Abstract
Strong evidence suggests that the macaque monkey perirhinal cortex is involved in both the initial formation as well as the long-term storage of associative memory. To examine the neurophysiological basis of associative memory formation in this area, we recorded neural activity in this region as monkeys learned new conditional-motor associations. We report that a population of perirhinal neurons signal newly learned associations by changing their firing rate correlated with the animal's behavioral learning curve. Individual perirhinal neurons signal learning of one or more associations concurrently and these neural changes could occur before, at the same time, or after behavioral learning was expressed. We also compared the associative learning signals in the perirhinal cortex to our previous findings in the hippocampus. We report global similarities in both the learning-related and task-related activity seen across these areas as well as clear differences in the within and across trial timing and relative proportion of different subtypes of learning-related signals. Taken together, these findings emphasize the important role of the perirhinal cortex in new associative learning and suggest that the perirhinal cortex together with the hippocampus contribute importantly to conditional-motor associative memory formation.
Keywords: changing cells, conditional-motor associations, electrophysiology, medial temporal lobe, primates, stimulus–response learning
Introduction
The perirhinal cortex is an area of strong multisensory convergence that receives inputs from widespread unimodal and polymodal association areas (Suzuki and Amaral 1994). It also has strong and reciprocal projections with the hippocampus via the entorhinal cortex (Insausti et al. 1987; Witter et al. 1989; Suzuki and Amaral 1994). Consistent with these anatomical connections, findings from the experimental literature show that damage to the perirhinal cortex impairs long-term associative memory for a variety of different kinds of information (Murray et al. 1993, 1998; Liu et al. 2004). Although its role in the long-term storage of associative information is well established (Sakai and Miyashita 1991; Sobotka and Ringo 1993; Murray et al. 1993; Naya et al. 1996; Booth and Rolls 1998; Naya et al. 2003), findings from lesion studies also suggest an important role of the perirhinal cortex in the initial formation of new long-term associative memories (Murray et al. 1993, 1998).
Consistent with these lesion findings, neurophysiological studies showed that perirhinal neurons modify their activity during the performance of various associative learning tasks (Erickson and Desimone 1999; Messinger et al. 2001). Although these studies provided strong evidence that perirhinal neurons signal new associations, much less is known about the time course of these changes in neural activity relative to learning. To characterize the pattern and time course of both learning-related and task-related activity in the perirhinal cortex, we recorded the responses of neurons in this region as monkeys performed a conditional-motor associative learning task (location-scene association task) known to be sensitive to damage to the medial temporal lobe (MTL) (Gaffan 1992; Murray et al. 1993, 1998). A major advantage of this task is that robust learning of multiple new associations can be achieved within a single session. We have previously used this same task to characterize associative learning-related activity in the hippocampus (Wirth et al. 2003).
Another important issue in the current literature concerns delineating the relative contributions of individual MTL structures in declarative memory. Early neurophysiological studies addressed this question in the context of the study of recognition memory, widely viewed as consisting of 2 components, recollection and familiarity. These studies reported striking differences between perirhinal neurons that signaled stimulus familiarity with a decreased neural response (Baylis and Rolls 1987; Brown et al. 1987; Riches et al. 1991; Miller et al. 1993; Li et al. 1993; Fahy et al. 1993; Xiang and Brown 1998) and hippocampal neurons that showed little or no such familiarity signal (Brown et al. 1987; Riches et al. 1991; Xiang and Brown 1998). These physiological findings provided some of the key evidence for the influential proposal that familiarity depends on the perirhinal cortex, whereas the recollective aspects of recognition depend on the hippocampus (Aggleton and Brown 1999). Since that time, several related “dual process” proposals have been put forth (Mishkin et al. 1997; Vargha-Khadem et al. 1997; Yonelinas 2001; Diana et al. 2007; Eichenbaum et al. 2007). Although the neurophysiological data focused on familiarity signals provided key evidence in support of the dual process theory of recognition memory, less is known about the neurophysiological signals across different MTL structures underlying forms of memory beyond recognition memory, including associative learning and memory. To address this question, in the present study we quantitatively compared the detailed patterns and timing of both learning-related and task-related activity in the perirhinal cortex during a conditional-motor associative learning task to our previous finding in the hippocampus using the same task (Wirth et al. 2003).
Materials and Methods
Behavioral Task
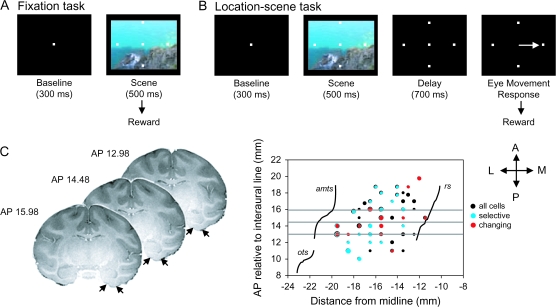
Two rhesus monkeys (Macaca mulatta, Monkey A: 11.5 kg and Monkey Z: 6.9 kg) participated in this experiment. Monkey A also participated in our earlier study of hippocampal learning-related activity using the same task (Wirth et al. 2003). Each day, the experimenter selected a set of 2 to 4 novel visual scenes from a large database of naturalistic color scenes. The scenes were first presented in a fixation only task, which served to pre-expose the animals to the novel scenes (Fig. 1A). Animals initiated each trial by fixating a central point for 300 ms (baseline period). The animal then saw a complex visual scene superimposed with 4 identical target locations for 500 ms. If fixation was successfully maintained throughout the scene/target presentation, animals were rewarded with a drop of juice. Each correct trial was followed by a 1500-ms intertrial interval (ITI). Error trials were followed by a 2000-ms ITI. Typically animals performed 10–15 fixation only trials for each novel scene used on that day of recording. Following the fixation only trials, the experimenter then used the same novel set of scenes in the context of the location-scene association task, a kind of conditional-motor associative learning task (Wise and Murray 1999; Fig. 1B). For the location-scene association task, animals also initiated each trial by fixating a central fixation spot for 300 ms. Animals then saw 4 targets superimposed on a complex visual scene for 500 ms followed by a 700-ms delay interval during which the scene disappeared but the targets remained on the screen. The trial always ended with the disappearance of the fixation spot which served as a cue for the animal to make an eye movement response to one of the 4 targets. Animals were rewarded with a drop of juice for choosing the correct target location. Only one of the 4 target locations was designated correct for each novel scene. Correct trials were followed by a 1500-ms ITI. Error trials were followed by a 2000-ms ITI. Animals learned new sets of 2–4 new location-scene associations during each daily recording session. Each new scene in the set was always associated with a different rewarded target location. Animals learned 405 of 606 new location-scene associations presented and typically improved from chance performance at the beginning of the session to 96% correct for Monkey A and 77% correct for Monkey Z. These new scenes in each set were randomly intermixed with a set of 4 highly familiar “reference” scenes where each reference scene was associated with a different rewarded target location. Both animals performed at or near 100% correct on all reference scenes.
Figure 1.
(A) Schematic representation of trial events during the fixation task. Animals initiated each trial by fixating a central fixation point after which they were shown a complex visual scene superimposed with 4 identical target locations. Animals were rewarded if fixation was successfully maintained throughout the scene/target presentation. (B) Schematic representation of the location-scene task. After initiating the trial by fixating a central fixation, animals were then presented with 4 targets superimposed on a complex visual scene followed by a delay interval where the scene disappeared but the targets remained on the screen. The trial ended with the disappearance of the fixation spot which served as a cue for the animal to make an eye movement response to one of the 4 targets. (C) The left panel shows 3 coronal MRI images from Monkey A. The black arrows indicate the locations of the medial and lateral boundaries of the perirhinal cortex. The right panel shows a flattened representation of the ventral surface of the monkey brain showing the locations of the rhinal sulcus (rs; medial boundary of the perirhinal cortex), the anterior middle temporal sulcus (amts; approximate lateral boundary of the perirhinal cortex), and the occipitotemporal sulcus (ots). Superimposed on the surface map are the locations of the nonselective (black), selective (blue), and changing (red) cells from monkeys A and Z. The size the dots in this graph are directly proportional to the density of cells recorded from that location. The 3 horizontal gray lines superimposed on the flat map correspond to the AP levels of the 3 coronal MRI images shown to the left. Additional abbreviations: A, anterior; L, lateral; M, medial; P, posterior.
Monkey Z performed the task with only 3 of the 4 possible spatial targets shown on the screen (north, east and west) for each new scene together with the 3 corresponding reference scenes. We removed the south target to alleviate this animal's strong bias to select this target for all new scenes. Following this modification, this animal's overall learning rate for new scenes improved considerably. Each day, Monkey Z learned only one set of 2–3 new scenes. Monkey A performed the task with all 4 possible spatial targets and in addition, in 69 of 128 total daily sessions, Monkey A was given a second set of 4 new scenes to learn after the first set. Animals from the hippocampal study (including monkey A and another monkey C) typically performed the task between 2 and 4 new scenes together with the corresponding number of reference scenes (Wirth et al. 2003).
Recording Locations
The position of the recording chamber for each animal was calculated using stereotaxic coordinates derived from the animals’ individual magnetic resonance imaging (MRI) images (Fig. 1C). Once the chamber was in place, the MRI images allowed us to estimate the anterior–posterior, medial–lateral and dorsal–ventral recording locations within the perirhinal cortex in each animal for the entire experiment. The depth of the bottom of the brain was also estimated by measuring the distance between the dorsal surface of the brain to the bottom of the brain directly using a thin microelectrode probe. We then used this depth landmark to estimate the location of the perirhinal cortex just dorsal of the bottom of the brain. A description of the recording locations for the hippocampal database is given in Wirth et al. (2003).
Recording Techniques
Following initial behavioral training, the animals were implanted with a scleral search coil, head post and recording chamber under aseptic conditions using isoflurane anesthesia. All surgical procedures were done in accordance with National Institutes of Health (NIH) guidelines and approved by the New York University Animal Welfare Committee. We used standard methods for single-unit extracellular recoding (Wirth et al. 2003; Yanike et al. 2004). Briefly, tungsten microelectrodes were advanced through a stainless steel guide tube inserted into a grid with 1 mm spacing (Crist Instruments, Hagerstown, MD). Neuronal signals were isolated using 2 different spike sorting systems. For 47% of the perirhinal dataset, action potentials were isolated and sorted on line using MSD (Israel). For the remaining 53% of the dataset, neural signals were collected and sorted online using Plexon (Dallas, TX). These signals were stored for additional off-line sorting analysis. If the number of spikes with interspike intervals shorter than 2 ms exceeded 5% of the total for a given unit, this unit was discarded or re-sorted. We made no attempt to prescreen isolated neurons. Instead, once we succeeded in isolating any neuron determined to be within the bounds of the perirhinal cortex according to our MRI localization, we started a new recording session. We verified the stability of neuronal isolation by analyzing waveform parameters using Plexon off-line sorting tools and by checking stability of baseline neuronal activity across the session for each neuron recorded with MSD.
Data Analysis
All analyses were done with custom written Matlab programs (MathWorks, MA). For both the perirhinal and hippocampal databases, we analyzed neuronal activity during each trial in 4 separate time periods: baseline (300 ms before stimulus onset), scene (100–500 ms after stimulus onset), early delay (Delay 1; 0–350 ms after stimulus offset) and late delay (delay 2; 350–700 ms after stimulus offset). In our original analysis of the hippocampal database, only a single delay interval of 700 ms following stimulus offset was used (Wirth et al. 2003). Here we split the delay interval into 2 smaller time periods because we found that it maximized the sensitivity of the subsequent analyses to identifying learning-related changing cells (see below).
For both the perirhinal and hippocampal database, we focus only on those cells that were recorded during successful learning of new associations. To determine whether a scene was learned, we constructed a learning curve, together with 90% confidence bounds, using a Bayesian state-space model (Smith et al. 2007). Because Monkey A performed the task with 4 spatial targets and Monkey Z performed the task with only 3 spatial targets we used 0.25 and 0.33, respectively, as the priors for chance probability for the first trial. We defined the trial of behavioral learning as the trial when the lower 90% confidence bound crossed the median estimated probability at trial 1. For cells recorded during successful learning, we analyzed both correct and error trials and excluded all trials in which the animal broke fixation.
Defining Changing and Nonchanging Cells
We modified the original criterion used in Wirth et al (2003) to define changing cells and used the modified criterion on both the population of perirhinal neurons as well as on the original population of hippocampal cells. For cells recorded during successful learning, as defined using a Bayesian state-space model (Smith et al. 2007), we first identified the subset of cells that responded significantly during either the scene and/or delay periods of the task relative to baseline (paired t-test with Bonferroni correction, P < 0.0167). Of those responsive cells, we identified the subset that responded selectively (i.e., differentially) to both the new and reference scenes during the scene and/or delay period of the task (permutation test with Bonferroni correction, P < 0.0167). Here we refer to a particular learned location-scene association as a “condition”. We then identified those new individual conditions where the firing rate during the scene and/or delay periods was significantly different relative to the baseline activity (paired t-test, P < 0.05). That population of scene and/or delay selective cells was further divided into changing (i.e., learning-related) and nonchanging cells. To determine whether the firing rate was significantly correlated with the behavioral learning curve, we performed a shuffled correlation test on raw firing rates and behavioral learning curve. For each condition, we correlated behavioral learning curve with shuffled firing rates and verified the level of significance by computing 2000 correlations on shuffled data. Changing cells were defined as the subset of cells with a significant relationship between neuronal activity and behavioral learning using the shuffled correlation test (P < 0.05; range of r-values for negative correlations: −0.3424 to −0.8045 and for positive correlations: 0.2951–0.872). We only selected those conditions with a significant difference between the firing rate during the first 10 trials and last 10 trials of the session (t-test, P < 0.05). For conditions when learning occurred in less than 10 trials, we compared the firing rate during the first 5 trials and last 5 trials of the session (t-test, P < 0.05). Those selective cells that showed no significant relationship between neuronal activity and behavioral learning using the shuffled correlation test were classified as nonchanging cells. Here we analyze both the changing as well as the nonchanging cells populations in both the perirhinal cortex and the hippocampus.
Estimating the Relative Timing of Changes in Neural Activity and Learning
To estimate changes in behavioral performance (i.e., learning) and neuronal activity across trials we used a Bayesian state-space model (Smith et al. 2007). For both behavioral performance and neuronal activity, we assumed a simple random walk model for the underlying state and assumed the observations were made from this state according to Bernoulli and Poisson-gamma distributions, respectively. Both models were estimated using Monte Carlo Markov chain software (WinBUGS, Lunn et al. 2000; Spiegelhalter 2004). To estimate neuronal activity we assumed a broad uninformative prior for the first trial for both Monkey A and Z. We used a prior of chance probability of 0.25 for Monkey A and 0.33 for Monkey Z, because they performed the task with 4 and 3 possible spatial targets, respectively. Once estimated, from these models we computed trial-by-trial estimates of median and 90% confidence bounds for behavioral performance and neuronal activity during either the scene and/or delay periods of the task. We defined the trial of behavioral learning as the trial when the lower 90% confidence bound crossed the median estimated probability at trial 1. To define a trial of neuronal change consistent with the behavioral change estimate, we first computed the median neuronal activity at the first trial, calling this the median firing rate, and then identified the decreasing (increasing) neuronal change trial on which the upper (lower) 90% confidence bound crossed the median firing rate.
Receiver Operating Characteristics Analysis
To examine the strength of the learning-related signal across each of the task periods, we performed a receiver operating characteristics (ROC) analysis (Green and Swets 1966; Newsome et al. 1989). For each neuron, we compared 2 distributions of neuronal activity: one constructed from activity including all trials before the learning criterion was reached (“before learning” trials) and another including all trials after the learning criterion was achieved (“after learning” trials). An ROC curve was constructed by taking the proportion of each firing rate in the distribution of “before learning” that exceeded the proportion of this rate in the distribution of “after learning.” An ROC area was calculated in each trial period by comparing average firing rate for “before learning” trials and “after learning” trials. In this way, for each task period with learning-related change, we calculated one ROC area. We also applied the ROC analysis to multiple time bins using the same “before learning” and “after learning” comparison as classifiers to obtain an estimated latency of when the learning signals developed within the trial. For each trial, we generated a spike density function by convolving the spike train with a Gaussian probability function (1-ms step, σ = 40 ms). An ROC area was calculated in 50 millisecond time bins starting from the beginning of the trial and shifted by 10-ms steps until the end of the trial.
Selectivity Analysis
We calculated a selectivity index (SI) for each selective nonchanging cell to measure how well the cell could discriminate between different scenes. The SI was calculated using the following formula (Moody et al. 1998; Yanike et al. 2004):
where λi is response to ith scene, λmax is maximal response for a cell, and n is the total number of scenes. Both reference and new scenes were included in this analysis. The SI was calculated separately during the scene and delay periods of the task including both correct and error trials.
Habituation
To determine whether cells showed a habituation-like decrease in response during the presentation of the fixation only trials, the firing rate for each condition was plotted as a function of trial number. We fit a simple regression line and estimated the slope. A negative slope indicated a habituation response. We then used a bootstrap test to estimate whether the slope of the regression line was significantly different from zero.
Results
General Response Properties
We recorded a total of 140 neurons in the perirhinal cortex (113 neurons from Monkey A and 27 neurons from Monkey Z). Most recording sites were at mid to posterior levels of the perirhinal cortex and extended throughout the full medial–lateral extent of the area (Fig. 1C). Our analysis focused on the 130 of 140 (Monkey A: 110 cells and Monkey Z: 20 cells) cells recorded during successful learning of new location-scene associations (see Methods). We found 77% of these perirhinal neurons (100/130) were responsive during the scene period, the first half of delay, and/or the second half of delay periods of the task relative to the baseline period (paired t-test with Bonferroni correction, P < 0.0167). We next used a permutation test (with Bonferroni correction, P < 0.0167) with scene identity as a main factor to determine how many of the responsive cells were also selective to particular visual stimuli (i.e., individual visual scenes). A total of 68 perirhinal neurons (68% of responsive cells) responded selectively during either scene and/or delay periods of the task.
We also compared the learning-related responses of the perirhinal neuronal population to the population of hippocampal learning-related neurons described in Wirth et al. (2003) using the same time periods and statistical analyses. We analyzed 137 hippocampal neurons recorded during successful learning of new location-scene associations. This comparison yielded similar proportions of responsive and selective cells across the 2 areas (Hippocampus [HPC]: 123/137 or 90% responsive and 85 of the 123 responsive cells, or 69% were selective; χ2 test Perirhinal [PR] vs. HPC: P = 0.39).
Learning-Related Neural Signals
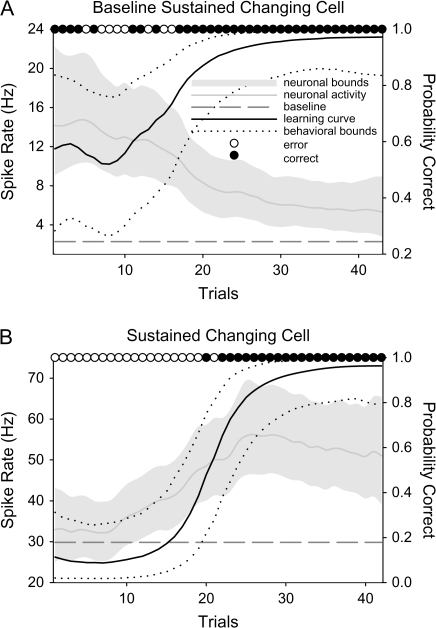
Of the 68 selective perirhinal neurons recorded during successful learning we identified 23 perirhinal cells (Monkey A: 18 cells and Monkey Z: 5 cells; 32% including 35 conditions) that signaled new associative learning with changes in their neural activity that paralleled behavioral learning (changing cells; Wirth et al. 2003). These changes in firing rate in the perirhinal neurons were seen across both scene and delay periods, with some changing cells showing changes during both time periods (“combined” in Table 1). Consistent with our previous study in the hippocampus (Wirth et al. 2003) we identified 2 categories of perirhinal changing cells (both categories were observed in both animals). More than half of the changing cells observed in the perirhinal cortex were of the baseline sustained type (14/23 cells, 61%). Baseline sustained changing cells initially responded selectively during the trials before learning and signaled learning by returning to baseline levels of activity. For the majority of baseline sustained cells, this change in activity was negatively correlated with learning. An example of a representative perirhinal baseline sustained cell is shown in Figure 2A. The majority of the perirhinal baseline sustained cells showed an elevated firing rate relative to the baseline activity early in learning (10/14 cells, 11/17 conditions), with few examples showing initial suppression in neuronal activity relative to the baseline firing rate and then returned to the baseline level with learning (4/14 cells, 6/17 conditions). Six of the 23 perirhinal changing cells (26%) were categorized as sustained changing cells. These cells typically showed little or no response to the task early in the session, but typically signaled learning with an increase (n = 5 of 6 sustained cells) in their firing rate (Fig. 2B). Three perirhinal changing cells were categorized as “mixed” because they showed both baseline sustained and sustained changes to different learned conditions. The overall proportion of baseline sustained conditions in the perirhinal cortex (including those from the mixed cells) was significantly greater than the overall proportion of sustained conditions (χ2 test, P < 0.03).
Table 1.
Number of examples of learning-related changes across different task periods for cells in the PR cortex and HPC
| Cells | Conds | Scene only | Delay1 only | Delay2 only | Combined | |
| PR changing cells | ||||||
| Sustained | 6 | 8 | 4 | 1 | 1 | 2 |
| Baseline sustained | 14 | 17 | 6 | 2 | 6 | 3 |
| Mixed | 3 | 10a | 2 | 1 | 2 | 5 |
| Total | 23 | 35 | 12 | 4 | 9 | 10 |
| HPC changing cells | ||||||
| Sustained | 18 | 22 | 7 | 7 | 4 | 4 |
| Baseline sustained | 7 | 11 | 5 | 2 | 1 | 3 |
| Total | 25 | 33 | 12 | 9 | 5 | 7 |
2 conds = sustained; 6 conds = baseline sustained; 1 cond = both sustained and baseline sustained signals in different task periods (this condition was counted twice). Both sustained and baseline sustained changing cells were found in Monkey A and in Monkey Z.
Figure 2.
(A) Illustration of the behavioral learning curve (solid black line, with 90% upper and lower confidence bounds shown as dotted black lines) and neural activity (solid gray line with upper and lower confidence bounds shown shaded in lighter gray) during the late delay period for a baseline sustained perirhinal neuron. The filled black circles at the top of the graph indicate correct trials, whereas the open circles indicate error trials. The dashed gray line indicates the average baseline firing rate. Early in learning when behavioral performance was poor, this cell responded with a higher firing rate during the late delay period of the task (Delay 2). However, with learning the activity of this cell decreased back to baseline levels of activity. The shuffled correlation test showed a significant relationship between the change in neural activity and the change in behavior with learning (r = −0.63, P < 0.0001). (B) An example of a perirhinal sustained changing cell. This cell did not respond to the task during the scene period early in the session when performance was near chance. However, this neuron exhibited a dramatic increase in activity that paralleled the animal's behavioral learning curve. There was a significant correlation between neural activity and behavioral learning (r = 0.66, P < 0.0001). All conventions are the same as in (A).
In the hippocampus, of the 85 selective cells recorded during successful learning, we identified 25 hippocampal changing cells (Monkey A: 7 cells and Monkey C: 18 cells; 28% including 33 conditions; 18 sustained and 7 baseline sustained changing cells). Although there were more hippocampal sustained conditions compared with baseline sustained conditions, this numerical difference did not reach significance (χ2 test, P = 0.06). A direct comparison of the relative proportion of sustained and baseline sustained changing conditions in the perirhinal cortex and hippocampus revealed a significant difference (P < 0.02; Table 1). Further analyses of the major features of the sustained and baseline sustained changing cells including baseline firing rate and magnitude of change showed no difference between the perirhinal cortex and hippocampus (Table 2). However, we found that the visual response latency of sustained cells in the perirhinal cortex (but not baseline sustained cells) was shorter compared with sustained cells in the hippocampus (Table 2). This latter finding is consistent with the anatomical connections of these areas relative to the input from the ventral visual pathway (Suzuki and Amaral 1994).
Table 2.
Basic response properties of changing cells
| Perirhinal cortex |
Hippocampus |
|||
| Baseline sustained | Sustained | Baseline sustained | Sustained | |
| Baseline firing rate (spikes/s) | 12.5 ± 2.0 | 19.6 ± 3.7 | 29.7± 13.0 | 18.9 ± 4.3 |
| Magnitude of change (spikes/s) | 6.6 ± 2.8 | 6.2 ± 1.2 | 6.0 ± 1.2 | 7.2 ± 2.1 |
| Visual response latency (ms) | 143 | 95a | 136 | 250a |
There is a significant difference in visual response latency between sustained cells in the perirhinal cortex and hippocampus (P < 0.02).
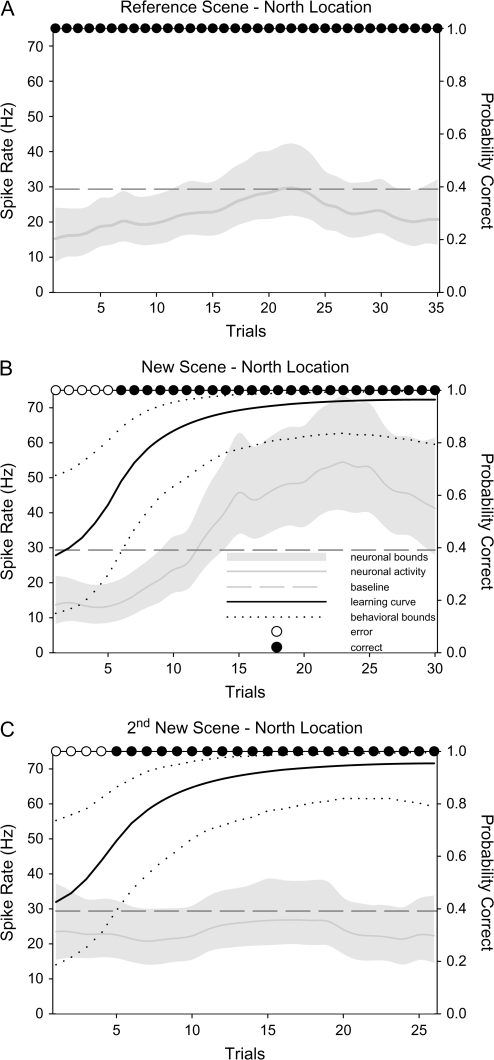
We next asked whether learning-related changes in the perirhinal cortex were specific to particular motor responses or particular learned target locations. To address these questions, we first compared the responses of changing cells during new learning to the response of the same cells during the performance of the reference scene with the same rewarded target location. In no case did the perirhinal changing cells give similar responses to the corresponding reference scenes suggesting that changing cells are not simply conveying information about the direction or location of the reward target (Fig. 3A). We also examined conditions in which the changing cells were recorded during learning of 2 sets of novel associations. We were particularly interested in the response of the changing cells during learning of a second new association with the same rewarded target location. Out of 6 perirhinal cells tested (11 conditions) none changed its activity to the second learned scene with the same rewarded target location (Fig. 3B,C). Taken together, these results indicate that the signals of the perirhinal changing cells do not represent a pure motor signal and are not made in a motor-based or location-based frame of reference. Similar to the results in the perirhinal cortex, we have previously shown that hippocampal changing cells did not respond similarly to the reference scene with the corresponding target location and did not signal multiple learned associations with the same rewarded target location (Wirth et al. 2003).
Figure 3.
Response of a prototypical perirhinal sustained changing cell to various control manipulations. (A) This panel illustrates a perirhinal cell's response to a reference scene with a north reward target location. (B) Response of the same perirhinal changing cell during learning of a new location-scene association to the north direction. Comparison of (A) and (B) confirm that the changing signal is not due to a motor-related response. (C) Response of the same neuron shown in (A) and (B) during learning of a second novel association with a north rewarded target location. Comparison of graphs (B) and (C) shows that this perirhinal changing cell did not signal new learning in a direction-based or location-based frame of reference. All conventions are the same as in Figure 2.
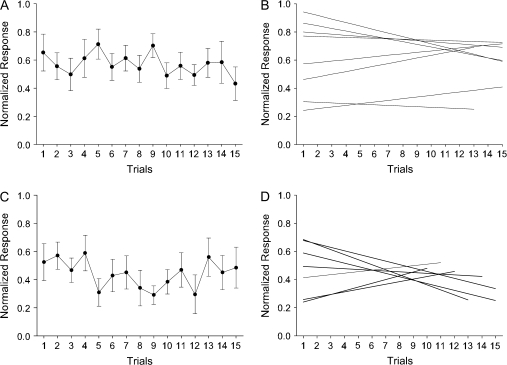
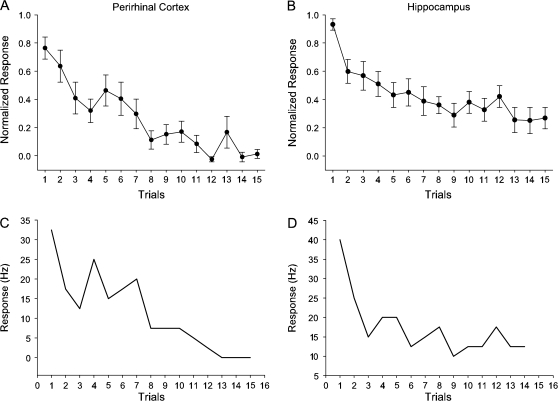
Because many previous studies have shown that perirhinal neurons decrease their stimulus-evoked response over time as initially novel stimulus becomes familiar (Miller et al. 1991; Riches et al. 1991), the perirhinal baseline sustained cells might reflect a passive habituation effect rather than an active associative learning signal. To address this possibility we analyzed the response of perirhinal baseline sustained changing cells during fixation only trials when the animals were required to simply fixate during the presentation of novel scenes for reward. We focused this analysis on the population of perirhinal baseline sustained cells that showed change during the scene period and also had a complete set of fixation only trials (n = 5 of 10 perirhinal baseline sustained cells, 8/22 conditions). We found that these perirhinal baseline sustained cells did not change their firing rate with repetition during the early fixation trials (Fig. 4A). The slopes of the regression lines (Fig. 4B) were close to zero across all cells (distribution mean = −0.01 ± 0.007, range = −0.03 to 0.01; P = 0.4) suggesting that baseline sustained changing cells do not exhibit habituation. None of the individual cells showed significant regression line slopes. We applied the same analysis to the hippocampal baseline sustained changing cells (n = 6 of 7 hippocampal baseline sustained cells, 7/11 conditions) and found that they also did not change their firing rates with repetition (Fig. 4C) and the slopes of the regression lines in the hippocampus were also not different from zero (Fig. 4D: mean slope = −0.01 + 0.01, range = −0.04 to 0.03; P = 0.61). Moreover, we also analyzed responses of 11 sustained cells during fixation only trials (n = 3 perirhinal sustained cells or 3 conditions; n = 8 hippocampal sustained cells or 9 conditions). Similar to the baseline sustained cells, the sustained cells in both the perirhinal cortex and hippocampus did not show habituation responses during fixation only trials.
Figure 4.
(A) Illustration of the average neural activity (plus standard error) of 5 perirhinal baseline sustained cells (8 conditions) seen over the 15 repetitions of the visual images shown during fixation only trials. The firing rate during the scene period was normalized by first subtracting baseline firing rate and then dividing by the maximum response. The response of these perirhinal baseline sustained cells did not change with repetition (i.e., no habituation effect seen). (B) Individual slopes for the 8 different conditions averaged in (A). Slopes were estimated using the least squares method. (C) The average neural activity and the standard error of 6 hippocampal baseline sustained cells (7 conditions) plotted over the 15 repetitions of the visual images during fixation only trials. Similar to the perirhinal cells in (A), hippocampal baseline sustained cells did not show habituation effect. (D) Individual slopes for 7 conditions included in the average in (C).
Further analysis showed that 35% (8/23) of the perirhinal changing cells signaled learning of 2 or more associations learned concurrently (6 cells = 2 associations and 2 cell = 4 associations, Table 3). All of these examples of multiple learned associations came from Monkey A. From a behavioral perspective, Monkey A generally performed at a very high level, learning an average of 3.9 new associations per session (range 2–12). In contrast, Monkey Z learned an average of 1.9 new associations per session (range 1–3). Although 5 of 8 multiple changing cells in the perirhinal cortex exhibited either sustained or baseline sustained changes for all of the learned associations, 3/8 exhibited a mixed response. Figure 5 illustrates an example of a perirhinal cell that exhibited a baseline sustained signal for 3 different conditions (Fig. 5A–C) and a sustained changing signal for one learned association (Fig. 5D). These findings illustrate that perirhinal changing cells are both flexible and highly selective in their learning-related signaling. In the hippocampus, there were fewer examples of changing cells that signaled 2 associations learned concurrently (5 cells and 10 conditions). All of the hippocampal multiple learning cell examples exhibited either sustained or baseline sustained changes for both of the changing conditions and unlike the perirhinal database, there were no examples of an individual hippocampal cell exhibiting both sustained and baseline sustained patterns of activity. However, because the hippocampal database included fewer examples of multiple new associations learned per session, it is difficult to determine if this difference is meaningful.
Table 3.
Changing cells that signal multiple new associations
| Perirhinal cortex |
Hippocampus |
|||
| Cells | Condsa | Cells | Condsa | |
| Sustained | 2 | 2/2 | 3 | 2/2/2 |
| Baseline sustained | 3 | 2/2/2 | 2 | 2/2 |
| Mixed | 3 | 2/2/4 | — | — |
| Total | 8 | 20 | 5 | 10 |
Number of changing conditions listed for each cell.
Figure 5.
(A) An example of a perirhinal changing cell that exhibited a baseline sustained pattern of activity during the late delay period of the task for a particular learned association. (B and C) Response of the same perirhinal changing cell illustrated in (A) with the baseline sustained pattern during the late delay period of the task for 2 other learned associations. (D) The same perirhinal changing cell showed a sustained changing pattern of activity during the late delay period of the task for a different learned association. All conventions are the same as Figure 2.
Time Course of Neuronal Changes Relative to Behavioral Learning
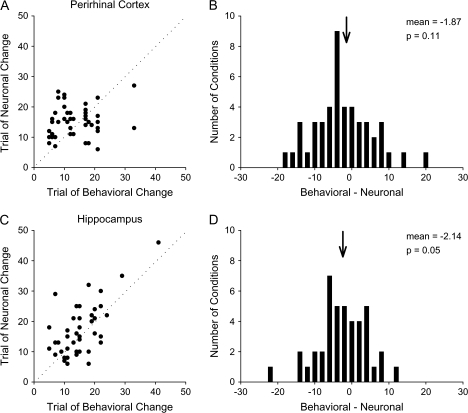
Although the results presented above suggest learning-related signals are seen throughout the perirhinal cortex and hippocampus, in this next analysis we ask if the timing of the learning signal relative to behavioral learning can differentiate the 2 areas. To address this question we estimated the trial number of behavioral learning and the trial number of the change in neural activity using a Bayesian state-space model (Smith et al. 2007). Next, we compared the trial on which behavioral performance changed with the trial on which neuronal activity changed. The neuronal activity leads behavior when change in neuronal activity occurs prior to change in behavioral performance. The neuronal activity lags behavior when changes in neuronal activity occur once animals acquired new associations. Figure 6A shows the distribution of lead/lag values for the perirhinal cortex. There was no significant difference in lag values between the sustained (median: −3.5; range: – 14 to + 10) and baseline sustained (median: −2.0; range: −17 to + 20) examples for any task period (t-test, P = 0.54). The changes in neuronal activity occurred before learning in 16 periods (lead values), after learning in 29 periods (lag values), and at the same time in 2 periods. The distribution of lead/lag values in the perirhinal cortex was centered on zero (Fig. 6B, mean = −1.87, t-test, P = 0.11) suggesting that, on average, changes in the perirhinal neuronal activity occurred parallel to changes in behavioral performance.
Figure 6.
(A) Distribution of trials of behavioral change relative to trials of neural change for perirhinal changing cells (n = 23). For each condition the trial of behavioral change is plotted against the trial of neural change. Conditions where learning lags changes in neural activity appear below the unity line, whereas condition where learning leads changes in neural activity appear above the unity line. For this population the difference between behavioral and neural trials of change is normally distributed around a zero difference and includes both positive and negative difference values. (B) Histogram showing the number of conditions with particular lead/lag values. Positive differences between behavioral and neural change values indicate conditions when learning lags changes in neural activity. Negative differences between behavioral and neural change values represent conditions when learning leads changes in neural activity. The distribution of difference values for perirhinal changing cells was not significantly different from zero (P = 0.11). (C) Distribution of difference values for hippocampal changing cells (n = 25). (D) Similar to the values observed in the perirhinal cortex, distribution of difference values for the hippocampal population peaked at near zero (P = 0.05).
Figure 6C illustrates results for the hippocampal changing cells. The results were similar to those in the perirhinal cortex. There was no significant difference between the sustained (median: −3.0; range: - 13 to + 9) and baseline sustained (median: −0.5; range: −22 to + 12) examples for any task period (t-test, P = 0.74). The neuronal activity changed before learning in 14 periods, at the same time in 3 periods and after learning in 25 periods and the distribution of lead/lag values was not significantly different from zero (Fig. 6D, t-test, mean = −2.14, P = 0.05). There were no significant differences in the distribution of lead/lag values between the perirhinal cortex and the hippocampus (Kolmogorov–Smirnov test, P = 0.99). Taken together, these results suggest that the perirhinal and hippocampal neurons are recruited at the same time, with changes in neuronal activity occurring on average, around the time of new behavioral learning.
Profile and Time Course of the Learning Signal Within a Trial
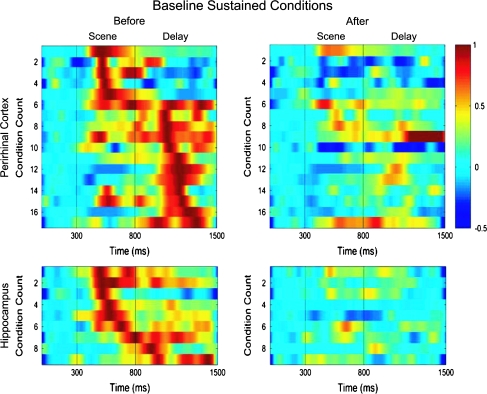
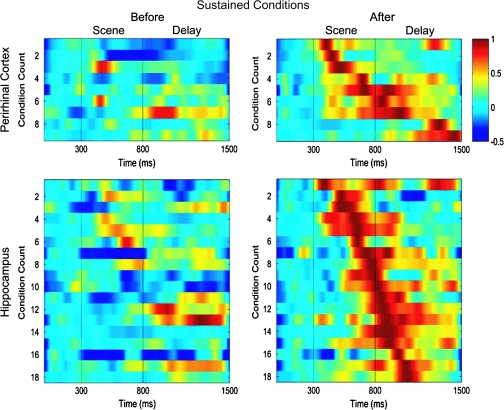
To examine the detailed response profiles of the baseline sustained and sustained changing cells within a trial (i.e., with respect to scene onset), we plotted the pattern of neural activity seen before behavioral learning when performance was at or near chance levels and after behavioral learning when performance was near 100% correct (see methods). Before learning, the perirhinal baseline sustained conditions (n = 18) exhibited a transient response with a peak activation that tended to focus either in the scene (6/18) or delay (12/18) periods of the task (Fig. 7 top panels). In contrast, after learning, the perirhinal baseline sustained cells showed little if any response. Hippocampal baseline sustained conditions (n = 9) also included examples with peak activations either in the scene (7/9) or delay (2/9) periods of the task. Although the baseline sustained perirhinal cells mostly responded during the delay period, the hippocampal baseline sustained cells tended to respond during the scene period (χ2 test, P < 0.03). Sustained changing perirhinal conditions (n = 9) showed little response before learning, but after learning, developed a transient response that could occur throughout the scene (5/9) or delay (4/9) periods of the task (Fig. 8 top panels). A similar overall pattern was also observed for hippocampal sustained changing conditions (n = 18, 9/18 in the scene and 9/18 in the delay, Fig. 8 lower panels). In contrast to the baseline sustained conditions, the proportions of sustained conditions with peak responses during the scene and delay periods were similar between the perirhinal cortex and hippocampus (χ2 test, P = 0.79).
Figure 7.
Illustration of the patterns of neural activity seen within trials for all baseline sustained conditions in the perirhinal cortex (top 2 panels) and hippocampus (bottom 2 panels). Each row represents the normalized firing rate for a single changing condition plotted over the trial period either before (left panels) or after (right panels) learning. The firing rate is normalized relative to the baseline rate and maximum firing rate observed before learning. This analysis is limited to conditions with excitatory responses relative to the baseline firing rate and the conditions are sorted by the latency to the maximum response before learning. The top panels show that perirhinal neurons (n = 17 conditions) tended to exhibit a bimodal distribution of activation peaks in either the scene or the delay period of the task before learning (top left panel) and this activity decreased dramatically after learning (top right panel). The distribution of activation peaks in the hippocampal baseline sustained neurons tended to include fewer examples of conditions with peak activations in the delay period of the task (n = 9 conditions).
Figure 8.
Illustration of the patterns of neural activity seen within trials for all sustained changing conditions in the perirhinal cortex (top 2 panels) and hippocampus (bottom 2 panels). All conventions are the same as Figure 7. Top panels show that perirhinal sustained changing conditions (n = 9) gave little or no response before learning but came to respond maximally during the scene or scene and delay periods of the task. A similar pattern of activity was seen in the population of 18 hippocampal sustained changing conditions (bottom 2 panels), with less activity before learning and maximal activity that typically included both the scene and delay periods of the task.
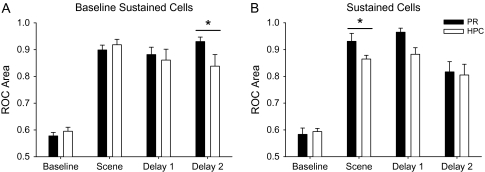
To quantify the time course of the learning signals illustrated in Figures 7 and 8, we used ROC analysis to compare neural activity before versus after learning. We computed the area under the ROC curve as a measure of the separation of the neuron's distribution of activity in these 2 time periods. To compare the time courses of the learning-related activity across task periods we separately plotted ROC area values for the scene, early delay (Delay 1) and late delay (Delay 2) periods for the baseline sustained and sustained cells (Fig. 9A,B). The ROC area values during the baseline period served as a control. The results in Figure 9A show that the baseline sustained cells in the perirhinal cortex and hippocampus signal learning during the scene, delay 1 and delay 2 periods relative to the baseline period (t-test, P < 0.002). The perirhinal learning signal remains strong across all task periods (no difference across task periods, t-test, P = 0.51). The hippocampal ROC area values are also highly significant relative to baseline control levels though the hippocampal ROC area value in the late delay period was significantly smaller than the corresponding value in the perirhinal cortex. Figure 9B shows results for the sustained cells. Sustained cells in both the perirhinal cortex and hippocampus signal learning in the scene, early and late delay periods. We also note that the relative representation in the scene period of the task in the perirhinal cortex was significantly larger than that seen in the hippocampus.
Figure 9.
(A) Bar graph showing the ROC area values and standard errors differentiating activity early in the trial before learning from activity late in the trial after learning for baseline sustained cells in both the perirhinal cortex (black bars) and the hippocampus (white bars). Also shown for references are control ROC area values in the baseline period of the task. Both the perirhinal and hippocampal baseline sustained cells differentiate activity from late activity in the scene, delay 1 and delay 2 periods of the task. In addition, the ROC area value for delay 2 was significantly larger in the perirhinal baseline sustained population compared with the hippocampal baseline sustained population (P > 0.05). (B) Bar graph showing the ROC area values for the sustained cells across the perirhinal cortex and hippocampus. All conventions are the same as (A). Here again both perirhinal and hippocampal sustained cells differentiate activity seen before from after learning in each of the 3 main task periods. For the sustained changing cells, the average ROC area value for the perirhinal cortex was significantly larger ROC value in the hippocampus in the scene period of the task (P > 0.05).
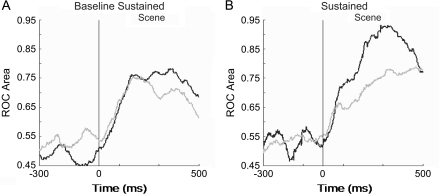
We next used ROC analysis to examine whether there are any differences in the onset latency of these signals during the scene period and when these cells signal learning relative to their response to visual stimuli. To determine the time at which changing cells started signaling learning, we calculated ROC area values in 50-ms bins slid in 10-ms steps across the entire length of the scene period. The results are summarized in Figure 10A,B. The baseline sustained cells in the perirhinal cortex and hippocampus start signaling learning at the same time during the scene period and the signals peak around 200 ms after scene onset in both areas (Fig. 10A). There was no difference in the latency to the onset of learning signal and that to the neuron's visual response for the perirhinal cortex and the hippocampus (t-test, P = 0.14).
Figure 10.
ROC analysis of the learning signal in the perirhinal cortex and hippocampus during the scene period of the task. ROC analysis was applied to the neural response for each condition in 50-ms windows in overlapping 10-ms steps. (A) The mean response latency of the learning signal for baseline sustained changing conditions in the perirhinal cortex (black, n = 13) and hippocampus (gray, n = 8) were not significantly different from each other (P = 0.12; t-test). Only conditions that showed changes during the scene period were included in this analysis. (B) Time course of the learning signal for sustained changing conditions in the perirhinal cortex (black, n = 5) and hippocampus (gray, n = 11). For this population of changing cells, perirhinal neurons differentiated between early and late learning significantly earlier than those in hippocampus (P < 0.03; t-test).
A comparison of ROC area values for the sustained cells across the perirhinal cortex and hippocampus showed that perirhinal neurons start signaling learning earlier compared with hippocampal cells (t-test, P < 0.03; Fig. 10B). Although the onset latencies for the perirhinal cortex ranged between 87 and 145 ms, the hippocampal onset latencies were longer between 120 and 456 ms (t-test, P < 0.04). These results show that compared with the population of sustained changing hippocampal cells, sustained changing perirhinal cells signal learning earlier with respect to scene onset, often as soon as they start responding to visual stimuli during the scene presentation (Table 2).
Selective Nonchanging Neural Signals
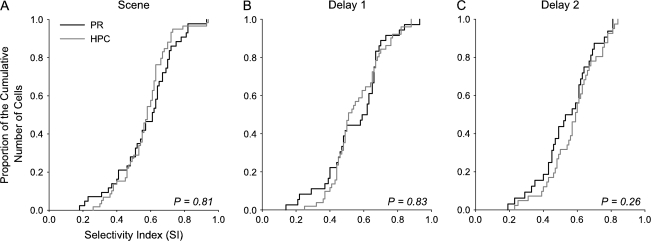
The results presented above show that there are global similarities as well as clear differences in the associative learning-related activity seen in the perirhinal cortex and hippocampus. To further compare the task-related response properties of neurons in these 2 areas, we evaluated the populations of selective, nonchanging cells (see Methods for definition). Overall we found similar proportions of selective nonchanging cells in the perirhinal cortex (74% or 50/68 selective cells) and the hippocampus (77% or 65/85 selective cells; χ2 test PR vs. HPC: P = 0.87). The average firing rate of the selective nonchanging cells during the baseline fixation period was significantly lower in the perirhinal cortex compared with the hippocampus (PR: 9.69 ± 1.28 Hz and HPC: 24.31 ± 3.46; t-test, P < 0.001). In the perirhinal cortex, a greater proportion of selective, nonchanging cells responded with an increased firing rate relative to baseline (84% or 42/50 cells) during either the scene or delay periods of the task compared with cells responding with a decrease in firing rate relative to baseline (16% or 8/50 cells). In contrast, in the hippocampus, 62% (40/65 cells) increased their firing rate during the scene and/or delay period with the remaining 38% (25/65 cells) exhibiting a decreased firing rate in either the scene or delay period. These relative proportions were significantly different (χ2 test, P = 0.05). Consistent with findings from the changing cell population, perirhinal selective nonchanging cells exhibited an earlier visual response latency compared with the hippocampal cells (PR: 117.9 ± 4.5 ms and HPC: 151.9 ± 10.3 ms; t-test, P < 0.02). We also asked if there were any differences in the degree of selectivity of these cells across the perirhinal cortex and hippocampus. To address this question we calculated a SI for each selective nonchanging cell (see Methods). We found that the perirhinal and hippocampal populations were not different in their ability to differentiate between different scenes during either the scene or the delay periods of the task (Fig. 11A-C).
Figure 11.
Comparison of selectivity of nonchanging cells in the perirhinal cortex and hippocampus. Proportion of the cumulative number of cells is plotted for each SI value for the perirhinal (black) and hippocampal (gray) nonchanging neurons separately for the scene (A), delay 1 (B), and delay 2 (C) periods of the task. The total number of cells included in this analysis was 50 in the perirhinal cortex and 65 in the hippocampus (PR: 39—scene, 36—delay 1, 32—delay 2; HPC: 59—scene, 51—delay 1, 41—delay 2). There was no difference in the SI values between the perirhinal cortex and hippocampus in any of the task periods (Kolmogorov–Smirnov test, P values in each panel).
Habituation Signals in the Selective Nonchanging Population
A common finding in the perirhinal cortex is that neurons in this area tend to fire maximally to novel stimuli and decrease their firing with stimulus repetition. This has been termed a habituation, familiarity signal, or repetition suppression (Baylis and Rolls 1987; Brown et al. 1987; Riches et al. 1991; Miller et al. 1993; Li et al. 1993; Fahy et al. 1993; Xiang and Brown 1998; see detailed discussion in Sohal and Hasselmo 2000). In contrast, few such signals have been seen in the hippocampus (Brown et al. 1987; Riches et al. 1991; Xiang and Brown 1998). We next examined the habituation/familiarity signals seen in the population of perirhinal and hippocampal selective nonchanging cells during the response to the first 15 repetitions of the novel stimuli shown in the fixation only trials at the beginning of each daily recording session (see Methods). We analyzed 32 cells (57 conditions) in the perirhinal cortex and 44 cells (93 conditions) in the hippocampus that responded selectively during the scene period and also had a complete set of fixation only trials. We identified a subset of 19% (6/32 cells or 10/57 conditions) of the perirhinal population that showed a clear habituation effect (Fig. 12A). This proportion of habituation-like cells is similar to the proportion that have been reported in other studies using simple recognition memory tasks (Brown et al. 1987; Riches et al. 1991; Miller et al. 1993; Li et al. 1993; Fahy et al. 1993; Xiang and Brown, 1998). In contrast to previous report by Li et al. (1993), we found no cells with increased activity during fixation only trials. Figure 12C shows an example of a perirhinal cell with a clear habituation signal. Surprisingly, we also found 16% (7/44 cells or 10/93 conditions) of the hippocampal population also exhibited a clear habituation/familiarity effect (Fig. 12B,D). Overall, the proportion of selective nonchanging cells with familiarity signals in the hippocampus did not differ from that in the perirhinal cortex (χ2 test, P = 0.34). Thus, we find that both perirhinal and hippocampal neurons represent information about the relative familiarity of the novel scene stimuli used in this task.
Figure 12.
(A and B) The average neural activity and the standard error of 6 perirhinal nonchanging cells (A) and 7 hippocampal nonchanging cells (B) plotted over the 15 repetitions of the visual images shown during fixation only trials. Only conditions with significant slopes as estimated using the least squares method were included in these averages (PR: 10 conditions, A; and HPC: 10 conditions, B). Similar notations as in Figure 4A,C. (C and D) Examples of a perirhinal neuron (C) and a hippocampal neuron (D) with reduced firing rate to stimuli repetition during the scene period over fixation only trials.
Discussion
We identified 2 subpopulations of perirhinal changing cells that signal learning during the performance of a conditional-motor associative learning task. Sustained changing cells signal learning with a significant change in their level of activity relative to baseline, whereas baseline sustained changing cells signaled learning by returning to baseline levels of activity. Both types of cells could change before, at the same time as well as after learning was expressed. Comparisons between the task-related and learning-related signals seen in the perirhinal cortex to those in our database of hippocampal recordings revealed both similarities as well as several notable differences. We discuss these patterns of similarities and differences relative to current models of MTL memory function.
Associative Learning Signals in the Perirhinal Cortex
We describe a population of perirhinal neurons that signal associative learning with clear changes in their firing rate. These findings confirm and extend previous studies of associative learning-related activity in the macaque perirhinal cortex (Erickson and Desimone 1999; Messinger et al. 2001). In the present study, monkeys learned multiple new location-scene associations to a high level of performance each day allowing us to characterize the detailed pattern and timing of learning-related changes in activity both within the trial as well as relative to behavioral learning. Some cells signaled learning by changing their level of activity relative to baseline (sustained changing cells), whereas others signaled learning by returning to baseline levels of activity (baseline sustained changing cells). The combined pattern of sustained and baseline sustained signals across the population of changing cells may represent the overall tuning of the perirhinal network to the newly learned location-scene associations. When we examined the timing of the changing neural activity relative to learning we found that the perirhinal changing cells could change either before learning, in parallel with learning as well as after learning. This is the general pattern one would expect from a network with feedback connections that participates in the learning process. That is, some cells change before learning is expressed, possibly playing a role in driving the early learning process, but with feedback, other cells in the network get recruited later in the learning process with the average population of cells changing around the time of learning.
Comparison between Perirhinal and Hippocampal Signals
Direct and quantitative comparisons between the associative learning-related signals seen in the monkey perirhinal cortex and the hippocampus revealed global similarities as well as several notable differences. First, both areas exhibited very similar overall patterns and proportions of learning-related changing cells. An ROC analysis showed that the sustained and baseline sustained changing populations in both areas differentiated between activity before and after learning across the entire length of the trial. The baseline sustained changing cells in neither the perirhinal cortex nor the hippocampus exhibited evidence of habituation-like effects. Not only were the overall patterns and strength of the learning-related activity across both areas similar, but both areas also exhibited similar proportions of changing signals before, at the same time and after learning suggesting that both these areas are engaged with similar timing relative to behavioral learning. These neurophysiological findings are also consistent with fMRI studies that have examined learning-related activity in the MTL during similar associative learning tasks that show similar changes in hemodynamic responses in both the hippocampus and perirhinal cortex with new associative learning (Toni et al. 1998, 2001; Law et al. 2005).
In addition to these similarities, we also described several notable differences. First, we found significantly more baseline sustained signals than sustained changing signals in the perirhinal cortex compared with the hippocampus. This finding suggests that the perirhinal cortex may signal learning with a different kind of population response compared with the hippocampus, namely with more suppression of neural activity. It will be important to use multielectrode recordings to further explore the nature of the network level interactions during associative learning within the 2 different areas. Second, we report a greater proportion of baseline sustained cells with peak responses during the delay period in the perirhinal cortex relative to the hippocampus. This finding of the greater delay activity in the perirhinal cortex has implications for several current models of MTL-dependent memory. For example, some models suggest that greater delay activity in the perirhinal cortex could be attained through a neural circuits dynamics via activation of several neurons (Durstewitz et al. 2000). Alternatively, it could be established through an intracellular mechanism, similar to a persistent activity in layer V neurons in slices of the entorhinal cortex (Klink and Alonso 1997; Egorov et al. 2002; Hasselmo and Stern 2006; Tahvildari et al. 2007). Additional experiments will be needed to differentiate between these possibilities. Third, we found that the population of perirhinal sustained changing cells signaled learning significantly earlier in the scene period of the task compared with the hippocampal sustained changing cells. This relatively early perirhinal learning activity for the sustained changing cells (but not for the baseline sustained changing cells), suggests that the sustained learning activity starts earliest within the trial in the perirhinal cortex. These findings raise the possibility that the entire learning effect may be driven by these early sustained changing perirhinal cells and this perirhinal signal then drives the learning activity seen in the hippocampus later in the trial. However, given the relatively small proportion of sustained changing perirhinal cells, this possibility seems unlikely. A second possibility is that the sustained changing cells in the perirhinal cortex and the hippocampus both participate in signaling new learning in parallel, with the perirhinal cortex more involved during the visual scene period of the task and the hippocampus more involved in signaling learning during the delay interval. It will be important to carry out simultaneous recording in both areas together with temporary inactivation procedures to differentiate between these 2 possibilities.
We also report similar proportions of neurons with habituation-like responses in the populations of perirhinal and hippocampal selective nonchanging cells during the performance of a fixation only task with novel stimuli. In contrast, several previous studies (Brown et al. 1987; Riches et al. 1991; Xiang and Brown 1998) reported few if any habituation-likes signals in the hippocampus during familiarity based recognition memory tasks. These differences could be due to the type of visual stimuli used in the studies. In the current experiment we used complex scene stimuli including both outdoor scenes as well as scenes of animals. The visual scene stimuli in particular have been shown to be highly effective at engaging the hippocampus in humans (Stern et al. 1996; Hartley et al. 2007; Blondin and Lepage 2007). Using these stimuli, we report that 90% of the hippocampal cells were responsive to the location-scene task and 73% of those responsive cells were selective. In contrast, previous studies that used simple geometrical shapes as stimuli (Brown et al. 1987; Riches et al. 1991) found a smaller proportion of responsive cells in the hippocampus and few if any familiarity signals in the hippocampus. Even a study by Xiang and Brown (1998) which used a combination of naturalistic scenes and visual object stimuli reported only 14% of their hippocampal population were responsive to the visual stimuli used in their task and none of those cells showed a familiarity signal. Our findings suggest that in certain situations, familiarity for complex scene stimuli is encoded by both the hippocampus as well as the perirhinal cortex in a similar proportion of cells.
Relation to Current Theories of MTL Function
There are currently numerous theories proposed to explain the relative contribution of different MTL areas to memory. Most, but not all of these theories have focused on understanding how the 2 major components of recognition memory, recollection and familiarity are represented by different MTL structures. One view suggests that the structures of the MTL work cooperatively in both recollection and familiarity (Squire et al. 2004). Several other studies, in contrast argue that the hippocampus is critical for recollection, relational or binding processes, whereas the perirhinal cortex is important for item familiarity (Mishkin et al. 1998; Brown and Aggleton 2001; Eichenbaum et al. 2007; Diana et al. 2007). Although these theories have provided a fruitful framework for understanding the role of the MTL in memory, it is also clear that the MTL contributes to a much wider range of mnemonic functions beyond just recognition memory, including both familiarity and recollection. Here we use neurophysiological approaches to examine a form of associative learning, also known as conditional-motor associative learning previously shown to be dependent on the MTL (Gaffan 1993; Murray et al. 1993; Murray et al. 1998). This form of conditional-motor associative memory can be considered a form of across domain association (Mayes et al. 2007; i.e., visual–motor association) or a domain-general association (Staresina and Davachi 2008) that have been attributed primarily to the hippocampus. Indeed this same task has also been used extensively to study conditional-motor learning signals in frontal motor areas (Mitz et al. 1991; Chen and Wise 1995a, 1995b), prefrontal cortex (Asaad et al. 1998) as well as the striatum (Brasted and Wise 2004; Pasupathy et al. 2004). These previous findings suggest a prominent motor learning component to the task.
Although several previous theories have stressed the important role of the perirhinal cortex in item–item associations (Diana et al. 2007; Eichenbaum et al. 2007) or item–feature association (Davachi 2006) they did not address the role of various MTL structure in stimulus–motor or stimulus–location associations likely used by the monkeys to learn the location-scene association task (Brasted et al. 2002; Brasted et al. 2003). For cross-domain conditional-motor associations studied here, we report global similarities in the overall patterns of associative learning-related signals as well as quantitative differences in the timing of across-trial and within-trial learning signals in the perirhinal cortex and hippocampus. These findings suggest that for conditional-motor associations, and possibly more generally for across domain associations, there appears to be a stronger interplay or interaction between different MTL areas than previously described for the recollection and familiarity components of recognition memory (Brown and Aggleton 2001; Eichenbaum et al. 2007; Diana et al. 2007). Moreover, although global similarities may be seen across different MTL areas during tasks requiring conditional-motor associations, the significant differences in timing seen across areas may reflect the particular anatomical connections and/or physiological properties of the individual MTL areas. The current findings taken together with findings from recognition memory studies (Brown and Aggleton 2001; Diana et al. 2007; Eichenbaum et al. 2007) suggests that there may exists a conditionally dynamic relationship between MTL structures that depends on the specific task demands. Thus, in some situations that require recognition memory, MTL structures may act more independently (but see Squire et al. 2004), whereas in other situations requiring cross-modal associations, there may be a stronger cooperation between the structures of the MTL. It will be of interest to test this hypothesis further with an even larger range of mnemonic tasks examined in parallel across different MTL structures.
Funding
National Institutes of Health grant (MH58847) to W.A.S.; a McKnight foundation grant to W.A.S.; R01 MH071847 to E.N.B. and National Institutes of Health grant (DA015644) to E.N.B. and W.A.S.
Supplementary Material
Acknowledgments
We thank Nilda Nystrom for expert animal care and Yuji Naya and Larry Squire for helpful comments on earlier versions of this manuscript.
Conflict of Interest: None declared.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET. Responses of neurons in the inferior temporal cortex in short term and serial recognition memory tasks. Exp Brain Res. 1987;65:614–622. doi: 10.1007/BF00235984. [DOI] [PubMed] [Google Scholar]
- Blondin F, Lepage M. An fMRI study on memory discriminability for complex visual scenes. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20455. Epub ahead of print September 25, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth MC, Rolls ET. View-invariant representations of familiar objects by neurons in the inferior temporal visual cortex. Cereb Cortex. 1998;8:510–523. doi: 10.1093/cercor/8.6.510. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Fornix transection impairs conditional visuomotor learning in tasks involving nonspatially differentiated responses. J Neurophysiol. 2002;87:631–633. doi: 10.1152/jn.00656.2001. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Role of the hippocampal system in associative learning beyond the spatial domain. Brain. 2003;126:1202–1223. doi: 10.1093/brain/awg103. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Wilson FAW, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409:158–162. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Neuronal activity in the supplementary eye field during acquisition of conditional oculomotor associations. J Neurophys. 1995a;73:1101–1121. doi: 10.1152/jn.1995.73.3.1101. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Supplementary eye field contrasted with the frontal eye field during acquisition of conditional oculomotor associations. J Neurophys. 1995b;73:1122–1134. doi: 10.1152/jn.1995.73.3.1122. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nat Neurosci. 2000;3(Suppl):1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;14:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA, Desimone R. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J Neurosci. 1999;19:10404–10416. doi: 10.1523/JNEUROSCI.19-23-10404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy FL, Riches IP, Brown MW. Neuronal activity related to visual recognition memory: long-term memory and the encoding of recency and familiarity information in the primate anterior and medial inferior temporal and rhinal cortex. Exp Brain Res. 1993;96:457–472. doi: 10.1007/BF00234113. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Amnesia for complex naturalistic scenes and for objects following Fornix transection in the rhesus monkey. Eur J Neurosci. 1992;4:381–388. doi: 10.1111/j.1460-9568.1992.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Normal forgetting, impaired acquisition in memory for complex naturalistic scenes by Fornix-transected monkeys. Neuropsychologia. 1993;31:403–406. doi: 10.1016/0028-3932(93)90163-t. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10(11):187–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol. 1997;77(4):1813–1828. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Law JR, Flanery MA, Wirth S, Yanike M, Smith AC, Frank LM, Suzuki WA, Brown EN, Stark CE. Functional magnetic resonance imaging activity during the gradual acquisition and expression of paired-associate memory. J Neurosci. 2005;25:5720–5729. doi: 10.1523/JNEUROSCI.4935-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophys. 1993;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- Liu Z, Richmond BJ, Murray EA, Saunders RC, Steenrod S, Stubblefield BK, Montague DM, Ginns EI. DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proc Natl Acad Sci USA. 2004;101:12336–12341. doi: 10.1073/pnas.0403639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modeling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Messinger A, Squire LR, Zola SM, Albright TD. Neuronal representations of stimulus associations develop in the temporal lobe during learning. Proc Natl Acad Sci USA. 2001;98:12239–12244. doi: 10.1073/pnas.211431098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Vis Neurosci. 1991;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Philos Trans R Soc Lond. 1997;352:1461–1467. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Vargha-Khadem F, Gadian DG. Amnesia and the organization of the hippocampal system. Hippocampus. 1998;8:212–216. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J Neurosci. 1991;11:1855–1872. doi: 10.1523/JNEUROSCI.11-06-01855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SL, Wise SP, di Pellegrino G, Zipser DA. A model that accounts for activity in primate frontal cortex during a delayed matching to sample task. J Neurosci. 1998;18:399–410. doi: 10.1523/JNEUROSCI.18-01-00399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci. 1998;112:1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Sakai K, Miyashita Y. Activity of primate inferotemporal neurons related to a sought target in pair-association task. Proc Natl Acad Sci USA. 1996;93:2664–2669. doi: 10.1073/pnas.93.7.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya Y, Yoshida M, Miyashita Y. Forward processing of long-term associative memory in monkey inferotemporal cortex. J Neurosci. 2003;23:2861–2871. doi: 10.1523/JNEUROSCI.23-07-02861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989;341:52–54. doi: 10.1038/341052a0. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Machon M, Histed MH, Miller EK. Comparison of the caudate nucleus (CN) and frontal eye fields (FEF) during two visuomotor tasks. Soc Nerosci Abstr. 2004 Program No. 87.8. [Google Scholar]
- Riches IP, Wilson FA, Brown MW. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354:152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Smith AC, Wirth S, Suzuki WA, Brown EN. Bayesian analysis of interleaved learning and response bias in behavioral experiments. J Neurophysiol. 2007;97:2516–2524. doi: 10.1152/jn.00946.2006. [DOI] [PubMed] [Google Scholar]
- Sobotka S, Ringo JL. Investigation of long-term recognition and association memory in unit responses from inferotemporal cortex. Exp Brain Res. 1993;96:28–38. doi: 10.1007/BF00230436. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Hasselmo ME. A model for experience-dependent changes in the responses of inferotemporal neurons. Network. 2000;11:169–190. [PubMed] [Google Scholar]
- Spiegelhalter D. 2004. WinBUGS v. 1.4.1. Imperial College and Medical Research Council (MRC) Available at: http://www.mrc-bsu.cam.ac.uk/bugs/. Accessed 1 July 2007. [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. J Cogn Neurosci. 2008;20(8):1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Tahvildari B, Fransén E, Alonso AA, Hasselmo ME. Switching between “on” and “off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17(4):257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- Toni I, Ramnani N, Josephs O, Ashburner J, Passingham RE. Learning arbitrary visuomotor associations: temporal dynamic of brain activity. Neuroimage. 2001;14:1048–1057. doi: 10.1006/nimg.2001.0894. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Role of the hippocampal system in conditional motor learning: mapping antecedents to action. Hippocampus. 1999;9:101–117. doi: 10.1002/(SICI)1098-1063(1999)9:2<101::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographic organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology. 1998;37:657–676. doi: 10.1016/s0028-3908(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Yanike M, Wirth S, Suzuki WA. Representation of well-learned information in the monkey hippocampus. Neuron. 2004;42:477–487. doi: 10.1016/s0896-6273(04)00193-x. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.