Abstract
The capacity of the immunomodulatory drug rapamycin (RAPA) to inhibit replication of the CCR5 strain of human immunodeficiency virus (HIV) in vitro prompted us to test its effects in a murine preclinical model of HIV infection. RAPA (0·6 or 6 mg/kg body weight) or its vehicle were administered daily, per os, to SCID mice reconstituted with human peripheral blood leucocytes (hu-PBL) starting 2 days before the intraperitoneal challenge with the R5 tropic SF162 strain of HIV-1 (1000 50% tissue culture infective dose/ml). Relative to hu-PBL-SCID mice that received no treatment, HIV-infected hu-PBL-SCID mice treated with the vehicle control for 3 weeks exhibited a severe depletion of CD4+ cells (90%), an increase in CD8+ cells and an inversion of the CD4+/CD8+ cell ratio. In contrast, treatment of HIV-infected mice with RAPA prevented a decrease in CD4+ cells and the increase of CD8+ cells, thereby preserving the original CD4+: CD8+ cell ratio. Viral infection also resulted in the detection of HIV-DNA within peritoneal cells and spleen, and lymph node tissues of the vehicle-treated mice within 3 weeks of the viral challenge. In contrast, treatment with RAPA decreased cellular provirus integration and reduced HIV-RNA levels in the blood. Furthermore, in co-cultivation assays, spleens from RAPA-treated mice exhibited a reduced capacity for infecting allogeneic T cells which was dose-dependent. These data show that RAPA possesses powerful anti-viral activity against R5 strains of HIV in vivo and support the use of additional studies to evaluate the potential application of this drug in the management of HIV patients.
Keywords: CD4/CD8, HIV, hu-PBL-SCID, immunotherapy, RAPA
Introduction
The progression of a human immunodeficiency virus (HIV) infection to development of acquired immunodeficiency syndrome in an individual depends upon multiple factors [1]. The entry of HIV into host cells requires multiple interactions between viral envelope proteins with primary (CD4) and secondary (CCR5 and CXCR4) cellular receptor complexes. Correspondingly, individuals lacking CCR5 (CCR5-Delta32 homozygous genotype) are resistant to infection by the R5 strain of HIV, while individuals expressing high levels of CCR5 co-receptor on their CD4+ T cells have increased progression of HIV infection and disease [2]. The expression of CCR5 on CD4+ T lymphocytes has been shown to correlate strongly with HIV viral loads [3]. As a result, novel anti-HIV strategies have included small molecules, monoclonal antibodies or modified ligands that target co-receptors of CCR5[4–6]. Mutations in CCR5, CCR2, CX(3)CR1, CXCL12 (SDF1) or CCL5 (regulated upon activation normal T cell expressed and secreted) have also been shown to influence the response of patients to anti-retroviral therapy [1].
Because a large majority of HIV-infected individuals possess biological variants that use CCR5 as a co-receptor for HIV infection, efforts have been made to develop non-functional antagonists of CCR5 [4]. Although the small molecule CCR5 inhibitors, maraviroc (UK-427 857) and vicriviroc (SCH-D), have already been proved to be effective in reducing viral load in HIV-infected individuals, secondary events have been associated with their prolonged clinical use, including the development of malignancies and the development of CXCR4-using HIV-1 variants. However, the demonstrated efficacy of CCR5 inhibitors in the clinical setting has provided a valuable proof of concept to justify additional efforts to identify other CCR5 inhibitors that may possess additional anti-HIV properties other than CCR5 inhibition.
Rapamycin [sirolimus (RAPA)] is a macrolide antibiotic already in clinical use for the prophylactic treatment of renal graft rejection. The drug inhibits T cell function selectively by interfering with molecular events secondary to the binding of interleukin (IL)-2 to its receptor. Upon binding of RAPA to its intracellular receptor FKBP12, this complex associates with fluorescence recovery after photobleaching to exert its cytostatic effects on T cells [7,8]. Recently, the capacity for RAPA to provide targeted treatment of HIV-associated Kaposi's sarcoma has been highlighted [9].
The RAPA was first identified to have anti-HIV effects when it was shown that it could selectively repress translation of a subset of mRNAs bearing a 5′-polypyrimidine motif in the presence of a suboptimal 5′-polypyrimidine tract, a site that is required for viral tat mRNA production [10–13]. Roy et al. demonstrated that RAPA represses HIV-1 replication powerfully in both human lymphoid T cells and human peripheral blood leucocytes (hu-PBLs). The inhibitory effect of RAPA on HIV-1 replication was associated with diminished basal HIV-1 long-terminal repeat gene expression independent of the virus-specific transactivating Tat protein [14]. In vitro inhibition of HIV replication in activated human T cells by RAPA has also been confirmed [15].
Further interest in the anti-HIV properties of RAPA emerged from work by Heredia et al. that showed RAPA reduces CCR5 expression on T cells and macrophages at the transcriptional level, and simultaneously up-regulates the synthesis of macrophage inflammatory protein (MIP)-1alpha and MIP1beta [16]. A recent study by Gilliam et al. has also shown that RAPA reduces CCR5 messenger RNA expression either in peripheral blood mononuclear cells (PBMCs) or in cervicovaginal tissue from cynomologous macaques [17].
These observations led us to perform an in vivo study with RAPA in the murine SCID–hu-PBL model of HIV infection that is used routinely by ourselves [18–21] and others [22,23] as an in vivo tool to gain insight into pathogenic mechanisms and evaluate novel anti-viral approaches worthy of being considered for translation to the clinical setting. Our data show that prolonged treatment with 6 mg/kg body weight (bd wt) of RAPA profoundly, and favourably, influences the course of HIV-1 infection by preventing decreases in CD4+ cells, increases the number of CD8+ cells, inhibits proviral integration and decreases the peripheral viral load increase associated typically with HIV-1 infection.
Materials and methods
The Hu-PBL-SCID mouse model
CB17 SCID/SCID female mice (Charles River Laboratories, Stone Ridge, NY, USA) were used at 4 weeks of age and were maintained under specific pathogen-free conditions as described elsewhere [18–21]. The handling of animals and the study protocol were in accordance with international guidelines and were approved by the local Institutional Animal Care and Use Committee.
The Hu-PBLs were obtained from the peripheral blood of healthy donors. All donors were screened for HIV-1 and hepatitis B and C viruses before donation. Four mice from each group were injected intraperitoneally (i.p.) with 40 × 106 cells resuspended in 0·5 ml RPMI-1640 medium.
Experimental design
CB.17 SCID/SCID mice were reconstituted initially by i.p. injections of 40 × 106 PBLs obtained as described [18–21], then treated per os with 0·6 or 6 mg/kg bd wt of RAPA or its vehicle as a control. These doses of RAPA were chosen based on the immunomodulatory effects shown for them in the non-obese diabetic mouse model of human type 1 diabetes [24]. Treatment was administered every day starting 2 days before i.p. challenge with R5 tropic SF162 virus (1000 50% tissue culture infective dose/ml) and was continued for a total of 23 consecutive days. Three additional groups of sex- and age-matched CB.17 SCID/SCID mice were reconstituted with 40 × 106 hu-PBLs, were not challenged with the virus, and were left untreated or were dosed with either RAPA (6 mg/kg) or its vehicle alone under the same experimental conditions used for the HIV-infected hu-PBL-SCID mice.
Cell recovery from the peritoneal cavity and other organs of the SCID mice
The HIV-infected, hu-PBL-SCID mice were killed 3 weeks after the viral challenge. Uninfected hu-PBL-SCID mice were killed 23 days after reconstitution. Only flow cytometry analyses were performed on samples from the uninfected mice. Cells were collected from both the peritoneal cavity and spleen. Spleen tissue was disrupted and connective tissue and debris were allowed to settle before single cell suspensions were washed twice in RPMI-1640 medium.
Detection of HIV-1 infection and flow cytometry analysis
Plasma HIV-1 RNA copy numbers were measured (Amplicor HIV-1 Monitor Assay; Roche Molecular Systems, Milano, Italy) 3 weeks after viral infection. For polymerase chain reaction detection of HIV-1 proviral sequences, DNA was extracted from peritoneal lavages and from spleen and lymph nodes tissues of the hu-PBL-SCID mice. The presence of human sequences was determined using specific primers for the human leucocyte antigen-DQα gene (data not shown). HIV-1 proviral DNA was detected by specific amplification of HIV-1 gag sequences as described previously [21]. In each experiment, the sensitivity of the assay was tested by amplifying DNA prepared from 8E5 cells (which harbour one proviral copy per cell) that was diluted serially into SCID mouse cell DNA (data not shown). At the end of the study human cells from the mouse peritoneal cavity were also analysed by flow cytometry using fluorescein isothiocyanate-labelled anti-CD45 and phycoerythrin anti-CD4, CD8 antibody (Becton Dickinson, San Jose, CA, USA) as described elsewhere [18–21].
Co-cultivation assay
Cells were recovered from the spleens of hu-PBL-reconstituted and HIV-infected mice treated with either RAPA or its vehicle as a control. Spleen cells (105) were co-cultured with 105 human allogeneic T cells which were activated preventively with 5 µg/ml phytohaemagglutinin and cultivated in the presence of 50 U/ml IL-2. Virus replication was determined after 10 days of culture by detection of p24 gag antigen in culture supernatant using a commercial enzyme-linked immunosorbent assay kit (Dupont, Wilmington, DE, USA).
Statistical analysis
Data are shown as the mean value ± standard deviation and are derived from two independent studies. Because the data from the two studies were reproducible, the data were merged and shown as the result of a single study. Each experimental group was composed of eight mice. Statistical analysis was performed using one-way analysis of variance. A P-value < 0·05 was considered significant. Undetectable values of p24 and HIV RNA were assigned, as a theoretical value, the lower limit of the assay in order to allow statistical analysis.
Results
In vivo treatment with RAPA prevents a decrease in CD4+ levels and increases CD8+ levels
Figure 1a shows the proportions of CD4+ and CD8+ cells in non-HIV-1 infected hu-PBL-SCID mice that were left untreated or received RAPA (6 mg/kg) or its vehicle and were killed 23 days after reconstitution. There were no significant differences in the proportions of CD4+ and CD8+ T cells and their ratio in the three groups of mice (Fig. 1a). In contrast, within 3 weeks following the infection of hu-PBL-SCID mice with the R5 tropic SF162 strain of HIV-1 and treatment with the vehicle of RAPA as a control, a severe depletion of CD4+ cells and an increase in CD8+ cells was observed that inverted the CD4+: CD8+ cell ratio. In contrast, when a viral challenge was applied to mice receiving RAPA treatment, both a decrease in CD4+ levels and an increase in CD8+ levels was prevented and the CD4+ : CD8+ cell ratio was preserved. The effect of RAPA was shown to be dose-dependent and the highest dose of the drug reduced CD4+ depletion to 59% compared with a 90% decrease in CD4+ levels observed in controls.
Fig. 1.
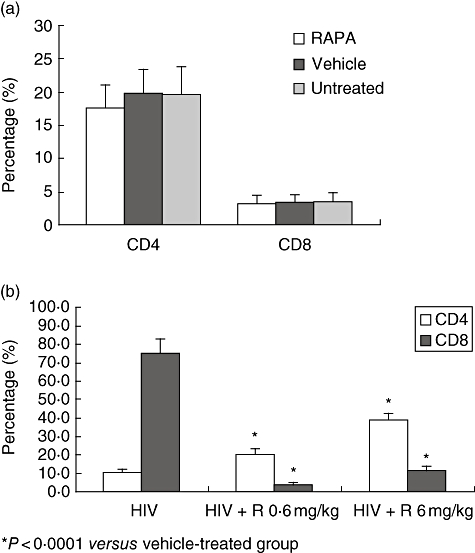
(a,b) The effect of rapamycin (RAPA) on the percentage of CD4+ and CD8+ T cells in human immunodeficiency virus (HIV)-infected human peripheral blood leucocytes (hu-PBL)-SCID mice. CB.17 SCID/SCID mice were reconstituted by intraperitoneal (i.p.) injections of 40 × 106 PBLs and treated per os with 0·6 or 6 mg/kg bd wt of RAPA, or its vehicle alone as a control. Treatment was administered every day starting 2 days before the i.p. challenge with R5 tropic SF162 virus (1000 50% tissue culture infective dose/ml), and continued thereafter for 3 consecutive weeks for a total of 23 consecutive days. Three additional groups of sex- and age-matched CB.17 SCID/SCID mice were reconstituted with 40 × 106 hu PBLs, were not challenged with the virus and were left untreated or were dosed with either RAPA (6 mg/kg) or its vehicle alone under the same experimental conditions used for the HIV-infected hu-PBL-SCID mice. The percentage of CD4+ and CD8+ T cells are shown for each of the groups. *P < 0·00001 compared with the vehicle-treated group.
Treatment with RAPA decreases cellular provirus integration, reduces HIV-RNA levels in the blood of hu-PBL-SCID mice and diminishes the capacity of spleen cells to infect allogeneic T cells
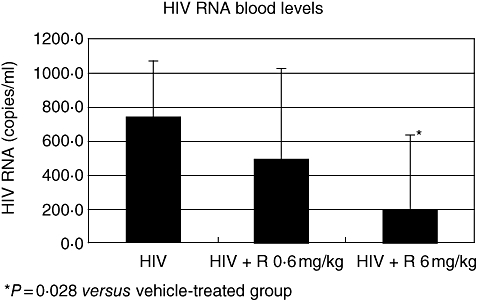
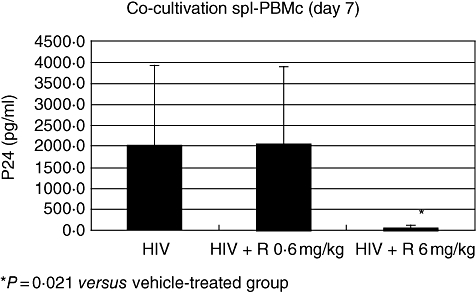
The HIV-1 infection of hu-PBL-SCID mice was associated with the presence of HIV-DNA within peritoneal cells, spleen and lymph node tissues (Fig. 2). Consistent with these results, HIV was detected in the blood of all the infected mice that were killed 21 days after inoculation (Fig. 3) In addition, co-cultivation of cells recovered from the spleens with allogenic human lymphocytes tested positive for the presence of HIV-p24 (Fig. 4). These readouts, known to be associated with HIV-1 infection in hu-PBL-SCID mice, were all affected by treatment with RAPA. Figure 2 shows the inhibition of provirus integration in peritoneal cells, spleen and lymph node tissues observed with treatment of RAPA, while Fig. 3 demonstrates the decrease in HIV detection for blood samples from RAPA-treated mice. Using co-cultivation assays, spleens from RAPA-treated mice were tested for their ability to infect allogeneic T cells. As shown by the absence of detectable HIV-p24 in only two of eight mice, spleens from RAPA-treated mice exhibited lower capacity of infecting allogeneic T cells than the spleens from the vehicle-treated mice (Fig. 4). Consistent with our findings of CD4+ and CD8+ modulation during the course of HIV-1 infection in hu-PBL-SCID mice, the effects of RAPA were found to be dose dose-dependent. In particular, seven of eight mice treated with the highest dose of RAPA had undetectable levels of HIV-RNA in their blood.
Fig. 2.
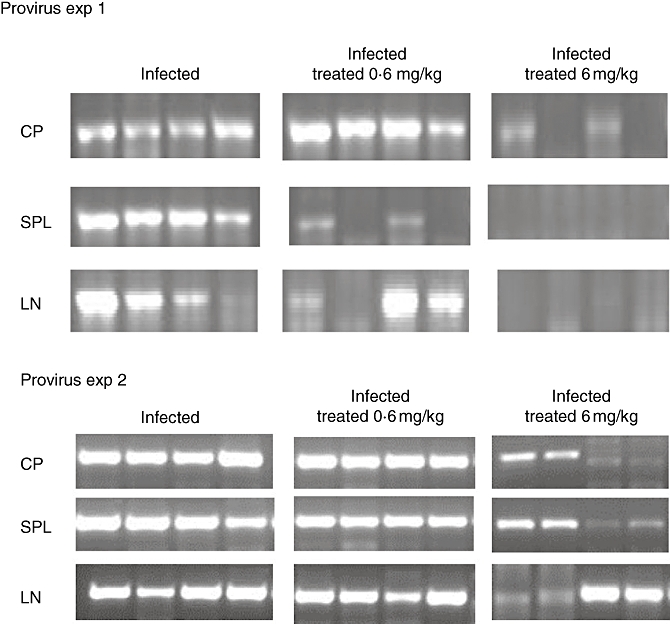
The effect of rapamycin (RAPA) on provirus integration in human immunodeficiency virus (HIV)-infected human peripheral blood leucocytes (hu-PBL)-SCID mice. For polymerase chain reaction detection of HIV-1 proviral sequences, DNA was extracted from peritoneal lavages (CP), spleen (SPL) and lymph node (LN) tissues of the hu-PBL-SCID mice that were killed 3 weeks after viral challenge. Each group consisted of samples from eight mice. Data from four representative animals are shown.
Fig. 3.
The effects of rapamycin (RAPA) on co-cultivation assays of spleen cells collected from human immunodeficiency virus (HIV)-infected human peripheral blood leucocytes (hu-PBL)-SCID mice. Cells were recovered from the spleens of hu-PBL-reconstituted and HIV-infected mice treated with either RAPA (0·6 versus 6 mg/kg) or its vehicle alone. Spleen cells (105) were co-cultured with 105 human allogeneic T cells which were activated preventively with 5 µg/ml phytohaemagglutinin (PHA) and cultivated in the presence of 50 U/ml interleukin-2. Virus replication was determined after 10 days of culture by detection of p24 gag antigen in culture supernatant using a commercial enzyme-linked immunosorbent assay kit. *P = 0·021 versus the vehicle-treated control. Each group consisted of eight mice.
Fig. 4.
The effects of rapamycin (RAPA) on human immunodeficiency virus (HIV) RNA blood levels in HIV-infected human peripheral blood leucocytes (hu-PBL)-SCID mice. Plasma samples were collected 3 weeks after viral infection to measure HIV-1 RNA copy numbers. HIV-1 proviral DNA was detected by specific amplification of HIV-1 gag sequences in samples collected from mice receiving RAPA treatment (0·6 versus 6 mg/kg) or vehicle treatment alone as a control. *P = 0·028 versus the vehicle-treated samples. Each group consisted of eight mice.
Clinical observations of RAPA-treated mice
Treatment with RAPA of both HIV-1 infected and uninfected hu-PBL-SCID mice appeared to be well tolerated by the animals, as judged by their general appearance and behaviour. In addition, there were no differences in bd wt between HIV-1-infected and uninfected HIV-1 mice treated with RAPA or its vehicle versus untreated mice (data not shown). In agreement with the lack of toxicity of RAPA that emerges from these clinical data, analysis of human peritoneal PBMCs from uninfected hu-PBL-SCID mice treated with RAPA, as assessed by trypan blue cell dye exclusion, exhibited a similar viability of the cells obtained from the untreated or vehicle-treated groups of mice (data not shown).
Discussion
Although highly active anti-retroviral therapy (HAART) is usually effective in reducing viral load and reconstituting CD4+ T cell count, latent virus reservoirs persist and patients are likely to experience drug-related toxicity and drug-related viral resistance during lifelong treatment. Recently, a type of anti-viral cytostatic drugs has been proposed to be included within the context of HAART. These drugs, such as hydroxyurea, mycophenolic acid and RAPA, are most often immunosuppressive substances that are used for other therapeutic indications. The potential for immunosuppressive drugs to represent a safe and effective adjuvant for HAART treatments in patients with stable HIV disease has already been reported [25,26]. An advantage of these drugs is their capacity to target host cellular proteins that are not susceptible to mutations to challenge HIV infection. Thus, their resistance profile seems to be promising.
The results presented herein show for the first time the capacity of the well-known immunosuppressive drug RAPA to significantly prevent virological and immunological events secondary to inoculation of the R5 tropic SF162 HIV strain in hu-PBL-SCID mice. In particular, the acute loss of CD4+ cells associated with the increase of CD8+ cells and the secondary alteration of the CD4+: CD8+ T cell ratio were prevented when the viral challenge was applied to mice treated with RAPA. Although we cannot exclude that the observed increase in the percentage of CD4 with RAPA may have been secondary to the decrease of percentage of CD8 cells, or to a toxic effect of the drug on this cell population, both these possibilities seem unlikely. In fact, RAPA did not alter the proportion of CD4+ and CD8+ cells in uninfected hu-PBL-SCID mice, which remained consistent with the CD4+ : CD8+ cell levels of the two reconstituted and uninfected control groups. In addition, another study proves that RAPA does not alter the proportion of CD8+ T cells in either Lewis or brown Norway rats [27]. Taken together, these data suggest that the prevention of the increase of the CD8+ T cells observed in the HIV-1-infected hu-PBL-SCID mice treated with RAPA is selectively secondary to the prevention of HIV infection of the CD4+ T cells from the drug and the subsequent modifications of the CD4 : CD8 ratio that are induced by the virus.
Other readouts known to be associated with HIV-1 infection in hu-PBL-SCID mice, such as the integration of HIV provirus in peritoneal cells, spleen and lymph node tissues and the appearance of circulating HIV in the inoculated mice, were also affected significantly by RAPA pretreatment. Spleen cells from these pretreated mice also exhibited a reduced capacity for infection of allogenic T cells in co-cultivation assays. All these effects were shown to be dose-dependent, with the highest dose of RAPA at 6 mg/kg being significantly more efficient than a 10-fold lower dose.
By using a well-known preclinical model of human HIV infection, our findings extend and validate independent in vitro studies [14–16] that demonstrated the capacity for RAPA to inhibit replication of R5 strains of HIV in leukaemic T cell lines, in human PBMCs and in macrophage. In these in vitro studies, the anti-viral effect of RAPA was associated with down-regulation of CCR5 in both PBMCs and monocytes at concentrations as low as 1 nM and 0·01 nM respectively [16]. Furthermore, infectivity assays showed that RAPA suppressed the replication of R5 strains of HIV both in PBMCs and macrophage cultures at a concentration (0·01 nM) remarkably lower than the therapeutic levels necessary for preventing kidney transplant rejection [16]. The efficacy of using low doses of RAPA to inhibit HIV replication is consistent with our present findings of apparent, although weaker, anti-HIV activity of RAPA even at a dose as low as 0·6 mg/kg.
Although the present experiments do not address directly the mechanism by which RAPA mediates an anti-viral effect in the hu-PBL-reconstituted mouse model of HIV infection, the combination of previously published in vitro evidence and the use of the R5 strain of SF162 for our viral challenges support strongly the hypothesis that down-regulation of CCR5 expression plays a key role in the anti-viral effects exhibited by RAPA. Studies are in progress in our laboratory to ascertain whether RAPA treatment down-regulates the expression of CCR5 in cells obtained from spleen, lymph or peritoneal cavity tissues during the course of HIV-1 infection of hu-PBL-SCID mice. Additional insight will be provided by determining whether the results of these in vivo studies will be consistent with previously reported in vitro data. However, although the existing literature data suggest strongly that inhibition of CCR5 expression might be one of the primary mechanisms mediating the anti-HIV activity of RAPA observed in this study, we cannot rule out the possibility that other immunomodulatory effects of the drug are contributing to prevent the development of HIV-1 infection in this preclinical model. In particular, as HIV-1 infection is facilitated by an up-regulation of tumour necrosis factor-alpha and hyperactivation of nuclear factor-κB activity [28,29], which mediate not only the host inflammatory response but also activate the HIV-1 long terminal repeat, and the fact that RAPA suppresses both events [30–32], it seems likely that these and possibly other immunopharmacological properties of RAPA reduce the infectivity of HIV-1.
The RAPA is a drug already in clinical use for the prevention of allograft rejection. Therefore, demonstration of its capacity to provide anti-viral effects against infection of the R5 HIV strain in vivo is of immediate relevance for the translation of RAPA treatments to the management of HIV patients. However, we have tested the effects of RAPA in a prophylactic fashion (the treatment was commenced by the time of SF162 inoculation) rather than in a therapeutic manner. Additional studies would be necessary to ascertain whether RAPA can also diminish actively ongoing viral replication, as is the case with established HIV infections. A possible therapeutic role for RAPA as a virostatic during HAART interruption has already been proposed [25]. The possibility that RAPA may have a role in the treatment of HIV infection also needs to be evaluated in the context of possible interactions with other anti-retroviral drugs within the HAART regimen. Heredia et al. have demonstrated recently that RAPA reduces CCR5 density on CD4+ T cells, thereby influencing synergistically the effect of the HIV fusion inhibitor, enfuvirtide, and enabling a 33-fold reduction of the latter drug's dosage [33]. Synergism of RAPA with the CCR5 antagonist, TAK-799[16], has also been reported from in vitro studies. Correspondingly, we are studying the possible synergism of RAPA with the small molecule, maraviroc, which is a small molecule inhibitor of CCR5 closest to gaining regulatory approval to enter the clinical setting.
Taken together, these data indicate that RAPA has important anti-viral properties against the R5 strain of HIV viruses and deserves further study to ascertain its possible utility in the management of HIV-infected patients.
Acknowledgments
The authors thank Dr Björn Hoffstedt, Christine Kreisl and Dr1 John Ryan for their valuable help with the design of the study, discussion of the data and critical reading of the manuscript. The technical and laboratory skills of Alberto Cesolini and Professor Filippo Palermo is also gratefully acknowledged.
References
- 1.Reiche EM, Bonametti AM, Voltarelli JC, Morimoto HK, Watanabe MA. Genetic polymorphisms in the chemokine and chemokine receptors: impact on clinical course and therapy of the human immunodeficiency virus type 1 infection. Curr Med Chem. 2007;14:1325–34. doi: 10.2174/092986707780597934. [DOI] [PubMed] [Google Scholar]
- 2.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS. 2001;15:1627–34. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 3.Reynes J, Portales P, Segondy M, et al. CD4 cell surface CCR5 density as a determining factor of viral load in HIV-infected individuals. J Infect Dis. 2000;181:927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 4.Repik A, Richards KH, Clapham PR. The promise of CCR5 antagonists as new therapies for HIV-1. Curr Opin Invest Drugs. 2007;8:130–9. [PubMed] [Google Scholar]
- 5.Fätkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–2. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 6.Gulick RM, Su Z, Flexner C, et al. AIDS Clinical Trials Group 5211 Team. J Infect Dis. 2007;196:304–12. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 7.Albers MW, Brown EJ, Tanaka A, Williams RT, Hall FL, Schreiber SL. An FKBP-rapamycin-sensitive, cyclin-dependent kinase activity that correlates with the FKBP-rapamycin-induced G1 arrest point in MG-63 cells. Ann NY Acad Sci. 1993;696:54–62. doi: 10.1111/j.1749-6632.1993.tb17142.x. [DOI] [PubMed] [Google Scholar]
- 8.Morice WG, Wiederrecht G, Brunn GJ, Siekierka JJ, Abraham RT. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J Biol Chem. 1993;268:22737–45. [PubMed] [Google Scholar]
- 9.Dittmer DP, Krown SE. Targeted therapy for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus. Curr Opin Oncol. 2007;19:425–27. doi: 10.1097/CCO.0b013e3281eb8ea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquenet S, Ropers D, Bilodeau PS, et al. Conserved stem-loop structures in the HIV-1 RNA region containing the A3 3′ splice site and its cis-regulatory element: possible involvement in RNA splicing. Nucleic Acids Res. 2001;29:464–78. doi: 10.1093/nar/29.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Si Z, Amendt BA, Stoltzfus CM. Splicing efficiency of human immunodeficiency virus type 1 tat RNA is determined by both a suboptimal 3′ splice site and a 10 nucleotide exon splicing silencer element located within tat exon 2. Nucleic Acids Res. 1997;25:861–7. doi: 10.1093/nar/25.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staffa A, Cochrane A. The tat/rev intron of human immunodeficiency virus type 1 is inefficiently spliced because of suboptimal signals in the 3′ splice site. J Virol. 1994;68:3071–9. doi: 10.1128/jvi.68.5.3071-3079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanga TÃ, Kjems J. SF2/ASF binds to a splicing enhancer in the third HIV-1 tat exon and stimulates U2AF binding independently of the RS domain. J Mol Biol. 2001;312:649–62. doi: 10.1006/jmbi.2001.4971. [DOI] [PubMed] [Google Scholar]
- 14.Roy S, Paquette JS, Fortin JF, Tremblay MJ. The immunosuppressant rapamycin represses human immunodeficiency virus type 1 replication. Antimicrob Agents Chemother. 2002;46:3447–55. doi: 10.1128/AAC.46.11.3447-3455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oswald-Richter K, Grill SM, Leelawong M, Unutmaz D. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur J Immunol. 2004;34:1705–14. doi: 10.1002/eji.200424892. [DOI] [PubMed] [Google Scholar]
- 16.Heredia A, Amoroso A, Davis C, et al. Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV beta-chemokines: an approach to suppress r5 strains of HIV-1. Proc Natl Acad Sci USA. 2003;100:10411–16. doi: 10.1073/pnas.1834278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilliam BL, Heredia A, Devico A, et al. Rapamycin reduces CCR5 mRNA levels in macaques: potential applications in HIV-1 prevention and treatment. AIDS. 2007;21:2108–10. doi: 10.1097/QAD.0b013e3282f02a4f. [DOI] [PubMed] [Google Scholar]
- 18.Rizza P, Santini SM, Logozzi M, et al. T-cell dysfunctions in hu-PBL-SCID mice infected with human immunodeficiency virus (HIV) shortly after reconstitution: in vivo effects of HIV on highly activated human immune cells. J Virol. 1996;70:7958–64. doi: 10.1128/jvi.70.11.7958-7964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santini SM, Lapenta C, Logozzi M, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garaci E, Aquaro S, Lapenta C, et al. Anti-nerve growth factor Ab abrogates macrophage-mediated HIV-1 infection and depletion of CD4+ T lymphocytes in hu-SCID mice. Proc Natl Acad Sci USA. 2003;22:8927–32. doi: 10.1073/pnas.1332627100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapenta C, Santini SM, Logozzi M, et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198:361–7. doi: 10.1084/jem.20021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth MD, Tashkin DP, Whittaker KM, Choi R, Baldwin GC. Tetrahydrocannabinol suppresses immune function and enhances HIV replication in the huPBL-SCID mouse. Life Sci. 2005;77:1711–22. doi: 10.1016/j.lfs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Del Real G, Jiménez-Baranda S, Mira E, et al. Statins inhibit HIV-1 infection by down-regulating Rho activity. J Exp Med. 2004;200:541–7. doi: 10.1084/jem.20040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeder WL, Sredy J, Sehgal SN, Chang JY, Adams LM. Rapamycin prevents the onset of insulin-dependent diabetes mellitus (IDDM) in NOD mice. Clin Exp Immunol. 1992;89:174–8. doi: 10.1111/j.1365-2249.1992.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Argyropoulos C, Mouraki A. Immunosuppressive drugs in HIV disease. Curr Top Med Chem. 2006;6:1769–89. doi: 10.2174/156802606778194271. [DOI] [PubMed] [Google Scholar]
- 26.Kelly LM, Lisziewitz J, Lori F. Virostatics as a potential new class of HIV drugs. Curr Pharm Des. 2004;10:4103–20. doi: 10.2174/1381612043382495. [DOI] [PubMed] [Google Scholar]
- 27.Damoiseaux JG, Beijleveld LJ, Schuurman HJ, van Breda Vriesman PJ. Effect of in vivo rapamycin treatment on de novo T-cell development in relation to induction of autoimmune-like immunopathology in the rat. Transplantation. 1996;62:994–1001. doi: 10.1097/00007890-199610150-00019. [DOI] [PubMed] [Google Scholar]
- 28.DeLuca C, Kwon H, Lin R, Wainberg M, Hiscott J. NF-kappaB activation and HIV-1 induced apoptosis. Cytokine Growth Factor Rev. 1999;10:235–53. doi: 10.1016/s1359-6101(99)00015-5. [DOI] [PubMed] [Google Scholar]
- 29.Kedzierska K, Crowe SM. Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother. 2001;12:133–50. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 30.Puzik A, Schultz C, Iblher P, Müller-Steinhardt M, Härtel C. Effects of ciclosporin A, tacrolimus and sirolimus on cytokine production in neonatal immune cells. Acta Paediatr. 2007;96:1483–9. doi: 10.1111/j.1651-2227.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 31.Giordano A, Avellino R, Ferraro P, Romano S, Corcione N, Romano MF. Rapamycin antagonizes NF-kappaB nuclear translocation activated by TNF-alpha in primary vascular smooth muscle cells and enhances apoptosis. Am J Physiol Heart Circ Physiol. 2006;290:H2459–65. doi: 10.1152/ajpheart.00750.2005. [DOI] [PubMed] [Google Scholar]
- 32.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heredia A, Gilliam B, Latinovic O, et al. Rapamycin reduces CCR5 density levels on CD4 T cells and this effect results in potentiation of Enfuvirtide (T-20) against R5 HIV-1 in vitro. Antimicrob Agents Chemother. 2007;51:2489–96. doi: 10.1128/AAC.01602-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


