Abstract
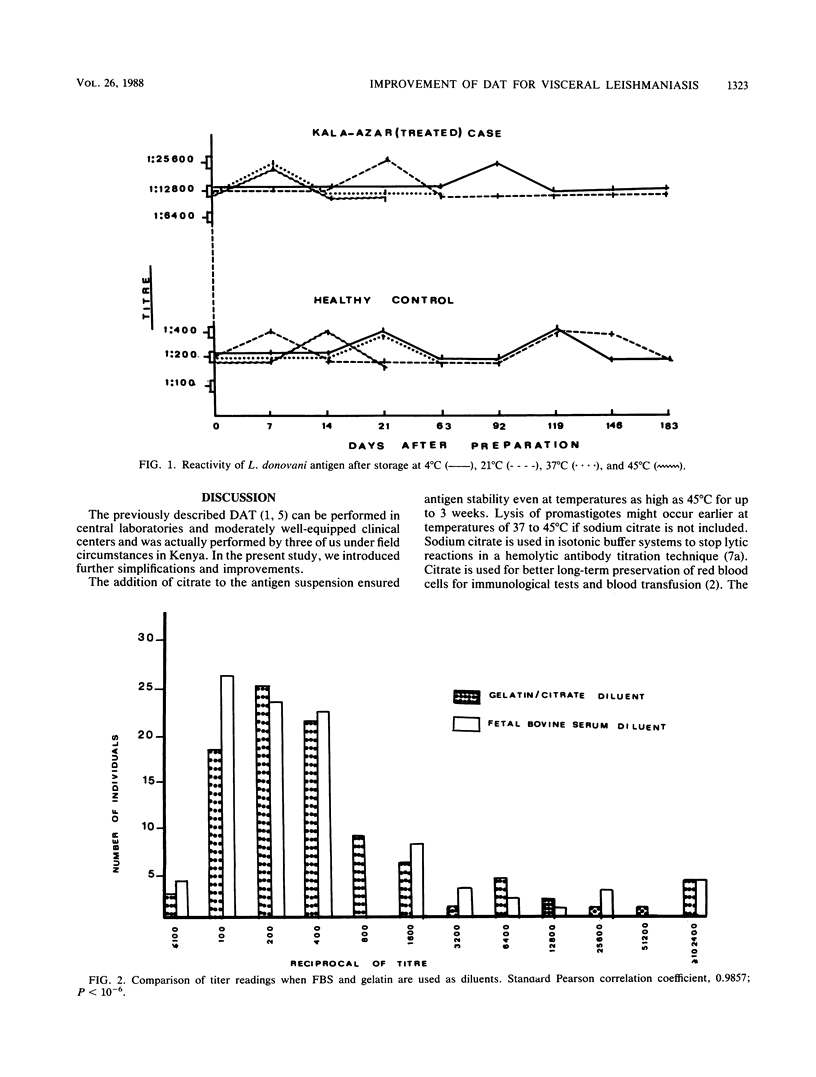
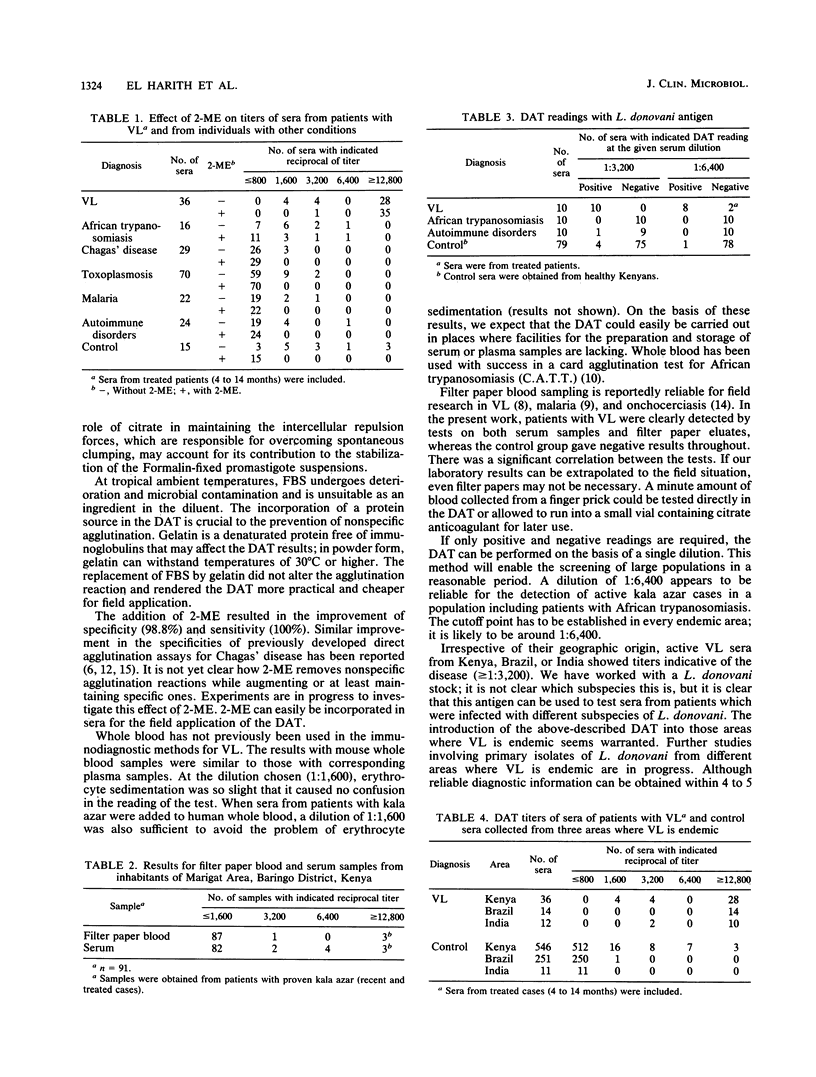
To increase the potential for the wide-scale application of our direct agglutination test for visceral leishmaniasis, modifications in the components and procedures were introduced. Supplementation with 0.056 M citrate of the suspension medium stabilized the antigen for 9 weeks at 37 degrees C. To circumvent the need for cooling systems in the field, 0.2% (wt/vol) gelatin was added to the serum diluent instead of fetal bovine serum, with reliable results. Specificity and sensitivity were improved by the incorporation of 0.1 M 2-mercaptoethanol in samples with borderline titers. The test could be performed on samples of whole blood; thus the difficulties of preparation and storage of serum, plasma, or filter paper blood are avoided. For mass screening programs, a single serum dilution of 1:6,400 could be employed, contributing to a further reduction in test expenses. Sera from different geographical areas showed equal reactivities in this direct agglutination test despite the nonhomologous Leishmania donovani antigens used.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade C. R., Silva O. A., Andrade P. P., Kolk A. H., Harith A. E. A direct agglutination test discriminative toward Chagas' disease for the diagnosis of visceral leishmaniasis in Brazil: preliminary results. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):457–459. doi: 10.1016/s0769-2625(87)80056-9. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. A monophasic medium for cultivating Leishmania donovani in large numbers. J Parasitol. 1972 Aug;58(4):847–848. [PubMed] [Google Scholar]
- Harith A. E., Kolk A. H., Kager P. A., Leeuwenburg J., Faber F. J., Muigai R., Kiugu S., Laarman J. J. Evaluation of a newly developed direct agglutination test (DAT) for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis: comparison with IFAT and ELISA. Trans R Soc Trop Med Hyg. 1987;81(4):603–606. doi: 10.1016/0035-9203(87)90423-8. [DOI] [PubMed] [Google Scholar]
- Harith A. E., Kolk A. H., Kager P. A., Leeuwenburg J., Muigai R., Kiugu S., Kiugu S., Laarman J. J. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80(4):583–536. doi: 10.1016/0035-9203(86)90149-5. [DOI] [PubMed] [Google Scholar]
- Ho M., Leeuwenburg J., Mbugua G., Wamachi A., Voller A. An enzyme-linked immunosorbent assay (ELISA) for field diagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 1983 Sep;32(5):943–946. doi: 10.4269/ajtmh.1983.32.943. [DOI] [PubMed] [Google Scholar]
- Jahn A., Diesfeld H. J. Evaluation of a visually read ELISA for serodiagnosis and sero-epidemiological studies of kala-azar in the Baringo District, Kenya. Trans R Soc Trop Med Hyg. 1983;77(4):451–454. doi: 10.1016/0035-9203(83)90110-4. [DOI] [PubMed] [Google Scholar]
- Kagan I. G. Evaluation of the indirect hemagglutination test as an epidemiologic technique for malaria. Am J Trop Med Hyg. 1972 Sep;21(5):683–689. doi: 10.4269/ajtmh.1972.21.683. [DOI] [PubMed] [Google Scholar]
- Magnus E., Vervoort T., Van Meirvenne N. A card-agglutination test with stained trypanosomes (C.A.T.T.) for the serological diagnosis of T. B. gambiense trypanosomiasis. Ann Soc Belg Med Trop. 1978;58(3):169–176. [PubMed] [Google Scholar]
- Pappas M. G., Hajkowski R., Hockmeyer W. T. Dot enzyme-linked immunosorbent assay (Dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J Immunol Methods. 1983 Nov 11;64(1-2):205–214. doi: 10.1016/0022-1759(83)90399-x. [DOI] [PubMed] [Google Scholar]
- Peralta J. M., Magalhães T. C., Abreu L., Manigot D. A., Luquetti A., Dias J. C. The direct agglutination test for chronic Chagas's disease. The effect of pre-treatment of test samples with 2-mercaptoethanol. Trans R Soc Trop Med Hyg. 1981;75(5):695–698. doi: 10.1016/0035-9203(81)90152-8. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Tada I., Korenaga M., Shiwaku K., Ogunba E. O., Ufomadu G. O., Nwoke B. E. Specific serodiagnosis with adult Onchocerca volvulus antigen in an enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1987 Mar;36(2):383–386. doi: 10.4269/ajtmh.1987.36.383. [DOI] [PubMed] [Google Scholar]
- Vattuone N. H., Yanovsky J. F. Trypanosoma cruzi: agglutination activity of enzyme-treated epimastigotes. Exp Parasitol. 1971 Dec;30(3):349–355. doi: 10.1016/0014-4894(71)90098-1. [DOI] [PubMed] [Google Scholar]
- el Harith A., Laarman J. J., Minter-Goedbloed E., Kager P. A., Kolk A. H. Trypsin-treated and coomassie blue-stained epimastigote antigen in a microagglutination test for Chagas' disease. Am J Trop Med Hyg. 1987 Jul;37(1):66–71. doi: 10.4269/ajtmh.1987.37.66. [DOI] [PubMed] [Google Scholar]


