Abstract
In humans, a mutation in the tyrosyl-DNA phosphodiesterase (Tdp1) is responsible for the recessively inherited syndrome spinocerebellar ataxia with axonal neuropathy (SCAN1). Tdp1 is a well-conserved DNA repair enzyme, which processes modified 3′ phospho-DNA adducts in vitro. Here, we report that in the yeast Schizosaccharomyces pombe, tdp1 mutant cells progressively accumulate DNA damage and rapidly lose viability in a physiological G0/quiescent state. Remarkably, this effect is independent of topoisomerase I function. Moreover, we provide evidence that Tdp1, with the polynucleotide kinase (Pnk1), processes the same naturally occurring 3′-ends, produced from oxidative DNA damage in G0. We also found that one half of the dead cells lose their nuclear DNA. Nuclear DNA degradation is genetically programmed and mainly depends on the two DNA damage checkpoint responses, ATM/Tel1 and ATR/Rad3, reminiscent to programmed cell death. Diminishing the respiration rate or treating cells with a low concentration of antioxidants rescues the quiescent tdp1 mutant cells. These findings suggest that mitochondrial respiration causes neuronal cell death in the SCAN1 syndrome and in other neurological disorders.
Keywords: apoptosis, ATM/Tel1, fission yeast, SSB, Tdp1
Introduction
Single-strand breaks (SSBs) are a physiological common source of DNA lesions. They are often associated with ‘dirty' ends and hinder essential biological processes. Endogenous reactive oxygen species (ROS), such as hydroxyl radicals and superoxide, are natural by products and if not rapidly neutralized, they may chemically attack the DNA and induce SSBs with 3′-phosphate or 3′-phosphoglycolate termini. Topoisomerase I (Top I) also introduces transient SSBs to relax the DNA during DNA replication and transcription. If a DNA polymerase or RNA polymerase encounters the DNA–Top I intermediate complex before DNA religation, then Top1 remains covalently trapped to the 3′-ends of the DNA breaks (Pommier et al, 2003).
Cells have evolved several specialized and partially overlapping efficient DNA repair processes to handle a broad range of SSB DNA lesions. However, it was estimated that about 1% of the SSBs escape from the DNA repair machineries and are transformed into double-strand breaks (DSBs) during DNA replication (Vilenchik and Knudson, 2003). Such DSBs are efficiently repaired by the homologous recombination (HR) machinery and errors in this process can be detrimental to cell viability or contribute to genetic instability. (Vilenchik and Knudson, 2003; Roseaulin et al, 2008). Taken together, it appears that SSB production and their repair are two critical parameters for genetic stability.
Tyrosyl-DNA phosphodiesterase 1 (TDP1) is a well-conserved SSB repair enzyme (Pouliot et al, 1999) cleaving in vitro the phosphodiester bond linking Top 1 to the 3′-end of DNA ends (Yang et al, 1996; Pouliot et al, 2001; Inamdar et al, 2002; Liu et al, 2002; Interthal et al, 2005b). Additionally, Tdp1 hydrolyses a variety of 3′ lesions, including 3′-phosphoglycolate (Interthal et al, 2001, 2005a; Debethune et al, 2002; Inamdar et al, 2002) a DNA adduct produced by H2O2, the drug bleomycin (BLM) and ionizing radiation. Following the Tdp1 reaction, a 3′-phosphate and a 5′-hydroxyl remain at the ends of the SSB, which are further processed by the polynucleotide kinase 3′ phosphatase (PNKP), restoring the conventional 3′-hydroxyl and 5′-phosphate DNA ends (Vance and Wilson, 2001). In mammals, Tdp1 interacts with ligase III and in concert with PNKP, the scaffold protein XRCC1 and the SSB sensor PARP1, repair the SSBs (Plo et al, 2003; El-Khamisy et al, 2005).
Consistent with this, lymphoblastoid cell lines from spinocerebellar ataxia with axonal neuropathy 1 (SCAN1) patients and budding yeast cells, mutated in tdp1, are sensitive to the anticancer drug camptothecin (CPT), which specifically traps Topo1 on DNA (Pouliot et al, 1999; Vance and Wilson, 2002; El-Khamisy et al, 2005; Miao et al, 2006). Furthermore, SCAN1 mutant cells are sensitive to H2O2, and low doses of ionizing radiation and cellular extracts from these cells were found deficient in the removal of 3′-phosphoglycolate adducts (Zhou et al, 2005; El-Khamisy and Caldecott, 2007). Current models propose that physiological Topo1 or ROS-induced SSB is responsible for neuronal dysfunction in the absence of the DNA end processing enzyme, Tdp1, but there is as yet no conclusive evidence for this hypothesis (El-Khamisy and Caldecott, 2007; Rass et al, 2007).
Although DSBs and blocked replication forks are known to alert the DNA damage checkpoints, including the ataxia telangiectasia mutated (ATM or Tel1 in Schizosaccharomyces pombe) and ATM- and Rad3-related (ATR or Rad3 in S. pombe) checkpoint kinases (Bartek and Lukas, 2007; Lavin, 2008), little is known about whether and how simple lesions, such as SSBs, alert the DNA damage checkpoints. However, it was recently reported in human cells that XRCC1 is phosphorylated in an ATM-dependent manner, suggesting that SSBs can also trigger checkpoint responses in G1 (Chou et al, 2008).
In this study, we studied the function of tdp1 mutant cells in the fission yeast, S. pombe, in vegetative and quiescent states. We further analysed the impact of CPT, oxidative stress and cellular respiration on the viability of the tdp1Δ and pnk1Δ mutant strains in the physiological quiescent conditions, a state relevant for SCAN1. We also considered the role of the Rad3/ATR and Tel1/ATM DNA damage checkpoint kinases in the phenotypes observed. This study shows that tdp1Δ mutant cells progressively accumulate unrepaired oxidative DNA damage, which ultimately induces an ATM/Tel1-dependent apoptotic-like programme in G0.
Results
Tdp1 mutant cells die in the quiescent state with an apoptotic-like phenotype
To evaluate the role of Tdp1 in DNA repair in fission yeast, we constructed a tdp1Δ-null mutant and analysed chronic sensitivity to CPT. We found that tdp1Δ cells grow as fast as wild-type cells but are sensitive to CPT (Figure 1A). Then, we compared the CPT sensitivity of tdp1Δ mutants in the presence or absence of the top1 gene. The CPT resistance of the top1Δ and tdp1Δ top1Δ mutant strains confirmed the importance of Tdp1 in the repair of Top1-induced lesions (Figure 1A) (Pommier, 2006).
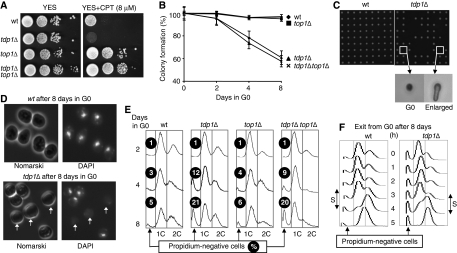
Figure 1.
Mortality of tdp1Δ cells in the quiescent state. (A) Spot assay showing that the tdp1Δ strain is sensitive to camptothecin in a topoisomerase I-dependent manner. Ten-fold serial dilutions of wild-type, tdp1Δ, top1Δ and tdp1Δ top1Δ cells grown on YES (rich) media and spotted on YES and YES + 8 μM CPT agar plates. (B) Loss of viability of the tdp1Δ strain; in the quiescent state is topoisomerase I independent. Plating efficiencies of quiescent wild-type, tdp1Δ, top1Δ and tdp1Δ top1Δ strains (more than 500 colonies are counted for each strain, n>3 independent experiments). (C) The 8-day starved wild-type and tdp1Δ cells were re-seeded on YES plates by micromanipulation and allowed to grow for 3 days at 32°C. Microscopic images show, that among the tdp1Δ cells unable to form colonies, about one-half are dead and the other half are enlarged but never divide. (D) DAPI staining of the 8-day quiescent wild-type and tdp1Δ cells (white arrows indicate the DAPI-negative tdp1Δ cells). (E) FACS profiles of wild-type, tdp1Δ, top1Δ and tdp1Δ top1Δ strains as a function of time in G0 (2-, 4- and 8-day starved cells) and quantification of the accumulation of propidium iodide-negative cells. The percentage (%) is indicated. (F) FACS profiles of wild-type and tdp1Δ strains, during the five first hours of vegetative cycle re-entry, show that the tdp1Δ cells enter into the first S phase. Note that the percentage of propidium iodide-negative cells remains constant during this period.
Next, we attempted to understand, why in humans, TDP1 dysfunction specifically affects non-dividing neuronal cells and allows normal development. Notably, it was recently shown that SCAN1 lymphoblastoid cells blocked in S phase, to mimic the postmitotic state, are also impaired in SSB repair following CPT treatments, supporting the idea that Tdp1 has an important role in SSB repair, independently of DNA replication (El-Khamisy et al, 2005; Miao et al, 2006). To evaluate the contribution of Tdp1 in non-dividing S. pombe cells, we grew cells in nitrogen starvation medium, which arrests cells in the G0/quiescent state (Su et al, 1996). In this state, wild-type cells are metabolically active, remain fully viable for several weeks and are able to respond and efficiently repair DNA damage (Mochida and Yanagida, 2006; Shimanuki et al, 2007).
We assessed cell viability by counting the number of colonies as a function of time in nitrogen-starved conditions. Only about 60% of the tdp1Δ mutant cells remained viable after 8 days in G0, whereas nearly all wild-type cells survived (Figure 1B). Microscopic observation of wild-type and tdp1Δ cells starved for 8 days and then re-seeded on rich media confirmed that about 40% of the tdp1Δ cells could not form colonies and remained as single cells after 3 days of incubation. Among the non-dividing tdp1Δ cells, about one-half were enlarged, indicating an attempt to re-enter into the vegetative cycle, whereas the other half did not change their appearance, suggesting that they died during the G0 period (Figure 1C). Because Tdp1 is involved in SSB repair, we analysed the nuclear DNA by fluorescence microscopy of the wild-type and tdp1Δ cells by 4′,6′-diamino-2-phenylindole (DAPI) staining and show that DAPI-negative cells accumulate in the tdp1Δ population (Figure 1D). To quantify this observation, we examined the cellular DNA content by FACS. After 2 days of nitrogen starvation, the FACS profile of the wild-type and tdp1Δ strains is not different (Figure 1E). However, increasing the incubation time in G0 (4 and 8 days), which does not affect the wild-type FACS profiles, revealed a new population of propidium iodide-negative tdp1Δ cells that reaches 21% after 8 days in G0 (Figure 1E).
The loss of viability in G0 is independent of Top I
To assess the role of Top I in fission yeast death, we analysed the viability and FACS profile of the starved top1Δ and tdp1Δ top1Δ mutant strains (Figure 1B and E). We show that in the presence or absence of Top I, the tdp1Δ strain accumulates dead cells and propidium iodide-negative cells in G0, whereas the single top1Δ mutant strain remains viable. Altogether, these results demonstrate that Top 1 is not responsible for the loss of viability observed in the quiescent tdp1Δ fission yeast mutant strain. Following nutrient addition and regardless of the anucleated cell fraction, the FACS profiles of the 8-day starved wild-type and tdp1Δ cells show that both strains were able to replicate their DNA (Figure 1F).
Tdp1 mutant cells accumulate DNA damage in G0
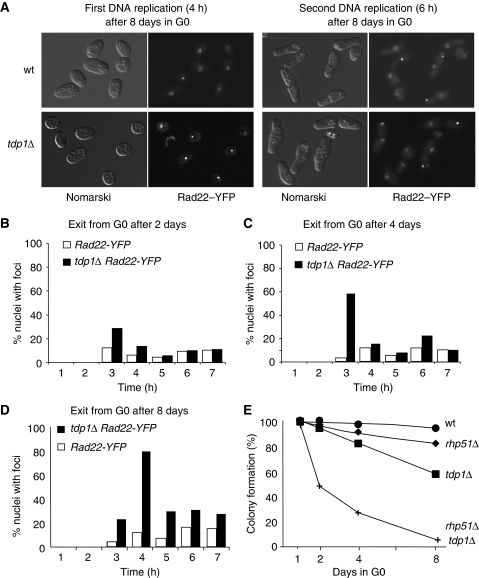
Given that Tdp1 is involved in SSB repair and that the HR process functions as a backup pathway during DNA replication in the absence of Tdp1 (Pouliot et al, 2001; Vance and Wilson, 2002; El-Khamisy and Caldecott, 2007), we next analysed the re-entry into the vegetative phase of starved wild-type and tdp1Δ cells for spontaneous formation of Rad22–YFP (the homologue of RAD52 in S. pombe) foci, a marker for the HR repair process (Lisby et al, 2001; Meister et al, 2003). Following addition of rich medium, the G0 cells re-entered into the vegetative cycle synchronously (Su et al, 1996) and the number of Rad22–YFP foci per cell as a function of time was determined by microscopic analysis (Figure 2A). The first DNA replication is readily assessed by FACS analysis and the second DNA replication coincides with the formation of the first septum of division, prior to the first cell division (data not shown). For the wild-type strain, extending the G0 incubation period had little effect on the proportion of nuclei containing Rad22–YFP foci during the first and the second DNA replication periods. However, the tdp1Δ strain exhibited a progressive and dramatic increase in the proportion of nuclei containing Rad22–YFP foci during the first DNA replication (Figure 2A–D). During the second DNA replication (time point 6 and 7 h in Figure 2D), the percentage of tdp1Δ cells with Rad22–YFP foci consistently decreased, although it did not reach the level observed in wild-type cells, indicating that many, but not all of the lesions, are repaired between the first and second DNA replication periods. The pivotal role of the HR process in DNA repair in the tdp1 mutant was confirmed by the rapid and extensive death of the tdp1Δ rhp51Δ double mutant strain (Figure 2E). Because one Rad22–YFP focus can recruit more than one DSB (Lisby et al, 2001), the number of foci per cell underestimates the real level of unrepaired DNA lesions, which might overload the capacity of the HR machinery to repair all of the damage. However, the lack of cancer predisposition in SCAN1 (Takashima et al, 2002) suggests that most of the lesions are repaired without gross genetic alterations.
Figure 2.
tdp1Δ cells in the quiescent state accumulate unrepaired DNA lesions. (A) Rad22RAD52–YFP foci of tdp1Δ cells dramatically increases during the first replication of cell cycle re-entry. Fluorescence microscopy of 8-day-starved wild-type and tdp1Δ cells during the first and second DNA replication periods after cell cycle re-entry. (B–D) Quantification of the Rad22–YFP foci of 2-, 4- and 8-day-starved wild-type and tdp1Δ cells during cell cycle re-entry (more than 500 cells are counted for each time point; n=2 independent experiments). (E) The DNA damage accumulating in quiescent tdp1Δ cells is repaired by the HR machinery. Plating efficiencies of quiescent wild-type, rhp51Δ, tdp1Δ and rhp51Δ tdp1Δ strains (more than 200 colonies are counted for each strain; n>2 independent experiments).
DNA damage checkpoints control the apoptotic-like cellular response
In an attempt to determine whether the nuclear DNA degradation observed for tdp1Δ cells is genetically controlled, we designed experiments to test the role of the DNA damage checkpoints. Wild-type and tdp1Δ strains have been treated with 2 mM caffeine in G0, a known inhibitor of the phosphoinositide 3-kinase-like kinases involved in cell cycle arrest, DNA repair and apoptosis in higher eukaryotes. Both the wild-type and tdp1Δ strains, when treated with 2 mM caffeine, exhibit a similar decrease in viability in the G0 state (Figure 3A and data not shown). When the DNA content of these strains is analysed by FACS, after 8 days in G0, we observed that caffeine abolished DNA degradation of quiescent tdp1Δ cells (Figure 3B).
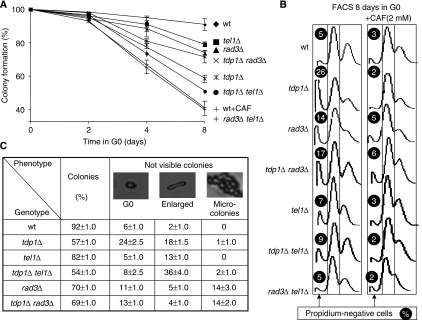
Figure 3.
Tel1 and Rad3 regulate nuclear DNA degradation. (A) Viability of the DNA damage checkpoints rad3Δ and tel11Δ in the presence or absence of tdp1Δ. More than 200 G0 cells from each strain are plated in rich media after 2, 4 and 8 days in G0 conditions and counted after 3 days at 33°C. The wild-type strain is also incubated with 2 mM caffeine (wt+CAF). (B) Tel1 and Rad3 control the nuclear DNA degradation in G0. The DNA content for each mutant after 8 days in G0, with and without caffeine (2 mM CAF) is analysed by FACS. The proportion of propidium iodide-negative cells is shown for each mutant, this experiment was repeated two times with similar results. (C) The 8-day-starved mutant cells, analysed in (B), were re-seeded on YES (rich) plates by micromanipulation and allowed to grow for 3 days at 32°C. Microscopic images show the proportion (%) of cells forming colonies, blocked in G0, enlarged or forming micro-colonies (containing less than 100 cells). More than 90 cells are analysed for each mutant; n>2 independent experiments.
As the DNA damage checkpoints (ATR/rad3 and ATM/tel1) are sensitive to caffeine, we directly addressed the role of the two checkpoint kinases with and without Tdp1 individually. We observed that the tel1Δ and tdp1Δ tel1Δ mutants are slightly more sensitive in G0 than the rad3Δ and tdp1Δ rad3Δ mutants, respectively (Figure 3A and C). We then followed by microscopic observation, the return to growth of the 8-day-starved mutant cells (Figure 3C). For the rad3Δ and tdp1Δ rad3Δ mutants, a new population of cells is able to form microcolonies (2–100 cells) and the individual cells from these colonies are not able to grow further, suggesting that they accumulate lethal chromosomal aberrations. These results are consistent with an important function of ATR/Rad3 in the S and G2/M DNA damage checkpoints, arresting the cells after S phase and before their first divisions (Figures 1F and 3C). For the tel1Δ mutant, compared with wild type, we observed a slight increase in the proportion of enlarged cells, which further increases in the tdp1Δ tel1Δ mutant concomitant with the decrease of the G0 dead cells (Figure 3C).
FACS analyses of these checkpoint mutants after 8 days in G0 indicate that both rad3Δ and tel1Δ mutants accumulate propidium iodide negative cells (Figure 3B). In contrast, this feature is abrogated in the rad3Δ tel1Δ double mutant strain or after caffeine treatment (Figure 3A and B). This result indicates that both kinases control nuclear DNA degradation in G0, and participate in the DNA repair of physiological lesions. This dual role is consistent with the reduced proportion of propidium negative tdp1Δ cells in either checkpoint mutant background (Figure 3B). However, the more pronounced decrease in propidium iodide signals found for tdp1Δ tel1Δ than for tdp1Δ rad3Δ mutant strains, indicates that Tel1 is having a predominant role in the G0 death response. Therefore, this new cell death programme appears genetically determined and functional during the G0 state.
Role of Tdp1 and Pnk1 in the oxidative DNA damage repair pathway
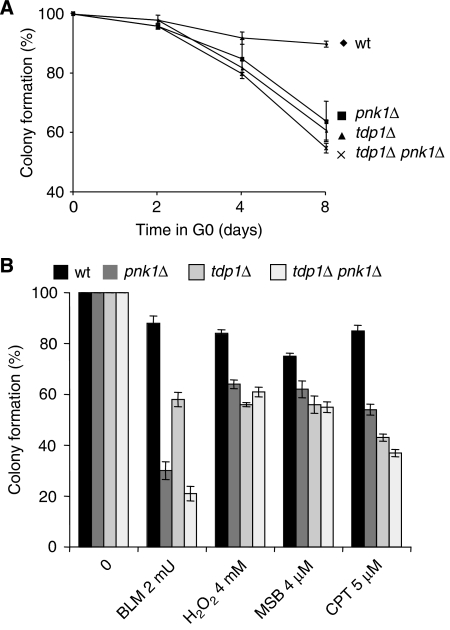
Given the limitation of our approach to directly identify the DNA lesion(s) accumulating in the absence of Tdp1, we have compared the relative contribution of Tdp1 and Pnk1, the DNA kinase/phosphatase of fission yeast (Meijer et al, 2002), in the quiescent state and after treatments inducing DNA breaks. We show that the viabilities of the tdp1Δ, pnk1Δ single mutants and tdp1Δ pnk1Δ double mutant in G0 are very similar, consistent with both enzymes sequentially processing the same type of DNA lesions (see Introduction). We then compared the cellular viabilities of these mutant strains following treatments with agents inducing SSBs, such as CPT, hydrogen peroxide (H2O2), menadione sodium bisulphite (MSB a generator of superoxide) or with BLM, a radiomimetic drug inducing DSBs. As mentioned above, CPT induces 3′-Top I termini, whereas H2O2 or MSB produces SSBs with 3′-end DNA lesions, including 3′-phosphoglycolates, 3′-abasic sites and 3′-phosphates. A similar relative sensitivity was found in G0 (Figure 4A) and following CPT, H2O2 and MSB treatments, for the single and the double mutants, indicating that the physiological and induced DNA lesions are similarly processed by both enzymes (Figure 4B). When the same mutants are treated with BLM, known to produce DSBs with 3′-phosphoglycolate ends (Povirk, 1996; Demple and DeMott, 2002), such as ionizing radiation (Strumberg et al, 2000), we found that the tdp1Δ mutant is less sensitive than the pnk1Δ and tdp1Δ pnk1Δ mutants (Figure 4B). This result indicates a predominant processing role for Pnk1 in such breaks.
Figure 4.
Quiescent tdp1Δ cells accumulate SSBs with 3′-phosphoglycolate termini. (A) Viability of tdp1Δ and pnk1Δ single and double mutants in G0. More than 200 G0 cells from each strain are plated in rich media after 2, 4 and 8 days in G0 conditions and counted after 3 days at 33°C. (B) Sensibility of tdp1Δ and pnk1Δ single and double mutants following treatment with bleomycin (BLM, 2mU), hydrogen peroxide (H2O2, 4 mM), menadione sodium bisulphite (MSB, 4 mM) or camptothecin (CPT, 5 mM). Each drug concentration sensitized the wild-type strain similarly. The strains are incubated for 24 h in G0 and then treated for 24 h before being plated on rich media for 3 days at 32°C and counted colonies are reported in percentage. More than 200 colonies are counted for each condition and the experiments have been repeated two times independently.
Oxidative DNA damages are chemically complex, as most of the atoms comprising DNA are subject to attack, which can undergo secondary chemical rearrangements. The end products are commonly described as 3′-phosphoglycolates, 3′-abasic sites and 3′-phosphates. Tdp1 is described as a general 3′-DNA phosphodiesterase and was shown to remove a number of the naturally occurring 3′-mononucleosides, but not 3′-phosphates (Interthal et al, 2005a; Dexheimer et al, 2008). Our data show that tdp1 and pnk1 single and double mutants are equally sensitive in G0 and exhibit the same sensitivity to H2O2 (and MSB). Altogether, we propose that the 3′-phosphate lesion is proportionally a minor physiological or H2O2-induced oxidative DNA damage and that Tdp1 and Pnk1 are sequentially processing 3′-mononucleosides at SSBs.
Tdp1 protects against physiological oxidative DNA damage in G0
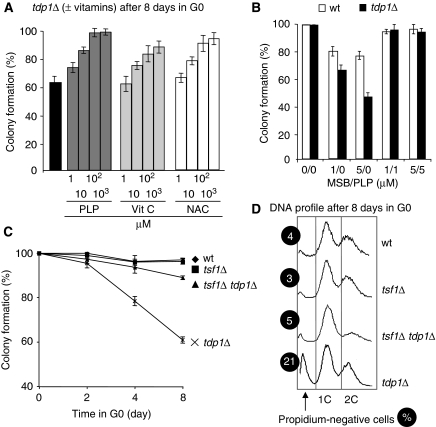
The central role of oxygen in metabolism, together with the high level of oxygen consumption of brain cells and their post-mitotic nature, has led to the hypothesis that cumulative oxidative DNA lesions might be responsible for neuronal cell death, as observed in SCAN1 patients (El-Khamisy et al, 2005; El-Khamisy and Caldecott, 2007; Rass et al, 2007). Great importance has been attributed to vitamins as antioxidants in the prevention and treatment of conditions associated with oxidative stress for many years. We found that 100 μM of vitamin B6 derivative, pyridoxal 5′-phosphate (PLP) protected the 8-day-starved tdp1Δ mutant cells, more efficiently than vitamin C or N-acetyl-L-cysteine antioxidants (Figure 5A). When the cells were simultaneously treated, in the same conditions as above, with an equimolar ratio of MSB to PLP, viability was fully maintained in both strains (Figure 5B). Because PLP does not react with MSB in liquid medium at pH 5.5 or 7.0 (Chumnantana et al, 2005), we conclude that the antioxidant effect of PLP is intracellular.
Figure 5.
Mitochondrial-generated ROS induce the death of tdp1Δ cells. (A) Vitamin B6 (or PLP), vitamin C (vit C) and N-acetyl-L-cysteine (NAC) protect the quiescent tdp1Δ cells. The strains are incubated for 24 h in G0 and then treated for 24 h with pyridoxal 5′-phosphate (PLP), vitamin C or N-acetyl-L-cysteine before being plated and incubated for 3 days. (B) PLP counteracts the deleterious effects of the superoxide generator MSB. PLP was introduced before MSB in the culture. The procedure is the same as above (for (A, B), more than 200 colonies are counted for each condition and the experiments have been repeated three times independently). (C) The low respiration rate observed in the tsf1Δ mutant protects the quiescent tdp1Δ cells. Plating efficiencies after 2, 4 and 8 days in G0 of quiescent wild-type, tdp1Δ, tsf1Δ and tsf1Δ tdp1Δ strains (more than 500 colonies are counted for each strain, n=3 independent experiments). (D) FACS analyses of the 8-day-starved tsf1Δ and tsf1Δ tdp1Δ mutant strains showing the lack of propidium iodide-negative cells. The percentage (%) of propidium iodide-negative cells is indicated for each strain.
As mitochondrial respiration by default generates endogenous ROS, we assessed the contribution of mitochondria for the death of cells lacking Tdp1 by using the tsf1Δ strain (Chiron et al, 2005). The tsf1 gene encodes the mitochondrial translational nucleotide exchange EF-Ts protein. S. pombe is a petite-negative yeast and loss of its mitochondrial DNA or translational machinery is lethal. However, the absence of Tsf1 allows residual protein synthesis, low respiration, healthy growing cells in glucose and no flocculation phenotypes. In addition, tsf1Δ cells contain a wild-type amount of mitochondrial DNA (Chiron et al, 2005). Plating experiments show that the tsf1Δ strain, and more importantly, the tdp1Δ tsf1Δ double-mutant strain, maintains almost full viability and do not accumulate propidium iodide-negative cells in G0 (Figure 5C and D). This result clearly shows that low cellular respiration levels protect the tdp1Δ mutant cells and indicates that the production of endogenous ROS by-products, generated by mitochondria, is a major cause for the accumulation of DNA lesions in the absence of Tdp1 (Zhou et al, 2005; Interthal et al, 2005b).
Discussion
Because only few unrepaired DNA lesions are sufficient to kill S. pombe, it becomes very difficult to directly identify the physiological substrate for Tdp1. We indirectly asked this question by comparing the relative sensitivity of tdp1Δ and pnk1Δ single and double mutants for known DNA lesions. Our results indicate a similar sensibility of tdp1Δ, pnk1Δ and tdp1Δ pnk1Δ mutants after prolonged incubation in G0 or after H2O2, MSB or CPT treatments, but not after BLM treatment. However, Top I is clearly not involved in the physiological death observed after several days in G0, despite the fact that CPT treatment sensitized tdp1Δ mutant cells during vegetative and quiescent states. Consistent with this, two recent reports have shown the hypersensitivity to CPT and BLM of Tdp1−/− mice, but they could not reproduce the ataxia phenotype observed in SCAN1 patients, and observed only a modest age-dependent decrease in cerebellar size, with several interpretations proposed therein (Hirano et al, 2007; Katyal et al, 2007). Our model system allowed us to genetically test the impact of Top I in combination with Tdp1 in G0, ruling out its putative role, at least in S. pombe. Moreover, we were able to reveal a specific sensitivity in G0 in the absence of Tdp1, in physiological conditions, during a 1-week period, instead of 2 years in mouse and 15 years in humans (Takashima et al, 2002). This is probably due to a high respiration metabolism and compact haploid genome (Wood et al, 2002), in which each essential gene is present in one copy and is coded by less than 3 Mb in this yeast. Even though the main DNA repair enzymes are well conserved between yeast and mammals, some important factors involved in DNA end processing and SSB repair, such as PARP1, XRCC1, Polβ and ligase 3, are not found in simple eukaryotes. This might indicate the need to increase the rate of SSB repair to cope with the increasing size of the genomic message and the complexity of cellular metabolism, organization and developmental fate arising during evolution (Caldecott, 2008).
It is well recognized that ATM/Tel1 checkpoint responses are triggered by DSBs, thus maintaining the integrity of the genome, minimizing the risk of cancer and neurodegeneration (Lavin, 2008) and consequently one concern is whether SSBs or DSBs are accumulating in G0 in the absence of Tdp1. The spectrum of drug sensitivities discussed above for the tdp1Δ mutant argues that the initial lesions are SSBs, but we cannot exclude that such SSBs are slowly transformed into gaps and ultimately into DSBs, by some unknown process. However, there is growing evidence that the initial stimulus for ATM activation correlates with relaxation of chromatin structure. For examples, low doses of irradiation, oxidative stress or agents altering the chromatin structure, not known to introduce DSBs, activate ATM and stabilize the tumour suppressor p53, but fail to activate other substrates, including SMC1 and NBS1, involved in DSB repair (Lavin, 2008). Moreover, it was recently reported that oxidative damage activates the ATM/Tel1 pathway independently of DSB formation (Bencokova et al, 2009; Chou et al, 2008), a phenomenon still poorly understood in higher eukaryotes (Goodarzi et al, 2008; Soutoglou and Misteli, 2008).
The nuclear DNA degradation results observed in the individual (rad3Δ or tel1Δ) checkpoint mutants, suggested that each checkpoint mutant accumulates lesions, which are detected by the remaining checkpoint. In the absence of both checkpoints, the number of lesions presumably increases, as observed by the loss of viability, but without a nuclear degradation response. In the absence of Tdp1, the unrepaired lesions accumulated in an age-dependent manner and both checkpoints trigger DNA degradation. When either checkpoint is absent, in tdp1Δ strain, the amplitude of DNA degradation diminished correspondingly, and reached a baseline level in the presence of caffeine. The cellular aspect of the cells during their re-entry in the vegetative state provides some additional information. When we compared the tdp1Δ and tdp1Δ tel1Δ mutants, we observed that the double mutant decreases the proportion of small G0 cells, concomitantly increasing the proportion of enlarged cells (Figure 3C). This result indicates that the tdp1Δ tel1Δ cells are not dead and are still able to re-enter into the vegetative cycle, albeit not successfully, despite the proficient ATR/Rad3 checkpoint and HR processes. Taken together, these results suggest that the ATR and ATM checkpoints are partially redundant and that the ATM/Tel1 checkpoint is the main candidate to monitor the threshold and severity of oxidative DNA damage, to trigger cell death by nuclear DNA degradation during quiescence. How the repair complexes sense damaged DNA and activate the ATM/Tel1 checkpoint response are poorly understood and several models have been proposed, which include the DNA lesions by themselves, a change in the chromatin structure or transcription abortion (Ljungman and Lane, 2004; Soutoglou and Misteli, 2008). In addition, although the ATR/Rad3 pathways during S and G2 are quite well understood in S. pombe, involving the Chk1 and Cds1 kinases, the specific downstream effectors of ATM/Tel1, with the exception of the Nbs1 protein, are still unknown (Subramanian et al, 2008). The absence of the tumour suppressor p53 protein in simple eukaryotes, and particularly in S. pombe, reveals a novel response to DNA damage and further analysis of the downstream targets of ATM/Tel1 will hopefully shed light on this intriguing cell death programme (Shimanuki et al, 2007).
In conclusion, the natural hypersensitivity of the tdp1Δ fission yeast cells in quiescent conditions is due to the accumulation of oxidative unrepaired SSBs, and lowering respiration or treatment with the vitamin B6 derivative, PLP, abrogates this sensitivity. We consistently observed that vitamin B6 showed a better protective effect in the absence of Tdp1 than the other antioxidant molecules used in this study and a similar relative effect was reported during vegetative growth upon exogenous oxidant treatments (Chumnantana et al, 2005). We do not know the reason for this, but we propose that PLP might be specifically imported inside the cell to function directly as an antioxidant. Moreover, PLP is built from ribose 5-phosphate, a precursor common with other important pathways and is the main cofactor of enzymes involved in amino-acid metabolism, a critical function in G0 (Amadasi et al, 2007).
This study may impact the prevention or treatment of human autosomal recessive ataxias, such as the SCAN1 syndrome (Rass et al, 2007) and other neurodegenerative diseases linked to defects in genes influencing physiological levels of ROS (Bohr et al, 2007; Navarro and Boveris, 2007). We also showed that the S. pombe quiescent state (Su et al, 1996) provides a model to revisit the roles of the different DNA repair pathways in non-dividing physiological conditions, a poorly understood physiological state, and to establish a new hierarchy among them. Relevant to cancer, Tdp1 overexpression is commonly observed in primary non-small cell lung cancers (Liu et al, 2007; Rosell et al, 2007), indicating an increased DNA repair requirement, potentially due to high oxidative stress in these frequent solid tumours (Dexheimer et al, 2008).
Materials and methods
Strains and growth conditions
Wild-type and mutant haploid heterothallic strains are all isogenic and prototrophic. The tdp1Δ mutant was constructed by substituting the tdp1 gene by the kanamycin gene by one-step homologous insertion. The strains are grown in minimal well-defined medium (MM) (Moreno et al, 1991) containing 3 or 5% glucose. Yeast cells are grown in MM to a density of 6 × 106 cells/ml at 32°C, harvested by centrifugation (3000 r.p.m./5 min) washed in MM-N (without nitrogen) and re-suspended in MM-N at a density of 106 cells/ml at 32°C, reaching after two rounds of cell division 4 × 106 cells/ml. This density of cell bodies is stable for several weeks and was used as a source of G0 cells. The re-entry into the vegetative cycle is performed by replacing the MM-N by one volume of fresh rich medium (YES). Samples were taken before or after the addition of YES at various incubation times for assays. Vitamins (B6, PLP and C) and drugs (BLM, H2O2, CPT and MSB) were obtained from Sigma-Aldrich and added to yeast cultures in the dark for the indicated time periods.
Cell viability, FACS analysis and fluorescence microscopy
Cell density was determined by the particle counter Z1 (Beckman). Cell viability was determined by plating on rich (YES) plates, and after 3 days of incubation at 32°C, colonies were counted. Viability is expressed as a fraction (%) of colonies divided by particles plated. Haploid S. pombe cells spend more time in G2 in growing conditions and the DNA content of a cell is 2C. In nitrogen-starved conditions, cells accumulate in G1 after two cell divisions with a 1C DNA content. For flow cytometry analysis (FACS), 4 × 106 cells (1 ml) were harvested and resuspended in 1 ml of cold 70% ethanol overnight at 4°C. To improve the propidium iodide staining, after removal of the ethanol, the cells are resuspended in 1 ml of 0.1 N HCl containing 2 mg/ml pepsin for 1 h at room temperature, washed in 1 ml of 50 mM sodium citrate (pH 7.0) and resuspended in 0.5 ml of 50 mM sodium citrate buffer, containing 0.1 mg/ml RNAase A for 1 h at 37°C. Next, we added 0.5 ml of 50 mM sodium citrate, containing 4 μg/ml propidium iodide followed by moderate sonication before FACS analysis. For the Rad22–YFP foci analysis, following replacement in rich medium, samples were taken at various time intervals and directly observed by fluorescence microscopy. For DAPI staining, the cells are fixed with 70% cold ethanol, washed in 1 ml of 50 mM sodium citrate (pH 7.0), resuspended in 0.5 ml of 50 mM sodium citrate buffer containing DAPI and observed with a fluorescence microscope using a UV filter. All of the assays were independently repeated several times (at least two times).
Acknowledgments
We thank A Holmes, J Weitzman, R Young and C Mann for a critical reading of the paper, as well as all members of the Arcangioli laboratory, C Miled and N Bonnefoy for help and encouragement. This study was supported by grants from the FRM and CSC to SBH and ANR-06-BLAN-0271 to BA.
References
- Amadasi A, Bertoldi M, Contestabile R, Bettati S, Cellini B, di Salvo ML, Borri-Voltattorni C, Bossa F, Mozzarelli A (2007) Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr Med Chem 14: 1291–1324 [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19: 238–245 [DOI] [PubMed] [Google Scholar]
- Bencokova Z, Kaufmann MR, Pires IM, Lecane PS, Giaccia AJ, Hammond EM (2009) ATM activation and signalling under hypoxic conditions. Mol Cell Biol 29: 526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA, Ottersen OP, Tonjum T (2007) Genome instability and DNA repair in brain, ageing and neurological disease. Neuroscience 145: 1183–1186 [DOI] [PubMed] [Google Scholar]
- Caldecott KW (2008) Single-strand break repair and genetic disease. Nat Rev Genet 9: 619–631 [DOI] [PubMed] [Google Scholar]
- Chiron S, Suleau A, Bonnefoy N (2005) Mitochondrial translation: elongation factor tu is essential in fission yeast and depends on an exchange factor conserved in humans but not in budding yeast. Genetics 169: 1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Wang HC, Wong FH, Ding SL, Wu PE, Shieh SY, Shen CY (2008) Chk2-dependent phosphorylation of XRCC1 in the DNA damage response promotes base excision repair. EMBO J 27: 3140–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumnantana R, Yokochi N, Yagi T (2005) Vitamin B6 compounds prevent the death of yeast cells due to menadione, a reactive oxygen generator. Biochim Biophys Acta 1722: 84–91 [DOI] [PubMed] [Google Scholar]
- Debethune L, Kohlhagen G, Grandas A, Pommier Y (2002) Processing of nucleopeptides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Res 30: 1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B, DeMott MS (2002) Dynamics and diversions in base excision DNA repair of oxidized abasic lesions. Oncogene 21: 8926–8934 [DOI] [PubMed] [Google Scholar]
- Dexheimer TS, Antony S, Marchand C, Pommier Y (2008) Tyrosyl-DNA phosphodiesterase as a target for anticancer therapy. Anticancer Agents Med Chem 8: 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khamisy SF, Caldecott KW (2007) DNA single-strand break repair and spinocerebellar ataxia with axonal neuropathy-1. Neuroscience 145: 1260–1266 [DOI] [PubMed] [Google Scholar]
- El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW (2005) Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature 434: 108–113 [DOI] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA (2008) ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 31: 167–177 [DOI] [PubMed] [Google Scholar]
- Hirano R, Interthal H, Huang C, Nakamura T, Deguchi K, Choi K, Bhattacharjee MB, Arimura K, Umehara F, Izumo S, Northrop JL, Salih MA, Inoue K, Armstrong DL, Champoux JJ, Takashima H, Boerkoel CF (2007) Spinocerebellar ataxia with axonal neuropathy: consequence of a Tdp1 recessive neomorphic mutation? EMBO J 26: 4732–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar KV, Pouliot JJ, Zhou T, Lees-Miller SP, Rasouli-Nia A, Povirk LF (2002) Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J Biol Chem 277: 27162–27168 [DOI] [PubMed] [Google Scholar]
- Interthal H, Chen HJ, Champoux JJ (2005a) Human Tdp1 cleaves a broad spectrum of substrates, including phosphoamide linkages. J Biol Chem 280: 36518–36528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interthal H, Chen HJ, Kehl-Fie TE, Zotzmann J, Leppard JB, Champoux JJ (2005b) SCAN1 mutant Tdp1 accumulates the enzyme—DNA intermediate and causes camptothecin hypersensitivity. EMBO J 24: 2224–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interthal H, Pouliot JJ, Champoux JJ (2001) The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc Natl Acad Sci USA 98: 12009–12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal S, El-Khamisy SF, Russell HR, Li Y, Ju L, Caldecott KW, McKinnon PJ (2007) TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J 26: 4720–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF (2008) Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9: 759–769 [DOI] [PubMed] [Google Scholar]
- Lisby M, Rothstein R, Mortensen UH (2001) Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci USA 98: 8276–8282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Pouliot JJ, Nash HA (2002) Repair of topoisomerase I covalent complexes in the absence of the tyrosyl-DNA phosphodiesterase Tdp1. Proc Natl Acad Sci USA 99: 14970–14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhou S, Begum S, Sidransky D, Westra WH, Brock M, Califano JA (2007) Increased expression and activity of repair genes TDP1 and XPF in non-small cell lung cancer. Lung Cancer 55: 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman M, Lane DP (2004) Transcription—guarding the genome by sensing DNA damage. Nat Rev Cancer 4: 727–737 [DOI] [PubMed] [Google Scholar]
- Meijer M, Karimi-Busheri F, Huang TY, Weinfeld M, Young D (2002) Pnk1, a DNA kinase/phosphatase required for normal response to DNA damage by gamma-radiation or camptothecin in Schizosaccharomyces pombe. J Biol Chem 277: 4050–4055 [DOI] [PubMed] [Google Scholar]
- Meister P, Poidevin M, Francesconi S, Tratner I, Zarzov P, Baldacci G (2003) Nuclear factories for signalling and repairing DNA double strand breaks in living fission yeast. Nucleic Acids Res 31: 5064–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao ZH, Agama K, Sordet O, Povirk L, Kohn KW, Pommier Y (2006) Hereditary ataxia SCAN1 cells are defective for the repair of transcription-dependent topoisomerase I cleavage complexes. DNA Repair (Amst) 5: 1489–1494 [DOI] [PubMed] [Google Scholar]
- Mochida S, Yanagida M (2006) Distinct modes of DNA damage response in S.pombe G0 and vegetative cells. Genes Cells 11: 13–27 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A (2007) The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292: C670–C686 [DOI] [PubMed] [Google Scholar]
- Plo I, Liao ZY, Barcelo JM, Kohlhagen G, Caldecott KW, Weinfeld M, Pommier Y (2003) Association of XRCC1 and tyrosyl DNA phosphodiesterase (Tdp1) for the repair of topoisomerase I-mediated DNA lesions. DNA Repair (Amst) 2: 1087–1100 [DOI] [PubMed] [Google Scholar]
- Pommier Y (2006) Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6: 789–802 [DOI] [PubMed] [Google Scholar]
- Pommier Y, Redon C, Rao VA, Seiler JA, Sordet O, Takemura H, Antony S, Meng L, Liao Z, Kohlhagen G, Zhang H, Kohn KW (2003) Repair of and checkpoint response to topoisomerase I-mediated DNA damage. Mutat Res 532: 173–203 [DOI] [PubMed] [Google Scholar]
- Pouliot JJ, Robertson CA, Nash HA (2001) Pathways for repair of topoisomerase I covalent complexes in Saccharomyces cerevisiae. Genes Cells 6: 677–687 [DOI] [PubMed] [Google Scholar]
- Pouliot JJ, Yao KC, Robertson CA, Nash HA (1999) Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science 286: 552–555 [DOI] [PubMed] [Google Scholar]
- Povirk LF (1996) DNA damage and mutagenesis by radiomimetic DNA-cleaving agents: bleomycin, neocarzinostatin and other enediynes. Mutat Res 355: 71–89 [DOI] [PubMed] [Google Scholar]
- Rass U, Ahel I, West SC (2007) Defective DNA repair and neurodegenerative disease. Cell 130: 991–1004 [DOI] [PubMed] [Google Scholar]
- Roseaulin L, Yamada Y, Tsutsui Y, Russell P, Iwasaki H, Arcangioli B (2008) Mus81 is essential for sister chromatid recombination at broken replication forks. EMBO J 27: 1378–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, Rzyman W, Puma F, Kobierska-Gulida G, Farabi R, Jassem J (2007) BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS ONE 2: e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimanuki M, Chung SY, Chikashige Y, Kawasaki Y, Uehara L, Tsutsumi C, Hatanaka M, Hiraoka Y, Nagao K, Yanagida M (2007) Two-step, extensive alterations in the transcriptome from G0 arrest to cell division in Schizosaccharomyces pombe. Genes Cells 12: 677–692 [DOI] [PubMed] [Google Scholar]
- Soutoglou E, Misteli T (2008) Activation of the cellular DNA damage response in the absence of DNA lesions. Science 320: 1507–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y (2000) Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol Cell Biol 20: 3977–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SS, Tanaka Y, Samejima I, Tanaka K, Yanagida M (1996) A nitrogen starvation-induced dormant G0 state in fission yeast: the establishment from uncommitted G1 state and its delay for return to proliferation. J Cell Sci 109(Part 6): 1347–1357 [DOI] [PubMed] [Google Scholar]
- Subramanian L, Moser BA, Nakamura TM (2008) Recombination-based telomere maintenance is dependent on Tel1-MRN and Rap1 and inhibited by telomerase, Taz1, and Ku in fission yeast. Mol Cell Biol 28: 1443–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima H, Boerkoel CF, John J, Saifi GM, Salih MA, Armstrong D, Mao Y, Quiocho FA, Roa BB, Nakagawa M, Stockton DW, Lupski JR (2002) Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat Genet 32: 267–272 [DOI] [PubMed] [Google Scholar]
- Vance JR, Wilson TE (2001) Uncoupling of 3′-phosphatase and 5′-kinase functions in budding yeast. Characterization of Saccharomyces cerevisiae DNA 3′-phosphatase (TPP1). J Biol Chem 276: 15073–15081 [DOI] [PubMed] [Google Scholar]
- Vance JR, Wilson TE (2002) Yeast Tdp1 and Rad1–Rad10 function as redundant pathways for repairing Top1 replicative damage. Proc Natl Acad Sci USA 99: 13669–13674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilenchik MM, Knudson AG (2003) Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA 100: 12871–12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, Basham D, Bowman S, Brooks K, Brown D, Brown S, Chillingworth T, Churcher C, Collins M, Connor R, Cronin A et al. (2002) The genome sequence of Schizosaccharomyces pombe. Nature 415: 871–880 [DOI] [PubMed] [Google Scholar]
- Yang SW, Burgin AB Jr, Huizenga BN, Robertson CA, Yao KC, Nash HA (1996) A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci USA 93: 11534–11539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lee JW, Tatavarthi H, Lupski JR, Valerie K, Povirk LF (2005) Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1). Nucleic Acids Res 33: 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]