Abstract
Scrapie is a transmissible spongiform encephalopathy in sheep and goats. Susceptibility to this neurodegenerative disease is mainly controlled by point mutations at the PRNP locus. Other genes, apart from PRNP, have been reported to modulate resistance/susceptibility to scrapie. On the basis of several studies in Alzheimer and different transmissible spongiform encephalopathy models, HSP90AA1 was chosen as a putative positional and functional candidate gene that might be involved in the polygenic variance mentioned above. In the present work, the ovine HSP90AA1 gene including the promoter and other regulatory regions has been isolated and characterized. Several sequence polymorphisms have also been identified. FISH-mapping localized the HSP90AA1 gene on ovine chromosome OAR19q24dist, which was confirmed by linkage analysis. This chromosome region has been shown to include a quantitative trait loci (QTL) for scrapie incubation period in sheep. Expression analyses were carried out in spleen and cerebellum samples. No differences in the expression of the HSP90AA1 gene were found in any of these tissues (p > 0.05) between control and infected animal samples. Nevertheless, association analyses revealed that several polymorphisms in the 5′ and 3′ regions of the HSP90AA1 gene were differentially distributed among animals with different responses to scrapie infection. Thus, results presented here support the hypothesis that HSP90AA1 could be a positional and functional candidate gene modulating the response to scrapie in sheep.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-007-0004-2) contains supplementary material, which is available to authorized users.
Introduction
Scrapie (SC) is a transmissible spongiform encephalopathy (TSE) of sheep and goats. TSEs are neurodegenerative diseases caused by transmissible proteinaceous particles (prions) devoid of nucleic acid that affect man and various animals. The key event in the pathogenesis of prion diseases is the conversion of the host encoded and naturally expressed cellular prion protein (PrPC) into its aberrant counterpart PrPSc. During the conversion process, PrPSc acquires new biophysical and biochemical characteristics, which lead to its accumulation in the form of amyloid plaques in nervous and lymphoid tissues (Prusiner 1998). However, the mechanism by which PrPSc causes neurodegeneration remains unclear. Like SC, most of the neurodegenerative diseases are associated with events of protein misfolding. The accumulation of misfolded protein aggregates can overwhelm the ubiquitin-proteasome system inducing apoptosis and increasing neuronal vulnerability to subsequent insults (Kang et al. 2004).
Susceptibility to SC is associated with polymorphisms in the amino acid sequence of the PrP protein. Twenty-five polymorphic codons and 40 haplotypes have been described to date in the open reading frame of the gene encoding the PrP (PRNP; Goldmann et al. 2005). In a slightly simplified assessment of genetic susceptibility for a sheep to develop SC, polymorphisms are classified attending to amino acid changes at positions V136A, R154H, and QH171R (Baylis et al. 2004). However, significant differences in the incubation period for this neurodegenerative disease have been reported in mice and sheep with the same PRNP genotype (Carlson et al. 1988; Diaz et al. 2005; Dickinson 1975; Westaway et al. 1987). Additionally, evidence for other genomic regions containing genes that influence the incubation period for SC in mice (Lloyd et al. 2001; Lloyd et al. 2002; Manolakou et al. 2001; Moreno et al. 2003a,b; Stephenson et al. 2000) and sheep (Moreno et al. 2003a) have been reported. Thus, a reasonable hypothesis is that a major gene (PRNP) controlling resistance/susceptibility to SC coexists with a number of other genes that modulate its effect.
Chaperones are essential proteins involved in the formation and maintenance of the proper conformation of other proteins and promoting cell survival after a large variety of environmental stresses. They protect other proteins against aggregation, solubilize initial, loose protein aggregates, assist in the folding of nascent proteins or in the refolding of damaged proteins, target severely damaged proteins to degradation and in case of excessive damage, sequester damaged proteins to larger aggregates (Soti et al. 2005).
A QTL interval modulating SC incubation period in sheep has been described on ovine chromosome OAR18 (Moreno et al. 2003a). By comparing this ovine region with the human and murine genomes, herein we have inferred that it should include the Hsp90α (cytosolic) class A member 1 gene (HSP90AA1).
The chaperone protein known as 90 kDa heat shock protein, Hsp90, is one of the most abundant proteins in eukaryotic cells, comprising 1–2% of cellular proteins under nonstress conditions. There are two major cytoplasmic isoforms of Hsp90, which have arisen by gene duplication, Hsp90α (inducible form) and Hsp90β (constitutive form). It is also known that additional Hsp90 paralogs are localized in the endoplasmic reticulum and mitochondria, denoted Grp94/gp96 and Hsp75/TRAP1, respectively. Since separating biochemically the Hsp90α and Hsp90β isoforms is rather difficult because they share 85% of sequence identity in higher eukaryotes, most studies have been carried out with a mixture of both isoforms (Sreedhar et al. 2004).
Cytosolic Hsp90’s contribution to various cellular processes including signal transduction, protein folding, protein degradation, cell survival, and morphological evolution has been extensively studied.
It has been shown that Hsp90 specifically influences aggregation at “early” stages in Alzheimer disease avoiding the production of toxic oligomers (Evans et al. 2006). Furthermore, Kakimura et al. (2002) reported that Hsp90 induced microglial activation participating in compensatory neuroprotection through the production of cytokines, enhancement of phagocytosis and clearance of Aβ in this human disease.
Although the exact role of this chaperone in SC pathology is unknown, it could participate in several aspects of the disease. Thus, Hsp90α acts as a repressor of HSF1, which is an activator of heat shock genes. Under stress conditions, nonnative proteins accumulate and compete with HSF1 for binding to Hsp90. As a result, HSF1 is released from this complex and becomes active (Zou et al. 1998). In addition, it is well known that Hsp90 maintains the functional integrity of the 26S proteasome and participates in its assembly. It is also responsible for refolding stress-damaged proteins and thereby might be sequestered into such damaged proteins after severe insults. In this way, the disassembly of the 26S proteasome could be a consequence of changes in the physiological state of Hsp90 due to stress conditions (Imai et al. 2003). It has been demonstrated that wild-type PrP is subjected to ubiquitination and degradation by the proteasome and that a significant fraction of PrP enriched in unglycosylated forms accumulates in the cytoplasm when the activity of the proteasome is compromised (Ma and Lindquist 2001; Yedidia et al. 2001). Furthermore, proteasomal activity decreases in mouse SC brains (Kang et al. 2004). Taking into account these reports and that both unglycosylated PrP forms and cytoplasmic environment favor the formation of PrPSc-like forms, it can be hypothesized that after SC infection, PrPSc starts to accumulate because its degradation is impaired. Hsp90 could contribute to the correct folding of PrPSc instead of participating in other functions and thus result in an increased expression of other heat shock proteins and in a minor proteasomal function. Additionally, as a consequence of the reduced proteasomal activity, the PrPC, which in normal circumstances is efficiently degraded by this machinery, would accumulate in the cytoplasm, increasing the amount of substrate susceptible of being transformed to the PrPSc isoform. These arguments led us to study HSP90AA1 as a possible positional and functional candidate gene contributing to ovine SC resistance/susceptibility modulation.
The first objective of this work was to isolate and characterize the gene, and determine the cytogenetic position of HSP90AA1 on the ovine genome to verify if its localization was in concordance with previous reports describing QTLs associated with resistance/susceptibility and incubation period for different TSEs in ovine (Moreno et al. 2003a) and murine models (Lloyd et al. 2001; Lloyd et al. 2002; Manolakou et al. 2001; Moreno et al. 2003b; Stephenson et al. 2000). The next step was to verify if there was any difference in the expression of this gene between SC infected and uninfected sheep presenting the same genotype for PRNP (ARQ/ARQ). Since, as it has been described above, there is no need of Hsp90α overproduction to trigger a stress response, the aim of this point was to gain insight into the possible role of Hsp90α in SC and in the way it participates in this neurodegenerative disease. This comparison was performed using real-time RT-PCR on samples of spleen and cerebellum tissues. These are important organs in the development of SC and other TSEs since, after oral infection, prions replicate in the spleen and subsequently migrate to the central nervous system causing a profound neurodegeneration. Finally, the HSP90AA1 polymorphism was studied to perform further association analyses to identify putative mutations in the ovine HSP90AA1 locus that could explain, at least in part, the polygenic response to SC in sheep.
Materials and methods
Isolation of sheep specific DNA fragments
Genomic DNA was extracted from ovine lymphocytes according to the salting-out procedure described by Miller et al. (1988). BAC DNA was purified by a Maxipreparation with the Nucleobond PC 100 Kit (Macherey-Nagel) according to the manufacturer’s instructions.
To isolate and sequence HSP90AA1 fragments, 12 heterologous primers from human (GenBank NT_026437), murine (GenBank NT_039551) and bovine sequences (GenBank NW_930044.1, NW_270059 and NM_001012670.1), and thirteen specific primers based on the sequence of the ovine fragments previously isolated with the primers described above, were synthesized (ESM Appendix 1). Bovine HSPCAB mRNA sequence (AB072369) was also taken into account to avoid unspecific amplifications due to the high homology level between HSP90AA1 and HSPCAB (gene encoding Hsp90β protein) coding regions. HSP90AA1 regulatory regions were inferred by sequencing both the BAC DNA insert and PCR amplified fragments from genomic and BAC DNA. Genomic (60–100 ng) and BAC DNA (100 ng) were amplified in a final volume of 25 μl containing 0.5 μM of each primer, 200 μM of dNTPs, 1.5–2 mM MgCl2, 2.5 μl of 10× buffer MgCl2 free (Biotools) and 1 U Taq polymerase (Biotools). In some cases, it was necessary to add DMSO to the PCR mix (see ESM Appendix 1). The following PCR conditions were used: denaturation at 94°C for 5 min, 30 amplification cycles of denaturation at 94°C for 45 s, annealing at 52°C to 59°C for 30 s, and extension at 72°C for 30 s to 1 min 30 s followed by a final 5–10 min extension at 72°C. Primer pairs and their amplification conditions are shown in ESM Appendix 1. The resulting PCR fragments were purified with the GFX PCR DNA and Gel Band Purification Kit (Amersham Bioscience) and bidirectionally sequenced with the PCR primers. The identity of the fragments was confirmed by BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST/). As indicated in ESM Appendix 1, the fragment generated with primers that hybridized with regions at −570 and intron 2 had to be sequenced with different primers from those used to perform the PCR reaction due to the secondary structures that they formed. Additional primers also had to be used to sequence the region amplified between −700 and +400 positions because of the presence of insertion/deletion polymorphisms.
Chromosomal location
Ovine HSP90AA1 gene was localized in the ovine genome by two approaches: cytogenetic and genetic mapping.
Cytogenetic mapping: Primers located in HSP90AA1 intron 10 and exon 11 (see ESM Appendix 1) were used to screen an ovine BAC library (Vaiman et al. 1999). DNA from the identified BAC clone was labelled by nick-translation in the presence of biotin-14-dATP (BioNick TM 18247-015 labelling system) and used as probe for in situ hybridisation on RBP-banded ovine chromosome preparations following the protocol described by Hayes et al. (1991).
Genetic mapping: Genomic DNA from five domestic sheep breeds [Latxa (n = 2), Manchega (n = 5), Awassi (n = 1), Assaf (n = 1) and Rasa Aragonesa (n = 1)] was amplified and sequenced as previously described and analyzed using CHROMAS 1.43 and ClustalW (http://www.ebi.ac.uk/clustalw/) software to detect polymorphisms along the isolated sequences of HSP90AA1, without taking into account either their PRNP genotype or their pattern of SC resistance/susceptibility. Animals were chosen from different breeds to avoid any problem due to allele fixation because of the selective status of these breeds in our country (Spain), and the identification of a representative polymorphism at the species level.
To follow the distribution of the gene within the pedigrees of the AgResearch International Mapping Flock (IMF), the SNP (A/G) located at position 84 in the HSP90AA1 intron 10 was analyzed by PCR-RFLP using primers that hybridized HSP90AA1 exons 10 and 11 (see ESM Appendix 1). The reaction product (8 μl) was digested with 0.2 U of Tsp509I at 65°C over 19 h in a final volume of 18 μl and then electrophoresed on a 3% agarose gel for visualization.
Thus, cytogenetic localization was confirmed by linkage mapping in nine sheep families. HSP90AA1 gene was mapped against markers on the sheep framework map (Maddox et al. 2001). Multipoint linkage analysis of the IMF pedigrees (Crawford et al. 1995) was performed using CRI-MAP (Lander and Green 1987).
Gene expression
A total of 13 Rasa Aragonesa female sheep (aged 3–5 years) with the same PRNP genotype (ARQ/ARQ) were included in this study. Eight of them exhibited clinical signs of SC in a terminal state. PRNP genotypes were determined according to Acin et al. (2004). The diagnosis was confirmed using a rapid test and immunohistochemistry to detect PrPSc (Bolea et al. 2005). These animals belong to a flock of sheep, conserved for research purposes by the Prion Research Centre of the University of Zaragoza and where several SC cases have appeared in the last 4 years. Control animals (n = 5) were selected from a different flock belonging to the same breed, where no SC had been reported to date.
The aim of this sample design was to determine if SC disease might cause any change in the expression pattern of HSP90AA1. Thus, small fragments of spleen and cerebellum were included in RNAlater (Ambion). Total mRNA was purified from spleen and cerebellum with the RNeasy Mini Kit and the RNeasy Lipid Tissue Mini Kit (Qiagen), respectively. Complementary DNA (cDNA) was synthesized from 1 μg of each RNA preparation using random hexamer primers with the SuperScript First-Stranded Synthesis System for RT-PCR (Invitrogen). Gene expression levels were subsequently determined by real-time RT-PCR. All RT-PCR reactions were run in duplicate. Two tissue-specific housekeeping genes were used to normalize each set of results. Data from the spleen were normalized for succinate dehydrogenase complex, subunit A (SDHA) and glyceraldehyde-3-phosphate deshydrogenase (GAPDH) mRNA. Hexose-6-phosphate dehydrogenase (G6PDH) and 18S rRNA mRNA were used in the case of cerebellum. Primers and probes used for gene expression analysis, their concentrations and amplicon sizes are shown in ESM Appendix 2. Amplification was carried out in a final volume of 25 μl containing SYBRGreen PCR Master Mix (Applied Biosystems) or TaqMan Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems), depending on the gene analyzed (see ESM Appendix 2). After preheating the mix at 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 30 s were carried out.
It was necessary to sequence a fragment of the cDNA containing part of exon 10 through intron 11 to synthesize a pair of primers adequate to carry out the real-time RT-PCR. These primers were F: 5′- TTGGTGACATCCCCATGCTG -3′ and R: 5′- CAACAGTCATCTGTTTAGG -3′, which hybridized with HSP90AA1 exons 10 and 11, respectively. Amplification conditions were those described in the first section of Materials and methods. The annealing temperature was 56°C, the extension time 30 s and MgCl2 concentration 2 mM. All the resulting PCR fragments were purified with ExoSAP-IT (USB) and bidirectionally sequenced using the PCR primers and the BigDye Terminator V 3.1 Cycle Sequencing kit (Applied Biosystems). The identity of genes was confirmed by BLAST comparison with the GenBank database.
The normalization factor (NF; Vandesompele et al. 2002) was calculated as the geometric mean of the quantity (Q) of the two housekeeping genes of each tissue (SDHA and GAPDH for spleen and G6PDH and 18S mRNA for cerebellum). The normalized expression level of HSP90AA1 mRNA (n HSP90AA1) was calculated as the ratio between the Q values of HSP90AA1 and the NF calculated for each sample. Later, this ratio (Q HSP90AA1/NF) was transformed according to the expression arc sin √ (n HSP90AA1 × 100–1) as recommended for parametric tests on relative data. Finally, a t test was run to compare the means of the two samples. The test was constructed to determine whether the differences between the means of expression data from naturally infected and control animals were equal to zero.
Polymorphism detection
The cDNA from the 13 Rasa Aragonesa breed sheep with the same PRNP genotype (ARQ/ARQ) used in the expression analysis was used in an attempt to identify polymorphism in the coding region of HSP90AA1 gene that might cause an amino acid change. In addition, genomic DNA from 80 ARQ/ARQ, Rasa Aragonesa sheep (see association analysis section and Tables 1, 2, 3) was examine and used to perform association analysis. Thirty-one of them (infected) exhibited clinical and immunohistochemical signs of SC while the remaining forty-nine (healthy) did not. All these animals belong to six flocks of sheep, conserved for research purposes by the Prion Research Centre of the University of Zaragoza and where several SC cases have appeared in the last 4 years. PRNP genotypes and SC diagnosis were assessed as explained before (Acin et al. 2004; Bolea et al. 2005). To determine if the SNP’s genotype distribution found in this group of animals was representative of other breeds or exclusive of the Rasa Aragonesa group, an additional genotyping of 38 ARQ/ARQ sheep belonging to the Manchega and Assaf breeds, which had no contact with SC, was carried out.
Table 1.
Genotypic frequencies and confidence interval for the SNPs in the 5′ flanking region of the HSPCAA1 gene in animals of several sheep breeds
| Position | Genotype | Whole (n = 67) | Rasa Aragonesa frequency (%) Healthy (n = 40) | Disease (n = 27) | Manchega and Assaf frequency (%; n = 38) |
|---|---|---|---|---|---|
| −660 | CC | 59.4 (47.3–71.4) | 76.3 (62.8–89.8) | 34.6 (16.3–52.9) | 50.0 (34.1–65.9) |
| CG | 35.9 (24.2–47.7) | 21.1 (8.1–34.0) | 57.7 (38.7–76.7) | 34.2 (19.1–49.3) | |
| GG | 4.7* | 2.6* | 7.7* | 15.8 (4.2–27.4) | |
| −528 | AA | 59.4 (47.3–71.4) | 76.3 (62.8–89.8) | 34.6 (16.3–52.9) | 60.5 (45.0–76.0) |
| AG | 35.9 (24.2–47.7) | 21.1 (8.1–34.0) | 57.7 (38.7–76.7) | 31.6 (16.8–46.3) | |
| GG | 4.7* | 2.6* | 7.7* | 7.9* | |
| −524 | TT | 75.0 (64.4–85.6) | 71.0 (56.6–85.4) | 80.8 (65.6–95.9) | 68.4 (53.6–83.2) |
| GT | 25.0 (14.4–35.6) | 29.0 (14.5–43.3) | 19.2 (4.1–34.4) | 26.3 (12.3–40.3) | |
| GG | 0.0 | 0.0 | 0.0 | 5.3* |
Confidence interval at 95% in brackets
*Frequency estimates no different from zero.
Table 2.
Genotypes detected for the SNPs at positions −660, −528 and −524 in the 5′ flanking region of the HSP90AA1 gene
| Genotype LYF SIF SP1a | Number of animals | Number of healthy animals | Number of infected animals | Probability of healthy animals | Probability of infected animals |
|---|---|---|---|---|---|
| CC AA GGb | 2 | 2 | 0 | 0.050 | 0.000 |
| CC AA GT | 11 | 10 | 1 | 0.250 | 0.037 |
| CC AA TT | 27 | 19 | 8 | 0.475 | 0.296 |
| CG AA TTb | 1 | 0 | 1 | 0.000 | 0.037 |
| CG AG GTb | 5 | 1 | 4 | 0.025 | 0.149 |
| CG AG TT | 18 | 7 | 11 | 0.175 | 0.407 |
| GG GG TTb | 3 | 1 | 2 | 0.025 | 0.074 |
| 67 | 40 | 27 |
The number of healthy and infected Rasa Aragonesa breed sheep and the probability of developing the disease based on the genotype are shown. χ2 = 15.9452 for the contingency table and p value = 0.014 (p < 0.05)
aPutative regulatory motif that could be affected by the SNP
bThe estimated probabilities for these genotypes should be cautiously considered due to the small number of animals found in the analyzed sample.
Table 3.
Genotypes detected for the SNPs at positions 40, 165, 178, 205, 220, and 239 of the ovine HSP90AA1 intron 10
| Genotype | n animals | n healthy animals | n infected animals | Probability of healthy | Probability of infected |
|---|---|---|---|---|---|
| GG CC GG CC TT TT | 75 | 49 | 26 | 1.000 | 0.839 |
| AG CT CG CT CT CT | 5 | 0 | 5 | 0.000 | 0.161 |
| 80 | 49 | 31 |
The number of healthy and infected Rasa Aragonesa breed sheep and the probability of developing the disease based on the genotype are shown.
χ2 = 8.4301 for the contingency table and p value = 0.004 (p < 0.005)
Association analysis
It is well established that there are significant differences among breeds in which PRNP genotypes are attacked by SC. For instance, the ARQ/ARQ genotype shows different resistance to SC infection depending on the breed studied (Baylis et al. 2004). Nevertheless, there is no evidence of any correlation between the occurrence of a specific ARQ haplotype, considering all the SNPs between codons 112 and 241 of the PRNP, and the SC disease status of a flock (Goldmann et al. 2005).
HSP90AA1 polymorphism was studied on a total of 80 Rasa Aragonesa breed sheep displaying the same PRNP genotype (ARQ/ARQ).
Since one of the major sources of variability in SC resistance/susceptibility and/or incubation period associated with Hsp90α might be explained by differences of expression of this chaperone, we decided to look for polymorphism in the 5′ and 3′ flanking regions of HSP90AA1 gene. There were 67 sheep (27 infected and 40 healthy) sequenced to study the 5′ flanking region (13 sheep from the 80 initial sample were not available in this case). To analyze the 3′ flanking region, 80 animals (31 infected and 49 healthy) were studied. Thus, genomic DNA from these sheep was amplified, sequenced, and analyzed as previously described. The primer pairs used hybridized with regions located at −700 and +400 bp from the transcription start site and in exons 10 and 11.
Finally, association analysis was performed with the CATMOD procedure of the SAS statistical package (SAS 1989), which is a procedure for categorical data modeling. In this case, the standard response function (generalized logits) was used because there were only two response levels (infected vs healthy). The Maximum Likelihood was the estimation method used. Analysis took into account response to SC (presence/absence of clinical signs and positive immunohistochemistry for PrPSc) as the dependent variable and the genotypes for the SNPs tested as the independent factor.
Results
Isolation of the ovine HSP90AA1 gene and polymorphism detection
Two cDNA fragments corresponding to exons 2 to 5 (921 bp) and exons 7 to 11 (1,123 bp) (Genbank accession number EF091713), and a sequence of 5,917 bp containing the complete coding sequence, interrupted by 10 introns, and 1,000 bp of the 5′ flanking region of HSP90AA1 gene DQ983231 were isolated.
Exons were identified by comparison with the ovine and bovine mRNA sequences (EF091713 and AB072368.1, respectively). Each HSP90AA1 intron showed the GT/AG consensus splice junction described by Breathnach and Chambon 1981. Thus, the whole HSP90AA1 ovine gene comprises 11 exons and 10 introns. The precise length and full sequence of each one were determined (GenBank accession no DQ983231).
In addition to the coding region of the HSP90AA1 gene, a sequence of more than 1,000 bp corresponding to the 5′ flanking region of the gene was analyzed. Several consensus sequences typical of promoter regions including a putative TATA box and several SP1 consensus sites (Dale et al. 1996) were identified in this 5′ flanking region. More interesting is the presence of several heat shock elements (HSEs), which are binding sites for the heat shock factor (HSF-1) involved in the inducible gene expression of hsp90α. The human HSP90AA1 core promoter described by Zhang et al. (1999) is also conserved in sheep. Additional cis-regulatory elements were identified with the informatics programs TFSEARCH and Signal Scan (http://www.cbrc.jp/research/db/TFSEARCH.html and http://bimas.dcrt.nih.gov/molbio/signal/, respectively), which predicted several putative binding sites for various trans-acting factors, the most important ones being Lyf-1, CdxA, SIF, and of course, SP-1, TATA binding protein and HSF-1.
There were 34 polymorphisms identified within the isolated fragments: 12 in the coding region (exons 4, 7, 8, 9, 10 and 11), 14 in the 5′ flanking region, and 8 in the HSP90AA1 intron 10 (see Fig. 1 and DQ983231). None of the SNPs located in the HSP90AA1 coding region produced any change in the amino acid sequence. The SNP (A/G) located at position 84 in the ovine HSP90AA1 intron 10 was further analyzed within the IMF families for linkage mapping.
Fig. 1.
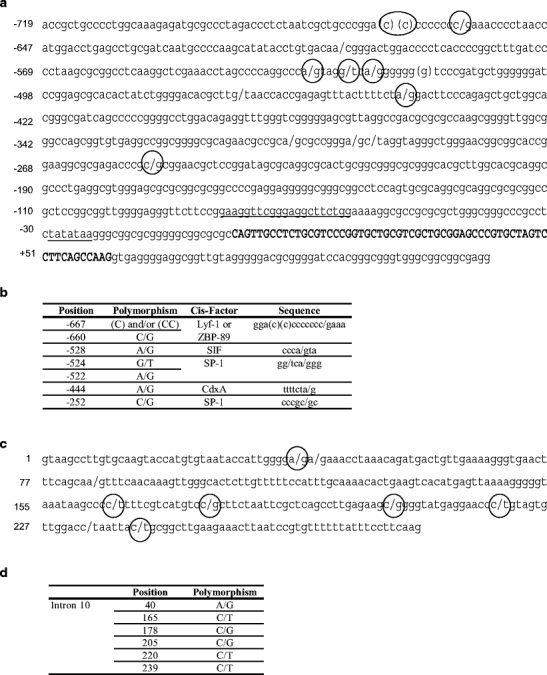
Polymorphisms and cis-acting elements localized within ovine HSP90AA1 5′ (a and b) and intron 10 (c and d) flanking regions. a Sequence amplified with –700 and +400 primers. Here are represented the polymorphisms found within this region and putative binding sites for Lyf-1 (−667 to −657), and CdxA (−450 to −444) factors predicted with the TFSEARCH program. Cis-acting elements previously described for the HSP90AA1 murine gene (Dale et al. 1996) are also indicated: SP-1 sites (−525 to −520 and −256 to −251), HSE (−82 to −62) and the TATA box (−29 to −23). The binding sites for ZBP-89 (−667 to −660) and SIF (−531 to −526) factors described in the promoters of other genes are also shown (Wagner et al. 1990; Zhang et al. 2003). Exon 1 is shown in bold capital letters. b Summary of the most interesting polymorphism found within the HSP90AA1 5′ flanking region. Their location, the trans-acting factor that binds to them and the sequence of the cis-element are shown. c Intron 10 is represented. d Summary of the most interesting polymorphism found within HSP90AA1 intron 10
Chromosomal localization of ovine HSP90AA1 gene
The ovine HSP90AA1 gene was mapped to OAR18q24dist by FISH using the ovine BAC library (Vaiman et al. 1999) at INRA-CRJ (Jouy-en-Josas, France). These results were confirmed by linkage mapping using the IMF pedigrees. The best supported linkage map of the relevant region of chromosome 18 including the HSP90AA1 gene is shown in Fig. 2 and was obtained by CRI-MAP multipoint analysis, after performing the two-point and flips options. HSP90AA1 was located at the end of ovine chromosome 18, next to VNTR16.
Fig. 2.
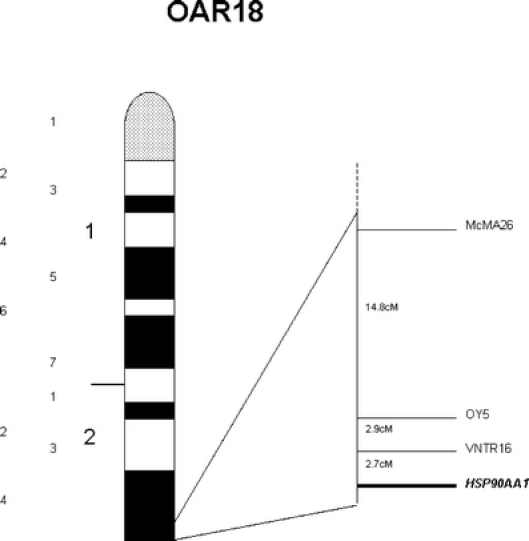
Cytogenetic (left) and genetic (right) maps showing HSP90AA1 gene localization on sheep chromosome 18
Gene expression analyses
The expression of Hsp90α was studied by real-time RT-PCR in spleen and cerebellum tissues. Standard curves for the genes studied present the appropriate slopes and correlation values (slope > -4, r2 = 0.99). No significant differences in the expression of HSP90AA1 between affected and healthy animals were found either in spleen or in cerebellum (p > 0.05).
Polymorphism detection and association analysis
Considering the many pathways in which this chaperone is involved, it is possible that a polymorphism affecting HSP90AA1 expression could explain part of the inter-individual response to SC that is not related with the PRNP genotype.
The polymorphism at 5′ flanking region and intron 10 of HSP90AA1 was studied on animals belonging to Rasa Aragonesa (n = 80) and Manchega and Assaf (n = 38) breeds sheep, displaying the same PRNP genotype (ARQ/ARQ).
5′ flanking region Fourteen polymorphisms were detected in the 5′ flanking region. As previously mentioned, part of these polymorphisms were located within possible trans-acting factors binding sites. For instance, the C/G SNP at −660 is in a putative Lyf-1 site, the −528 and −524 A/G and G/T substitutions are present in SIF and SP-1 consensus sites, respectively, and an additional A/G polymorphism was found at −444 affecting a CdxA site. These represent a small sample of the putative cis-regulatory elements identified within this region. The 14 polymorphisms identified in the HSP90AA1 5′ flanking region are represented in Fig. 1a and b. After discarding the SNPs presenting extreme allelic frequencies, only the genotype of the three SNPs located at positions –660, –528, and –524 were analyzed. Table 1 showed genotypic frequencies and its confidence intervals (95%) for the SNPs identified at positions −660, −528 and −524 in Rasa Aragonesa healthy and disease animals and in animals of Manchega and Assaf breeds. Since samples had enough size (n > 30), we can assume that binomial proportions trend to normal distributions, and therefore, confidence intervals for the frequencies can be calculated. In those cases in which proportions were no different from cero, confidence intervals could not be calculated. Similar genotypic frequencies in Rasa Aragonesa, Manchega, and Assaf breeds were observed for the three SNPs studied (Table 1). However, between healthy and disease animals of Rasa Aragonesa breed clear differences between genotypic frequencies at positions –660 and –528 were detected. Thus, the CCAA linked genotype at these positions, was more frequent in the healthy than in the disease group. Conversely, the heterozygous genotype CGAG, for these SNPs, was predominant in the disease sample. This event was independent from the genotype obtained for the SNP at –524 position. The grouped genotypes for the three SNPs at positions −660, −528, and −524 of the HSP90AA1 5′ flanking region, in Rasa Aragonesa healthy and disease animals, are shown in Table 2. Although it is not possible to know the ancestral allele, taking into account the frequencies of genotypes found in the analyzed sample (see Table 2), the existence of the CAT, CAG, GGT, and GAT alleles could be inferred. It is worthy to mention that despite the GAT allele appeared just one within the Rasa Aragonesa sample, within the Manchega and Assaf group it appears three times, confirming the real existence of such allele. Distribution of SC cases among genotypes indicate that the SNP located at −524 has not influence in the probability to develop the disease (see Tables 1 and 2). For this reason, only the two first SNPs at the 5′ flanking region (−660 and −528) were used to carry out the association analyses. In this way, maximum likelihood variance analyses revealed that predicted haplotypes for these two positions were statistically associated (p value < 0.0002) with the presence/absence of the disease. Thus, the CA allele was overrepresented in healthy sheep, whereas the GG was more common in infected animals. The GA allele was not considered due to its reduced representation in this case.
3′ flanking region After discarding the SNPs presenting extreme allelic frequencies, six SNPs located at positions 40, 165, 178, 205, 220, and 239 of HSP90AA1 intron 10, which showed a complete linkage, were analyzed. Frequencies for the homozygous genotype (GG CC GG CC TT TT) were 93.8% of Rasa Aragonesa and 94.8% of the Manchega and Assaf sheep breeds. The remaining 6.2% of Rasa Aragonesa and 5.2% of the Manchega and Assaf sheep breeds, were heterozygous with a AG CT CG CT CT CT genotype. No animal with the AA TT CC TT CC CC genotype was identified.
Maximum likelihood variance analyses of the genotypes for this six SNPs showed statistical significance (p value = 0.0022) with the presence/absence of the disease. Within Rasa Aragonesa breed, the 100% of the heterozygous sheep were infected, while only the 35% of the homozygous animals catch the disease.
Discussion
We have isolated and characterized the ovine HSP90AA1 gene, which consists of 11 exons ranging in size from 60 to 367 bp interrupted by 10 introns ranging in size from 92 to 456 bp. This gene organization is conserved in the human (at least in the region isolated in the present work, see below), murine, and bovine homologues (Dale et al. 1996; NW_930044.1, NW 270059, and NM001012670.1). The relative position of each intron is also conserved including the position of the first intron exactly at the boundary between exon 1 and the initiation of translation site (ATG). This unusual positioning of the first intron is a feature shared by other members of the HSP90 gene family (Dale et al. 1996). Additionally, various regulatory regions, also present in man, mouse and cattle, have been determined.
The coding region is very similar among these three species (>90% sequence homology), except that the human sequence has three nucleotides less than the ruminant counterpart. Thus, the protein in cattle and sheep contains 733 amino acids, whereas the human homolog lacks a glutamic acid at position 266. Amino acid sequences in cattle and sheep are 100% identical. At this point, it is interesting to mention that five different transcript variants have been described for this gene in man, the larger form consisting of 854 amino acids whereas the shortest has only 413 amino acids (Chen et al. 2005). Although we could not identify the coding region for all these variants neither in cattle nor in sheep, the sequence isolated in the present work contains the variant that is expressed most ubiquitously in man (Chen et al. 2005).
The localization of the ovine HSP90AA1 gene on OAR18q24dist, next to the marker OY5 (Fig. 2), is consistent with comparative mapping information since this gene maps to human chromosome 14 and mouse chromosome 12. Ovine chromosome 18 has already been reported as containing a QTL region involved in incubation period to SC (Moreno et al. 2003a) and it is interesting to note that the HSP90AA1 gene is located within the proposed interval. This result supports the hypothesis that HSP90AA1 may be a positional candidate gene modulating the response to SC infection in sheep.
Expression results revealed no differences in HSP90AA1 mRNA concentration between the two groups of sheep (infected and uninfected) analyzed. This suggests that SC infection is not the cause of any change at the HSP90AA1 mRNA level. However, there are several things that should be taken into account to avoid inferring erroneous conclusions: (1) the number of tissues analyzed (spleen and cerebellum) was limited. It is possible that HSP90AA1 mRNA varies in other regions not studied in the present work. (2) the existence of transcript variants that could not be detected with the primers used (they hybridise with exons 10 and 11), (3) Jacquier-Sarlin et al. (1995) have suggested that under conditions associated with sustained Hsps expression such as inflammation or ischemia, Hsps synthesis results from a posttranscriptional regulatory mechanism. Thus, the regulation of HSP90AA1 during SC could occur at the translational level, and the increase in Hsp90α synthesis might be via the activation of transcription of stored mRNA. This is conceivable since the HSP90AA1 mRNA level decreases by 20 and 10%, in spleen and cerebellum, respectively, in SC infected sheep as compared with controls (data not shown).
Although it is real that Hsp90α does not change during SC infection, we suggest that any cause leading to an up or down regulation of cytosolic Hsp90 could have important consequences along the SC neurodegeneration process. Genetic polymorphism is an acknowledged source of phenotypic variation. Considering that Hsp90α is the inducible form of cytoplasmic Hsp90s, any polymorphism at the HSP90AA1 locus modifying the activity of Hsp90α could affect the neurodegeneration process in SC infected animals. This is why we decided to study the polymorphism in the regulatory regions of these genes among sheep with the same PRNP genotype (ARQ/ARQ), which, although exposed to SC infection, present different responses to it (infected vs healthy).
Among the 34 polymorphisms identified within the isolated fragments, 3 SNPs in the 5′ flanking region (positions: −660, −528, −524) and 6 SNPs in the 3′ end (positions: 40, 165, 178, 205, 220, 239 of intron 10) were included in the association analyses. These analyses showed significant results for the C/G and A/G substitutions at –660 and −528 (p < 0.001) and for the A/G C/T C/G C/G C/T C/T linked SNPs in the HSP90AA1 intron 10 (p < 0.01).
A survey of the literature and the use of the programs TFSEARCH and Signal Scan to determine if all these changes affect any regulatory element showed that the region upstream of the HSP90AA1 minimal promoter contains apparently multiple elements controlling HSP90AA1 gene transcription (Fig. 1a). Thus, the (C)(C)CCCCCCC/gA sequence, located between –667 and −659 positions, is very similar to the consensus motif for IKAROS/LYF-1 family members described in the human B29 promoter (CCTCCCCCA) by Thompson et al. (1996). In addition, this ovine sequence is identical with a ZBP-89 binding site described in the 5′ region of the vimentin gene (GGACCCCCCCC) by Zhang et al. (2003). ZBP-89 has been reported to bind regulatory regions of various genes, and it appears to act both as transcriptional activator and repressor (Yamada et al. 2001). In addition, ZBP-89 is known to interact with SP elements and the SNPs (G/T and A/G) at locations −524 and −522 in the HSP90AA1 5′flanking region are both affecting an SP1 binding site described by Dale et al. (1996) in the HSP90AA1 murine promoter. On the other hand, the SNP (A/G) at −528 affects a putative Sis-Inducing Factor (SIF) binding site (CCCG/aTM). The sequence of this putative regulatory element is very similar to the core sequence of the SIF binding site described by Wagner et al. (1990) in the c-fos promoter (CCCGTC). SIF complexes are dimerized forms of signal transducers and activators of transcription (SAS Institute Inc.) factors which, after being activated by different ligands, translocate into the nucleus to direct the transcription of specific target genes (Wang et al. 2006). Thus, mutations at these sites might yield different rates of ovine HSP90AA1 transcription due to positive or negative interactions between different transcriptional regulatory elements. These different transcription levels might have important effects on SC incubation period.
Sequence analysis of intron 10 and exon 11 revealed eleven other polymorphisms. In this case, results from the association analysis were not as clear as those reported above because only five infected individuals presented the genotype (AG CT CG CT CT CT) for the six linked SNPs in HSP90AA1 intron 10. Nevertheless, since it has been shown that efficiency of RNA 3′-end formation is correlated with the efficiency of the final intron removal, and that the influence of the 3′-terminal intron on 3′-end formation can be attributable to the determinants of splicing efficiency (Nesic and Maquat 1994), these SNPs are not less interesting than those described in the 5′ flanking region. Thus, some of the mutations found within the ovine HSP90AA1 intron 10 may interfere with these post-transcriptional regulatory steps affecting the level of HSP90AA1 expression. To sum up, individuals capable of producing more Hsp90α under different conditions would have a minor proteasomal activity inhibition when they are submitted to SC infection. As a consequence, in these individuals, cytoplasmic PrPC accumulation would be lower, decreasing the conversion and aggregation process and extending the incubation period.
Although additional experiments should be performed to determine the exact implication of these polymorphisms in SC development, it can be concluded that HSP90AA1 is a good positional and functional candidate gene modulating SC incubation period in sheep. Thus, the GG and ATCTCC alleles, for positions described at 5′ flanking region and intron 10 are associated with higher susceptibility to SC. In a new project, which we have just started with the aim of studying in depth the HSP90AA1 polymorphism, we have found the third genotype for the intron 10 region (AA TT CC TT CC CC). Additionally, it seems that some of the polymorphisms are differentially distributed within different breeds. Nevertheless, further analyses should be perform to see if there are any differences in Hsp90α expression that could be explained by the polymorphisms described in the present work, and to test if combinations of the alleles described for the HSP90AA1 5′ flanking region and intron 10 can interact to modulate the response to SC in sheep.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 55.5 KB)
(DOC 42.0 KB)
Acknowledgment
We thank the CERSYRA-Valdepeñas and AGRAMA breeders association, CSIC-León, CITA-Aragón, Prion Research Centre of the University of Zaragoza—and INIA-Madrid for kindly providing Manchega, Awassi, Assaf, Rasa Aragonesa and Mouflon samples. We are very grateful to Dr. K.G. Dodds for his suggestions and English correction of the manuscript, to Dr. MA Roca for helping us with the PCRs improvement, to Dr. C.R. Moreno for her suggestions in the statistical area, to Dr. M.E.F. Alves for her continuous help and to Dr. E.P. Cribiu and Dr. P. Zaragoza for allowing us to perform the cytogenetic mapping and the expression analysis in their respective laboratories. This work was supported by the RTA2006–00104 INIA project, and a Predoctoral Grant from the INIA.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-007-0004-2) contains supplementary material, which is available to authorized users.
References
- Acin C, Martin-Burriel I, Goldmann W et al (2004) Prion protein gene polymorphisms in healthy and scrapie-affected Spanish sheep. J Gen Virol 85:2103–2110 [DOI] [PubMed]
- Baylis M, Chihota C, Stevenson E, Goldmann W, Smith A, Sivam K, Tongue S, Gravenor MB (2004) Risk of scrapie in British sheep of different prion protein genotype. J Gen Virol 85:2735–2740 [DOI] [PubMed]
- Bolea R, Monleon E, Schiller I et al (2005) Comparison of immunohistochemistry and two rapid tests for detection of abnormal prion protein in different brain regions of sheep with typical scrapie. J Vet Diagn Invest 17:467–469 [DOI] [PubMed]
- Breathnach R, Chambon P (1981) Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem 50:349–383 [DOI] [PubMed]
- Carlson GA, Goodman PA, Lovett M, Taylor BA, Marshall ST, Peterson-Torchia M, Westaway D, Prusiner SB (1988) Genetics and polymorphism of the mouse prion gene complex: control of scrapie incubation time. Mol Cell Biol 8:5528–5540 [DOI] [PMC free article] [PubMed]
- Crawford AM, Dodds KG, Ede AJ, et al (1995) An autosomal genetic linkage map of the sheep genome. Genetics 140:703–724 [DOI] [PMC free article] [PubMed]
- Chen B, Piel WH, Gui L, Bruford E, Monteiro A (2005) The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 86:627–637 [DOI] [PubMed]
- Dale EC, Yang X, Moore SK, Shyamala G (1996) Cloning and characterization of the promoter for murine 84-kDa heat-shock protein. Gene 172:279–284 [DOI] [PubMed]
- Diaz C, Vitezica ZG, Rupp R, Andreoletti O, Elsen JM (2005) Polygenic variation and transmission factors involved in the resistance/susceptibility to scrapie in a Romanov flock. J Gen Virol 86:849–857 [DOI] [PubMed]
- Dickinson AG (1975) Host-pathogen interactions in scrapie. Genetics 79(Suppl):387–395 [PubMed]
- Evans CG, Wisen S, Gestwicki JE (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid beta (1–42) aggregation in vitro. J Biol Chem 281:33182–33191 [DOI] [PubMed]
- Garcia-Crespo D, Juste R, Hurtado A (2005) Selection of ovine housekeeping genes for normalisation by real-time RT-PCR; analysis of PrP gene expression and genetic susceptibility to scrapie. BMC Veterinary Research 1:3 [DOI] [PMC free article] [PubMed]
- Goldmann W, Baylis M, Chihota C, Stevenson E, Hunter N (2005) Frequencies of PrP gene haplotypes in British sheep flocks and the implications for breeding programmes. J Appl Microbiol 98:1294–1302 [DOI] [PubMed]
- Hayes H, Petit E, Dutrillaux B (1991) Comparison of RBG-banded karyotypes of cattle, sheep, and goats. Cytogenet Cell Genet 57:51–55 [DOI] [PubMed]
- Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K (2003) The molecular chaperone Hsp90 plays a role in the assembly and maintenance of the 26S proteasome. Embo J 22:3557–3567 [DOI] [PMC free article] [PubMed]
- Jacquier-Sarlin MR, Jornot L, Polla BS (1995) Differential Expression and Regulation of hsp70 and hsp90 by Phorbol Esters and Heat Shock. J Biol Chem 270:14094–14099 [DOI] [PubMed]
- Kakimura J-I, Kitamura Y, Takata K et al (2002) Microglial activation and amyloid-{beta} clearance induced by exogenous heat-shock proteins. FASEB J 16:601–603 [DOI] [PubMed]
- Kang SC, Brown DR, Whiteman M et al (2004) Prion protein is ubiquitinated after developing protease resistance in the brains of scrapie-infected mice. J Pathol 203:603–608 [DOI] [PubMed]
- Lander ES, Green P (1987) Construction of Multilocus Genetic Linkage Maps in Humans 10.1073/pnas.84.8.2363. PNAS 84:2363–2367 [DOI] [PMC free article] [PubMed]
- Lyahyai J, Bolea R, Serrano C et al (2006) Correlation between Bax overexpression and prion deposition in medulla oblongata from natural scrapie without evidence of apoptosis. Acta Neuropathol (Berl) 112:451–460 [DOI] [PubMed]
- Lloyd SE, Onwuazor ON, Beck JA, Mallinson G, Farrall M, Targonski P, Collinge J, Fisher EM (2001) Identification of multiple quantitative trait loci linked to prion disease incubation period in mice. Proc Natl Acad Sci U S A 98:6279–6283 [DOI] [PMC free article] [PubMed]
- Lloyd SE, Uphill JB, Targonski PV, Fisher EM, Collinge J (2002) Identification of genetic loci affecting mouse-adapted bovine spongiform encephalopathy incubation time in mice. Neurogenetics 4:77–81 [DOI] [PubMed]
- Ma J, Lindquist S (2001) Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. PNAS 98:14955–14960 [DOI] [PMC free article] [PubMed]
- Maddox JF, Davies KP, Crawford AM et al (2001) An enhanced linkage map of the sheep genome comprising more than 1000 Loci 10.1101/gr.GR-1350R. Genome Res 11:1275–1289 [DOI] [PMC free article] [PubMed]
- Manolakou K, Beaton J, McConnell I, Farquar C, Manson J, Hastie ND, Bruce M, Jackson IJ (2001) Genetic and environmental factors modify bovine spongiform encephalopathy incubation period in mice. PNAS 98:7402–7407 [DOI] [PMC free article] [PubMed]
- Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res 16:1215 [DOI] [PMC free article] [PubMed]
- Moreno CR, Cosseddu GM, Andreoletti O et al (2003a) Identification of quantitative trait loci (QTL) modulating prion incubation period in sheep. (Identification de QTL affectant la durée d'incubation de la tremblante chez les ovins.). (Toulouse: Proceedings of the International Workshop on Major Genes and QTL in Sheep and Goat), Tolouse, France,8–11 December 2002, Communication No. 2–27
- Moreno CR, Lantier F, Lantier I, Sarradin P, Elsen JM (2003b) Detection of new quantitative trait Loci for susceptibility to transmissible spongiform encephalopathies in mice. Genetics 165:2085–2091 [DOI] [PMC free article] [PubMed]
- Nesic D, Maquat LE (1994) Upstream introns influence the efficiency of final intron removal and RNA 3″-end formation. Genes Dev 8:363–375 [DOI] [PubMed]
- Prusiner SB (1998) Prions. PNAS 95:13363–13383 [DOI] [PMC free article] [PubMed]
- SAS Institute Inc., SAS/STAT® Users's Guide, version 6, Fourth Edition, Volume1, Cary, NC:, 1989
- Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P (2005) Heat shock proteins as emerging therapeutic targets. Br J Pharmacol 146:769–780 [DOI] [PMC free article] [PubMed]
- Sreedhar AS, Kalmar E, Csermely P, Shen YF (2004) Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett 562:11–15 [DOI] [PubMed]
- Stephenson DA, Chiotti K, Ebeling C, Groth D, DeArmond SJ, Prusiner SB, Carlson GA (2000) Quantitative trait loci affecting prion incubation time in mice. Genomics 69:47–53 [DOI] [PubMed]
- Thompson AA, Wood WJ Jr., Gilly MJ, Damore MA, Omori SA, Wall R (1996) The promoter and 5′-flanking sequences controlling human B29 gene expression. Blood 87:666–673 [PubMed]
- Vaiman D, Billault A, Tabet-Aoul K, Schibler L, Vilette D, Oustry-Vaiman A, Soravito C, Cribiu EP (1999) Construction and characterization of a sheep BAC library of three genome equivalents. Mamm Genome 10:585–587 [DOI] [PubMed]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034 [DOI] [PMC free article] [PubMed]
- Wagner BJ, Hayes TE, Hoban CJ, Cochran BH (1990) The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. Embo J 9:4477–4484 [DOI] [PMC free article] [PubMed]
- Wang XD, Chen XM, Wang JZ et al (2006) Signal transducers and activators of transcription 3 mediates up-regulation of angiotensin II-induced tissue inhibitor of metalloproteinase-1 expression in cultured human senescent fibroblasts. Chin Med J (Engl) 119:1094–1102 [PubMed]
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB (1987) Distinct prion proteins in short and long scrapie incubation period mice. Cell 51:651–662 [DOI] [PubMed]
- Yamada A, Takaki S, Hayashi F, Georgopoulos K, Perlmutter RM, Takatsu K (2001) Identification and Characterization of a Transcriptional Regulator for the lck Proximal Promoter. J Biol Chem 276:18082–18089 [DOI] [PubMed]
- Yedidia Y, Horonchik L, Tzaban S, Yanai A, Taraboulos A (2001) Proteasomes and ubiquitin are involved in the turnover of the wild-type prion protein. EMBO J 20:5383–5391 [DOI] [PMC free article] [PubMed]
- Zhang SL, Yu J, Cheng XK, Ding L, Heng FY, Wu NH, Shen YF (1999) Regulation of human hsp90alpha gene expression. FEBS Lett 444:130–135 [DOI] [PubMed]
- Zhang X, Diab IH, Zehner ZE (2003) ZBP-89 represses vimentin gene transcription by interacting with the transcriptional activator, Sp1. Nucl Acids Res 31:2900–2914 [DOI] [PMC free article] [PubMed]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94:471–480 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 55.5 KB)
(DOC 42.0 KB)


