatsugi A, et al. Facilitation of NMDAR-independent LTP and spatial learning in mutant mice lacking ryanodine receptor type 3. Neuron. 1999;24:701–713. doi: 10.1016/s0896-6273(00)81123-x. [DOI] [PubMed] [Google Scholar]
- 190.Jouvenceau A, et al. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- 191.Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci USA. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Holahan MR, Honegger KS, Tabatadze N, Routtenberg A. GAP-43 gene expression regulates information storage. Learn Mem. 2007;14:407–415. doi: 10.1101/lm.581907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shumyatsky GP, et al. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 194.Nolan MF, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 195.Nolan MF, et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- 196.Slane JM, Lee HS, Vorhees CV, Zhang JH, Xu M. DNA fragmentation factor 45 deficient mice exhibit enhanced spatial learning and memory compared to wild-type control mice. Brain Res. 2000;867:70–79. doi: 10.1016/s0006-8993(00)02258-7. [DOI] [PubMed] [Google Scholar]
- 197.McQuade JM, Vorhees CV, Xu M, Zhang JH. DNA fragmentation factor 45 knockout mice exhibit longer memory retention in the novel object recognition task compared to wild-type mice. Physiol Behav. 2002;76:315–320. doi: 10.1016/s0031-9384(02)00716-3. [DOI] [PubMed] [Google Scholar]
- 198.Giese KP, et al. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kv beta 1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. [PMC free article] [PubMed] [Google Scholar]
- 199.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Thiels E, et al. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20:7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]