Abstract
Signaling by polypeptide hormone prolactin (PRL) is mediated by its cognate receptor (PRLr). The PRLr is commonly stabilized in human breast cancer due to decreased phosphorylation of residue Ser 349, which when phosphorylated recruits the βTrcp E3 ubiquitin ligase and facilitates PRLr degradation. Here we demonstrate that an impaired PRLr turnover results in an augmented PRL signaling and PRL-induced transcription. Human mammary epithelial cells harboring degradation-resistant PRLr display accelerated proliferation and increased invasive growth. Conversely, a decrease in PRLr levels achieved by either pharmacologic or genetic means in human breast cancer cells dramatically reduced transformation and tumorigenic properties of these cells. Consequences of alteration of PRLr turnover for homeostasis of mammary cells and development of breast cancers as well as the utility of therapies that target PRLr function in these malignancies are discussed.
Keywords: prolactin receptor, breast cancer, tumorogenesis, cannabinoid
INTRODUCTION
Malignant transformation of cells and development of tumors result from a number of key events that include stimulation of cell proliferation and inhibition of cell death (1). The pituitary hormone prolactin (PRL), which is also secreted by mammary epithelia, plays a central role in mammary gland development and function. In addition, several lines of evidence strongly implicate the role of PRL in breast tumorigenesis (reviewed in (2)). First, PRL promotes growth of human breast cancer cells acting as a survival agent and as a mitogen (3, 4), and up to 95% of primary human breast cancers are positive for PRL and its receptors (5–7). Second, transgenic mice locally expressing PRL within mammary epithelia develop tumors (8, 9), whereas genetic ablation of PRL receptors severely delays the development of SV40 large T antigen-induced breast carcinomas (10). Third, mutant prolactin receptors that are characterized by high levels of constitutive signaling have been recently identified in human breast tumors (11, 12). Finally, epidemiologic studies link elevated levels of circulating PRL with increased risk of breast cancer (13, 14), and its metastases (15), as well as with decreased taxane therapeutic efficacy (16–18) that could be reversed by pharmacological suppression of PRL levels (17).
Prolactin acts via cell surface receptors that exist as long/ΔS1 isoforms (hereafter referred to as PRLr) and several shorter alternatively spliced variants that often exert dominant-negative effects on signaling via the PRLr. PRL activates the PRLr-associated Jak2 tyrosine kinase (19) and a series of downstream signaling pathways, including signal transducers and activators of transcription (Stats), Erk1/2, PI3K-Akt and others. Since a high proportion of human breast cancer cells secrete their own PRL, the autocrine effects of PRL may account for the limited success of inhibitors of pituitary PRL synthesis/release against human breast cancers (reviewed in (2)). Antagonists of PRLr kill human breast cancer cells in vitro and abrogate the tumorigenesis in the xenograft models demonstrating that persistent signaling induced by locally secreted PRL is essential for growth and survival of these cells (20, 21).
However, PRL also induces proteolytic degradation of PRLr via receptor ubiquitination facilitated by the SCFβTrcp E3 ubiquitin ligase that is recruited to the substrate in a manner that requires phosphorylation of Ser349 within the phosphodegron (22, 23). Given that this ligand-induced PRLr down regulation limits the extent of PRL signaling (2), it is not clear how PRL maintains the survival of breast cancer cells. While levels of PRLr are decreased in the breast cancer intratumoral stromal compartment, the levels of PRLr in tumor cells are not decreased in comparison with benign mammary cells (5, 24) suggesting a possibility that down regulation and degradation of PRLr in tumor cells might be impaired. Indeed, we have reported that phosphorylation of PRLr on Ser349 within its phosphodegron is impaired in breast cancer cells and tissues that exhibit increased stability of PRLr and ensuing high levels of its expression (25).
Our previous reports outlined a mechanism by which PRLr might get stabilized and accumulated in breast cancers. We found that glycogen synthase kinase 3β (GSK3β) mediates the recruitment of βTrcp and receptor ubiquitination and degradation through phosphorylation of PRLr on Ser349. Constitutive oncogenic signaling downstream of the Ras pathway inactivates GSK3β through phosphorylation of GSK3β on Ser9. Inhibition of GSK3β activity prevents phosphorylation of PRLr on Ser349 and PRLr ubiquitination, ultimately leading to PRLr stabilization (26).
Here we sought to investigate the outcomes of PRLr stabilization in breast cancer. Our studies reveal that abrogation of PRLr phosphorylation on Ser349 in near normal human mammary epithelial cells contributes to the development of a transformed phenotype. Furthermore, decreasing the levels of PRLr in human breast cancer cells is detrimental for their growth, invasion and tumorigenicity. Collectively these findings suggest that an altered degradation (and resulting accumulation) of PRLr might play a role in human breast cancers and could be targeted for anti-cancer therapies.
MATERIALS AND METHODS (additional details are provided in Supplemental data)
Cell lines, DNA constructs and gene delivery
MCF10AΔp53 derivative cell line, in which p53 expression is knocked down (MCF10A) were a generous gift of Alan Eastman (27). Generation of the MCF10AΔp53 cells stably expressing wild type or S349A mutant PRLr was previously described (28). Human breast cancer MCF7 and T47D cells (gift of Ze’ev Ronai) were cultured as previously described (29). Negative control shRNA (Sigma, # SHC002) is a lentiviral pLKO.1-puro vector containing an irrelevant shRNA insert. ShPRLr subcloned within the same vector was purchased (Open Biosystems, #RHS3979-98492771) and used for transduction of T47D cells followed by selection in puromycin (2μg/ml). CISH promoter-driven firefly luciferase reporter (30) was kindly provided by C. V. Clevenger (Northwestern University, Chicago, IL). Renilla luciferase expression vector was purchased (Promega).
Chemicals and antibodies
Human recombinant prolactin (PRL) was kindly provided for a fee by Dr. A.F. Parlow, National Hormone and Peptide Program, Torrance, CA, USA. Antibody against pSer349-PRLr was previously described (21). Antibodies against Flag tag (M2; Sigma St Louis, MO), PRLr (H300, Santa Cruz Biotechnology), Actin (Affinity BioReagents), phospho-Erk, Erk, phospho-Stat5, Stat5 (Cell Signaling Technology, Inc, Danvers, MA), Cyclin D1 (AB-3, Calbiochem Inc., San Diego, CA) were purchased. Secondary antibodies conjugated with horseradish peroxidase (Chemicon) were purchased. Methylamine hydrochloride, cycloheximide and anandamide as well as other chemicals were purchased from Sigma (Saint Lois, MO). Immunoprecipitations and immunoblotting analyses that were carried out as previously described (29). Immunohistochemical analysis on tumors was carried out using the anti-PRLr antibody (H-300, Santa Cruz) as previously described (25).
Analysis of cell growth, invasion and tumorigenesis
Growth in two-dimension culture was analyzed using the staining with trypan blue. The number of live cells in each well was counted. Results from three independent experiments are presented as average ±S.E. For the analysis of cell growth in 3D culture, cells were mixed with Matrigel™ Basement Membrane Matrix (BD Bioscience, Bedford, MA) and cultured in complete medium for indicated number of days. Growth of cells in soft agar was carried out as previously described (31).
Invasion assays were done in a Boyden chambers supplied with polyethylene terephthalate filter inserts containing 0.8-μm pores (BD Company, Franklin Lakes, NJ). Filters were coated on ice with 50μl of MatrigelTM Basement Membrane Matrix (BD Bioscience, Bedford, MA) and incubated for 30min (37°C). 5x104 of either MCF10AΔp53 or T47D-derived cells were plated in 300μl of Matrigel (diluted in 0.1% bovine serum albumin (BSA)-DMEM/F-12 (1:3)) into the upper chamber. The lower chamber was filled with 700μl of DMEM/F-12 medium supplied with 10% FBS. Non-invaded cells in the inserts were removed with cotton swabs after either 48h (for MCF10AΔp53) or 72h (for T47D) of incubation. Invaded cells on the underside were fixed with absolute methanol for 2 min, stained with Eosin-Hematoxylin solution (Sigma, St Louis, MO) and photographed using either 5X or 10X objectives.
For zymography analysis, cells were seeded as described in “Invasion assay”. The medium was collected at 24h after the seeding, centrifuged, concentrated using “Amicon Ultra” (Millipore, #UFC901024, cut off 10kDa) and 10μg of the proteins were resolved by gelatin-contained Novex 10% Zymogram (Gelatin) gel (Invitrogen #EC61755BOX) and analyzed in accordance to manufacture’s protocol. Tumorigenesis assays was carried out in NCRNU-M (Taconic) or in NSG mouse model (NOD-SCID, IL2Rgnull, The Jackson Laboratory) female mice that also obtained pellets of 17β-Estradiol and prolactin (purchased from Innovative Research of America). Cells were implanted subcutaneously or into abdominal mammary glandsand the growth of tumors was measured by caliper at indicated days after cell injection.
Signal quantification and statistical analysis
Digital images were processed with Adobe Photoshop 7.0 software. For some experiments, band intensities and percent of surface covered by cell growth were quantified by densitometry (ImageJ software). The statistical differences were analyzed using two-tailed t-Students test.
RESULTS
Stabilization of PRLr augments the extent of PRL signaling in human mammary epithelial cells
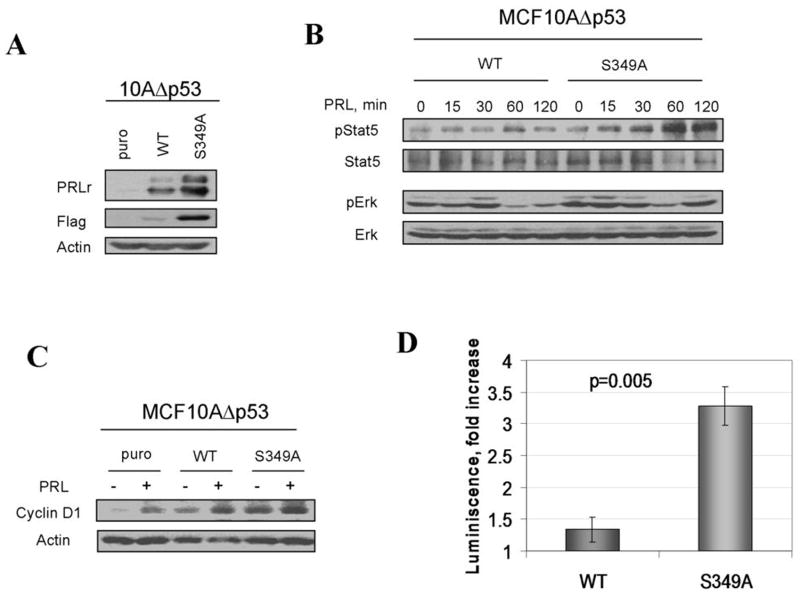
Stabilization of PRLr in human breast cancers occurs via oncogene-mediated inhibition of PRLr phosphorylation on Ser349 by GSK3β (26). We sought to investigate the consequences of impaired PRLr phosphorylation on Ser349 in human mammary epithelial cells by expressing PRLrS349A mutant that cannot be phosphorylated by GSK3β (26). This approach (rather than modulating overall GSK3β activity, which affects cell functions via numerous diverse mechanisms, reviewed in (32)) was implemented in MCF10AΔp53 cells (27), which express low levels of endogenous PRLr (Figure 1A). Stable cell lines transduced with PRLrS349A mutant (S349A) exhibited higher levels of PRLr expression compared to those that received wild type PRLr (WT) or empty vector (puro, Figure 1A). This result is consistent with inefficient degradation of PRLr proteins whose phosphorylation within the phosphodegron is impaired (22, 25, 26). Abrogated phosphorylation of PRLr on Ser349 is expected to impair receptor ubiquitination leading to impaired PRLr endocytosis and, therefore, increased stability (23, 28, 33). Indeed, turnover of PRLr was noticeably impaired in S349A cells (Figure S1).
Figure 1. Stabilized PRLr mediates augmented PRL signaling in human mammary epithelial cells.
A. Mass cultures of MCF10AΔp53 cells transduced with either empty retrovirus (puro) or retroviruses for expression of indicated Flag-PRLr proteins were harvested and analyzed by immunoblotting using indicated antibodies (upper panels). Analysis of β-actin levels was used as a loading control (lower panel).
B. Phosphorylation and levels of Stat5 and Erk proteins in indicated MCF10AΔp53-derived cell lines pulsed with PRL (100ng/ml) for 15 min following ligand removal and incubation for indicated times were analyzed using indicated antibodies
C. Levels of cyclin D1 in indicated cell lines treated with PRL (100ng/ml) for 24hr were analyzed by immunoblotting. Same assay using anti-β-actin antibody is used as a loading control.
D. PRL-induced fold activation of the CISH promoter-driven luciferase reporter expressed in indicated MCF10AΔp53 cells was performed as described in the Materials and Methods.
It is plausible that stabilization and accumulation of PRLr are expected to augment the magnitude and duration of PRL signaling (22, 23). Indeed, expression of PRLrS349A led to a robust increase in magnitude and duration of signaling events including activation of Stat5 and Erk triggered by a pulse treatment with PRL when compared to cells expressing wild type PRLr (Figure 1B). Furthermore, the S349A cells displayed the highest levels of cyclin D1 (which is a key regulator of cell cycle progression and is known to be induced by PRL (34)) among all cell lines tested. Accordingly, these cells exhibited much higher levels of PRL-induced transcriptional activity as evident from analysis of the CISH promoter-driven luciferase reporter (Figure 1D). Together, these results indicate that abrogation of PRLr phosphorylation on Ser349 augments the cellular responses of mammary epithelial cells to PRL.
Stabilized PRLr contributes to transformation of human mammary epithelial cells
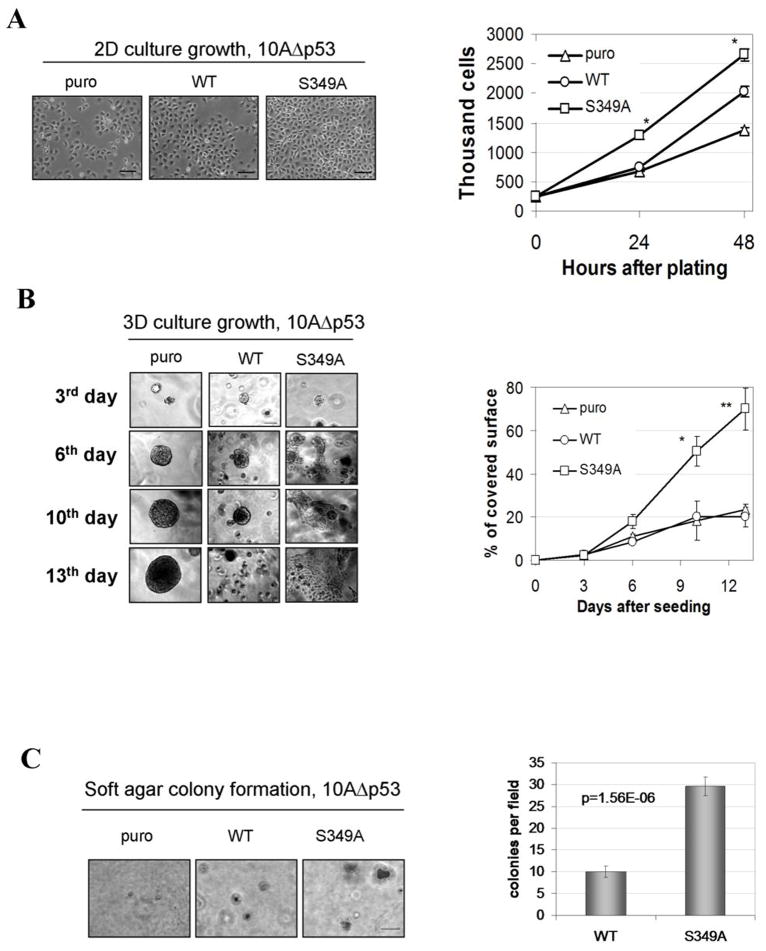
We have noticed that MCF10AΔp53 derivatives that express stabilized PRLr grow faster in tissue culture (Figure 2A). Furthermore, analysis of cell growth in three-dimension cultures in Matrigel revealed significant differences in both the rate of growth and morphology between all examined cell types. While vector-transduced puro cells grew slowly and formed well-defined spherical aggregates, WT cells formed numerous smaller spheroids. Remarkably, cells expressing mutant PRLrS349A rapidly deviated from spherical growth to a pattern of irregular and poorly defined masses forming a network of branches and meshes and, eventually, filling the entire culture space (Figure 2B). Three other independent S349A individual clones displayed similarly fast tumor-like growth and morphology (Figure S2) indicating that differences in cell growth were not clone-specific but mediated by the PRLrS349A mutant. A greater transformed phenotype of cells expressing stabilized receptor was further tested in another transformation assay such as growth in semi-solid medium. Cells expressing PRLr but not parental MCF10AΔp53 cells formed colonies in soft agar. Furthermore, consistent with the results obtained in 2D culture or in Matrigel, S349A clones formed larger colonies and demonstrated statistically significant increase in colonies number when compared to cells expressing PRLrWT (Figure 2C). In all, these data indicate that increased stability of PRLr contributes to a transformed phenotype in human mammary epithelial cells.
Figure 2. Expression of stabilized PRLr mutant augments growth of human mammary epithelial cells.
A. Representative pictures of indicated MCF10AΔp53 cells at 24h after the seeding; scale bar - 100μM. Graph represents the numbers of live cells ± SE that were calculated using trypan blue at 24 and 48h after the seeding. The differences between number of S349A and WT cells were statistically significant (p≤0.01).
B. Representative morphology of indicated MCF10AΔp53 cell lines growth in three-dimensional cultures at the indicated day after plating. The experiment was repeated twice. Scale bar - 15μM. The graph represents percent surface ± SE covered by cell growth calculated from nine of 10x magnification pictures (triplicates). The differences between the surfaces covered either by S349A or WT cells were statistically significant at 10 (p≤0.05) and 13 (p≤0.01) days.
C. Growth of MCF10AΔp53-derived cells in soft agar. Colonies were photographed at 14 days after plating. Scale bar - 50μM. The graph represents the number of colonies of WT or S349A cells ± SE calculated from nine fields (of 10X magnification, each in triplicates). The difference between the number of colonies formed by WT and S349A cells was statistically significant (p≤0.01).
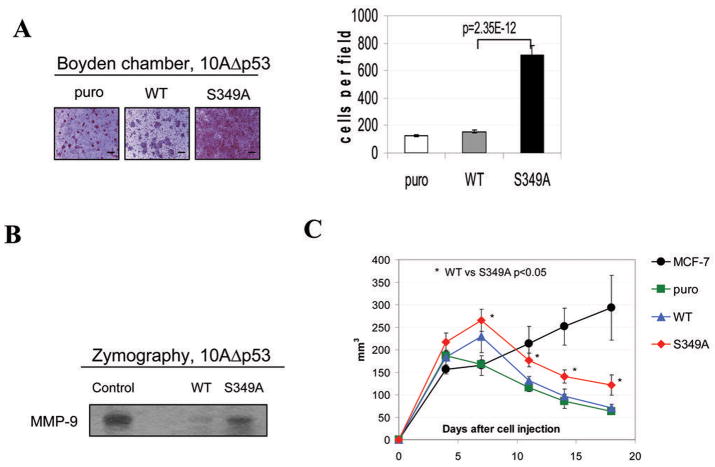
Aggressive and irregular growth of S349A cells in Matrigel and their ability to form colony in soft agar points to changes in their ability to grow invasively. Indeed, in vitro invasion assays revealed a superior ability of S349A cells (in comparison to puro or WT cells) to penetrate through Matrigel and insert pores in Boyden chamber assays (Figure 3A). Cell motility and invasiveness is a complex process positively regulated among other by pathways that involve MAPK, PI3K and Rho-family GTPases all of which are known to be activated by PRL (reviewed in (2, 35, 36)). One of the consequences of PRL signaling may be an increased expression of metalloproteinases 2 and 9 (MMPs) that are the critical enzymes for cell invasiveness (37). Zymography analysis of levels of MMP-2/9 expression in MCF10AΔp53 derived cell revealed that S349A cells expressed significantly higher level of MMP-9 compared to cells harboring wild type PRLr (Figure 3B). Expression of MMP-2 followed a similar pattern (data not shown). Together these data suggest that stabilization and increased levels of PRLr in breast cells contribute to a transformed in vitro phenotype that is reflected by accelerated cell growth and increased motility and/or invasive abilities.
Figure 3. Analysis of invasiveness, MMP activity, and tumorogenicity of MCF10AΔp53-derived cell lines.
A. Migration of indicated MCF10AΔp53 cells through Boyden chambers was analyzed as described in the Materials and Methods. The undersides of membrane inserts containing invaded cells fixed and stained with Eosin-Hematoxylin were photographed 48h after the plating using a 5X objective. Scale bar - 100μM. The graph represents number of invasive cells ± SE calculated from nine of 10x magnification pictures (triplicates).
B. Relative activity of MMP-9 in the indicated MCF10AΔp53 cells was analyzed by zymography as described in the Material and Methods.
C. Volume of growth of indicated MCF10AΔp53 mammary epithelial cells and MCF7 breast cancer cells (positive control) injected into the flanks of the NCRNU-M mice were calculated as described in the Materials and Methods. Asterisks denote statistically significant (p<0.05) growth difference between WT and S349A clones.
We next compared the tumorigenic growth of various MCF10AΔp53 derivatives injected into the flanks of the NCRNU-M immunocompromised mice that were implanted with pellets releasing estradiol and PRL. MCF7 breast cancer cells (positive control) grew rapidly and continuously, and the mice that were injected with these cells developed large tumors and had to be sacrificed by day 24. Although MCF10AΔp53 derivatives displayed a period of growth and formed distinct tumors (Figure S3), this growth was relatively short and was followed by tumor regression within four weeks after injection. Intriguingly, tumor regression proceeded significantly slower in S349A cells compared to either WT or puro cells (p<0.05, Figure 3C). Similar results were obtained when NSG immunodeficient mice were used as hosts upon either intra-flank or intra-mammary gland injection of human cells (data not shown). These data suggest that stabilization of PRLr promotes growth of MCF10AΔp53 cells in vivo but is not sufficient for maintaining the tumorigenic phenotype.
Downregulation of PRLr is detrimental for growth and tumorigenicity of human breast cancer cells
Our observations in non-tumorigenic human mammary epithelial cells demonstrate that stabilization and accumulation of PRLr augments PRL signaling and contributes to the transformed phenotype suggesting that high levels of PRLr might be important for development of breast cancers. To corroborate this conclusion we undertook a diametrically opposite approach by seeking to investigate whether downregulation of PRLr in human breast cancer cells that otherwise display a stabilized PRLr would affect their transformed properties.
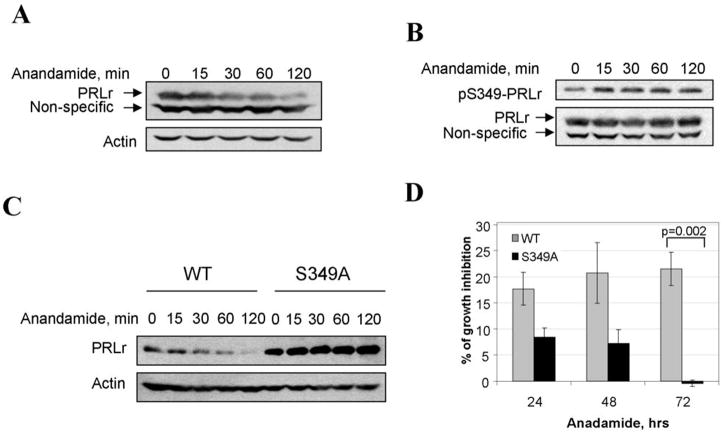
We initially used pharmacologic approaches based on the published observation that endogenous cannabinoid anandamide decreased the levels of PRLr and slowed down growth of MCF7 human breast cancer cells (38–41). Similarly, treatment with anandamide inhibited growth of T47D breast cancer cells (Figure S4) and also led to a rapid downregulation of PRLr (Figure 4A) in these cells known to contain hypophosphorylated and stabilized PRLr (25, 26). Intriguingly, an increase in the level of S349 phosphorylation was observed in T47D cells upon treatment with anandamide (along with a lysosomal inhibitor to prevent the degradation of phosphorylated PRLr species). These data are consistent with a hypothesis that anandamide affects growth of breast cancer cells via accelerating the phosphorylation-dependent degradation of PRLr.
Figure 4. Anandamide promotes phosphorylation and downregulation of PRLr and inhibits growth of human breast cells.
A. Effect of anandamide on PRLr expression in T47D cells. T47D cells were treated with 10μM of anandamide and harvested at indicated times. The lysates (100μg of protein) were separated on SDS PAGE and analyzed by immunoblotting with antibodies against PRLr (H-300,) upper panels; and Actin (Affinity BioReagents) for loading control, bottom panels.
B. Effect of anandamide on S349-PRLr phosphorylation in T47D cells. T47D cells were pretreated with 20mM of methylamine for 2 hours, and then with 10μM of anandamide for indicated times. The lysates (100μg of protein) were separated on SDS PAGE and analyzed by immunoblotting with antibodies against pSer349-PRLr upper panel; and PRLr, bottom panel.
C. Effect of anandamide on PRLr expression in 53-MCF10A cells. 53-MCF10A stable cell lines expressing flag-tagged wild type PRLr (WT) or S349A mutant (S349A) were treated with 10μM of anandamide and harvested at indicated times. The lysates (100μg of protein) were separated on SDS PAGE and analyzed by immunoblotting with antibodies against Flag, upper panels; and Actin for loading control, bottom panels.
D. Effect of anandamide on 53-MCF10A cell growth. The graph represents the percent of the difference in the number of cells between ethanol and Anandamide treated groups ± SE at indicated times after the treatment calculated as described in the Materials and Methods. The differences between groups were statistical significant (p < 0.01) at 72h after the cell seeding.
To test this hypothesis, we investigated the effect of anandamide on MCF10AΔp53-derived cells expressing PRLr. Treatment of WT cells with anandamide led to an increase in Ser349 phosphorylation of PRLr when its degradation was blocked by lysosomal inhibitor (Figure S5). Accordingly anandamide stimulated PRLr degradation upon blocking protein synthesis by cycloheximide treatment (Figure S6). Intriguingly, anandamide dramatically downregulated PRLr in WT cells but did not affect PRLr levels in S349A cells (Figure 4C and Figure S7). Furthermore, an inhibitory effect of anandamide on cell growth was much more pronounced in WT cells than in S349A cells (Figure 4D). This result indicates that downregulation of PRLr in response to anandamide is mediated by Ser349 phosphorylation-dependent degradation of PRLr and that impaired degradation of PRLr contributes to the resistance of mammary cells to cannabinoid-induced growth inhibition.
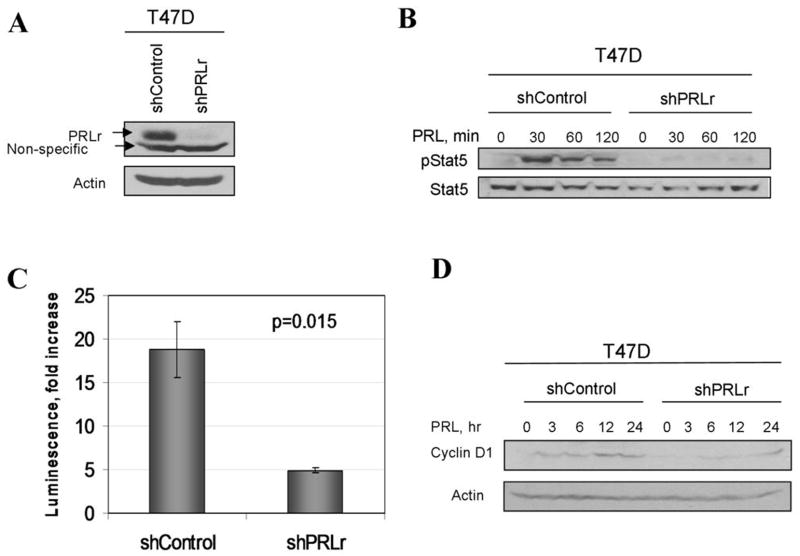
We next used a genetic approach to independently verify potential link between the levels of PRLr expression with growth of human breast cancer cells. To this end, we investigated an effect of knocking down the PRLr levels by RNAi on the extent of PRL signaling and transformed phenotype. Human breast cancer T47D cells harboring shRNA against PRLr displayed noticeably decreased levels of PRLr (Figure 5A). Knockdown of PRLr almost entirely prevented ligand-induced phosphorylation of Stat5 (Figure 5B) and dramatically attenuated an activation of PRL-responsive CISH promoter (Figure 5C). Accordingly, PRL-induced induction of cyclin D1 was noticeably impaired in T47D cells that harbor shRNA against PRLr (shPRLr, Figure 5D).
Figure 5. Knockdown of PRLr inhibits PRL signaling in T47D cells.
A. Expression of PRLr in T47D cells transduced with indicated shRNA constructs was analyzed by immunoblotting using indicated antibodies (upper panel). Analysis of β-actin levels was used as a loading control (lower panel).<br>B. Phosphorylation and levels of Stat5 proteins in T47D cell lines treated with PRL (100ng/ml) for indicated time were analyzed using indicated antibodies.
C. PRL-induced CISH promoter-driven luciferase activity in indicated T47D cell lines was performed as described in the Materials and Methods.
D. Levels of cyclin D1 in indicated T47D cell lines treated with PRL for indicated times were analyzed by immunoblotting. Same assay using anti-β-actin antibody is used as a loading control.
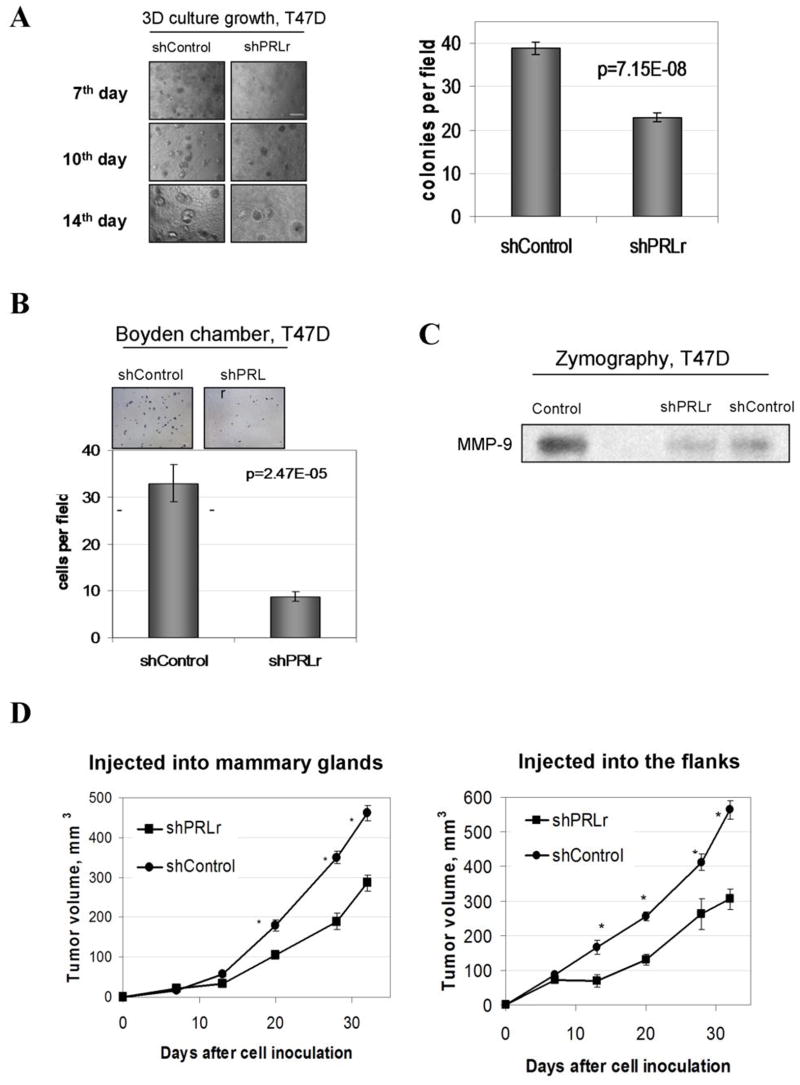
We next determined how downregulation of PRLr affect the transformed phenotype of T47D cells. We noticed that shPRLr-containing cells grew slower when cultured under normal conditions (Figure S8). Conversely, an ability to form colonies in three-dimensional culture was noticeably attenuated (Figure 6A). Knockdown of PRLr dramatically impaired the ability of T47D cells to migrate through Matrigel-covered filters in Boyden chambers (Figure 6B) and decreased the expression of MMP-9 (Figure 6C) confirming the role of PRLr levels in promoting invasiveness and motility of human breast cells seen in Figure 3A. Furthermore, cells harboring shPRLr formed visibly smaller tumors when injected either in the flank or into the mammary gland of immunocompromized mice (Figure S9). Cells transduced with shRNA constructs against PRLr formed tumors that displayed a pronounced knockdown of PRLr levels (Figure S10) and exhibited a statistically significant decrease in growth rate (Figure 6D). These results indicate that maintenance of high levels of PRLr plays an important role in tumorigenicity of human breast cancer cells.
Figure 6. Analysis of growth, invasiveness, and tumorigenicity of T47D cell lines.
A. Representative morphology of indicated T47D cell lines growth in three-dimensional cultures at the indicated day after plating. The experiment was repeated twice. Scale bar - 15μM. The graph represents percent surface ± SE covered by cell growth calculated from nine of 10x magnification pictures (triplicates).
B. Migration of indicated T47D cells through Boyden chambers was analyzed as described in the Materials and Methods. The undersides of membrane inserts containing invaded cells fixed and stained with Eosin-Hematoxylin were photographed 72h after the plating using a 10X objective. Scale bar - 100μM. The graph represents number of invasive cells ± SE calculated from nine of 20x magnification pictures (triplicates).
C. Relative activity of MMP-9 in the indicated T47D-derived cells was analyzed by zymography as described in the Material and Methods.
D. Tumor volumes of indicated T47D breast cancer cells injected into either mammary glands or the flanks of the NRS mice were calculated as described in the Materials and Methods. Asterisks denote statistically significant (p<0.01) between the volume of tumors formed by T47D-derivatives that harbor indicated shRNA constructs.
DISCUSSION
While numerous epidemiologic and experimental data support important roles of PRL signaling in human breast cancers, the mechanisms that lead to constitutive activation of PRLr signaling that occurs in primary human mammary tumors are poorly understood. Recent identification of gain-of-function mutations in PRLr in women with benign breast tumors (12) together with the fact that PRLr levels are elevated in human breast carcinoma (5) suggest that PRLr and PRL signaling are conducive to tumor cells grow and survival in at least a subset of breast cancer cases.
However, besides activating diverse signaling pathways, PRL also stimulates downregulation of its own receptors. We previously found that PRLr is stabilized in some human breast cancers and tissues due to an impaired phosphorylation of PRLr on Ser349, which is required for recruitment of βTrcp ubiquitin ligase followed by PRLr ubiquitination and degradation (22, 25). Ras-mediated inhibition of ability of GSK3β to phosphorylate PRLr contributes to PRLr stabilization in human breast cancers (26). Here we investigated the consequences of PRLr stabilization and accumulation that was expected to contribute to elevated PRL signaling (25).
In this study, we used two converging approaches: stabilization of PRLr in non-tumorigenic mammary epithelial cells (by expressing the PRLrS349A mutant) and decreasing the levels of PRLr in human breast cancer cells (by either treatment with endogenous cannabinoid that stimulated phosphorylation-dependent degradation of PRLr or by knocking down this receptor). Data from experiments using both approaches clearly demonstrate that increased levels of PRLr in human mammary cells play a key role in developing and maintaining their transformed phenotype.
These data indicate that human breast cancers gain growth and invasive advantages by stabilizing the PRLr and suggest that PRL signaling in general and regulation of PRLr in particular are important for mammary tumorigenesis. This hypothesis is consistent with published data demonstrating that knockout of PRLr in mice prevents mammary tumorigenesis induced by SV40 large T antigen (10) and that either PRLr antagonists (20) or endogenous cannabinoids that decrease levels of PRLr ((38) and this study) suppress growth of human breast cancer cells. As shown here, an augmented PRL signaling increases the expression of cyclin D1 and MMP9. Given that the loss of Stat5 activation detected during metastatic progression of human breast cancer was correlated with poor prognosis (42) and reduced differentiation (43, 44), it is plausible that PRLr stabilization along with further alterations in modulatory signals might skew elevated PRL signaling toward activation of Src, MAPK and PI3K-Akt rather than toward the canonical Jak2-Stat5 pathway. Additional studies aimed at defining the mechanisms, by which stabilized PRLr stimulate growth and invasiveness of human mammary epithelial cells, are underway.
While our current data clearly point to the importance of maintaining PRLr levels for breast cancer cell tumorigenicity, future studies should reveal additional genetic events that cooperate with stabilized PRLr during formation of tumors. Although stabilization of PRLr along with knockdown of p53 tumor suppressor protein temporarily allowed near normal MCF10A to grow in nude mice, these genetic changes were clearly insufficient to sustain tumorigenesis (Figure 3C). Given an aggressive phenotype of these cells in vitro, they seem to lack a systemic factor when implanted in mice. It is plausible that activation of other oncogenes (e.g., c-Myc) is required for angiogenesis in these tumors; under this scenario, stabilized PRLr is likely to promote survival of tumor cells deprived of nutrition and oxygen. On the other hand, the fact that expression of stabilized PRLr slowed down tumor regression may reflect prolonged PRL signaling, which might be insufficient in transplanted human cells given that mouse PRL poorly activates human PRLr (45). Generation of human PRL knock-in mice will enable testing of this possibility.
Data demonstrating that decreasing levels of PRLr in breast cancer cells was detrimental for their tumorigenicity provide a justification for development of the agents that promote PRLr degradation. Treatment of cells with endogenous cannabinoid anandamide stimulated downregulation of PRLr via promoting phosphorylation of this receptor on Ser349 (Figures 4 and S5).
Furthermore, although anandamide can affect human breast cancer cell growth by various pathways (for example by affecting cAMP/protein kinase or MAPK pathways (39)), the phosphorylation-dependent degradation of PRLr appeared to be important for the anti-proliferative effects of anandamide. Intriguingly, anandamide did not stimulate activity of GSK3β (Plotnikov and Fuchs, unpublished data) indicating the existence of another kinase pathway that promotes Ser349 phosphorylation and PRLr degradation. As opposed to constitutively active GSK3β, this alternate kinase is likely to be induced by PRL in a Jak2-depdenden manner to mediate ligand-stimulated phosphorylation, ubiquitination, endocytosis and degradation of PRLr (23, 28, 33). We speculate that anandamide may activate this yet to be identified ligand-sensitive kinase to promote Ser349 phosphorylation and subsequent PRLr ubiquitination and degradation. Studies aimed at the identification of this putative kinase and the mechanisms of its activation might be of translational value; these studies are currently in progress. Although endogenous cannabinoids are too unstable and pleiotropic to be used as drugs, identification of other types of small molecules that stimulate PRLr phosphorylation and turnover in a GSK3-independent manner should benefit those patients whose malignancies depend on PRL signaling.
Supplementary Material
Acknowledgments
We are grateful to V.S. Spiegelman, C.V. Clevenger and the members of his lab for their critical comments and discussions. We thank C.V. Clevenger, A. Eastman, A.F. Parlow and Z. Ronai for reagents, and V.M. Weaver and A. Dagvadorj for technical advice. This work was supported by the NCI grants CA115281 (to S.Y.F.) and CA118740 (to H.R.).
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24(1):1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das R, Vonderhaar BK. Prolactin as a mitogen in mammary cells. J Mammary Gland Biol Neoplasia. 1997;2(1):29–39. doi: 10.1023/a:1026369412612. [DOI] [PubMed] [Google Scholar]
- 4.Perks CM, Keith AJ, Goodhew KL, Savage PB, Winters ZE, Holly JM. Prolactin acts as a potent survival factor for human breast cancer cell lines. Br J Cancer. 2004;91(2):305–11. doi: 10.1038/sj.bjc.6601947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds C, Montone KT, Powell CM, Tomaszewski JE, Clevenger CV. Expression of prolactin and its receptor in human breast carcinoma. Endocrinology. 1997;138(12):5555–60. doi: 10.1210/endo.138.12.5605. [DOI] [PubMed] [Google Scholar]
- 6.Gill S, Peston D, Vonderhaar BK, Shousha S. Expression of prolactin receptors in normal, benign, and malignant breast tissue: an immunohistological study. J Clin Pathol. 2001;54(12):956–60. doi: 10.1136/jcp.54.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatavdekar JM, Patel DD, Shah NG, et al. Prolactin as a local growth promoter in patients with breast cancer: GCRI experience. Eur J Surg Oncol. 2000;26(6):540–7. doi: 10.1053/ejso.2000.0943. [DOI] [PubMed] [Google Scholar]
- 8.Wennbo H, Gebre-Medhin M, Gritli-Linde A, Ohlsson C, Isaksson OG, Tornell J. Activation of the prolactin receptor but not the growth hormone receptor is important for induction of mammary tumors in transgenic mice. J Clin Invest. 1997;100(11):2744–51. doi: 10.1172/JCI119820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22(30):4664–74. doi: 10.1038/sj.onc.1206619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakes SR, Robertson FG, Kench JG, et al. Loss of mammary epithelial prolactin receptor delays tumor formation by reducing cell proliferation in low-grade preinvasive lesions. Oncogene. 2007;26(4):543–53. doi: 10.1038/sj.onc.1209838. [DOI] [PubMed] [Google Scholar]
- 11.Canbay E, Degerli N, Gulluoglu BM, Kaya H, Sen M, Bardakci F. Could prolactin receptor gene polymorphism play a role in pathogenesis of breast carcinoma? Curr Med Res Opin. 2004;20(4):533–40. doi: 10.1185/030079904125003232. [DOI] [PubMed] [Google Scholar]
- 12.Bogorad RL, Courtillot C, Mestayer C, et al. Identification of a gain-of-function mutation of the prolactin receptor in women with benign breast tumors. Proc Natl Acad Sci U S A. 2008;105(38):14533–8. doi: 10.1073/pnas.0800685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629–34. doi: 10.1093/jnci/91.7.629. [DOI] [PubMed] [Google Scholar]
- 14.Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res. 2004;64(18):6814–9. doi: 10.1158/0008-5472.CAN-04-1870. [DOI] [PubMed] [Google Scholar]
- 15.Mujagic Z, Mujagic H. Importance of serum prolactin determination in metastatic breast cancer patients. Croat Med J. 2004;45(2):176–80. [PubMed] [Google Scholar]
- 16.Frontini L, Lissoni P, Vaghi M, et al. Enhancement of the efficacy of weekly low-dose taxotere by the long acting anti-prolactinemic drug cabergoline in pretreated metastatic breast cancer. Anticancer Res. 2004;24(6):4223–6. [PubMed] [Google Scholar]
- 17.Lissoni P, Bucovec R, Malugani F, et al. A clinical study of taxotere versus taxotere plus the antiprolactinemic agent bromocriptine in metastatic breast cancer pretreated with anthracyclines. Anticancer Res. 2002;22(2B):1131–4. [PubMed] [Google Scholar]
- 18.Lissoni P, Vaghi M, Ardizzoia A, et al. Efficacy of monochemotherapy with docetaxel (taxotere) in relation to prolactin secretion in heavily pretreated metastatic breast cancer. Neuro Endocrinol Lett. 2001;22(1):27–9. [PubMed] [Google Scholar]
- 19.Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994;269(7):5364–8. [PubMed] [Google Scholar]
- 20.Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26(3):400–22. doi: 10.1210/er.2004-0016. [DOI] [PubMed] [Google Scholar]
- 21.Fuh G, Wells JA. Prolactin receptor antagonists that inhibit the growth of breast cancer cell lines. J Biol Chem. 1995;270(22):13133–7. doi: 10.1074/jbc.270.22.13133. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Kumar KG, Tang W, Spiegelman VS, Fuchs SY. Negative regulation of prolactin receptor stability and signaling mediated by SCF(beta-TrCP) E3 ubiquitin ligase. Mol Cell Biol. 2004;24(9):4038–48. doi: 10.1128/MCB.24.9.4038-4048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swaminathan G, Varghese B, Fuchs SY. Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(1):81–91. doi: 10.1007/s10911-008-9068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clevenger CV, Chang WP, Ngo W, Pasha TL, Montone KT, Tomaszewski JE. Expression of prolactin and prolactin receptor in human breast carcinoma. Evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146(3):695–705. [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Clevenger CV, Minkovsky N, et al. Stabilization of prolactin receptor in breast cancer cells. Oncogene. 2006;25(13):1896–902. doi: 10.1038/sj.onc.1209214. [DOI] [PubMed] [Google Scholar]
- 26.Plotnikov A, Li Y, Tran TH, et al. Oncogene-mediated inhibition of glycogen synthase kinase 3 beta impairs degradation of prolactin receptor. Cancer Res. 2008;68(5):1354–61. doi: 10.1158/0008-5472.CAN-07-6094. [DOI] [PubMed] [Google Scholar]
- 27.Levesque AA, Kohn EA, Bresnick E, Eastman A. Distinct roles for p53 transactivation and repression in preventing UCN-01-mediated abrogation of DNA damage-induced arrest at S and G2 cell cycle checkpoints. Oncogene. 2005;24(23):3786–96. doi: 10.1038/sj.onc.1208451. [DOI] [PubMed] [Google Scholar]
- 28.Varghese B, Barriere H, Carbone CJ, et al. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol. 2008;28(17):5275–87. doi: 10.1128/MCB.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang W, Li Y, Yu D, Thomas-Tikhonenko A, Spiegelman VS, Fuchs SY. Targeting beta-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res. 2005;65(5):1904–8. doi: 10.1158/0008-5472.CAN-04-2597. [DOI] [PubMed] [Google Scholar]
- 30.Fang F, Antico G, Zheng J, Clevenger CV. Quantification of PRL/Stat5 signaling with a novel pGL4-CISH reporter. BMC Biotechnol. 2008;8:11. doi: 10.1186/1472-6750-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spiegelman VS, Tang W, Chan AM, et al. Induction of homologue of Slimb ubiquitin ligase receptor by mitogen signaling. J Biol Chem. 2002;277(39):36624–30. doi: 10.1074/jbc.M204524200. [DOI] [PubMed] [Google Scholar]
- 32.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116(Pt 7):1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan G, Varghese B, Thangavel C, et al. Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J Endocrinol. 2008;196(2):R1–7. doi: 10.1677/JOE-07-0554. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder MD, Symowicz J, Schuler LA. PRL modulates cell cycle regulators in mammary tumor epithelial cells. Mol Endocrinol. 2002;16(1):45–57. doi: 10.1210/mend.16.1.0762. [DOI] [PubMed] [Google Scholar]
- 35.Clevenger CV, Kline JB. Prolactin receptor signal transduction. Lupus. 2001;10(10):706–18. doi: 10.1191/096120301717164949. [DOI] [PubMed] [Google Scholar]
- 36.Clevenger CV, Zheng J, Jablonski EM, Galbaugh TL, Fang F. From bench to bedside: future potential for the translation of prolactin inhibitors as breast cancer therapeutics. J Mammary Gland Biol Neoplasia. 2008;13(1):147–56. doi: 10.1007/s10911-008-9074-8. [DOI] [PubMed] [Google Scholar]
- 37.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52(4):255–64. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 38.De Petrocellis L, Melck D, Palmisano A, et al. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci U S A. 1998;95(14):8375–80. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melck D, Rueda D, Galve-Roperh I, De Petrocellis L, Guzman M, Di Marzo V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999;463(3):235–40. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- 40.Melck D, De Petrocellis L, Orlando P, et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141(1):118–26. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 41.Di Marzo V, Melck D, De Petrocellis L, Bisogno T. Cannabimimetic fatty acid derivatives in cancer and inflammation. Prostaglandins Other Lipid Mediat. 2000;61(1–2):43–61. doi: 10.1016/s0090-6980(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 42.Nevalainen MT, Xie J, Torhorst J, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22(11):2053–60. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 43.Yamashita H, Nishio M, Ando Y, et al. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr Relat Cancer. 2006;13(3):885–93. doi: 10.1677/erc.1.01095. [DOI] [PubMed] [Google Scholar]
- 44.Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer. 2004;108(5):665–71. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- 45.Utama FE, LeBaron MJ, Neilson LM, et al. Human prolactin receptors are insensitive to mouse prolactin: implications for xenotransplant modeling of human breast cancer in mice. J Endocrinol. 2006;188(3):589–601. doi: 10.1677/joe.1.06560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.