Abstract
Synaptotagmin 2 (Syt2) functions as a low affinity, fast exocytic Ca2+ sensor in neurons, where it is activated by Ca2+ influx through voltage-gated channels. Targeted insertion of lacZ into the mouse syt2 locus reveals expression in mucin-secreting goblet cells of the airways. In these cells, rapid Ca2+ entry from the extracellular medium does not contribute significantly to stimulated secretion (Davis, C. W., and Dickey, B. F. (2008) Annu. Rev. Physiol. 70, 487–512). Nonetheless, Syt2–/– mice show a severe defect in acute agonist-stimulated airway mucin secretion, and Syt2+/– mice show a partial defect. In contrast to Munc13-2–/– mice (Zhu, Y., Ehre, C., Abdullah, L. H., Sheehan, J. K., Roy, M., Evans, C. M., Dickey, B. F., and Davis, C. W. (2008) J. Physiol. (Lond.) 586, 1977–1992), Syt2–/– mice show no spontaneous mucin accumulation, consistent with the inhibitory action of Syt2 at resting cytoplasmic Ca2+ in neurons. In human airway goblet cells, inositol trisphosphate receptors are found in rough endoplasmic reticulum that closely invests apical mucin granules, consistent with the known dependence of exocytic Ca2+ signaling on intracellular stores in these cells. Hence, Syt2 can serve as an exocytic sensor for diverse Ca2+ signaling systems, and its levels are limiting for stimulated secretory function in airway goblet cells.
Mucin secretion in the airways of the lungs is crucial for clearance of inhaled particulates and pathogens (1). However, mucin hypersecretion is a leading cause of mortality in common diseases such as asthma and cystic fibrosis (2). Thus, tight control of mucin secretion is critical for lung homeostasis. Airway mucin secretion is stimulated by triphosphate nucleotides secreted into the extracellular lumenal liquid layer (3). These bind to epithelial apical P2Y2 receptors that activate Gq, which in turn activates phospholipase Cβ1 generating the intracellular second messengers diacylglycerol and inositol trisphosphate (IP3).3 Diacylglycerol directly induces mucin granule exocytosis by activating the priming protein Munc13-2 (4), and indirectly regulates exocytosis by activating protein kinase Cε (5). IP3 induces the release of Ca2+ from intracellular stores, resulting in a rise in cytoplasmic Ca2+ that rapidly triggers mucin granule exocytosis (6–9). However, the precise mechanism by which a rise in cytoplasmic Ca2+ is coupled to exocytosis in goblet cells is not known.
Synaptotagmins (Syts) are a family of structurally related proteins of which several are known to mediate Ca2+-dependent exocytosis. Syts are composed of a short intravesicular amino terminus, a transmembrane domain, a variable linker region, and two conserved C2 domains near the carboxyl terminus (10, 11). There are at least 15 Syt family members encoded in mammalian genomes. Of these, eight (Syt1–3, -5–7, -9, and -10) display Ca2+-dependent phospholipid binding that is thought to be essential for Ca2+-dependent exocytosis (12–16). A subset of three of these (Syt1, -2, and -9) binds Ca2+ with low affinity (∼10 μm) and high cooperativity (n = 5) and functions as fast, synchronous Ca2+ sensors in neurons (16). Syt1 mediates synchronous synaptic vesicle release in forebrain neurons, and also mediates rapid exocytosis in adrenal chromaffin cells (15, 16). Like neurons, chromaffin cells express voltage-gated Ca2+ channels activated by neurotransmitter-induced depolarization. Syt2 mediates synchronous synaptic vesicle release in hindbrain neurons and at the neuromuscular junction (14, 17), but it has not been previously known to function outside the nervous system. Syt9 mediates synchronous synaptic vesicle release from limbic and striatal neurons (16), and it also functions in dense core granule release from the PC12 chromaffin cell line (18–20) and insulin release from pancreatic islet cells (21, 22). In islet cells, membrane depolarization and opening of voltage-gated Ca2+ channels is induced by closure of KATP channels when blood glucose is elevated. To our knowledge, there has been no analysis of the function of a low Ca2+ affinity, fast Syt in a nonexcitable cell (i.e. a cell not expressing voltagegated Ca2+ channels).
EXPERIMENTAL PROCEDURES
Materials—Affinity-purified pan-IP3-R (23) and Rab3D antibodies (24) and ankyrin-B (25) and Clara cell secretory protein (CCSP) antibodies (24) have been described. Rabbit antibodies against mature MUC5AC (26) were from John Sheehan (University of North Carolina, Chapel Hill), and against Syt2 (A320) (14) were from Thomas Sudhof (University of Texas Southwestern Medical Center). We purchased mouse monoclonal antibodies against protein disulfide isomerase (PDI) (S34200; Molecular Probes), immature MUC5AC (MAB201; Chemicon), and acetylated tubulin (T6793; Sigma); goat antibodies against β-galactosidase (4600-1409; Biogenesis); fluorescein isothiocyanate-conjugated goat anti-mouse IgG, and Texas Red-conjugated goat anti-rabbit IgG secondary antibodies (Jackson ImmunoResearch); bovine serum albumin (A7906; Sigma); VectaShield mounting medium (Vector Laboratories); Superfrost/Plus microscope slides and coverglasses (Fisher); Liquid Blocker Super Pap Pens (Daido Sangyo); and Polybed resin (Polysciences).
Mice—Generation of a syt2 null allele was described previously (14). Mice carrying this allele were backcrossed for 11 generations onto a C57BL/6 background and studied at the M. D. Anderson Cancer Center in accordance with its Institutional Animal Care and Use Committee. Mice with an ankyrin-B null allele have been carried on a C57BL/6 background (27) and were studied at the University of North Carolina, Chapel Hill, in accordance with its Institutional Animal Care and Use Committee. Experimental animals and wild-type (WT) littermate controls were obtained from heterozygous crosses. For immunoblot and histochemical analyses, mice were euthanized by CO2 asphyxiation, and their lungs were harvested.
Human Tissue—Human lung tissue was obtained from normal and cystic fibrosis patients according to the guidelines of the Committee on the Protection of the Rights of Human Subjects of the University of North Carolina, Chapel Hill. The lung tissue was either immediately frozen in OCT embedding medium (Sakura) on dry ice, fixed in 10% neutral buffered formalin, or fixed in buffered aldehyde (2% glutaraldehyde and 2% paraformaldehyde).
Immunoblots—Lung tissue was homogenized in PBS containing a mixture of protease inhibitors in DMSO (P8340; Sigma), diluted 1:1,000, and consisted of 4-(2-aminoethyl)benzenesulfonyl fluoride, N-(trans-epoxysuccinyl)-l-leucyl-amido-(4-guanidino)butylamide, pepstatin A, bestatin, leupeptin, and aprotinin. Protein concentrations were determined by bicinchoninic acid assay (Pierce); equal amounts of protein were loaded for SDS-PAGE, and the resolved proteins were electrotransferred to nitrocellulose. Blots were probed with antibodies against Syt2 to measure its expression in lungs (14) or against IP3-R, ankyrin-B, and PDI (23) to determine antibody specificity in lung tissue, all as described.
Histochemistry of Mouse Tissues—For β-galactosidase staining, lungs from adult Syt2 heterozygous mutant mice and WT controls were fixed in 0.2% glutaraldehyde, incubated with X-gal, dehydrated, embedded in paraffin, sectioned, deparaffinized, rehydrated, and counterstained with treosin as described (14) or co-stained immunohistochemically for CCSP as described (24) and then examined by bright field microscopy. For immunocolocalization of β-galactosidase with cell lineage markers, tissues from neonatal homozygous mutant mice and WT controls were fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, deparaffinized, and labeled with antibodies against β-galactosidase, CCSP, and acetylated tubulin as described (28) and then examined by fluorescence microscopy.
Mucin Secretion in Mouse Airways—The apical secretory cells of the intrapulmonary airways of mice and the small distal airways of humans lack histochemically apparent mucin under base-line healthy conditions, although intracellular mucin can be demonstrated using sensitive immunohistochemical techniques (4, 24, 29, 30). Under base-line conditions, these airway secretory cells are often termed Clara cells (24). When production of gel-forming polymeric mucins is increased by allergic or infectious inflammation, intracellular mucin becomes histochemically apparent under the rubric “mucous metaplasia,” and these airway secretory cells are generally termed goblet or mucous cells although they continue to express the Clara cell marker CCSP (24, 30). The secretory cells of the large airways of humans often contain histochemically apparent mucin even under base-line conditions (30). To induce mucous metaplasia in mouse airways for the measurement of mucin secretion, post-natal day 16 pups and their mothers were exposed for 60 min to an aerosolized solution of 0.225 mg/ml human IL-13 (Wyeth) in PBS generated by an AeroMist CA-209 nebulizer (CIS-US) driven by 10 liters/min of 5% CO2 in air to increase ventilation, with a calculated deposition of 0.5 μg of IL-13 per mouse. Three days later, mice were exposed to a 100 mm aerosol of ATP in PBS for 5 min to induce mucin secretion (24), then euthanized by CO2 asphyxiation, and their lungs harvested. Mucous metaplasia was also induced in adult heterozygous mice by ovalbumin sensitization and challenge as described (24), and mucin secretion was stimulated with aerosolized ATP as for the pups. Intracellular epithelial mucin was measured before and after ATP stimulation by staining with periodic acid-fluorescent Schiff's (PAFS) reagent, as described (24). In brief, lungs were fixed with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.2) infused through a tracheal cannula at 21 °C, then removed from the thoracic cavity, and further fixed overnight at 4 °C, embedded in paraffin, sectioned, and stained for fluorescence microscopy. Data are presented as the volume of intracellular mucin per surface area of the basement membrane, which is derived as the ratio of epithelial area with red fluorescence staining to total epithelial area divided by a boundary length measurement that is a product of the total epithelial area, the basement membrane length, and the geometric constant 4/π to yield an idealized cylindrical cell (24).
Confocal Microscopy of Human Tissues—For IP3-R immunohistochemistry, formalin-fixed human lung tissue was dehydrated and embedded in paraffin; sections were cut at 5 μm, deparaffinized, and rehydrated. For the other antibodies, frozen sections were fixed in cold 100% methanol for 10 min, washed with PBS, blocked with 3% bovine serum albumin in PBS for 2 h at 21 °C, and incubated with primary antibody overnight at 4 °C and then the appropriate secondary antibody for 1 h at 21 °C, both diluted in blocking solution. Immunofluorescence was visualized using a Leica SP2 or Zeiss 510 laser scanning confocal microscope, and oil immersion 40× Apochromat and 63× plan-Apochromat objectives (1.25 and 1.4 NA, respectively), with excitation from a 488-nm argon and a 568-nm krypton laser (Leica SP2) or a 543-nm HeNe laser (Zeiss 510). Photo-multiplier gains and offsets for the two fluorescence channels were adjusted to minimize cross-talk, and differential interference microscopy was used in a third channel for general morphology. Simple PCI (Compix) and Zeiss 510 imaging software were used for image analysis and quantitation. For each experiment, the immunostaining procedure was repeated a minimum of three times using tissue from at least three subjects, at least one of which came from a patient with non-cystic fibrosis. The images and protein distributions presented are fully representative, and there were no apparent differences in the described parameters between cystic fibrosis and non-cystic fibrosis tissues.
Electron Microscopy—Aldehyde-fixed human lung tissue was post-fixed in 1% OsO4 for 1 h at 4 °C, dehydrated, immersed two times in propylene oxide for 20 min, then infiltrated with Epon resin, and polymerized. Sections of 90 nm were cut onto copper grids, stained with uranyl acetate followed by lead citrate, and viewed with a Zeiss EM900 transmission electron microscope.
RESULTS
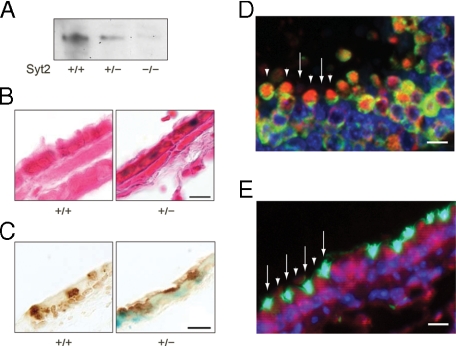
Syt2 Expression in Airway Epithelium—We found Syt2 in the lungs of WT but not null mice by immunoblot analysis (Fig. 1A), raising the possibility that Syt2 serves as an exocytic Ca2+ sensor in secretory epithelial cells. Syt2 null mice had been generated by knocking IRES-lacZ with a nuclear localization signal into the syt2 locus under the transcriptional control of the native Syt2 regulatory elements (14). We found X-gal staining in the nuclei of non-ciliated cells of the bronchial epithelium of neonatal (Fig. 1B) and adult (data not shown) heterozygous mice. Localization of Syt2 to airway secretory (Clara) cells was confirmed by colocalization of X-gal+ nuclei within CCSP+ cells (24) (Fig. 1C). By dual immunofluorescence colocalization, β-galactosidase was detected in the nuclei and cytoplasm of virtually all CCSP-labeled airway epithelial secretory cells (Fig. 1D and supplemental Fig. 1) but no acetylated tubulin-labeled ciliated cells (Fig. 1E). Despite localizing Syt2 expression to airway secretory cells through analysis of lacZ expression, we were unable to detect Syt2 immunohistochemically in the airways of WT mice even though it can be readily detected in neurons (14, 17). This suggests that the level of Syt2 protein in airway secretory cells is less than in neurons.
FIGURE 1.
Syt2 is expressed in airway secretory (Clara) cells. A, Western-blotted lung homogenates from WT (+/+), heterozygous (+/–), and null (–/–) mutant mice were probed with rabbit polyclonal antibodies against Syt2. A single band was observed in WT and heterozygous mouse lung tissue at ∼65 kDa. B, bronchial airways of WT and heterozygous mutant mice with lacZ knocked into the syt2 locus were stained with X-gal (blue) and then counterstained with treosin (red). Blue staining is observed only in heterozygous mice in cells that do not contain ciliated tufts. Scale bar in all tissue sections = 10 μm. C, bronchial airways of WT and heterozygous mutant mice were stained with X-gal (blue) and then immunostained with rabbit polyclonal antibodies against mouse CCSP developed with diaminobenzidine (brown). Blue staining is observed in association with brown-stained Clara cells. D, bronchial airways of null mice were labeled with antibodies against β-galactosidase (red) and CCSP (green), and nuclei were labeled with 4′,6-diamidino-2-phenylindole (blue). Both red and green labeling are observed in Clara cells (arrowheads), alternating with ciliated cells that are not labeled with either antibody (arrows). Control WT mice show no red labeling (supplemental Fig. 1). E, bronchial airways of null mice were labeled with antibodies against β-galactosidase (red) or acetylated tubulin (green), and nuclei were labeled with 4′,6-diamidino-2-phenylindole (blue). Red labeling is observed in Clara cells that are not labeled with acetylated tubulin antibodies (arrowheads), whereas green labeling is observed in ciliated cells that are not labeled with β-galactosidase antibodies (arrows).
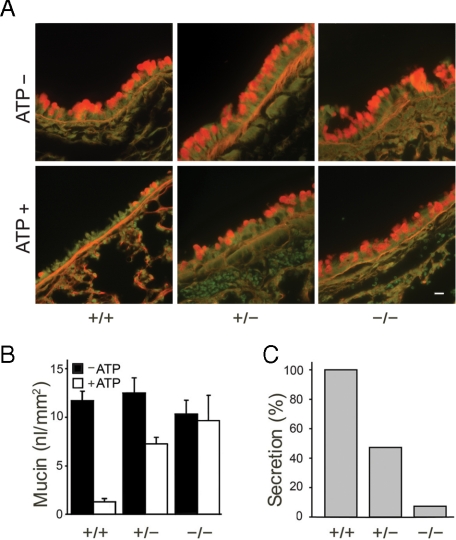
Mucin Secretory Function in Syt2 Null Mice—We analyzed airway secretory cell function by measuring the release of intracellular mucin after stimulation with the strong secretagogue ATP. Because the small amount of intracellular mucin present within the secretory cells of mouse airways under base-line uninflamed conditions is not visible histochemically (see “Experimental Procedures”), we first increased polymeric gel-forming mucin production with IL-13 (i.e. induced mucous metaplasia) (24). syt2 null mice have severe motor dysfunction and die at the time of weaning (P19–P24) (14), so post-natal day 16 pups of mixed genotypes and their heterozygous mothers were exposed to an IL-13 aerosol. This led to robust mucin gene expression in all genotypes, apparent as abundant intracellular PAFS staining 3 days after IL-13 exposure (Fig. 2A, top row), compared with faint patchy PAFS staining in mice of all genotypes not exposed to IL-13 (data not shown). We then stimulated mucin secretion by activating P2Y2 receptors with an ATP aerosol. The airway epithelial cells of WT pups secreted most of their PAFS-positive intracellular mucin within 5 min. In contrast, the epithelial cells of homozygous null pups showed a severe mucin secretory defect, and those of heterozygous pups showed an intermediate phenotype (Fig. 2). The heterozygous mothers exposed to the IL-13 aerosol showed a similar mucin secretory defect in response to the ATP aerosol (data not shown), and heterozygous adult mice of both sexes with mucous metaplasia induced by ovalbumin sensitization and challenge also showed a comparable secretory defect (Fig. 3).
FIGURE 2.
Syt2 mediates stimulated mucin secretion. A, bronchial airways of WT, heterozygous, and homozygous mutant P19 mice that had had been exposed 3 days earlier to aerosolized IL-13 to induce mucous metaplasia (top row), then exposed to aerosolized ATP to induce mucin secretion (bottom row), were stained for intracellular mucin with PAFS (orange). Scale bar, 10 μm. B, intracellular mucin content of bronchial airways from three experiments such as that in Fig. 2A, which included at least three pups of each genotype and five sections from each airway, was measured and analyzed. C, data from B are replotted as the percentage of intracellular mucin released for each genotype relative to WT.
FIGURE 3.
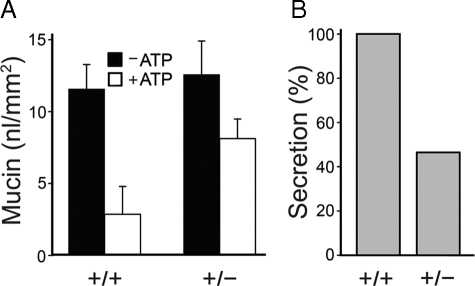
Airway mucin secretion is impaired in heterozygous Syt2 mutant adult mice. A, mucous metaplasia was induced in the airways of WT and heterozygous adult mice of both sexes by intraperitoneal sensitization and aerosol challenge with ovalbumin. Three days later, secretion was stimulated by exposure to aerosolized ATP, and retained intracellular mucin was measured by PAFS staining as in Fig. 2. There was no difference in intracellular mucin content between heterozygous and WT mice in the absence of mucous metaplasia (∼1 nl/mm2, not shown) or in mice with mucous metaplasia prior to stimulation with the ATP aerosol (∼12 nl/mm2, black bars). However, heterozygous mice released only half as much mucin as WT mice after ATP stimulation (white bars). Shown are the results of a representative experiment that was performed three times with similar results. B, data from A are replotted as the percentage of intracellular mucin released for each genotype relative to WT.
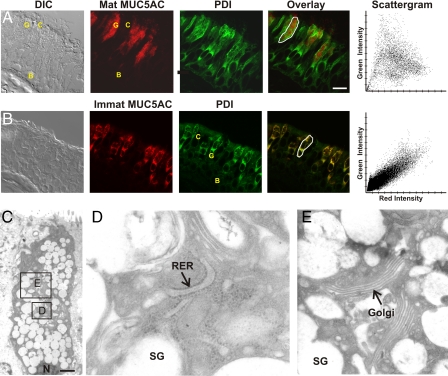
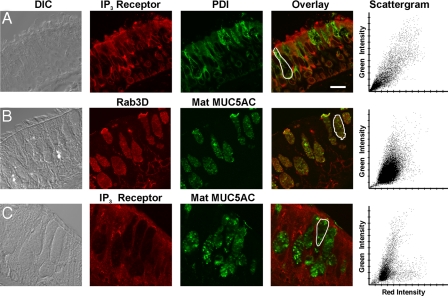
Proximity of ER to Mucin Secretory Granules—ATP-stimulated elevations in cytoplasmic Ca2+ in airway goblet cells are due primarily to IP3-mediated release from intracellular stores (3, 7, 9). IP3 typically acts on Ca2+-release channels in the ER in non-muscle cells, so we examined the localization of ER in cells of human airway epithelium using antibodies to PDI whose specificity in lung tissue was verified by immunoblot analysis (supplemental Fig. 2A). Immunostaining of lung sections showed ER distributed around the nuclei of basal, ciliated, and goblet cells and throughout the cytoplasm of goblet cells apical to the nuclei (Fig. 4A). The heavy staining of goblet cells for PDI is consistent with the demands of synthesizing disulfide-linked polymeric mucins (31). Ca2+ is heavily buffered in the cytoplasm, so changes in concentration are highly localized. Because Syt proteins are localized on the cytoplasmic surface of secretory vesicles (3), we examined the proximity of ER to mucin granules in goblet cells using confocal microscopy and antibodies against mature MUC5AC (matMUC5AC), the predominant secreted polymeric mucin of human airway epithelium (2). The PDI-positive ER apical to the nuclei of goblet cells surrounded matMUC5AC-positive mucin granules with little overlap of the two signals in the scattergram generated from the merged images (Fig. 4A).
FIGURE 4.
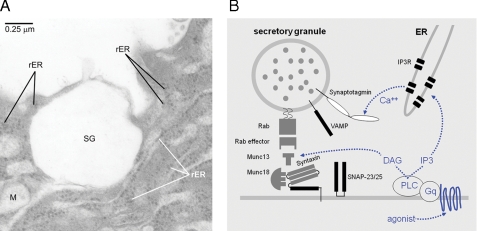
ER apical to the nucleus of airway secretory cells is closely apposed to mucin secretory granules. A, frozen human lung section imaged by differential interference contrast (DIC) microscopy (left) and labeled with antibodies against the secretory granule glycoprotein mature MUC5AC (red) and the lumenal ER resident protein PDI (green). An overlay image and a scattergram of the outlined goblet cell indicating the relative intensity of red and green labels for each pixel show a lack of colocalization of the two antibodies. Basal (B), ciliated (C), and goblet (G) cells are marked. Scale bar, 16 μm. B, ER in airway goblet cells is predominantly rough ER. A frozen human lung section labeled with antibodies against the newly synthesized cargo protein immature (nonglycosylated) MUC5AC (red) that labels only rough ER and antibodies against PDI (green) that labels both smooth and rough ER (31) shows a high degree of colocalization in the overlay image and scattergram. Scale identical to A. C, electron micrograph of a goblet cell from normal human lung, with the nucleus (N) marked. Scale bar, 2.5 μm. D, higher magnification of the area indicated in C, with rough ER (RER) and a mucin secretory granule (SG) marked. E, neighboring area from C at the same magnification, with Golgi apparatus (Golgi) and a mucin secretory granule (SG) marked.
ER in Goblet Cells Is Predominantly Rough ER—Airway goblet cells specialize in synthesis, storage, and secretion of polymeric mucins, so they could be expected to contain predominantly rough ER. To identify rough ER, human lung sections were labeled with antibodies to the immature, nonglycosylated form of MUC5AC (immatMUC5AC) (31). Confocal microscopy showed extensive colocalization of immatMUC5AC and PDI (Fig. 4B). By electron microscopy, the apical ER was seen to closely invest mucin granules, and confirmed to be predominantly rough ER (Fig. 4C and Fig. 6A). Golgi cisternae (Fig. 4E) and mitochondria (Fig. 6A) were interspersed among the ER and granules, similar to what we have observed in mouse airways (24).
FIGURE 6.
Juxtaposition of apical endoplasmic reticulum and mucin secretory granules. A, electron micrograph of the apical membrane region of a goblet cell, showing the juxtaposition of a mucin secretory granule (SG) and rough endoplasmic reticulum (rER). This human lung specimen was fixed ∼24 h after harvest for transplantation, and hence fixation is not optimal and internal membrane structures are not well preserved. Nonetheless, the arrays of ribosomes reveal the rough ER and show the intimate relationships between the plasma membrane, granule, rough ER, and mitochondria (M). B, pathway for activation of regulated secretion in airway goblet cells. Extracellular ligands in the airway surface liquid layer (bottom) bind to heptahelical receptors in the apical membrane that activate Gq and phospholipase (PLC) β1, generating the second messengers diacylglycerol (DAG) and inositol trisphosphate (IP3). Diacylglycerol activates the exocytic priming protein Munc13-2 (dotted blue arrow, left fork) and the exocytic regulator protein kinase Cε (data not shown). IP3 induces the release of Ca2+ from endoplasmic reticulum (ER) in the vicinity of mucin-containing secretory granules, activating Syt2 on the surface of secretory granules (dotted blue arrow, right fork) and coactivating Munc13 proteins (data not shown).
Localization of IP3-R to ER Adjacent to Mucin Granules—IP3-Rs have generally been localized to ER in secretory cells, but they have also been reported on secretory granules and plasma membrane (32–34). Human lung sections probed with a pan-IP3-R antibody that is highly specific in lung tissue (supplemental Fig. 2B) showed a broad distribution of immunoreactivity in basal, goblet, and ciliated cells (Fig. 5A). In goblet cells, IP3-R colocalized with the ER marker PDI (Fig. 5A), as expected. Presumably, membrane-localized IP3-R appears to colocalize with lumenal PDI because of the sub-microscopic nature of the ER. Because mucin secretory granules are much larger (∼0.5–1 μm), it was necessary to determine whether a granule surface marker would also appear to colocalize with matMUC5AC before testing whether IP3-R is expressed on granule membranes. We found that Rab3D, a small GTPase implicated in regulated exocytosis that resides on the surface of secretory granules (24), has a distribution that correlated closely with that of matMUC5AC (Fig. 5B), indicating that granule lumenal and membrane proteins are indistinguishable under the optical conditions of these experiments. Finally, we found that IP3-R and matMUC5AC resolve independently in co-stained images (Fig. 5C), indicating they populate distinct organelles. Hence, these data suggest that Ca2+ is released predominantly from the ER. Notably, the ER distributes to the vicinity of the apical membrane (Fig. 6A), positioned to provide Ca2+ locally to activate granules near exocytic docking sites.
FIGURE 5.
IP3-Rs are concentrated in apical ER adjacent to mucin secretory granules but not on granule membranes. A, frozen human lung section labeled with antibodies against IP3-R (red) and PDI (green), with the scattergram showing colocalization in the goblet cell outlined in the overlay image. Scale bar, 16 μm. DIC, differential interference contrast. B, mucin granule membrane and lumenal proteins are not resolved under the optical conditions of our experiments. Paraffin-embedded human lung section labeled with antibodies against the secretory granule surface protein Rab3D (red) and antibodies against the secretory granule lumenal protein mature MUC5AC (green), with the scattergram showing colocalization in the cell outlined in the overlay image. C, IP3-R are not detected on mucin granules. Paraffin-embedded human lung section labeled with antibodies against IP3-R (red) and mature MUC5AC (green) is shown, with the scattergram showing a lack of colocalization in the cell outlined in the overlay image (note the splaying of two clearly separated populations of points to their respective axes).
DISCUSSION
Syt2 as the Major Stimulated Exocytic Ca2+ Sensor in Airway Goblet Cells—Our results indicate that Syt2 mediates >90% of acute mucin secretion in response to extracellular signals in airway goblet cells. Syt2 is one of three low affinity Ca2+ sensors that trigger fast synaptic vesicle release in neurons, together with Syt1 and Syt9 (16, 17). Of these, Syt2 is the fastest and is expressed at synapses specialized for precise responses such as the calyx of Held (17). It is striking that Syt2 also serves as an exocytic Ca2+ sensor in goblet cells where the kinetics of stimulated exocytosis are measured in hundreds of milliseconds (35, 36) rather than the <2-ms time course of synchronous evoked synaptic vesicle release (10, 37). In goblet cells, the low affinity and high cooperativity of Ca2+ binding by Syt2 might ensure primarily the fidelity rather than the speed of signaling, in view of the danger of airway obstruction from excessive acute mucin release. For example, in death from asthma, asphyxiation occurs because of widespread plugging of airways with mucus (2). Similarly, airway liquid volume is tightly regulated by purinergic signaling with serious consequences to dysregulation in diseases such as cystic fibrosis (1).
Limitation of Goblet Cell Secretory Function by Syt2 Expression—Another striking finding of our studies is that acute airway mucin secretion in response to extracellular signals is reduced by ∼50% in heterozygous null mice. The dependence of regulated exocytosis on Syt2 levels in goblet cells (Figs. 2 and 3) but not neurons (14, 17) could reflect a lower level of expression in goblet cells, differences in accessory proteins, or differences in physical properties of the systems. One such latter possibility is the greater size of mucin granules (∼500 nm diameter) relative to synaptic vesicles (∼40 nm diameter) if Syt2 is distributed over the granule surface, and the cooperation of several SNARE-Syt complexes is required for membrane fusion, as suggested (10, 11, 38). In addition, a relatively low level of expression of Syt2 in goblet cells is indicated by our inability to detect Syt2 immunohistochemically in the airway, although we are readily able to detect it in neurons (14, 17) and mast cells.4 Because Syt2 inhibits exocytosis when not fully liganded by Ca2+ (14, 17, 39, 40), a low level of Syt2 expression in airway goblet cells could have adaptive value by allowing base-line mucin secretion through attenuation of this inhibitory function at resting cytoplasmic Ca2+ concentration (see comparison with Munc13-2 null mice, below). In contrast, spontaneous secretion by neurons reduces the fidelity of neural signaling, and spontaneous secretion by mast cells would induce inflammation, so there could be adaptive value to tightly constraining unstimulated release from these cell types through high levels of Syt2 expression.
Coupling of Syt2 to Intracellular Ca2+ Stores—Despite the different mechanisms of Ca2+ elevation in goblet cells compared with neurons and other excitable cells, the Ca2+ dependence (EC50 = 5.5 μm) of Syt2 binding to phospholipids (41) fits well with IP3-induced Ca2+ concentrations (10 μm) in the vicinity of the exocytic machinery we have estimated in goblet cells (6). IP3-Rs localized in the ER of goblet cells are in close apposition to secretory granules that presumably contain the Ca2+ sensor Syt2 (Figs. 4, 5, 6) (42), consistent with prior evidence in goblet cells for a proximity of Ca2+ channels to the exocytic Ca2+ sensor <50 nm using the fast Ca2+ buffer 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (6). These findings, together with the presence of mitochondria (Fig. 6A) (24, 43) that have well established roles in Ca2+ homeostasis (33, 44), indicate that the machinery for generating and responding to an exocytic Ca2+ signal are colocalized in the apical pole of goblet cells, where extracellular signals are sensed and mucins secreted (Fig. 6). The reliance of airway secretory cells on intracellular Ca2+ stores may reflect the instability of Ca2+ concentrations in airway lining fluid (45, 46), which is directly exposed to the external environment and to large amounts of Ca2+ released as counterions during the decondensation of highly anionic secreted mucins (34). In contrast, non-exocrine secretory cells are bathed in interstitial fluid or plasma with tightly controlled Ca2+ concentrations. Indeed, in airway secretory cells, capacitative Ca2+ entry occurs exclusively at the basolateral surface that is exposed to interstitial fluid (9).
Lack of Base-line Mucin Accumulation in syt2 Null Mice—The airway secretory phenotype of syt2 null mice, which show no spontaneous accumulation of intracellular mucin in the absence of inflammatory metaplasia but a severe defect in stimulated mucin secretion (Fig. 2), is strikingly different from that of Munc13-2 null mice, which spontaneously accumulate intracellular mucin but have only a moderate defect in stimulated mucin secretion (4). Possible explanations include differential distribution of Syt2 and Munc13-2 on distinct secretory granule populations, or intrinsic differences between Syt2 and Munc13-2 in their mechanisms of control of a single granule population. We favor the latter based upon the known physiology of Syt2 in neurons (14, 16, 17), as follows.
Fast, synchronous synaptic vesicle release occurs in close temporal and spatial proximity to the elevated cytoplasmic Ca2+ (∼10 μm) associated with an action potential (10, 37). Slow, asynchronous release occurs after voltage-gated Ca2+ channels have closed and is thought to be triggered by residual elevations of cytoplasmic Ca2+ (∼1 μm). Spontaneous release occurs at resting levels of cytoplasmic Ca2+ (∼0.1 μm). Whereas fast, synchronous release depends upon low affinity Syts (Syt1, -2, and -9), slow, asynchronous, and spontaneous releases are instead inhibited by low affinity Syts (14, 17, 39, 40). Nonetheless, in calyx of Held neurons, synchronous (Syt2-dependent) and asynchronous (Syt2-independent) evoked releases act on the same synaptic vesicle pool (17). By analogy, we consider it most likely that Syt2 and Munc13-2 regulate a single pool of mucin granules in airway goblet cells. Furthermore, the increase in spontaneous synaptic vesicle release at neuromuscular junctions of Syt2 null mice (14) suggests that the lack of base-line mucin accumulation in these mice might reflect a similar increase in spontaneous release because of the loss of the inhibitory action of Syt2. In contrast, unactivated Munc13-2 has no inhibitory function, so its absence results only in a partial loss of secretory function at both base-line and stimulated levels of cytoplasmic Ca2+, with no apparent gain of function. Residual priming function at both Ca2+ levels is likely mediated by Munc13-4 that is also expressed in airway goblet cells (4, 47). This provides a parsimonious explanation of the available results, although additional experiments are required to further test this model.
Supplementary Material
Acknowledgments
We thank Wyeth for the gift of IL-13 and Christopher M. Evans for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants HL072984, HL094848, CA105352, CA016672, and HL063756. This work was also supported by grants from the North American Cystic Fibrosis Foundation and American Heart Association.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: IP3, inositol trisphosphate; IP3-R, inositol trisphosphate receptor; Syt2, synaptotagmin 2; CCSP, Clara cell secretory protein; PDI, protein disulfide isomerase; MUC5AC, human mucin 5AC; X-gal, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; PAFS, periodic acid-fluorescent Schiff; WT, wild type; ER, endoplasmic reticulum; PBS, phosphate-buffered saline; IL, interleukin.
E. Melicoff and R. Adachi, unpublished data.
References
- 1.Knowles, M. R., and Boucher, R. C. (2002) J. Clin. Investig. 109 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams, O. W., Sharafkhaneh, A., Kim, V., Dickey, B. F., and Evans, C. M. (2006) Am. J. Respir. Cell Mol. Biol. 34 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis, C. W., and Dickey, B. F. (2008) Annu. Rev. Physiol. 70 487–512 [DOI] [PubMed] [Google Scholar]
- 4.Zhu, Y., Ehre, C., Abdullah, L. H., Sheehan, J. K., Roy, M., Evans, C. M., Dickey, B. F., and Davis, C. W. (2008) J. Physiol. (Lond.) 586 1977–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehre, C., Zhu, Y., Abdullah, L. H., Olsen, J., Nakayama, K. I., Nakayama, K., Messing, R. O., and Davis, C. W. (2007) Am. J. Physiol. 293 C1445–C1454 [DOI] [PubMed] [Google Scholar]
- 6.Rossi, A. H., Sears, P. R., and Davis, C. W. (2004) J. Physiol. (Lond.) 559 555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi, A. H., Salmon, W. C., Chua, M., and Davis, C. W. (2007) Am. J. Physiol. 292 L92–L98 [DOI] [PubMed] [Google Scholar]
- 8.Kemp, P. A., Sugar, R. A., and Jackson, A. D. (2004) Am. J. Respir. Cell Mol. Biol. 31 446–455 [DOI] [PubMed] [Google Scholar]
- 9.Bahra, P., Mesher, J., Li, S., Poll, C. T., and Danahay, H. (2004) Br. J. Pharmacol. 143 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman, E. R. (2008) Annu. Rev. Biochem. 77 615–641 [DOI] [PubMed] [Google Scholar]
- 11.Rizo, J., Chen, X., and Arac, D. (2006) Trends Cell Biol. 16 339–350 [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson, N., Lao, Y., Maximov, A., Chuang, J. C., Kostromina, E., Repa, J. J., Li, C., Radda, G. K., Sudhof, T. C., and Han, W. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 3992–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maximov, A., Lao, Y., Li, H., Chen, X., Rizo, J., Sorensen, J. B., and Sudhof, T. C. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 3986–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pang, Z. P., Melicoff, E., Padgett, D., Liu, Y., Teich, A. F., Dickey, B. F., Lin, W., Adachi, R., and Sudhof, T. C. (2006) J. Neurosci. 26 13493–13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schonn, J. S., Maximov, A., Lao, Y., Sudhof, T. C., and Sorensen, J. B. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 3998–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu, J., Mashimo, T., and Sudhof, T. C. (2007) Neuron 54 567–581 [DOI] [PubMed] [Google Scholar]
- 17.Sun, J., Pang, Z. P., Qin, D., Fahim, A. T., Adachi, R., and Sudhof, T. C. (2007) Nature 450 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda, M., Kowalchyk, J. A., Zhang, X., Martin, T. F., and Mikoshiba, K. (2002) J. Biol. Chem. 277 4601–4604 [DOI] [PubMed] [Google Scholar]
- 19.Fukuda, M. (2004) Biochem. J. 380 875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch, K. L., and Martin, T. F. (2007) J. Cell Sci. 120 617–627 [DOI] [PubMed] [Google Scholar]
- 21.Iezzi, M., Kouri, G., Fukuda, M., and Wollheim, C. B. (2004) J. Cell Sci. 117 3119–3127 [DOI] [PubMed] [Google Scholar]
- 22.Iezzi, M., Eliasson, L., Fukuda, M., and Wollheim, C. B. (2005) FEBS Lett. 579 5241–5246 [DOI] [PubMed] [Google Scholar]
- 23.Mohler, P. J., Davis, J. Q., Davis, L. H., Hoffman, J. A., Michaely, P., and Bennett, V. (2004) J. Biol. Chem. 279 12980–12987 [DOI] [PubMed] [Google Scholar]
- 24.Evans, C. M., Williams, O. W., Tuvim, M. J., Nigam, R., Mixides, G. P., Blackburn, M. R., DeMayo, F. J., Burns, A. R., Smith, C., Reynolds, S. D., Stripp, B. R., and Dickey, B. F. (2004) Am. J. Respir. Cell Mol. Biol. 31 382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scotland, P., Zhou, D., Benveniste, H., and Bennett, V. (1998) J. Cell Biol. 143 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkham, S., Sheehan, J. K., Knight, D., Richardson, P. S., and Thornton, D. J. (2002) Biochem. J. 361 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohler, P. J., Schott, J. J., Gramolini, A. O., Dilly, K. W., Guatimosim, S., duBell, W. H., Song, L. S., Haurogne, K., Kyndt, F., Ali, M. E., Rogers, T. B., Lederer, W. J., Escande, D., Le Marec, H., and Bennett, V. (2003) Nature 421 634–639 [DOI] [PubMed] [Google Scholar]
- 28.Li, H., Cho, S. N., Evans, C. M., Dickey, B. F., Jeong, J. W., and DeMayo, F. J. (2008) Genesis 46 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen, L. P., Omoluabi, O., Parra, S., Frieske, J. M., Clement, C., Ammar-Aouchiche, Z., Ho, S. B., Ehre, C., Kesimer, M., Knoll, B. J., Tuvim, M. J., Dickey, B. F., and Bond, R. A. (2008) Am. J. Respir. Cell Mol. Biol. 38 256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans, C. M., Kim, K., Tuvim, M. J., and Dickey, B. F. (2009) Curr. Opin. Pulm. Med. 15 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehan, J. K., Kirkham, S., Howard, M., Woodman, P., Kutay, S., Brazeau, C., Buckley, J., and Thornton, D. J. (2004) J. Biol. Chem. 279 15698–15705 [DOI] [PubMed] [Google Scholar]
- 32.Dellis, O., Dedos, S. G., Tovey, S. C., Taufiq, U. R., Dubel, S. J., and Taylor, C. W. (2006) Science 313 229–233 [DOI] [PubMed] [Google Scholar]
- 33.Petersen, O. H., and Tepikin, A. V. (2008) Annu. Rev. Physiol. 70 273–299 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen, T., Chin, W. C., and Verdugo, P. (1998) Nature 395 908–912 [DOI] [PubMed] [Google Scholar]
- 35.Davis, C. W., Dowell, M. L., Lethem, M., and Van Scott, M. (1992) Am. J. Physiol. 262 C1313–C1323 [DOI] [PubMed] [Google Scholar]
- 36.Danahay, H., Atherton, H. C., Jackson, A. D., Kreindler, J. L., Poll, C. T., and Bridges, R. J. (2006) Am. J. Physiol. 290 L558–L569 [DOI] [PubMed] [Google Scholar]
- 37.Heidelberger, R. (2007) Nature 450 623–625 [DOI] [PubMed] [Google Scholar]
- 38.Zhang, B., Koh, Y. H., Beckstead, R. B., Budnik, V., Ganetzky, B., and Bellen, H. J. (1998) Neuron 21 1465–1475 [DOI] [PubMed] [Google Scholar]
- 39.Chicka, M. C., Hui, E., Liu, H., and Chapman, E. R. (2008) Nat. Struct. Mol. Biol. 15 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Littleton, J. T., Stern, M., Perin, M., and Bellen, H. J. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 10888–10892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy, G., Kim, J. H., Pang, Z. P., Matti, U., Rettig, J., Sudhof, T. C., and Sorensen, J. B. (2006) J. Neurosci. 26 632–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Vilar, J., Ribeiro, C. M., Salmon, W. C., Mabolo, R., and Boucher, R. C. (2005) J. Histochem. Cytochem. 53 1305–1309 [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro, C. M., Paradiso, A. M., Livraghi, A., and Boucher, R. C. (2003) J. Gen. Physiol. 122 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Brito, O. M., and Scorrano, L. (2008) Nature 456 605–610 [DOI] [PubMed] [Google Scholar]
- 45.Widdicombe, J. G. (1989) Eur. Respir. J. 2 107–115 [PubMed] [Google Scholar]
- 46.Effros, R. M., Peterson, B., Casaburi, R., Su, J., Dunning, M., Torday, J., Biller, J., and Shaker, R. (2005) J. Appl. Physiol. 99 1286–1292 [DOI] [PubMed] [Google Scholar]
- 47.Koch, H., Hofmann, K., and Brose, N. (2000) Biochem. J. 349 247–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.