Abstract
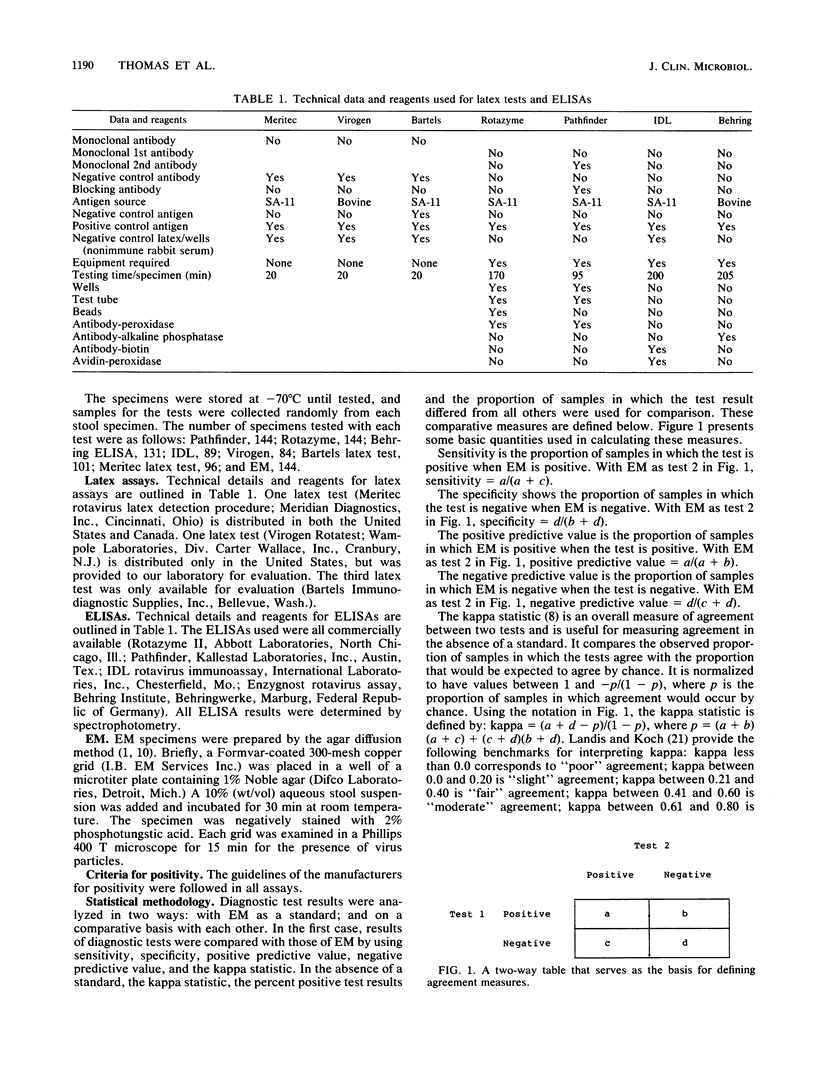
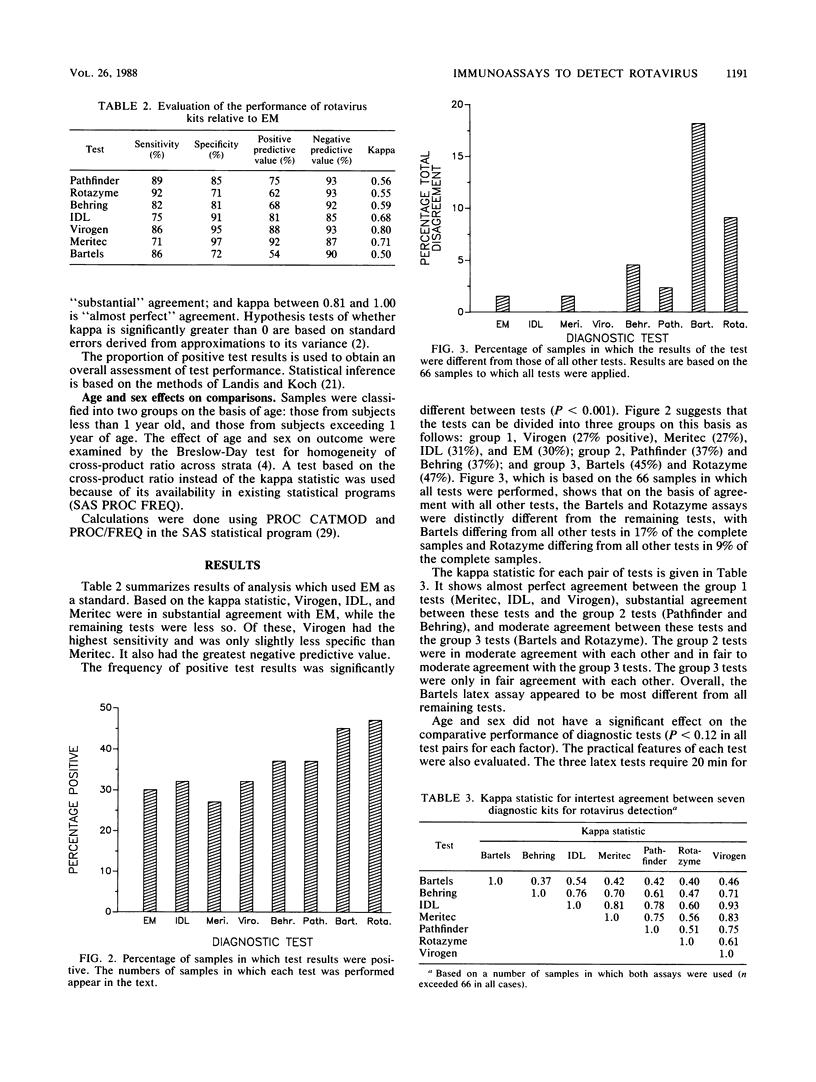
The performance of seven commercially manufactured rotavirus assays was evaluated with 144 pediatric stool specimens and compared with electron microscopy (EM) findings. The four enzyme-linked immunosorbent assays used were Rotazyme II, Pathfinder, IDL rotavirus immunoassay, and Enzygnost (Behring) rotavirus assay. The three latex tests were Meritec rotavirus detection test, Virogen Rotatest, and Bartels rotavirus latex test. Test outcomes were compared with EM on the basis of sensitivity, specificity, positive-negative predictive value, and the kappa statistic. Relative to EM, Meritec had the highest specificity (97%), followed by Virogen (95%), IDL (91%), Pathfinder (85%), Behring (81%), Bartels (72%), and Rotazyme (71%). The sensitivities were as follows: Rotazyme (92%), Pathfinder (89%), Bartels (86%), Virogen (86%), Behring (82%), Meritec (71%), and IDL (75%). Patient age and sex did not influence test results. Owing to the absence of a true standard, the tests were also compared with each other on the basis of the kappa statistic, the frequency of positive test results, and the frequency of samples in which a test differed from all other tests. Using these measures, the assays could be classified into three groups with progressively decreasing utility: group 1 (Virogen, Meritec, IDL, and EM), group 2 (Pathfinder and Behring), and group 3 (Rotazyme and Bartels). Laboratory criteria were also compared. Latex tests were faster and required less equipment than enzyme-linked immunosorbent assays. The Virogen latex assay showed the best overall performance, which made it our choice for rapid and accurate rotavirus diagnosis. However, in children who have gastrointestinal symptoms with negative rotavirus test results, EM will be useful until such time as immunological tests for other enteric viruses are available.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N., Doane F. W. Agar diffusion method for negative staining of microbial suspensions in salt solutions. Appl Microbiol. 1972 Sep;24(3):495–496. doi: 10.1128/am.24.3.495-496.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champsaur H., Questiaux E., Prevot J., Henry-Amar M., Goldszmidt D., Bourjouane M., Bach C. Rotavirus carriage, asymptomatic infection, and disease in the first two years of life. I. Virus shedding. J Infect Dis. 1984 May;149(5):667–674. doi: 10.1093/infdis/149.5.667. [DOI] [PubMed] [Google Scholar]
- Chernesky M., Castriciano S., Mahony J., DeLong D. Examination of the Rotazyme II enzyme immunoassay for the diagnosis of rotavirus gastroenteritis. J Clin Microbiol. 1985 Sep;22(3):462–464. doi: 10.1128/jcm.22.3.462-464.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystie I. L., Totterdell B. M., Banatvala J. E. False positive rotazyme tests on faecal samples from babies. Lancet. 1983 Oct 29;2(8357):1028–1028. doi: 10.1016/s0140-6736(83)91014-0. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Davies H., Bryden A. S., Robertson M. J. Diagnostic electron microscopy of faeces. II. Acute gastroenteritis associated with reovirus-like particles. J Clin Pathol. 1974 Aug;27(8):608–614. doi: 10.1136/jcp.27.8.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikala O. J., Kokkonen J. O., Leinonen M. K., Nurmi T., Mäntyjärvi R., Sarkkinen H. K. Rapid detection of rotavirus in stool by latex agglutination: comparison with radioimmunoassay and electron microscopy and clinical evaluation of the test. J Med Virol. 1983;11(2):91–97. doi: 10.1002/jmv.1890110202. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Ahluwalia G. S., Hazelton P. R. Detection of human rotaviruses in fecal specimens by a commercial latex-agglutination test. J Infect Dis. 1984 Jun;149(6):1021–1021. doi: 10.1093/infdis/149.6.1021. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Blacklow N. R., Perron D. M., Cukor G., Krause P. J., Hyams J. S., Barrett H. J., Ogra P. L. Enzyme immunoassay with monoclonal antibodies for the detection of rotavirus in stool specimens. J Infect Dis. 1985 Oct;152(4):830–832. doi: 10.1093/infdis/152.4.830. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Cline W. L., Arrobio J. O., Brandt C. D., Rodriguez W. J., Sack D. A., Chanock R. M., Parrott R. H. Human reovirus-like agent as the major pathogen associated with "winter" gastroenteritis in hospitalized infants and young children. N Engl J Med. 1976 Apr 29;294(18):965–972. doi: 10.1056/NEJM197604292941801. [DOI] [PubMed] [Google Scholar]
- Knisley C. V., Bednarz-Prashad A. J., Pickering L. K. Detection of rotavirus in stool specimens with monoclonal and polyclonal antibody-based assay systems. J Clin Microbiol. 1986 May;23(5):897–900. doi: 10.1128/jcm.23.5.897-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Hyams J. S., Middleton P. J., Herson V. C., Flores J. Unreliability of Rotazyme ELISA test in neonates. J Pediatr. 1983 Aug;103(2):259–262. doi: 10.1016/s0022-3476(83)80361-8. [DOI] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Miotti P. G., Eiden J., Yolken R. H. Comparative efficiency of commercial immunoassays for the diagnosis of rotavirus gastroenteritis during the course of infection. J Clin Microbiol. 1985 Nov;22(5):693–698. doi: 10.1128/jcm.22.5.693-698.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Shahrabadi M. S., Ince B. Rapid diagnosis of rotavirus gastroenteritis by a commercial latex agglutination test. J Clin Microbiol. 1985 Nov;22(5):846–850. doi: 10.1128/jcm.22.5.846-850.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. S., Miller M. F. Comparison of an enzyme immunoassay with electron microscopic procedures for detecting rotavirus. J Clin Microbiol. 1982 May;15(5):938–944. doi: 10.1128/jcm.15.5.938-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambourg M., Goudeau A., Courant C., Pinon G., Denis F. Direct appraisal of latex agglutination testing, a convenient alternative to enzyme immunoassay for the detection of rotavirus in childhood gastroenteritis, by comparison of two enzyme immunoassays and two latex tests. J Clin Microbiol. 1985 Apr;21(4):622–625. doi: 10.1128/jcm.21.4.622-625.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. C., Campbell A. D., Jenkins M. F. Routine detection of human rotavirus by latex agglutination: comparison with enzyme-linked immunosorbent assay, electron microscopy and polyacrylamide gel electrophoresis. J Virol Methods. 1986 Jul;13(4):285–290. doi: 10.1016/0166-0934(86)90053-4. [DOI] [PubMed] [Google Scholar]
- Sanekata T., Yoshida Y., Okada H. Detection of rotavirus in faeces by latex agglutination. J Immunol Methods. 1981;41(3):377–385. doi: 10.1016/0022-1759(81)90199-x. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Leister F. J. Evaluation of enzyme immunoassays for the detection of human rotavirus. J Infect Dis. 1981 Oct;144(4):379–379. doi: 10.1093/infdis/144.4.379. [DOI] [PubMed] [Google Scholar]


