Abstract
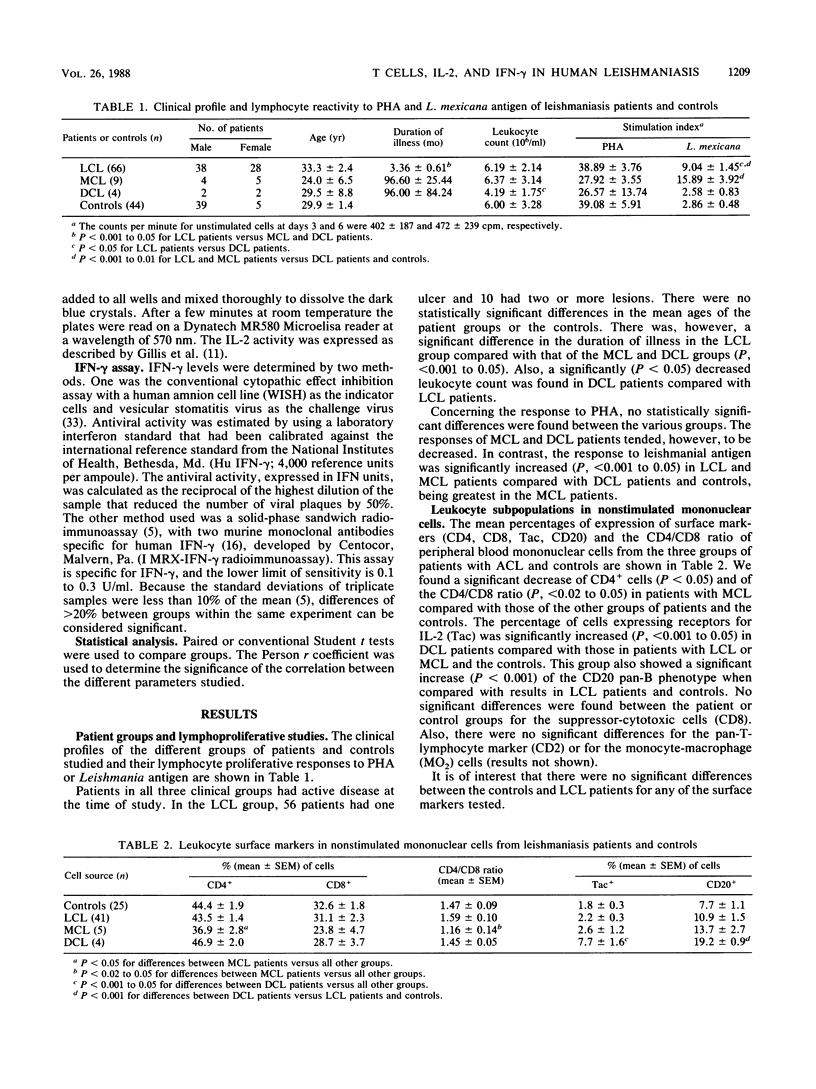
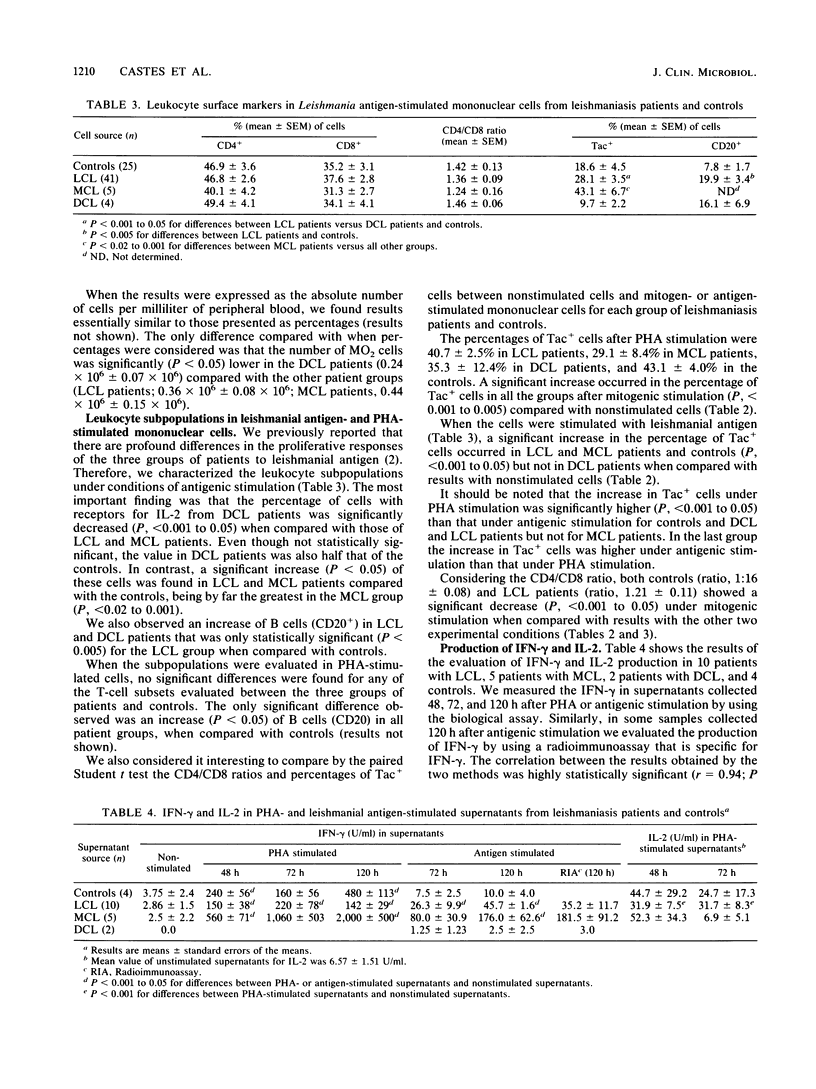
Leukocyte subpopulations, the expression of the interleukin-2 (IL-2) receptor, and the production of IL-2 and gamma interferon (IFN-gamma) were studied in the peripheral blood mononuclear cells of American cutaneous leishmaniasis patients that had been stimulated in vitro with either leishmanial antigen or mitogen (phytohemagglutinin M). The 75 patients examined were classified as having either the localized (LCL; 66 patients), mucocutaneous (MCL; 5 patients), or the rare diffuse (DCL; 4 patients) form of the disease. Patients with DCL, who are characterized by their defective cell-mediated immune response to leishmanial antigen, failed to express the IL-2 receptor and did not produce IFN-gamma when exposed to the antigen but did so when stimulated by phytohemagglutinin M. Both LCL and MCL patients showed strong proliferative responses to leishmanial antigen; these were by far the greatest in MCL patients. Both groups had significantly increased IL-2 receptor expression and IFN-gamma production after exposure to either antigen or mitogen, and these were highest in the MCL patients. Concerning the leukocyte subpopulations evaluated (CD2, CD4, CD8, CD20, MO2), the most significant findings were a decrease of both CD4+ cells and the CD4/CD8 ratio in MCL patients compared with the other groups. Considering IL-2 production, in response to phytohemagglutinin M both MCL and LCL patients showed amounts of IL-2 comparable to those of the controls. Our results help explain the anergy of T cells from DCL patients to leishmanial antigen, which could lead to a defective production of IFN-gamma and possibly contribute to their incapacity to kill the Leishmania parasite. Concerning MCL patients, the significantly increased expression of IL-2 receptor, decreased expression of the CD4 (helper-inducer of suppression) phenotype, and elevated IFV-gamma production might partially explains the state of hypersensitivity and mucosal damage exhibited by these patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carvalho E. M., Johnson W. D., Barreto E., Marsden P. D., Costa J. L., Reed S., Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985 Dec;135(6):4144–4148. [PubMed] [Google Scholar]
- Castes M., Agnelli A., Verde O., Rondón A. J. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol. 1983 May;27(2):176–186. doi: 10.1016/0090-1229(83)90068-5. [DOI] [PubMed] [Google Scholar]
- Castes M., Trujillo D., Scott D., Rondon A. J. Demonstration of an indomethacin-sensitive mechanism regulating immune reactivity in American cutaneous leishmaniasis patients. Clin Exp Immunol. 1987 Aug;69(2):280–290. [PMC free article] [PubMed] [Google Scholar]
- Castés M., Agnelli A., Rondón A. J. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clin Exp Immunol. 1984 Aug;57(2):279–286. [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., McKinney S., Liu V., Kung P. C., Vilcek J., Le J. Use of monoclonal antibodies as sensitive and specific probes for biologically active human gamma-interferon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5219–5222. doi: 10.1073/pnas.81.16.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rondón A. J. Diffuse cutaneous leishmaniasis: a disease due to an immunological defect of the host. Trans R Soc Trop Med Hyg. 1972;66(4):603–610. doi: 10.1016/0035-9203(72)90306-9. [DOI] [PubMed] [Google Scholar]
- Croll A. D., Wilkinson M. F., Morris A. G. Interleukin 2 receptor blockade by anti-Tac antibody inhibits IFN-gamma induction. Cell Immunol. 1985 Apr 15;92(1):184–189. doi: 10.1016/0008-8749(85)90076-0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Robb R. J., Waldmann T. A., Greene W. C. Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J Immunol. 1983 Aug;131(2):690–696. [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hofman F. M., Billing R. J., Parker J. W., Taylor C. R. Cytoplasmic as opposed to surface Ia antigens expressed on human peripheral blood lymphocytes and monocytes. Clin Exp Immunol. 1982 Aug;49(2):355–363. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lainson R., Shaw J. J. Epidemiology and ecology of leishmaniasis in Latin-America. Nature. 1978 Jun 22;273(5664):595–600. doi: 10.1038/273595a0. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Le J., Barrowclough B. S., Vilcek J. Monoclonal antibodies to human immune interferon and their application for affinity chromatography. J Immunol Methods. 1984 Apr 13;69(1):61–70. doi: 10.1016/0022-1759(84)90277-1. [DOI] [PubMed] [Google Scholar]
- Miles M. A., Lainson R., Shaw J. J., Póvoa M., de Souza A. A. Leishmaniasis in Brazil: XV. Biochemical distinction of Leishmania mexicana amazonensis, L. braziliensis braziliensis and L. braziliensis guyanensis--aetiological agents of cutaneous leishmaniasis in the Amazon Basin of Brazil. Trans R Soc Trop Med Hyg. 1981;75(4):524–529. doi: 10.1016/0035-9203(81)90191-7. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Tapia F. J., Bloom B. R., Gallinoto M. E., Castes M., Rondon A. J., Rea T. H., Convit J. In situ characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Exp Immunol. 1985 May;60(2):241–248. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Distaso J. A., Borel Y., Schlossman S. F., Reinherz E. L. Communicative interactions between subpopulations of human T lymphocytes required for generation of suppressor effector function in a primary antibody response. J Immunol. 1982 Apr;128(4):1645–1650. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S., Acosta A. M. Reversible defect in antigen-induced lymphokine and gamma-interferon generation in cutaneous leishmaniasis. J Immunol. 1984 Oct;133(4):2250–2254. [PubMed] [Google Scholar]
- Passwell J. H., Shor R., Shoham J. The enhancing effect of interferon-beta and -gamma on the killing of Leishmania tropica major in human mononuclear phagocytes in vitro. J Immunol. 1986 Apr 15;136(8):3062–3066. [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha R. A., Sampaio R. N., Guerra M., Magalhâes A., Cuba C. C., Barreto A. C., Marsden P. D. Apparent Glucantime failure in five patients with mucocutaneous leishmaniasis. J Trop Med Hyg. 1980 Aug;83(4):131–139. [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Steiger R. F., Steiger E. Cultivation of Leishmania donovani and Leishmania braziliensis in defined media: nutritional requirements. J Protozool. 1977 Aug;24(3):437–441. doi: 10.1111/j.1550-7408.1977.tb04771.x. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Kelso A., Louis J. A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Henriksen-Destefano D., Siegel D., Klion A., Robb R. J., Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985 Sep;135(3):1851–1856. [PubMed] [Google Scholar]


