Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA) has traditionally been associated with infections in hospitals. Recently, a new strain of MRSA has emerged and rapidly spread in the community, causing serious infections among young, healthy individuals. Preliminary reports imply that a particular clone (USA300) of a community-acquired MRSA (CA-MRSA) strain is infiltrating hospitals and replacing the traditional hospital-acquired MRSA strains. If true, this event would have serious consequences, because CA-MRSA infections in hospitals would occur among a more debilitated, older patient population.
Methods
A deterministic mathematical model was developed to characterize the factors contributing to the replacement of hospital-acquired MRSA with CA-MRSA and to quantify the effectiveness of interventions aimed at limiting the spread of CA-MRSA in health care settings.
Results
The model strongly suggests that CA-MRSA will become the dominant MRSA strain in hospitals and health care facilities. This reversal of dominant strain will occur as a result of the documented expanding community reservoir and increasing influx into the hospital of individuals who harbor CA-MRSA. Competitive exclusion of hospital-acquired MRSA by CA-MRSA will occur, with increased severity of CA-MRSA infections resulting in longer hospitalizations and a larger in-hospital reservoir of CA-MRSA.
Conclusions
Improving compliance with hand hygiene and screening for and decolonization of CA-MRSA carriers are effective strategies. However, hand hygiene has the greatest return of benefits and, if compliance is optimized, other strategies may have minimal added benefit.
Infections caused by methicillin-resistant Staphylococcus aureus (MRSA) cause considerable morbidity and mortality, with estimated mortality rates surpassing those caused by HIV infection [1, 2]. Until recently, MRSA has been a health care—associated pathogen that affects predominantly the elderly population and debilitated individuals [3]. In 1998, a new strain of MRSA emerged in the community setting that caused infections among young, healthy individuals with no exposure to the health care setting [4]. Since then, community-acquired MRSA (CA-MRSA) strains have rapidly spread throughout the world [5]. Outbreaks of CA-MRSA have been reported among children, athletes, nurseries, obstetrical wards, and in many other populations [4, 6–8]. Some strains of CA-MRSA have been implicated in severe infections, including necrotizing skin infections, septic thrombophelbitis, bacteremia, and infective endocarditis [9–12].
The expanding community reservoir of CA-MRSA has led to the inevitable infiltration of CA-MRSA into hospitals [11, 13–15). Several reports further suggest that CA-MRSA may be replacing the traditional hospital-acquired MRSA (HA-MRSA) [11, 13–15]. This event has the potential for catastrophic consequences, because CA-MRSA can cause severe infections, which will now occur among debilitated, immunocompromised hospitalized patients.
We hypothesized that CA-MRSA will replace the traditional HA-MRSA strain in the health care setting over time, and we sought to identify the epidemiological factors that would result in the dominance of CA-MRSA strains and the competitive exclusion of HA-MRSA strains. A deterministic mathematical model was developed to quantify the temporal patterns of CAMRSA spread into the hospital setting. Using this model, we quantified the consequences of the expanding community reservoir and the increased documentation of more-severe infections caused by CA-MRSA in the dissemination of this new strain into the hospital. The model was also extended to determine the optimal strategy or combination of strategies that would prevent the in-hospital cross-transmission of CA-MRSA.
METHODS
Mathematical model
The deterministic differential equations model describes the transmission dynamics of CA-MRSA within a 400-bed tertiary care hospital with ∼25,000 admissions per year. Individuals within the hospital are in 5 mutually exclusive states: susceptible (S), colonized with either CA-MRSA (CC) or HA-MRSA (CH), or infected with either CA-MRSA (IC) or HA-MRSA (IH). Individuals enter the hospital in one of these states and exit via death or hospital discharge. Steady states of MRSA colonization or infection are achieved over time. Within the hospital, susceptible individuals can become colonized with either CA-MRSA or HA-MRSA and can subsequently become infected with the respective MRSA strain. Transmission of MRSA between individuals occurs through contact with health care workers. To simplify the model, co-colonization with CA-MRSA and HA-MRSA and environmental contamination were not included. Control strategies for preventing the spread of MRSA include improving compliance with hand hygiene and placing individuals who are infected with MRSA on contact precautions [16]. Patients who are infected with either HA-MRSA or CA-MRSA are assumed to be placed on contact precautions, thereby reducing their transmission risk, compared with that for the unidentified reservoir of asymptomatically colonized patients. The model compartments are illustrated schematically in figure 1. Steady states of MRSA colonization or infection are achieved over time. Mathematical equations are provided in a separate publication [17]. A deterministic model was used to better present the basic reproduction number and to consider the long-term behavior of the models in these large patient populations. Patients were therefore aggregated into compartments and were considered to be homogenous. Using stochastic, individual-based models would allow for patient heterogeneity; however, the increase in behavioral detail provides data that are more difficult to interpret and apply, compared with data provided by deterministic models [18].
Figure 1.
A compartment model of the transmission dynamics of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and hospital-acquired MRSA (HA-MRSA) in a 400-bed hospital. The arrows and parameter values correspond to entry and exit from the 5 compartments. The number of hospital admissions per day is Λ, with the fractions of patients admitted with CA-MRSA colonization, CA-MRSA infection, HA-MRSA colonization, and HA-MRSA infection expressed as λCC, λCH, λIC, and λIH, respectively. The transition rates between compartments or exit rates from compartments are expressed as follows: γS, γC, and γH are exit rates of susceptible patients, patients colonized with CA-MRSA, and patients colonized with HA-MRSA, respectively (with the mean length of stay defined as 1/γS, 1/γC, and 1/γH, respectively); the colonization rates of susceptible patients to the CA-MRSA colonization compartment are (1-η)βCC/N and (1-η)βIC/N and to the HA-MRSA colonization compartment are (1-η)βCH/N and (1-η)βIC/N, where η is the compliance with hand washing hygiene (with η = 0 corresponding to 0% compliance and η = 1 corresponding to 100% compliance), βCC, βIC, βCH, and βICare the rates of colonization transmission to patients from health care workers contaminated by patients with CA-MRSA colonization, CA-MRSA infection, HA-MRSA colonization, and HA-MRSA infection, respectively, and N is the total number of patients in the hospital. The rates of infection of patients with CA-MRSA colonization and patients with HA-MRSA colonization are ϕC and ϕH, respectively. The cure rates of patients with CA-MRSA infection and HA-MRSA infection are τC and τH, respectively, and the death rates of these patients are δCand δH, respectively. The rates of decolonization of patients with CA-MRSA colonization and HA-MRSA colonization are αCC and αCH, respectively.
Baseline parameters
Parameter estimates are obtained from the Beth Israel Deaconess Medical Center’s computerized database system, which provides patient and infection-control data. Values were also extrapolated from population-based surveillance or multicenter studies of the USA300 and other strains (table 1). The length of stay (LOS) of patients colonized with CA-MRSA was assumed to equal the LOS of susceptible patients, because patients colonized with CA-MRSA are predominantly healthy individuals whose colonization status would not affect their LOS. Patients who are colonized with HA-MRSA, however, are a group of patients with multiple comorbidities and with longer LOS.
Table. 1.
Estimates and values for the transmission dynamics of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and hospital-acquired MRSA (HA-MRSA).
| Variable | Symbol | Baseline value | Source |
|---|---|---|---|
| Total no. of patients | N | 400 | … |
| No. of admissions per day | |||
| Total | Λ | 70 | BI |
| Patients colonized with CA-MRSA | λCC | 0.03 | [19, 20 ] |
| Patients colonized with HA-MRSA | λCH | 0.07 | BI, [19, 20 ] |
| Patients infected with CA-MRSA | λIC | 0.005 | [11] |
| Patients infected with HA-MRSA | λIH | 0.0017 | [11] |
| Length of stay, by patient group | |||
| Susceptible patients | γS=1/5 | 5 days | BI |
| Patients colonized with CA-MRSA | γCC=1/5 | 5 days | BI |
| Patients colonized with HA-MRSA | γHC=1/7 | 7 days | [14] |
| Patients infected with CA-MRSA | γIC=1/10 | 10 days | [14] |
| Patients infected with HA-MRSA | γIH=1/18 | 18 days | [14] |
| Hand hygiene compliance efficacy, % | η | 60 | [21] |
| Transmission rate per susceptible patienta | |||
| Patients colonized with CA-MRSA per patient colonized with CA-MRSA/N |
βCC | 0.36 per day | [22, 23] |
| Patients colonized with HA-MRSA per patient colonized with HA-MRSA/N |
βCH | 0.27 per day | [22, 23] |
| Patients colonized with CA-MRSA per patient infected with CA-MRSA/N |
βIC | 0.09 per day | [22, 23] |
| Patients colonized with HA-MRSA per patient infected with HA-MRSA/N |
βIH | 0.07 per day | [22, 23] |
| Rate of infection per colonized patient per day of length of stay, % |
|||
| CA-MRSA | 100 ϕC | 10 γCC | [24] |
| HA-MRSA | 100 ϕH | 10 γCH | [24] |
| Death rate per infected patient per day of length of stay, % |
|||
| CA-MRSA | 100 δC | 3.3 γIC | [1, 25] |
| HA-MRSA | 100 δH | 20.0 γIH | [1, 25] |
| Infection cure rate per infected patient per day of length of stay, % |
|||
| CA-MRSA | 100 τC | 96.7 γIC | [1, 25] |
| HA-MRSA | 100 τH | 80.0 γIH | [1, 25] |
| Decolonization rate per colonized patient per day of length of stay, % |
|||
| CA-MRSA | 100 αC | 0 γCC | [26, 27] |
| HA-MRSA | 100 αH | 0 γCH |
NOTE. BI, data obtained from the Beth Israel Deaconess Medical Center.
Transmission of MRSA from a colonized or infected patient to a susceptible patient with an assumption that hand hygiene compliance is 0%.
Unique differences in the biology of CA-MRSA, compared with HA-MRSA, are incorporated into the transmission parameter (β). In vitro studies have demonstrated that the growth rate of CA-MRSA is ∼1.33 times faster than the growth rate of HA-MRSA [22]. The decreased doubling time can provide CA-MRSA strains an advantage toward more-successful colonization and subsequent transmission [22, 23]. The ratios of the transmission parameters for patients colonized or infected with CA-MRSA and HA-MRSA (βCC/βCHand βIC/βIH) were therefore set at ∼4:3. A simulation was also performed to determine the transmission dynamics of CA-MRSA if there were no differences in growth rate between CA-MRSA and HAMRSA and, therefore, an equal risk of transmission.
RESULTS
Transmission dynamics of CA-MRSA in the hospital
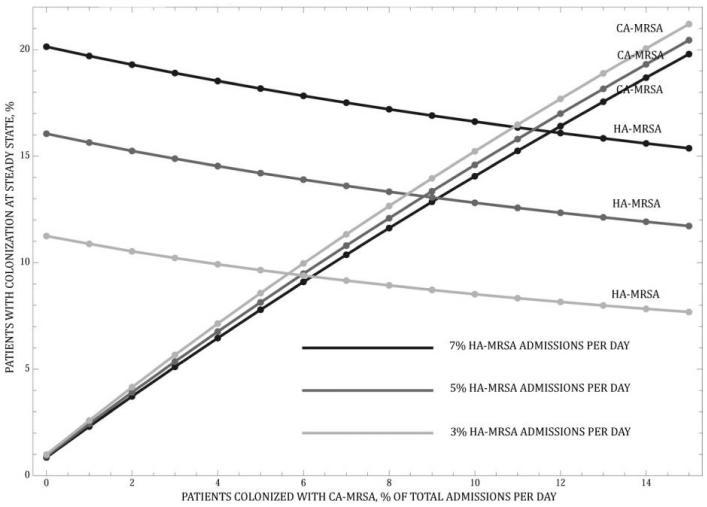
Simulations of the baseline model demonstrate that CA-MRSA becomes endemic in the hospital over time. At baseline, the endemic prevalence of HA-MRSA is higher that the prevalence of CA-MRSA, reflecting a greater admission rate and longer LOS among patients who harbor HA-MRSA, compared with patients who harbor CA-MRSA (figure 2). Increasing the influx into the hospital of patients who are colonized or infected with CA-MRSA, however, leads to a rapid reversal of dominance, with CA-MRSA strains surpassing the endemic prevalence of HA-MRSA (figure 3). The prevalence of CA-MRSA will further increase, given the feedback loop dynamics between the community and the hospital, whereby an increase in the influx of patients harboring CA-MRSA and a subsequent decrease in the prevalence of HA-MRSA in the hospital will lead to an overall decrease in the number of patients with HA-MRSA exiting and subsequently reentering the hospital. Figure 3 illustrates this concept.
Figure 2.
Numerical simulation using baseline parameter values and showing the proportion of hospitalized patients colonized or infected with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and hospital-acquired MRSA (HA-MRSA) over time. The patient subpopulations converge to endemic steady states. CC, patients colonized with CA-MRSA; CH, patients colonized with HA-MRSA; IC, patients infected with CA-MRSA; IH, patients infected with HA-MRSA; t, time.
Figure 3.
Effect of an increased influx of patients colonized with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and the feedback loop that creates a decrease in the influx of patients colonized with hospital-acquired MRSA (HA-MRSA). The percentage of admissions that are of patients colonized with HA-MRSA are held constant at 3%, 5%, and a baseline value of 7% as the percentage of admissions that are of patients colonized with CA-MRSA increases from 0% to 15%.
To determine whether the increase in the prevalence of CA-MRSA was attributable only to the greater value for the transmission parameter of CA-MRSA, based on the decreased doubling time, a simulation was performed with the transmission parameter of CA-MRSA equal to the transmission parameter of HA-MRSA. This simulation shows findings similar to those of the baseline model (figure 3); the prevalence of CA-MRSA continues to increase over time and surpasses the prevalence of HA-MRSA, but this requires that a greater number of patients who harbor CA-MRSA enter the hospital (figure 4).
Figure 4.
Effect of an increased influx of patients colonized with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and the feedback loop that creates a decrease in the influx of patients colonized with hospital-acquired MRSA (HA-MRSA) when the transmission risk of CA-MRSA is equal to that of HA-MRSA (βCC = βCH = .27 and βIC = βIH = .07).
Patients who harbor MRSA act as reservoirs for these pathogens and provide a constant source of transmission. Increasing the LOS of these individuals will, therefore, increase exposure to CA-MRSA among susceptible individuals. The baseline model assumed that the mean LOS among patients infected with CA-MRSA was 10 days. This value reflects the predominance of skin and soft-tissue infections caused by the community strain. CA-MRSA is also implicated in more-severe infections, which are reported with increasing frequency. These infections are associated with longer LOS and, therefore, result in greater exposure to the reservoirs of CA-MRSA [9–11]. The numerical simulations demonstrate that prolonging the LOS of patients infected with CA-MRSA increases the prevalence of CA-MRSA (figure 5). This increase is more marked if the LOS among colonized individuals increases, and it reflects the larger reservoir of colonized individuals, compared with infected individuals. Increasing the influx of patients who are colonized with CA-MRSA into the hospital, combined with an increased LOS of patients who are either colonized or infected with CA-MRSA, leads to even greater numbers of patients colonized with CA-MRSA over time (figure 5).
Figure 5.
The effect of increased admissions of patients colonized with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and the effect of an increased length of stay (LOS) among patients colonized with CA-MRSA (A) and patients infected with CA-MRSA (B) on the percentage of patients colonized with CA-MRSA.
Competitive exclusion principle
The competitive exclusion of HA-MRSA by CA-MRSA is demonstrated using the basic reproductive number (R0). R0 quantifies the mean number of secondary cases of MRSA colonization generated by 1 colonized individual. If R0< 1, the strain becomes extinct. Baseline R0 values for colonized CA-MRSA and HA-MRSA are and , respectively. In this case, with no influx of colonized or infected patients, both strains are eliminated. In general, the strain with the higher R0 value becomes endemic and drives the other strain to extinction, provided that its R0 value is >1. Numerical simulations demonstrate that competitive exclusion of HA-MRSA occurs when the LOS of patients who are either colonized or infected with CA-MRSA increases, resulting in exceeding the critical value of 1. The interpretation of the epidemic reproductive rates for HA-MRSA and CA-MRSA assumes that there are no admissions of colonized or infected individuals with either strain. Ongoing admission of either colonized or infected individuals guarantees that the epidemic is never eliminated. The level of endemicity is commensurate with admission rates and parameters in the model, as described by the formulas for the steady states.
Interventions
The effects of 3 standard control strategies were evaluated: (1) compliance with hand hygiene, (2) screening for MRSA colonization, and (3) decolonization of colonized individuals (figure 6). Screening results in the identification of previously unrecognized, asymptomatically colonized individuals, who are subsequently placed on contact precautions. Screening is therefore assumed to reduce the transmission risk of colonized individuals to the transmission risk of infected individuals, who are already on contact precautions (βCC = βIC and βCH = βIH). The identification of the unrecognized reservoirs with screening requires action, including not only the timely placement of newly identified colonized individuals on contact precautions, but also compliance with these precautions. Sensitivity analyses were performed to evaluate the “finding is not enough” concept of screening [26]. Decolonization of colonized individuals was simulated by adding the movement of colonized individuals into the susceptible compartment. The efficacy of decolonization with topical agents was set at 66% [27]. The parameter varied from 100% (to reflect potentially more-efficient decolonization strategies, such as whole-body antimicrobial washes, use of systemic antimicrobials, or combinations of such strategies) to 0% (to reflect the inevitable emergence of resistance to the antimicrobial agents) [28]. We also simulated the effect of these interventions and of combinations of these interventions as the rate of entry of patients with CA-MRSA increased.
Figure 6.
Comparison of the percentage of patients colonized with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) at steady state for 3 interventions (hand hygiene, screening, and decolonization). In the models shown, patients colonized with CA-MRSA account for 3% of admissions per day (baseline; A), 6% per day (B), 10% per day (C), and 20% per day (D).
Simulations demonstrate that all 3 interventions are effective in decreasing the spread of CA-MRSA. The magnitude of their effect, however, differs substantially, depending on the level of compliance or efficacy of each strategy. Decolonization of patients known to be colonized with CA-MRSA is the most effective strategy and would result in the lowest percentage of total patients colonized with CA-MRSA. The greater efficacy of decolonization, compared with that of hand hygiene or screening, reflects the first-order effect of decolonization, which would directly eliminate the reservoir of MRSA. The other 2 strategies, hand hygiene and screening, have a second-order effect, because they decrease MRSA transmission but do not eliminate the source. The impact of reduced efficacy of decolonization with the emergence of resistance to the decolonizing antimicrobial was also simulated [28].
Compliance with hand hygiene had the greatest return of benefits, with a decrease in compliance resulting in marked increases in the percentage of patients who were colonized with CA-MRSA; conversely, improved compliance dramatically decreased the percentage of colonized patients. In contrast with the other 2 interventions, there was no diminishing return as hand hygiene approached 100%, compared with improving the effectiveness of screening and decolonization toward 100%. The simulations also showed that, once compliance with hand hygiene surpassed 80%, it became a more effective strategy than screening.
The efficacy of these interventions as the influx of CA-MRSA into the hospital increased was also evaluated. The simulations showed that the relationship between the efficacies of the 3 interventions remained comparable, regardless of the rate of entry of patients colonized with CA-MRSA. The magnitude of effect however, increased with all 3 interventions as more individuals with CA-MRSA entered the hospital.
Combination of interventions
Combinations of 2 interventions and their impact on the spread of CA-MRSA were evaluated. These simulations further verified the importance of including improved hand hygiene compliance with other interventions and showed that, when compliance with hand hygiene was maximized, the additional benefit of effective screening or decolonization interventions was small. Screening interventions, for example, had minimal or no additional benefit if hand hygiene compliance was >90%. Conversely, if there was poor compliance with hand hygiene, effective screening strategies reduced the overall spread of CA-MRSA substantially (figure 7). Combining screening and decolonization strategies while maintaining a baseline hand hygiene compliance of 60% had a small additional benefit, even when the efficacy of these interventions was maximized (figure 7).
Figure 7.
Comparison of the percentage of patients colonized with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) at steady state for hand hygiene compliance, screening, and decolonization interventions. A, Hand hygiene compliance increases from 0% to 100% and the efficacy of decolonization increases from 0% to 25%, 50%, 75% and 100%. B, Hand hygiene compliance increases from 0% to 100% and the efficacy of the screening intervention increases from 0% to 25%, 50%, 75%, and 100%. C, The efficacy of the decolonization strategy increases from 0% to 100% and the efficacy of the screening strategy increases from 0% to 25%, 50%, 75%, and 100%.
DISCUSSION
The rapid emergence and spread of CA-MRSA in the community has become a major public health threat. Our model strongly suggests that CA-MRSA will become the dominant MRSA strain in hospitals, with competitive exclusion or near exclusion of the traditional HA-MRSA strain. Several hospitals have already documented the predominance of CA-MRSA over HA-MRSA strains as a cause of hospital-acquired infections, which provides preliminary validation of our model [11, 13, 15, 30].
Our model focused predominantly on the epidemiological factors contributing to the reversal of dominance between HA-MRSA and CA-MRSA strains in hospitals. Simulations demonstrate that the expanding community reservoir of CA-MRSA will lead to a greater influx of CA-MRSA strains in hospitals, with a rapid increase in the endemic hospital prevalence of CA-MRSA. The model strongly suggests that even small increases in the number of patients entering the hospital with CA-MRSA will contribute substantially to the in-hospital dissemination of CA-MRSA. The second factor that strongly contributes to the replacement of HA-MRSA by CA-MRSA is the expanding inhospital reservoir of CA-MRSA. Recent surveillance studies have documented that CA-MRSA strains are increasingly implicated in severe hospital-acquired infections, including blood stream and surgical site infections [11, 13, 15]. The morbidity and mortality associated with these nosocomial infections imply that the reservoir of patients who harbor CA-MRSA in the hospital will expand, because both the number of CA-MRSA– infected patients and their LOS will increase. A recent model of CA-MRSA transmission in a correctional facility also demonstrated that prolonged incarceration time would lead to a catastrophic outbreak [31]. Another mathematical model that described the population dynamics of antimicrobial-resistant bacteria among the community, hospitals, and long-term care facilities also demonstrated the important role of both the community reservoir and the LOS in the spread of antimicrobial-resistant organisms [32].
The replacement of the traditional HA-MRSA strain with CA-MRSA strains, as shown in our model, is further supported by unique bacterial characteristics of CA-MRSA that may provide these strains with a competitive advantage over HA-MRSA. CA-MRSA carries a smaller version of the staphylococcal cassette chromosomes mec (type IV SCCmec), which confers methicillin resistance, compared with larger cassettes carried by HA-MRSA (type 1-III SCCmec) [23]. CA-MRSA also tend to carry fewer antimicrobial resistance genes, compared with HA-MRSA [23]. The fitness cost of antibiotic resistance may, therefore, be minimized by the carriage of smaller or fewer genes, thereby providing CA-MRSA with a competitive advantage over HA-MRSA strains. The more rapid growth rate among CA-MRSA strains, compared with that among HA-MRSA strains, would further increase this advantage by potentially out-competing HA-MRSA strains and increasing the likelihood of colonization [22, 23]. Our model included the decreased doubling time of CA-MRSA in the transmission parameter but avoided the inclusion of greater biological fitness, because studies that directly compare the fitness between these 2 strains have not been performed to date. Our model simulations also showed that, even if an assumption is made that there is no difference in growth rate between CA-MRSA and HA-MRSA and, therefore, that the transmission risks are equal, the prevalence of CA-MRSA will still increase and surpass that of HA-MRSA over time.
Our model was extended to evaluate the efficacy of 3 control strategies aimed at limiting the spread of CA-MRSA strains. Decolonization was the most effective strategy. This intervention eliminated the reservoir of CA-MRSA, whereas the other 2 interventions only decreased the cross-transmission of CA-MRSA between patients. Although the decolonization strategy was the most effective, clarifications to this conclusion are warranted. First, CA-MRSA may preferentially colonize sites other than the nares, and therefore skin-to-skin or skin-to-fomite transmission may play a greater role in the spread of CA-MRSA than in the spread of HA-MRSA [29]. More-aggressive and costlier decolonization strategies, in addition to nasal decolonization (which is used predominantly for HA-MRSA), may therefore be indicated. Second, the emergence of resistance to the decolonizing agent, in addition to the high recolonization rates, need to be considered, because this strategy may provide only temporary benefit [28]. Improving compliance with hand hygiene was a very effective strategy and had the greatest return of benefits, with rapid decreases in the endemic prevalence as compliance increased and, conversely, rapid increases as compliance decreased. Improving compliance with this simple, inexpensive, and effective practice continues to be at the forefront of infection-control strategies. Interestingly, simulations that combined hand hygiene compliance and screening showed that the efficacy of a screening strategy is dependent on the level of hand hygiene compliance. In fact, when compliance with hand hygiene was maximized, screening had no additional benefit in decreasing the endemic prevalence of CA-MRSA. When compliance was poor, however, screening was effective. These data may provide some explanation of the contradictory conclusions of 2 recent studies that addressed the efficacy of MRSA screening [33, 34].
The transmission dynamics of CA-MRSA are complex. For CA-MRSA strains, outbreak investigations suggest that skin-to-skin and skin-to-fomite transmission may play a greater role in the spread of CA-MRSA than in the spread of HA-MRSA [29]. To simplify our model, environmental contamination and the potential for greater skin-to-skin transmission of CA-MRSA were not assessed. Inclusion of these routes would likely further increase the spread of CA-MRSA in hospitals. Previous models have also incorporated the transmission dynamics between health care workers and patients, and the models have included parameters that reflect the staffing ratio, the probability of health care worker contamination, and the rate of contact between health care workers and patients [35–37]. In this model, it was assumed that health care workers would contribute to the spread of HA-MRSA and CA-MRSA equally; therefore, separate compartments for health care workers were not included. The baseline parameters for our model were obtained from population-based surveillance and multicenter studies that defined CA-MRSA predominantly on the basis of the absence of risk factors for health care exposure. Recent data suggest that a substantial proportion of patients with health care exposure harbor CA-MRSA at admission to the hospital [38]. Thus, our baseline values may underestimate the extent of CA-MRSA burden in the hospital. Last, to simplify our model, cocolonization with CA-MRSA, HA-MRSA, or methicillin-susceptible S. aureus and colonization with multiple MRSA strains were not addressed and may lead to different results.
Our model strongly suggests that CA-MRSA will quickly replace the traditional HA-MRSA strain in hospitals. The expanding community reservoir of CA-MRSA, coupled with the greater growth rate and potentially greater biological fitness of this strain, support the conclusions of our model. The consequences of this reversal in dominance raise great concern, because the reported serious infections caused by CA-MRSA strains will now occur among hospitalized patients, who are a more debilitated and older patient population. Effective control is possible, but it necessitates compliance, especially with hand hygiene.
Acknowledgments
Financial support. The joint Division of Mathematical Sciences/National Institute of General Medicine Sciences Initiative through the National Institutes of Health (R01GM083607 to E.M.C.D., G.F.W., and S.R.) and the National Science Foundation (to M.A.H. and S.R.).
Potential conflicts of interest. R.C.M. has been a consultant for Pfizer, Cubist, and Targanta. All other authors: no conflicts.
Footnotes
Any opinion, findings, conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft EA. Antimicrobial resistance, it’s not just for hospitals. JAMA. 2007;298:1803–4. doi: 10.1001/jama.298.15.1803. [DOI] [PubMed] [Google Scholar]
- 3.Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11:868–72. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold BC, Immergluck LC, Maranan LC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 5.Tristan A, Bes M, Meugnier H, et al. Global distribution of panton-valentine leukocidin–positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis. 2007;13:594–600. doi: 10.3201/eid1304.061316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Methicillin-resistant Staphylococcus aureus infections among competitive sports participants-Colorado, Indiana, Pennsylvania, and Los Angeles county, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52:793–5. [PubMed] [Google Scholar]
- 7.Otter JA, French GL. Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2006;6:753–5. doi: 10.1016/S1473-3099(06)70636-3. [DOI] [PubMed] [Google Scholar]
- 8.Saiman L, O’keefe M, Graham PL, et al. Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis. 2003;37:1313–9. doi: 10.1086/379022. [DOI] [PubMed] [Google Scholar]
- 9.Millar BC, Prendergast BD, Moore JE. Community-associated MRSA (CA-MRSA): an emerging pathogen in infective endocarditis. J Anti-microb Chemother. 2008;61:1–7. doi: 10.1093/jac/dkm410. [DOI] [PubMed] [Google Scholar]
- 10.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. New Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 11.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 genotype as a major cause of health care—associated blood stream infections. Clin Infect Dis. 2006;42:647–66. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 12.Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006—January 2007. MMWR Morb Mortal Wkly Rep. 2007;56:325–29. [PubMed] [Google Scholar]
- 13.Patel M, Waites KB, Hoesley CJ, Stamm AM, Canupp KC, Moser SA. Emergence of USA-300 MRSA in a tertiary medical centre: implications for epidemiological studies. J Hosp Infect. 2008;68:208–13. doi: 10.1016/j.jhin.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Davis SL, Rybak MJ, Amjad M, Kaatz GW, McKinnon PS. Characteristics of patients with healthcare-associated infection due of SCCmec type IV methcillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2006;27:1025–31. doi: 10.1086/507918. [DOI] [PubMed] [Google Scholar]
- 15.Popovich KJ, Weinstein RA, Hota B. Are community-associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:795–8. doi: 10.1086/528716. [DOI] [PubMed] [Google Scholar]
- 16.Siegel JD, Rinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee Management of multidrug-resistant organisms in healthcare settings, 2006 Available at: http:// www.cdc.gov/ncidod/dhqp/pdf/ar/mdroGuideline2006.pdf Accessed 18 December 2008 [DOI] [PubMed]
- 17.Webb GF, Horn M, D’Agata EMC, Moellering RC, Jr, Ruan S.Competition of hospital-acquired and community-acquired methicillin-resistant Staphylococcus aureus strain in hospitals Available at: http:// www.math.miami.edu/~ruan/MyPapers/HAMRSA_CAMRSA_ Competition.pdf Accessed 15 October 2008 [DOI] [PMC free article] [PubMed]
- 18.D’Agata EMC, Magal P, Olivier D, Ruan S, Webb G. Modeling antibiotic resistance in hospitals: the impact of minimizing duration of treatment. J Theor Biol. 2007;249:487–99. doi: 10.1016/j.jtbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005;15:159–66. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 20.Jarvis WR, Schlosser J, Chinn RY, Tweeten S, Jackson M. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities, 2006. Am J Infect Control. 2007;35:631–7. doi: 10.1016/j.ajic.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Trick WE, Vernon MO, Welbel SF, Demarais P, Hayden MK, Weinstein RA. Chicago Antimicrobial Resistance Project. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infect Control Hosp Epidemiol. 2007;28:42–9. doi: 10.1086/510809. [DOI] [PubMed] [Google Scholar]
- 22.Okuma K, Iwakawa K, Turnidge JD, et al. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba T, Takeuchi F, Kuroda M, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–27. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 24.Burke JP. Infection control: a problem for patient safety. N Engl J Med. 2003;348:651–6. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 25.Selvey LA, Whitby M, Johnson B. Nosocomial methicillin-resistant Staphylococcus aureus bacteremia: is it any worse than nosocomial methicillin-sensitive Staphylococcus aureus bacteremia? Infect Control Hosp Epidemiol. 2000;21:645–8. doi: 10.1086/501707. [DOI] [PubMed] [Google Scholar]
- 26.Diekema DJ, Climo M. Preventing MRSA infections: finding it is not enough. JAMA. 2008;299:1190–2. doi: 10.1001/jama.299.10.1190. [DOI] [PubMed] [Google Scholar]
- 27.Wertheim HF, Melles DC, Vos MC, et al. Effect of mupirocin treatment on nasal, pharyngeal, and perineal carriage of Staphylococcus aureus in healthy adults. Antimicrob Agents Chemother. 2005;49:1465–7. doi: 10.1128/AAC.49.4.1465-1467.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conly JM, Johnston BL. Mupirocin: are we in danger of losing it? Can J Infect Dis. 2002;13:157–9. doi: 10.1155/2002/692581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:752–60. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 30.Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Pedrau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730–8. doi: 10.1086/425019. [DOI] [PubMed] [Google Scholar]
- 31.Kajita E, Okano JT, Bodine EN, Layne SP, Blower S. Modelling an outbreak of an emerging pathogen. Nat Rev Microbiol. 2007;5:700–9. doi: 10.1038/nrmicro1660. [DOI] [PubMed] [Google Scholar]
- 32.Smith DL, Dushoff J, Perencevich EN, Harris AD, Levin SA. Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: resistance is a regional problem. Proc Natl Acad Sci U S A. 2004;101:3709–14. doi: 10.1073/pnas.0400456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harbath S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA. 2008;299:1149–57. doi: 10.1001/jama.299.10.1149. [DOI] [PubMed] [Google Scholar]
- 34.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Int Med. 2008;148:409–18. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 35.Bootsma MCJ, Diekmann O, Bonten MJM. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of iterventions and rapid diagnostic testing. Proc Natl Acad Sci U S A. 2006;103:5620–5. doi: 10.1073/pnas.0510077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Agata EMC, Webb G, Horn M. A mathematical model quantifying the impact of antibiotic exposure and other interventions on the endemic prevalence of vancomycin-resistant enterococci. J Infect Dis. 2005;192:2004–11. doi: 10.1086/498041. [DOI] [PubMed] [Google Scholar]
- 37.Austin DJ, Bonten MJM, Weinstein RA, Slaughter S, Anderson R. Vancomycin-resistant enterococci in intensive-care hospital settings: transmission dynamics, persistence, and the impact of infection control programs. Proc Natl Acad Sci U S A. 1999;96:6908–13. doi: 10.1073/pnas.96.12.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197:1235–43. doi: 10.1086/533502. [DOI] [PubMed] [Google Scholar]