Abstract
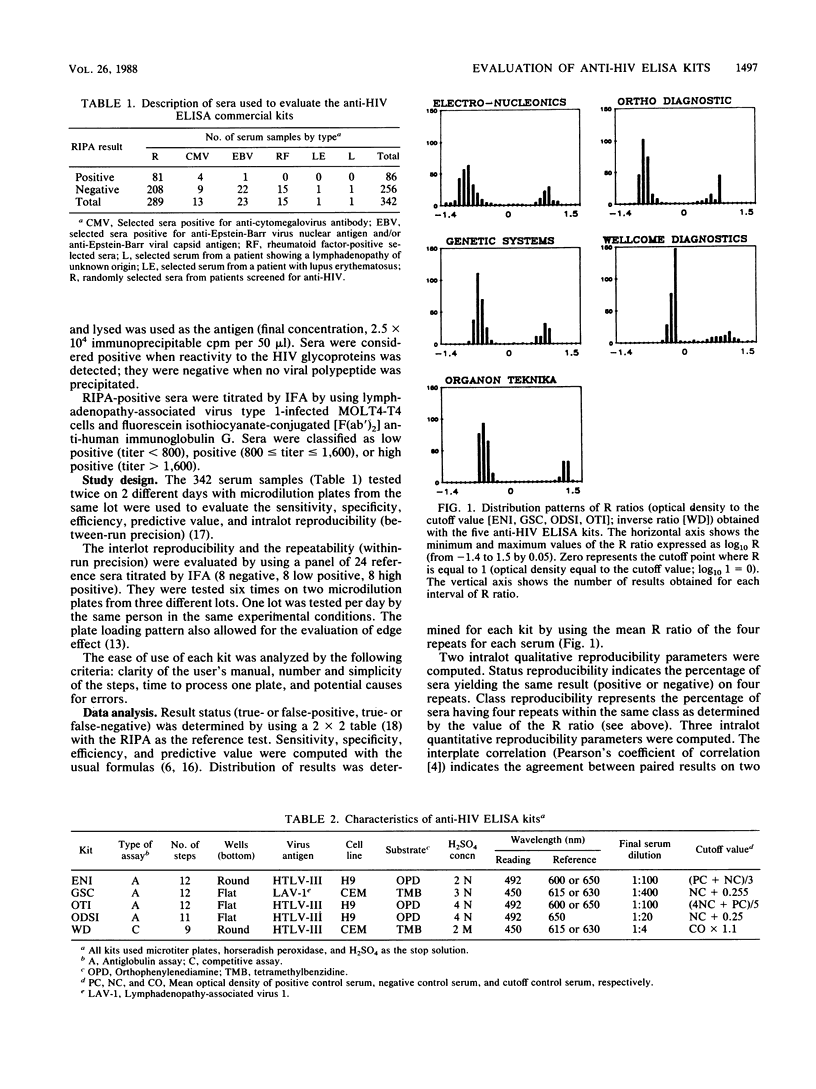
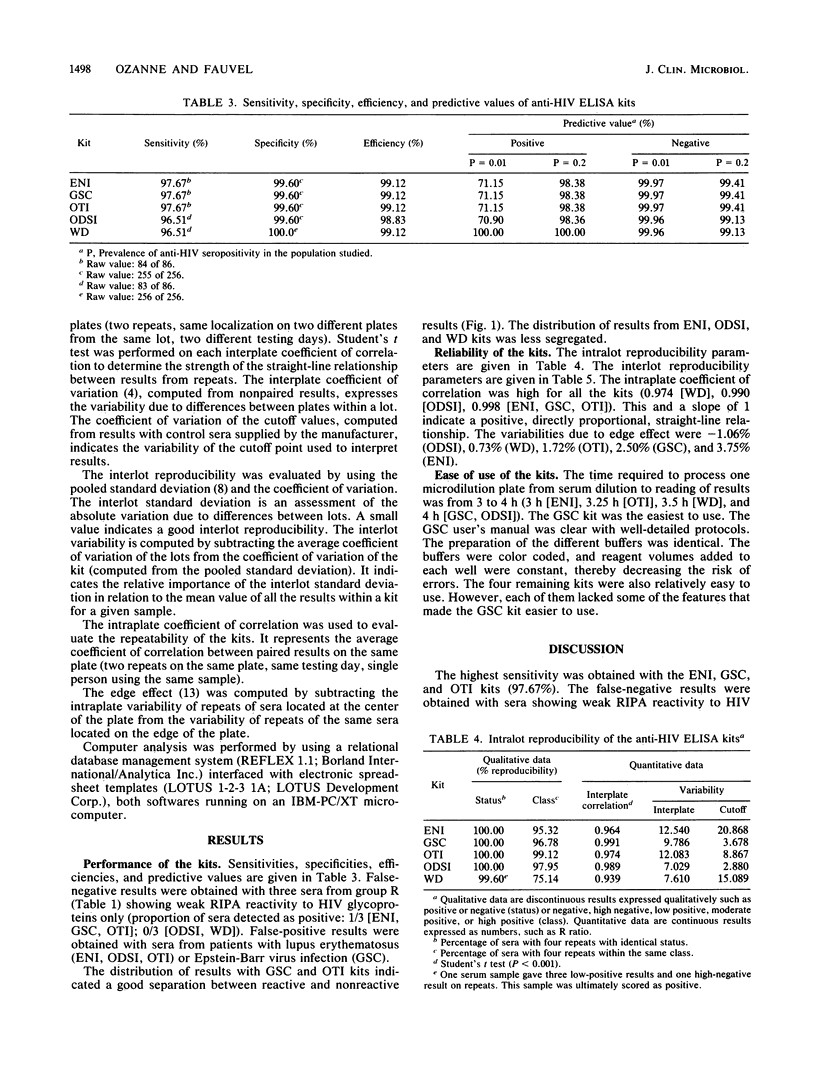
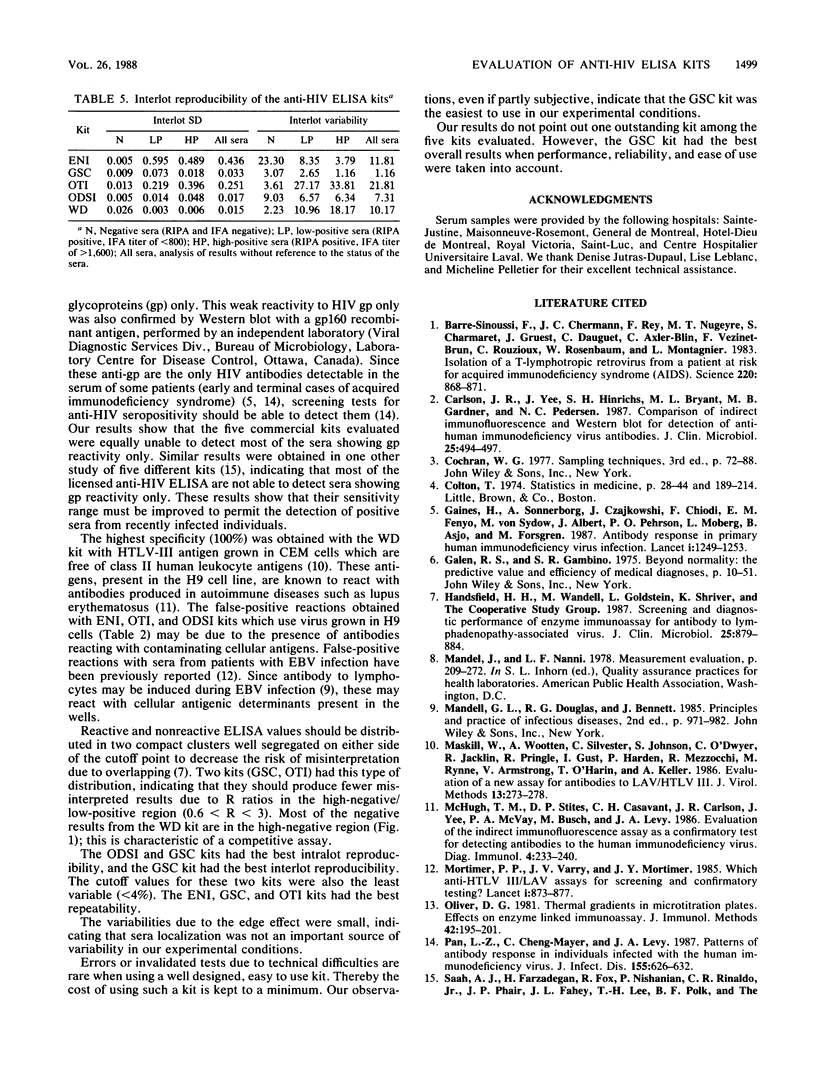
Anti-human immunodeficiency virus enzyme-linked immunosorbent assay kits marketed by Electro-Nucleonics Inc. (ENI), Genetic Systems Corp. (GSC), Organon Teknika Inc. (OTI), Ortho Diagnostic Systems Inc. (ODSI), and Wellcome Diagnostics (WD) were evaluated by using 289 randomly selected serum samples from a high-risk population and 53 serum samples likely to produce false-positive results. The radioimmunoprecipitation assay was used as the reference test. Sensitivities ranged from 96.51% (ODSI, WD) to 97.67% (ENI, GSC, OTI). Sera showing antibodies to viral glycoproteins only produced the false-negative results. Specificities ranged from 99.6% (ENI, GSC, ODSI, OTI) to 100% (WD). False-positive results were obtained with sera from patients with autoimmune disease or Epstein-Barr virus infection. Only results from GSC and OTI kits were distributed in two compact clusters well segregated on either side of the cutoff point. ODSI and GSC kits had the best intralot reproducibility. The GSC kit had the best interlot reproducibility. Cutoff values for ODSI and GSC kits were the least variable. Intraplate repeatability was good for all kits. Sample localization was not an important source of variability. Our results do not point out one outstanding kit among the five evaluated. However, the GSC kit showed the best overall results.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Yee J., Hinrichs S. H., Bryant M. L., Gardner M. B., Pedersen N. C. Comparison of indirect immunofluorescence and Western blot for detection of anti-human immunodeficiency virus antibodies. J Clin Microbiol. 1987 Mar;25(3):494–497. doi: 10.1128/jcm.25.3.494-497.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaines H., von Sydow M., Sönnerborg A., Albert J., Czajkowski J., Pehrson P. O., Chiodi F., Moberg L., Fenyö E. M., Asjö B. Antibody response in primary human immunodeficiency virus infection. Lancet. 1987 May 30;1(8544):1249–1253. doi: 10.1016/s0140-6736(87)92696-1. [DOI] [PubMed] [Google Scholar]
- Handsfield H. H., Wandell M., Goldstein L., Shriver K. Screening and diagnostic performance of enzyme immunoassay for antibody to lymphadenopathy-associated virus. J Clin Microbiol. 1987 May;25(5):879–884. doi: 10.1128/jcm.25.5.879-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskill W., Healey D., Wootten A., Silvester C., Johnson S., O'Dwyer C., Jacklin R., Pringle R., Gust I., Harden P. Evaluation of a new assay for antibodies to LAV/HTLV III. J Virol Methods. 1986 Jul;13(4):273–278. doi: 10.1016/0166-0934(86)90051-0. [DOI] [PubMed] [Google Scholar]
- McHugh T. M., Stites D. P., Casavant C. H., Carlson J. R., Yee J., McVay P. A., Busch M. P., Levy J. A. Evaluation of the indirect immunofluorescence assay as a confirmatory test for detecting antibodies to the human immunodeficiency virus. Diagn Immunol. 1986;4(5):233–240. [PubMed] [Google Scholar]
- Mortimer P. P., Parry J. V., Mortimer J. Y. Which anti-HTLV III/LAV assays for screening and confirmatory testing? Lancet. 1985 Oct 19;2(8460):873–877. doi: 10.1016/s0140-6736(85)90136-9. [DOI] [PubMed] [Google Scholar]
- Oliver D. G., Sanders A. H., Hogg R. D., Hellman J. W. Thermal gradients in microtitration plates. Effects on enzyme-linked immunoassay. J Immunol Methods. 1981;42(2):195–201. doi: 10.1016/0022-1759(81)90149-6. [DOI] [PubMed] [Google Scholar]
- Pan L. Z., Cheng-Mayer C., Levy J. A. Patterns of antibody response in individuals infected with the human immunodeficiency virus. J Infect Dis. 1987 Apr;155(4):626–632. doi: 10.1093/infdis/155.4.626. [DOI] [PubMed] [Google Scholar]
- Sivak S. L., Wormser G. P. Predictive value of a screening test for antibodies to HTLV-III. Am J Clin Pathol. 1986 Jun;85(6):700–703. doi: 10.1093/ajcp/85.6.700. [DOI] [PubMed] [Google Scholar]
- Weiss S. H., Goedert J. J., Sarngadharan M. G., Bodner A. J., Gallo R. C., Blattner W. A. Screening test for HTLV-III (AIDS agent) antibodies. Specificity, sensitivity, and applications. JAMA. 1985 Jan 11;253(2):221–225. [PubMed] [Google Scholar]


