Abstract
Data collected as part of a multi-year trial examining the efficacies of inactivated and live-attenuated influenza vaccines were used to evaluate the reported occurrence of post-vaccination reactions. Two cohorts were defined: (1) individuals who received the same vaccine over two consecutive years, and (2) individuals who first enrolled in year 2 of the study and received vaccine only in that year. For both vaccines there were significantly fewer reactions reported in year 2 in those subjects who were vaccinated both years. Declines were demonstrated when comparing year 1 and 2 reaction frequencies in subjects vaccinated both years, and differences were seen when comparing year 2 reaction frequencies in subjects vaccinated both years with those first vaccinated in year 2. Reaction reporting peaked on post-vaccination days 0 and 1 following receipt of the inactivated vaccine, and on day 2 following receipt of the live-attenuated vaccine.
Keywords: influenza vaccines, adverse reactions, inactivated influenza vaccine, live-attenuated influenza vaccine
Introduction
Vaccination is the primary means of preventing seasonal influenza and its serious complications. Annual vaccination is recommended for a large segment of the American population, including children 6 months to 18 years of age, all persons age greater than 49 years, adults and children with chronic health conditions, and health care workers. [1] Because of the health and economic burdens of this vaccine-preventable disease, the US is moving toward a recommendation of universal use of influenza vaccines. [2] Inactivated influenza vaccine, administered as an intramuscular injection, has been in use since the Second World War. More recently, a live-attenuated influenza vaccine administered as an intranasal spray, was licensed for use in healthy persons aged 2 to 49 years. Both the inactivated and live-attenuated vaccines are trivalent, containing influenza A/H3, A/H1 and B components, and are updated on an annual basis.
Adverse reactions associated with vaccination, whether mild or serious, real or simply perceived, may influence the decision to receive an influenza vaccination. [3, 4] This is of particular importance given the need to sustain vaccination programs from one year to the next. In a large study of adults conducted prior to widespread use of the live-attenuated vaccine, beliefs that influenza vaccination could cause illness and that side effects were not worth it, were two of the primary reasons for not receiving an annual influenza vaccination. [3] The live-attenuated vaccine is a new product to most consumers and little is known about the public’s perceptions of it; however, its route of administration was expected to increase its acceptability. Ironically, because it contains infectious virus, the live-attenuated vaccine may produce a greater number and/or more pronounced reactions than the inactivated vaccine [5], possibly lowering its acceptability for some potential recipients.
Frequencies of reactions reported annually following receipt of the inactivated and live-attenuated vaccines were examined in a placebo-controlled trial of influenza vaccine efficacies carried out during the 2004–2005 and 2005–2006 influenza seasons. In the first year, among solicited reactions, only arm soreness was significantly more likely to be reported by recipients of the inactivated vaccine than by recipients of the matching (injected) placebo; runny nose/congestion, cough, headache and muscle aches were all more likely to be reported by recipients of the live-attenuated vaccine than by recipients of the matching (intranasal) placebo. [6] In the second year, arm soreness, arm redness, muscle aches, trouble breathing and red eyes were all significantly associated with receipt of the inactivated vaccine; only sore throat and runny nose/congestion were associated with receipt of the live-attenuated vaccine. [7] In both study years, arm soreness in inactivated vaccine recipients and runny nose/congestion in live-attenuated vaccine recipients were the most frequently reported post-vaccination reactions and demonstrated the greatest absolute differences in frequency between vaccine and matching placebo groups. [6, 7] These results were consistent with those demonstrated in other safety assessments of the inactivated and live-attenuated vaccines. [5, 8, 9, 10, 11]
We used data collected as part of the multi-year vaccine efficacy trial to compare reported frequencies of solicited post-vaccination reactions by vaccine group, in those subjects who participated in both the first and second study years, and those who participated in only the second year. [6, 7] Our objectives were to evaluate whether repeated annual vaccination affected the reported occurrence of reactions, and to examine and compare the frequency and timing of reported reactions relative to vaccine administration.
Methods
The study was a placebo-controlled, community-based trial. Participants were healthy men and women 18 to 48 years of age; persons with any health condition for which the inactivated vaccine was recommended or for whom either vaccine was contraindicated, were excluded. Participants were randomly assigned to one of four intervention groups at study enrollment: the inactivated vaccine or saline placebo administered as an intramuscular injection; or, the live-attenuated vaccine or placebo (saline in the first year and normal allantoic fluid [the same egg fluid plus diluent that was used in preparing the live-attenuated vaccine] in the second year) administered as an intranasal spray. Participants and nurses who administered the intervention were unaware of whether vaccine or placebo was administered but were aware of the route of administration. Participants reported the occurrence of fourteen (including “other”) solicited local and systemic reactions to vaccine or placebo using diary cards [6, 7]; data were recorded the day of the intervention (day 0) and for the next 7 days. Reported reactions were also rated by the participants as mild (“It didn’t affect how I did my usual activities at all”), moderate (“It affected how I did my usual activities, but I was still able to do them”) or severe (“I could not work or do my usual activities”). The study was approved by the institutional review board at the University of Michigan Medical School and all participants provided written informed consent.
We included two groups in our analysis: a cohort of individuals who received the same vaccine (inactivated or live-attenuated) or placebo (injected or intranasal) over two consecutive years (study years 1 and 2), and a cohort of individuals who first enrolled in the second year of the study and received vaccine or placebo only in study year 2. We limited our evaluation to reactions that were both frequently reported (>10% of participants reported reaction in one or both study years) plus risk differences between vaccine and corresponding placebo group were >5% in either or both years regardless of statistical significance. [6, 7] Selected reactions included runny nose/congestion, cough, sore throat, headache, muscle aches, weakness, and arm soreness (selected for injected vaccine or placebo only). Since even mild but annoying symptoms could affect vaccine acceptability and the decision to get vaccinated, all reported reactions from the selected group were considered without regard to reported reaction severity.
Statistical analyses
The proportions of participants reporting any (one or more) of the selected reactions were graphically presented and compared between cohorts by vaccine or placebo group. To determine whether repeated annual vaccination affected reported occurrence of reactions, 1) reaction frequency was examined in study year 2 and compared for those subjects who participated over two consecutive years (and received the same vaccine each year) versus those who participated only in year 2; and 2) pair-wise analyses using McNemar’s test of discordant pairs were examined for consecutive year participants with comparisons of year 1 versus year 2 reaction reporting frequency. The frequency and timing of selected reactions reported in the days following vaccine receipt were graphically presented and compared for inactivated and live-attenuated vaccine recipients; this analysis was limited to first experiences for each cohort (year 1 for both year participants; year 2 for single year participants), and to any (one or more) reactions, and separately, to runny nose/congestion for the live-attenuated vaccine and arm soreness for the inactivated vaccine. Statistical analyses were generated using SAS (release 8.2, SAS Institute, 2000) and MedCalc (for calculation of 95% confidence intervals around risk differences) statistical software; P-values <0.05 and 95% confidence intervals around risk differences that excluded zero were considered statistically significant.
Results
Participants
Nine hundred six subjects participated in both study years 1 and 2 and provided reaction reports in both years. There were 384 (42%) inactivated and 375 (41%) live-attenuated vaccine recipients, and 147 (16%) placebo recipients (68 injected and 79 intranasal). An additional 990 subjects participated only in study year 2 and provided reaction reports that year. Among these, there were 419 (42%) inactivated and 408 (41%) live-attenuated vaccine recipients, and 163 (16%) placebo recipients (86 injected and 77 intranasal). Participant characteristics were similar across intervention groups; participant mean age was 27 years in year 1 and 25 years in year 2, and each year more than 60% of subjects were women.
Reported Reaction Frequency
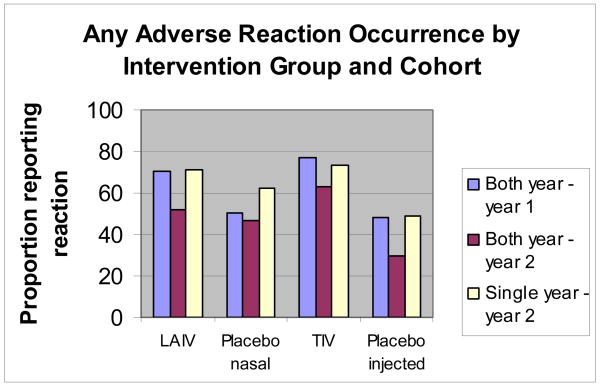
The proportion of participants reporting any (one or more) selected reactions is graphically presented in Figure 1 by vaccine and placebo group, and cohort (two year or single year). For both vaccines and for the injected placebo, a significant (P<0.01) decline in reaction reporting in year 2 was noted for those subjects who participated both years; reporting frequency among year 2 only participants was similar (P>0.05) to that reported in year 1 (with the exception of intranasal placebo).
Figure 1. The proportions of participants reporting occurrence of any (one or more) adverse reactions by vaccine or placebo group, and cohort (both year versus single year participants).
LAIV (live-attenuated influenza vaccine); placebo nasal (saline in year 1 and normal allantoic fluid in year 2); TIV (inactivated vaccine); placebo injected (saline both years)
Table 1 presents comparisons of the frequencies of reported reactions among inactivated vaccine recipients, by cohort. For all of the selected reactions, a decline in reporting frequency among both year participants in the second year of consecutive vaccine receipt was demonstrated (Group 1a vs. 1b); significant risk differences in reported frequencies were noted for runny nose/congestion, headache and arm soreness. Similarly, participants who received the inactivated vaccine in consecutive study years were less likely to report all selected reactions in study year 2 compared to those initially enrolled in year 2 (Group 1b vs. 2); significant risk differences in reported frequencies were noted for runny nose/congestion, sore throat and weakness. Reported frequencies at first vaccine receipt (Group 1a vs. 2, risk differences and 95% confidence intervals not shown) were similar with smaller, non-significant risk differences demonstrated.
Table 1.
Selected reactions* among participants who received the inactivated vaccine (TIV) over two consecutive seasons (Both Years) and those who received vaccine only in the second season (Single Year)
| Study Cohort: | Both Years | Both Years | Single Year | Group 1a vs. 1b | Group 1b vs. 2 |
|---|---|---|---|---|---|
| Study Year: | Year 1 | Year 2 | Year 2 | ||
| Group: | 1a | 1b | 2 | ||
| Number: | N=384 | N=384 | N=419 | ||
| Reported Reaction | Frequency (%) | Frequency (%) | Frequency (%) | Difference (95% CI)# | Difference (95% CI) |
| Runny nose or congestion | 119 (31.0) | 83 (21.6) | 118 (28.2) | 9.4 (3.6 to 14.7) | 6.6 (0.6 to 12.4) |
| Cough | 61 (15.9) | 52 (13.5) | 67 (16.0) | 2.4 (−2.8 to 7.3) | 2.5 (−2.5 to 7.2) |
| Sore throat | 73 (19.0) | 52 (13.5) | 79 (18.9) | 5.5 (−0.1 to 10.7) | 5.4 (0.2 to 10.2) |
| Headache | 135 (35.2) | 94 (24.5) | 128 (30.6) | 10.7 (4.7 to 16.2) | 6.1 (−0.1 to 12.1) |
| Muscle aches | 59 (15.4) | 47 (12.2) | 62 (14.8) | 3.2 (−1.6 to 7.6) | 2.6 (−2.2 to 7.1) |
| Weakness | 81 (21.1) | 69 (18.0) | 108 (25.8) | 3.1 (−2.0 to 8.1) | 7.8 (2.1 to 13.3) |
| Arm soreness | 211 (55.0) | 184 (47.9) | 221 (52.7) | 7.1 (1.4 to 12.3) | 4.8 (−2.1 to 11.7) |
| One or more reactions | 297 (77.3) | 243 (63.3) | 307 (73.3) | 14.0 (8.4 to 19.0) | 10.0 (3.6 to 16.3) |
Reported reaction selected from list of solicited reactions
McNemar Test: pairwise comparison of marginal homogeneity
TIV - Trivalent inactivated (Fluzone®) influenza vaccine
Table 2 presents similar comparisons of the frequencies of reported reactions among live-attenuated vaccine recipients, by cohort. For most selected reactions (exception: sore throat) a decline in reporting frequency among both year participants in the second year of consecutive vaccine receipt was demonstrated (Group 1a vs. 1b); significant risk differences were noted for runny nose/congestion, cough, headache, and weakness. Participants who received the live-attenuated vaccine in consecutive study years were less likely to report all selected reactions in study year 2 compared to those initially enrolled in year 2 (Group 1b vs. 2); significant risk differences were noted for runny nose/congestion, cough, headache, muscle aches and weakness. Reported frequencies at first vaccine receipt (Group 1a vs. 2, risk differences and 95% confidence intervals not shown) were similar with smaller, non-significant risk differences demonstrated.
Table 2.
Selected reactions* among participants who received the live-attenuated vaccine (LAIV) over two consecutive seasons (Both Years) and those who received vaccine only in the second season (Single Year)
| Study Cohort: | Both Years | Both Years | Single Year | Group 1a vs. 1b | Group 1b vs. 2 |
|---|---|---|---|---|---|
| Study Year: | Year 1 | Year 2 | Year 2 | ||
| Group: | 1a | 1b | 2 | ||
| Number: | N=375 | N=375 | N=408 | ||
| Reported Reaction | Frequency (%) | Frequency (%) | Frequency (%) | Difference (95% CI)# | Difference (95% CI) |
| Runny nose or congestion | 189 (50.4) | 141 (37.6) | 193 (47.3) | 12.8 (6.2 to 18.9) | 9.7 (2.8 to 16.5) |
| Cough | 66 (17.6) | 40 (10.7) | 86 (21.1) | 6.9 (1.9 to 11.5) | 10.4 (5.4 to 15.0) |
| Sore throat | 87 (23.2) | 95 (25.3) | 114 (27.9) | 2.1 (−3.4 to 7.6) | 2.6 (−3.6 to 8.7) |
| Headache | 143 (38.1) | 110 (29.3) | 151 (37.0) | 8.8 (2.6 to 14.6) | 7.7 (1.1 to 14.1) |
| Muscle aches | 50 (13.3) | 41 (10.9) | 71 (17.4) | 2.4 (−2.1 to 6.7) | 6.5 (1.6 to 11.0) |
| Weakness | 84 (22.4) | 61 (16.3) | 118 (28.9) | 6.1 (1.1 to 10.8) | 12.6 (6.8 to 18.1) |
| One or more reactions | 264 (70.4) | 195 (52.0) | 290 (71.1) | 18.4 (12.4 to 23.6) | 19.1 (12.3 to 25.6) |
Reported reaction selected from list of solicited reactions
McNemar Test: pairwise comparison of marginal homogeneity
LAIV - Live-attenuated (FluMist®) influenza vaccine
Timing of reactions
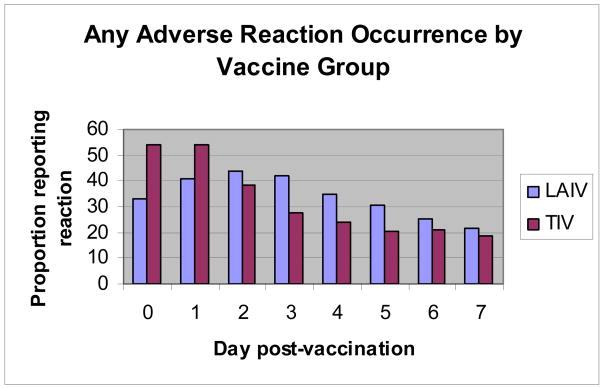
Figure 2 graphically presents the reported frequency and timing (days, post-vaccination) of any (one or more) reactions by vaccine group. Data considered were limited to first experiences for each cohort (year 1 for both year participants and year 2 for single year participants). For the inactivated vaccine, reporting of reactions peaked on days 0 and 1 and then declined; one or more reactions affected 54% of inactivated vaccine recipients on peak days. In contrast, reaction reporting frequency for the live-attenuated vaccine peaked on day 2 and gradually declined; 44% of vaccine recipients were affected by reactions on day 2.
Figure 2. The frequency and timing (days post-vaccination) of reported adverse reactions (any) by vaccine group.
LAIV (live-attenuated influenza vaccine [FluMist®]); TIV (inactivated vaccine [Fluzone®])
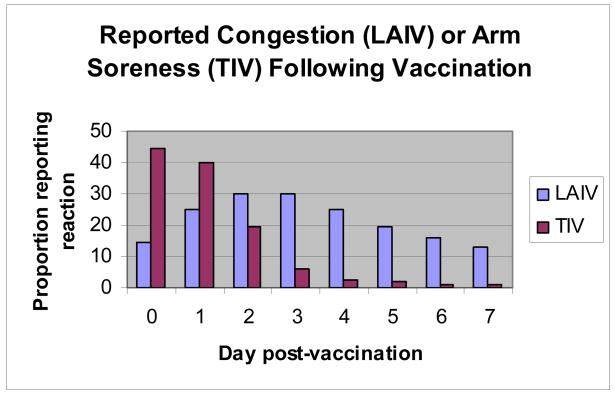
Figure 3 graphically presents the reported frequency and timing (days, post-vaccination) of the most consistently reported reactions for each vaccine – arm soreness (inactivated) and runny nose/congestion (live-attenuated). Of note, arm soreness among inactivated vaccine recipients was most frequently reported on days 0 and 1 post-vaccination and then rapidly declined with peak reporting on day 0 (44%). In contrast, runny nose/congestion was reported by greater than 12% of live-attenuated vaccine recipients each report day with peak reporting on days 2 and 3 (30%, both days) post-vaccination.
Figure 3. The frequency and timing (days post-vaccination) of reported congestion (LAIV) and arm soreness (TIV) by vaccine group.
LAIV (live-attenuated influenza vaccine [FluMist®]); TIV (inactivated vaccine [Fluzone®])
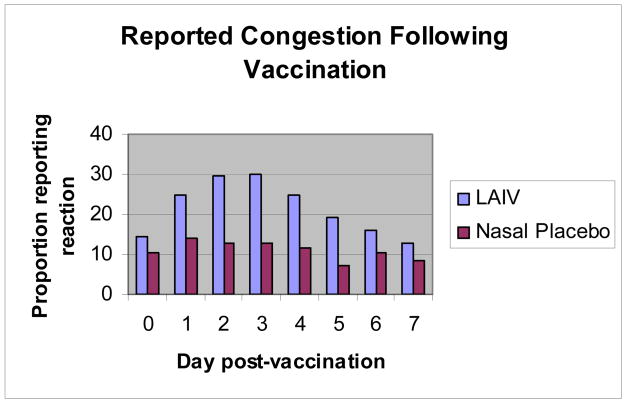
The delayed peak in reported reactions among live-attenuated vaccine recipients may be associated with vaccine virus replication. To explore this we examined and compared the reporting frequency and timing of runny nose/congestion for live-attenuated vaccine recipients compared with recipients of the intranasal placebo (Figure 4). A relatively flat and lower reporting frequency, by day, was noted for placebo recipients; this finding supports the notion that reported reactions among vaccine recipients were related to vaccine virus replication.
Figure 4. The frequency and timing (days post-vaccination) of reported congestion by recipients of the live-attenuated vaccine or intranasal placebo.
LAIV (live-attenuated influenza vaccine [FluMist®]); Intranasal Placebo (saline in year 1 and normal allantoic fluid in year 2)
Discussion
Influenza vaccination, whether it is administered by injection or intranasally, is unusual in that it needs to be given annually for protective benefit. Adverse reactions associated with vaccination, whether mild or serious, may influence an individuals’ decision to seek revaccination. [3, 4, 19, 20] Serious adverse events, including Guillian Barre syndrome, seizures, asthma exacerbation and Bell’s palsy are rare and often of questionable association, but have been reported following influenza vaccine receipt. [15, 16, 17, 18] Immediate, allergic reactions occur rarely after influenza vaccination; most are thought to result from hypersensitivity to residual egg protein from the vaccine production process. [12, 13, 14, 15] However, more minor reactions are relatively common. Reactions to the inactivated vaccine may result from the injection itself or from inflammatory responses to components of the vaccine (e.g. viral antigens), or with the live-attenuated vaccine, replication of the vaccine viruses; reactions can be local (e.g. nasal congestion, arm soreness) or systemic (e.g. fever, fatigue). [1, 5, 9]
Only limited examination of the effect of repeated annual vaccination on adverse reaction reporting has been reported, especially when the study is carried out in a blinded, placebo-controlled fashion. In a previous comparative study of the inactivated and live-attenuated vaccines no significant differences in the proportion of subjects reporting reactions between initial and repeated vaccination were noted; however, data were not presented. [5] Other investigators have noted that adverse reactions to vaccines are most likely to occur among those without prior experience, for example, young children. [21, 22]
In the present analyses, we found significant reductions in reaction reporting frequency with repeated vaccination with both the inactivated and live-attenuated vaccines. These reductions were demonstrated when examining second year report frequencies in subjects vaccinated over two consecutive years compared with those first vaccinated in the second year, and when examining first and second year report frequencies for the subjects vaccinated over two consecutive years. For these analyses, reaction data were solicited from adult subjects participating in a multi-year comparative trial of influenza vaccine efficacy who were randomly assigned to interventions. Declines in reaction reporting frequencies may have been due to so-called “immune tolerance” to vaccine virus antigens on repeated exposure with fewer reactions occurring, or alternatively, to reduced recognition or reduced reporting of temporally related symptoms with fewer reactions noted. The fact that adverse reactions to vaccines are most likely to occur among those without prior experience, for example, young children, supports the concept of immune tolerance on repeat exposure. [21, 22] However, in this study, reductions in reporting frequency, although not significantly different, were also demonstrated for consecutive year compared with single year placebo recipients; this supports the notion of reduced recognition or reporting due to prior experience. Post-vaccination reaction or symptom differences across study groups and years might also have been related to intercurrent upper respiratory infections.
The likelihood of reporting any reaction following vaccination was slightly higher for inactivated compared to live-attenuated vaccine recipients; however, the likelihood of reporting a moderate or severe reaction (data not shown) did not differ by vaccine group. The timing of post-vaccination reactions and the most frequently identified reactions differed by vaccine group; however, for both vaccines, reactions were generally mild, local rather than systemic and of limited duration.
Despite established efficacy and an acceptable safety profile, the uptake of influenza vaccine remains less than optimal, even among high-risk groups and health care workers. [1] The notion that the vaccine can cause illness and a lack of perceived need for vaccination remains common even among those at high-risk for complications from influenza infection. [3] Meeting vaccine coverage expectations is further complicated by the necessity for annual vaccine receipt for continuing protection. Strategies to increase annual vaccine use, including physician recommendations, employee health programs, considering vaccine coverage as a measure of health care facility quality assessment, and a national education campaign promoting vaccination, are necessary.
Acknowledgments
Supported by a grant from the National Institute of Allergy and Infectious Diseases (U01 AI057853)
Footnotes
Potential conflicts of interest: Dr Victor reports receiving consulting fees from Wyeth, and Dr. Monto reports receiving consulting fees from GlaxoSmithKline, MedImmune, Solvay, and Novartis, and an unrestricted research grant from Sanofi-Pasteur. No other potential conflicts of interest relevant to this article are reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov number, NCT00133523
References
- 1.Fiore AE, Shay DK, Haber P, et al. Prevention and Control of Influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Morb Mortal Wkly Rep. 2007;56(RR6):1–54. [PubMed] [Google Scholar]
- 2.Schwartz B, Hinman A, Abramson J, et al. Universal influenza vaccination in the United States: are we ready? Report of a meeting J Infect Dis. 2006;194:S147–54. doi: 10.1086/507556. [DOI] [PubMed] [Google Scholar]
- 3.Jones TF, Ingram LA, Craig AS, Schaffner W. Determinants of influenza vaccination, 2003–2004: shortages, fallacies and disparities. Clin Infect Dis. 2004;39:1824–8. doi: 10.1086/427153. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Influenza vaccination and self-reported reasons for not receiving influenza vaccination among medicare beneficiaries aged >65 years- United States, 1991–2003. MMWR Morb Mortal Wkly Rep. 2004;53:1012–5. [PubMed] [Google Scholar]
- 5.Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. A randomized, controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 6.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmit SE, Victor JC, Teich ER, et al. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live-attenuated vaccines. J Infect Dis. 2008 doi: 10.1086/589885. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govaert TM, Dinant GJ, Aretz K, et al. Adverse reactions to influenza vaccine in elderly people; randomized double blind placebo controlled trial. BMJ. 1993;307:988–90. doi: 10.1136/bmj.307.6910.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichol KL, Margolis KL, Lind A, et al. Side effects associated with influenza vaccination in healthy working adults; a randomized, placebo-controlled trial. Arch Int Med. 1996;156:1546–50. [PubMed] [Google Scholar]
- 10.Belshe RB, Nichol KL, Black SB, et al. Safety, efficacy and effectiveness of live attenuated, cold-adapted influenza vaccine in an indicated population aged 5–49 years. Clin Infect Dis. 2004;39:920–7. doi: 10.1086/423001. [DOI] [PubMed] [Google Scholar]
- 11.Nichol KL, Mendelman PM, Mallon KP, et al. Effectiveness of live attenuated intranasal influenza virus vaccine in healthy, working adults. JAMA. 1999;282:137–44. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 12.D’Heilly SJ, Blade MA, Nichol KL. Safety of influenza vaccinations administered in nontraditional settings. Vaccine. 2006;24:4024–7. doi: 10.1016/j.vaccine.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 13.Bohlke K, Davis RL, Marcy SM, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112:815–20. doi: 10.1542/peds.112.4.815. [DOI] [PubMed] [Google Scholar]
- 14.Zeiger RS. Current issues with influenza vaccination in egg allergy. J Alergy Clin Immunol. 2002;110:834–40. doi: 10.1067/mai.2002.129372. [DOI] [PubMed] [Google Scholar]
- 15.McMahon AW, Iskander J, Haber P, et al. Adverse events after inactivated influenza vaccination among children less than 2 years of age: analysis of reports from the vaccine adverse event reporting system, 1993–2003. Pediatrics. 2005;115:453–60. doi: 10.1542/peds.2004-1519. [DOI] [PubMed] [Google Scholar]
- 16.Juurlink DN, Stukel TA, Kwong J, et al. Guillain-Barre syndrome after influenza vaccination in adults: a population-based study. Arch Int Med. 2006;166:2217–21. doi: 10.1001/archinte.166.20.2217. [DOI] [PubMed] [Google Scholar]
- 17.Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005;294:2720–5. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT. A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. Pharmacoepidemiology Drug Safety. 2004;13:505–10. doi: 10.1002/pds.998. [DOI] [PubMed] [Google Scholar]
- 19.Nichol KL, Lofgren RP, Gapinski J. Influenza vaccination. Knowledge, attitudes and behavior among high-risk outpatients. Arch Int Med. 1992;152:106–10. doi: 10.1001/archinte.152.1.106. [DOI] [PubMed] [Google Scholar]
- 20.Fiebach NH, Viscoli CM. Patient acceptance of influenza vaccination. Am J Med. 1991;91:393–400. doi: 10.1016/0002-9343(91)90157-s. [DOI] [PubMed] [Google Scholar]
- 21.Belshe RB, Gruber WC. Safety, efficacy and effectiveness of cold-adapted, live attenuated, trivalent intranasal influenza vaccine in adults and children. Philosophical Transactions of the Royal Society of London-Series B: Biological Sciences. 2001;356:1947–51. doi: 10.1098/rstb.2001.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry DW, Mayner RE, Hochstein HD, et al. Comparative trial of influenza vaccines. II Adverse reactions in children and adults. Am J Epidemiol. 1976;104:47–59. doi: 10.1093/oxfordjournals.aje.a112273. [DOI] [PubMed] [Google Scholar]