Abstract
Cyclic nucleotide-regulated cation channels are ion channels whose activation is regulated by the direct binding of cAMP or cGMP to the channel protein. Two structurally related families of channels regulated by cyclic nucleotides have been identified, the cyclic nucleotide-gated channels and the hyperpolarization-activated cyclic nucleotide-gated channels. Cyclic nucleotide-gated channels play a key role in visual and olfactory transduction. Hyperpolarization-activated cyclic nucleotide-gated channels are present in the conduction system of the heart and are involved in the control of cardiac automaticity. Moreover, these channels are widely expressed in central and peripheral neurons, where they control a variety of fundamental processes.
Cyclic nucleotides exert their physiological effects by binding to four major classes of cellular receptors: cAMP- and cGMP-dependent protein kinases (1, 2), cGMP-regulated phosphodiesterases (3), cAMP-binding guanine nucleotide exchange factors (4), and cyclic nucleotide-regulated cation channels. Cyclic nucleotide-regulated cation channels are unique among these receptors because their activation is directly coupled to the influx of extracellular cations into the cytoplasm and to the depolarization of the plasma membrane. Two families of channels regulated by cyclic nucleotides have been identified, the CNG2 and HCN channels (5–9). The two channel classes differ from each other with regard to their mode of activation. CNG channels are opened by direct binding of cAMP or cGMP. In contrast, HCN channels are principally operated by voltage. These channels open at hyperpolarized membrane potentials and close upon depolarization. Apart from their voltage sensitivity, HCN channels are also activated directly by cyclic nucleotides, which act by increasing the channel open probability.
General Features of Cyclic Nucleotide-regulated Cation Channels
Structurally, CNG and HCN channels are members of the superfamily of voltage-gated cation channels (10). Like other subunits encoded by this large gene family, CNG and HCN channel subunits assemble into tetrameric complexes. The proposed structure and the phylogenetic relationship between mammalian CNG and HCN channel subunits are shown in Fig. 1 (see also Table 1). The transmembrane channel core consists of six α-helical segments (S1–S6) and an ion-conducting pore loop between S5 and S6. The N and C termini are localized in the cytosol. CNG and HCN channels contain a positively charged S4 helix carrying three to nine regularly spaced arginine or lysine residues at every third position. In HCN channels, as in most other members of the channel superfamily, the S4 helix functions as a “voltage sensor,” conferring voltage-dependent gating. However, inward movement of S4 charges through the plane of the cell membrane leads to opening of HCN channels, whereas it triggers the closure of depolarization-activated channels such as the Kv channels (11). The molecular determinants underlying the different polarity of the gating mechanism between HCN and depolarization-gated channels remain to be determined. In CNG channels, which are not gated by voltage, the specific role of S4 is unknown.
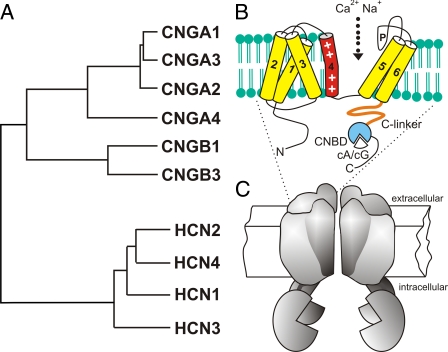
FIGURE 1.
A, phylogenetic tree and structural model of mammalian cyclic nucleotide-regulated cation channels. The CNG channel family comprises six members, which are classified into A subunits (CNGA1–4) and B subunits (CNGB1 and CNGB3). The HCN channel family comprises four members (HCN1–4). B, CNG and HCN channel subunits share a common transmembrane topology, consisting of six α-helical segments (S1–S6) and a pore loop (P). In the cytosolic C terminus, all subunits carry a CNBD that is functionally coupled to the transmembrane channel core via the C-linker domain. The S4 segment contains a series of positively charged residues and forms the voltage sensor in HCN channels. CNG channels are activated in vivo by binding of either cAMP (cA) or cGMP (cG), depending on the channel type. HCN channels activate upon membrane hyperpolarization. Binding of cAMP to the CNBD produces an allosteric conformational change that increases the open probability of the channel pore. C, four subunits assemble to form functional CNG and HCN channels, respectively. There is no evidence for the formation of mixed CNG-HCN channel tetramers.
TABLE 1.
Molecular properties of cyclic nucleotide-regulated cation channels
aa, amino acids; pS, picosiemens; NE, not established.
| Subunit | Primary sequence | Conductance | Glycosylation |
|---|---|---|---|
| CNGA1 | 683 aaa,b | 25-30 pSc | Yes (66) |
| CNGA2 | 664 aaa | 35 pSc | Yes (67) |
| CNGA3 | 631 aaa | 40 pSc | NE |
| CNGA4 | 575 aaa | No (67) | |
| CNGB1a | 1339 aad | No (66) | |
| CNGB1b | 858 aad | No (67) | |
| CNGB3 | 694 aaa | NE | |
| HCN1 | 910 aaa | NEe | Yes (68, 69) |
| HCN2 | 863 aaa | NEe | Yes (69) |
| HCN3 | 779 aaa | NEe | Yes (70) |
| HCN4 | 1201 aaa | NEe | Yes (69) |
This is the mouse isoform.
The proximal N terminus of CNGA1 is proteolytically cleaved in rod outer segments (71).
This was in calcium-free solution.
This is the rat isoform.
There is an ongoing controversy on the size of the single channel conductance of HCN channels. Originally, single channel conductance was found to be very low, being in the range of ∼1 pS (72). This estimate is in good agreement with very recent data (73). However, single channel conductances that are 10-30 times higher have been reported by Michels et al. (74).
CNG and HCN channels reveal different ion selectivities. CNG channels pass monovalent cations such as Na+ and K+ but do not discriminate between them. Ca2+ is also permeable but at the same time acts as a voltage-dependent blocker of monovalent cation permeability (12). By providing an entry pathway for Ca2+, CNG channels control a variety of cellular processes that are triggered by this cation. HCN channels conduct Na+ and K+ with permeability ratios of ∼1:4 and are blocked by millimolar concentrations of Cs+ (13–15). Despite this preference for K+ conductance, HCN channels carry an inward Na+ current under physiological conditions. HCN channels can also conduct Ca2+ but not as well as CNG channels. At 2.5 mm external Ca2+, the fractional Ca2+ current of HCN2 and HCN4 is ∼0.5%, whereas for native CNG channels, it is in the range of 10–80% (16).
In the C terminus, CNG and HCN channels contain a CNBD that has significant sequence similarity to the CNBDs of most other types of cyclic nucleotide receptors. The crystal structure of the CNBD has been determined for HCN2 (17), the HCN channel from sea urchin (18), and a bacterial cyclic nucleotide-regulated potassium channel (19). In CNG channels, the binding of cyclic nucleotides to the CNBD initiates a sequence of allosteric transitions that lead to the opening of the ion-conducting pore (7). In HCN channels, the binding of cyclic nucleotides is not required for activation. However, cyclic nucleotides shift the voltage dependence of channel activation to more positive membrane potentials and thereby facilitate voltage-dependent channel activation (13–15). Despite the fact that the CNBDs of HCN and CNG channels show significant sequence homology, the two channel classes reveal different selectivities for cyclic nucleotides. HCN channels display an ∼10-fold higher apparent affinity for cAMP than for cGMP, whereas CNG channels select cGMP over cAMP (5, 7). Recently, amino acid residues determining this difference have been identified (18, 20).
CNG Channels
CNG channels are expressed in retinal photoreceptors and olfactory neurons and play a key role in visual and olfactory signal transduction (5, 6, 8). CNG channels are also found at low density in some other cell types and tissues such as brain, testis, and kidney (5). Whereas the function of CNG channels in sensory neurons has been unequivocally demonstrated, the role of these channels in other cell types remains to be established. Based on the phylogenetic relationship, the six CNG channels identified in mammals are divided in two subfamilies, the A subunits (CNGA1–4) and the B subunits (CNGB1 and CNGB3) (Fig. 1). CNG channel A subunits (with the only exception of CNGA4) form functional homomeric channels in various heterologous expression systems. In contrast, the B subunits do not give rise to functional channels when expressed alone. However, when coexpressed with CNGA1–3, they confer novel properties (e.g. single channel flickering, increased sensitivity for cAMP and l-cis-diltiazem) that are characteristic of native CNG channels (5). Recent genetic studies in mice indicate that besides modulating intrinsic channel properties, the B subunits also play a key role in principal channel formation and channel targeting in native sensory neurons (21, 22). The subunit composition is known for three native CNG channels: the rod and cone photoreceptor channels and the olfactory channel. The CNG channel of rod photoreceptors consists of the CNGA1 subunit and a long isoform of the CNGB1 subunit (CNGB1a) (3:1 stoichiometry) (23–25). The cone photoreceptor channel consists of the CNGA3 and CNGB3 subunits (2:2 stoichiometry) (26). CNG channels control the membrane potential and the calcium concentration of photoreceptors. In the dark, both channels are maintained in the open state by a high concentration of cGMP. The resulting influx of Na+ and Ca2+ (“dark current”) depolarizes the photoreceptor and promotes synaptic transmission. Light-induced hydrolysis of cGMP leads to the closure of the CNG channel. As a result, the photoreceptor hyperpolarizes and shuts off synaptic glutamate release. Mutations in the CNGA1 (27) and CNGB1 (28) subunits have been identified in the genome of patients suffering from retinitis pigmentosa. The functional loss of either the CNGA3 (29, 30) or CNGB3 (31) subunit causes total color blindness (achromatopsia) and degeneration of cone photoreceptors.
The CNG channel expressed in cilia of OSNs consists of three different subunits: CNGA2, CNGA4, and a short isoform of the CNGB1 subunit (CNGB1b) (2:1:1 stoichiometry) (32). The channel is activated in vivo by cAMP, which is synthesized in response to the binding of odorants to their cognate receptors. The olfactory CNG channel mainly conducts Ca2+ under physiological ionic conditions (33). The increase in cellular Ca2+ activates a Ca2+-activated Cl- channel, which further depolarizes the cell membrane. Ca2+ is not only a permeating ion of the olfactory CNG channel but also an important modulator of this channel. By forming a complex with CaM, which binds to the CNGB1b and CNGA4 subunits, Ca2+ decreases sensitivity of the CNG channel to cAMP (33). Fast Ca2+/CaM desensitization of the CNG channel has been inferred to be the dominant mechanism of OSN adaptation to repeated stimulation and also to play a role in adaptation during sustained stimulation (34–36). However, analysis of a genetic mouse model lacking fast Ca2+/CaM desensitization (CNGB1ΔCaM mice) has challenged this hypothesis (37). CNGB1ΔCaM mice showed normal receptor current adaptation to repeated stimulation. Rather, the mice displayed slower response termination and, consequently, reduced ability to transmit olfactory information to the olfactory bulb. They also displayed reduced response decline during sustained odorant exposure. These results suggest that Ca2+/CaM-mediated CNG channel fast desensitization is less important in regulating the sensitivity to recurring stimulation than previously thought and instead functions primarily to terminate OSN responses.
HCN Channels
A cation current that is slowly activated by membrane hyperpolarization (termed Ih, If, or Iq) is found in a variety of excitable cells, including neurons, cardiac pacemaker cells, and photoreceptors (38). The best established function of Ih is to control heart rate and rhythm by acting as “pacemaker current” in the SA node (39). Ih is activated during the membrane hyperpolarization following the termination of an action potential and provides an inward Na+ current that slowly depolarizes the plasma membrane. Sympathetic stimulation of SA node cells raises cAMP levels and increases Ih by a positive shift of the current activation curve, thus accelerating diastolic depolarization and heart rate. Stimulation of muscarinic receptors slows down heart rate by the opposite action. In neurons, Ih fulfills diverse functions, including generation of pacemaker potentials, control of membrane potential, generation of rebound depolarizations during light-induced hyperpolarizations of photoreceptors, dendritic integration, and synaptic transmission (9, 40, 41).
HCN channels represent the molecular correlate of the Ih current (13–15). In mammals, the HCN channel family comprises four members (HCN1–4) that share ∼60% sequence identity with each other and ∼25% sequence identity with CNG channels. The highest degree of sequence homology between HCN and CNG channels is found in the CNBD. When expressed in heterologous systems, all four HCN channels generate currents displaying the typical features of native Ih: activation by membrane hyperpolarization, permeation of Na+ and K+, positive shift of the voltage dependence of channel activation by direct binding of cAMP, and channel blockade by extracellular Cs+. HCN1–4 mainly differ from each other with regard to their speed of activation and the extent by which they are modulated by cAMP. HCN1 is the fastest channel, followed by HCN2, HCN3, and HCN4. Unlike HCN2 and HCN4, whose activation curves are shifted by about +15 mV by cAMP, HCN1 and HCN3 are only weakly affected by cAMP, if at all. Site-directed mutagenesis experiments have provided initial insight into the complex mechanism underlying dual HCN channel activation by voltage and cAMP. As in other voltage-gated cation channels, activation of HCN channels is initiated by the movement of the positively charged S4 helix in the electric field (11). The resulting conformational change in the channel protein is allosterically coupled by other channel domains to the opening of the ion-conducting pore. Major determinants affecting channel activation are the intracellular S4-S5 loop, the S1 segment, and the extracellular S1-S2 loop (42–44). The CNBD fulfills the role of an autoinhibitory channel domain. In the absence of cAMP, the cytoplasmic C terminus inhibits HCN channel gating by interacting with the channel core and thereby shifting the activation curve to more hyperpolarizing voltages (45). Binding of cAMP to the CNBD relieves this inhibition. Differences in the magnitude of the response to cAMP among the four HCN channel isoforms are largely due to differences in the extent to which the CNBD inhibits basal gating. It remains to be determined if the inhibitory effect of the CNBD is conferred by a direct physical interaction with the channel core domain or by some indirect pathway. There is initial evidence that the so-called C-linker, a peptide of ∼80 amino acids that connects the last transmembrane helix (S6) to the CNBD, plays an important role in this process. The C-linker was also shown to play a key role in the gating of CNG channels, suggesting that the functional role of this domain has been conserved during channel evolution (7, 46, 47).
HCN channels are found in neurons and heart cells. In mouse and rat brains, all four HCN channel isoforms have been detected (48, 49). HCN2 is the most abundant channel and is found almost ubiquitously in the brain. In contrast, HCN1, HCN3, and HCN4 are enriched in specific regions of the brain such as the thalamus (HCN4), hippocampus (HCN1), and olfactory bulb and hypothalamus (HCN3). HCN channels have also been detected in the retina and some peripheral neurons such as dorsal root ganglion neurons. In SA node cells, HCN4 represents the predominantly expressed HCN channel isoform. In addition, minor amounts of HCN1 and HCN2 are also present in these cells. Insights into the (patho)physiological relevance of HCN channels have been gained from the analysis of mouse lines lacking individual HCN channel isoforms. Disruption of HCN1 impairs motor learning but enhances spatial learning and memory (50, 51). Deletion of HCN2 results in absence epilepsy, ataxia, and sinus node dysfunction (52). Mice lacking HCN4 die in utero because of the failure to generate mature SA pacemaker cells (53). Interestingly, mice in which HCN4 is deleted at the adult stage are viable but display cardiac arrhythmia characterized by recurrent sinus pauses (54). The key role of HCN4 in controlling heart rhythmicity is corroborated by genetic data from human patients. Mutations in the human HCN4 gene leading to mutated or truncated channel proteins have been found to be associated with sinus bradycardia (55–58) and complex cardiac arrhythmia (56).
HCN Channels as Therapeutic Targets
Given the key role of HCN channels in cardiac pacemaking, these channels are promising pharmacological targets for the development of drugs used in the treatment of cardiac arrhythmias and ischemic heart disease. HCN channels are not expressed in vascular and airway smooth muscles. As a consequence, specific HCN channel blockers are expected to have no side effect on the peripheral resistance. Notably, unlike the well established β-adrenoreceptor blockers, HCN channel blockers would not impair pulmonary function in patients with asthma or obstructive pulmonary disease. Recently, ivabradine (S16257, Procoralan) was approved as the first therapeutic Ih blocker (59). Ivabradine blocks cardiac Ih at low micromolar concentrations and is used in the treatment of stable angina pectoris. Other known Ih blockers with blocking mechanisms related to that of ivabradine are ZD7288, zatebradine, and cilobradine (60). These blockers were not introduced into therapy because they either lacked specificity or exerted unacceptable side effects, in particular visual disturbances due to the inhibition of retinal Ih. Interestingly, the well known α2-adrenoreceptor agonist clonidine also effectively blocks HCN channels (61). The block of cardiac Ih (mainly conferred by HCN4) contributes significantly to the bradycardic effect of clonidine. Modulation of Ih may also be a promising approach for treatment of disease processes in the central and peripheral nervous systems. For example, Ih is up-regulated in dorsal root ganglion neurons in response to nerve injury, making HCN channels interesting candidates for therapeutic modulation of inflammation and neuropathic pain (62). Moreover, agents acting on HCN channels may be utilized in the treatment of epilepsies (63). Finally, HCN1 and HCN2 channels are inhibited by clinically relevant concentrations (≤0.5 mm) of the inhalational anesthetics halothane and isoflurane (64). Similarly, the intravenous anesthetic propofol inhibits and slows the activation of native and expressed HCN channels (65). Thus, modulation of Ih may contribute to clinical actions of anesthetic agents.
Supplementary Material
This work was supported by the Deutsche Forschungsgemeinschaft and by European Union Research Project NORMACOR within the Sixth Framework Programme of the European Union. This minireview will be reprinted in the 2009 Minireview Compendium, which will be available in January, 2010.
Footnotes
The abbreviations used are: CNG, cyclic nucleotide-gated; HCN, hyperpolarization-activated cyclic nucleotide-gated; CNBD, cyclic nucleotide-binding domain; OSN, olfactory sensory neuron; CaM, calmodulin; SA, sinoatrial.
References
- 1.Taylor, S. S., Kim, C., Vigil, D., Haste, N. M., Yang, J., Wu, J., and Anand, G. S. (2005) Biochim. Biophys. Acta 1754 25–37 [DOI] [PubMed] [Google Scholar]
- 2.Hofmann, F., Feil, R., Kleppisch, T., and Schlossmann, J. (2006) Physiol. Rev. 86 1–23 [DOI] [PubMed] [Google Scholar]
- 3.Bender, A. T., and Beavo, J. A. (2006) Pharmacol. Rev. 58 488–520 [DOI] [PubMed] [Google Scholar]
- 4.Bos, J. L. (2006) Trends Biochem. Sci. 31 680–686 [DOI] [PubMed] [Google Scholar]
- 5.Kaupp, U. B., and Seifert, R. (2002) Physiol. Rev. 82 769–824 [DOI] [PubMed] [Google Scholar]
- 6.Hofmann, F., Biel, M., and Kaupp, U. B. (2005) Pharmacol. Rev. 57 455–462 [DOI] [PubMed] [Google Scholar]
- 7.Craven, K. B., and Zagotta, W. N. (2006) Annu. Rev. Physiol. 68 375–401 [DOI] [PubMed] [Google Scholar]
- 8.Biel, M., and Michalakis, S. (2007) Mol. Neurobiol. 35 266–277 [DOI] [PubMed] [Google Scholar]
- 9.Wahl-Schott, C., and Biel, M. (2009) CMLS Cell. Mol. Life Sci. 66 470–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu, F. H., Yarov-Yarovoy, V., Gutman, G. A., and Catterall, W. A. (2005) Pharmacol. Rev. 57 387–395 [DOI] [PubMed] [Google Scholar]
- 11.Männikkö, R., Elinder, F., and Larsson, P. H. (2002) Nature 419 837–841 [DOI] [PubMed] [Google Scholar]
- 12.Frings, S., Seifert, R., Godde, M., and Kaupp, U. B. (1995) Neuron 15 169–179 [DOI] [PubMed] [Google Scholar]
- 13.Gauss, R., Seifert, R., and Kaupp, U. B. (1998) Nature 393 583–587 [DOI] [PubMed] [Google Scholar]
- 14.Ludwig, A., Zong, X., Jeglitsch, M., Hofmann, F., and Biel, M. (1998) Nature 393 587–591 [DOI] [PubMed] [Google Scholar]
- 15.Santoro, B., Liu, D. T., Yao, H., Bartsch, D., Kandel, E. R., Siegelbaum, S. A., and Tibbs, G. R. (1998) Cell 93 717–729 [DOI] [PubMed] [Google Scholar]
- 16.Yu, X., Chen, X. W., Zhou, P., Yao, L., Liu, T., Zhang, B., Li, Y., Zheng, H., Zheng, L. H., Zhang, C. X., Bruce, I., Ge, J. B., Wang, S. Q., Hu, Z. A., Yu, H. G., and Zhou, Z. (2007) Am. J. Physiol. 292 C1147–C1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagotta, W. N., Olivier, N. B., Black, K. D., Young, E. C., Olson, R., and Gouaux, E. (2003) Nature 425 200–205 [DOI] [PubMed] [Google Scholar]
- 18.Flynn, G. E., Black, K. D., Islas, L. D., Sankaran, B., and Zagotta, W. N. (2007) Structure (Camb.) 15 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton, G. M., Silverman, W. R., Heginbotham, L., and Morais-Cabral, J. H. (2004) Cell 119 615–627 [DOI] [PubMed] [Google Scholar]
- 20.Zhou, L., and Siegelbaum, S. A. (2007) Structure (Camb.) 15 655–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hüttl, S., Michalakis, S., Seeliger, S., Luo, D., Acar, N., Geiger, H., Hudl, K., Mader, R., Haverkamp, S., Moser, M., Pfeifer A., Gerstner, A., Yau, K. W., and Biel, M. (2005) J. Neurosci. 25 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalakis, S., Reisert, S., Geiger, H., Wetzel, C., Zong, X., Bradley, J., Spehr, M., Hüttl, S., Gerstner, A., Pfeifer, A., Hatt, H., Yau, K. W., and Biel, M. (2006) J. Biol. Chem. 281 35156–35166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng, J., Trudeau, M. C., and Zagotta, W. N. (2002) Neuron 36 891–896 [DOI] [PubMed] [Google Scholar]
- 24.Weitz, D., Ficek, N., Kremmer, E., Bauer, P. J., and Kaupp, U. B. (2002) Neuron 36 881–889 [DOI] [PubMed] [Google Scholar]
- 25.Zhong, H., Molday, L. L., Molday, R. S., and Yau, K. W. (2002) Nature 420 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng, C., Rich, E. D., and Varnum, M. D. (2004) Neuron 42 401–410 [DOI] [PubMed] [Google Scholar]
- 27.Dryja, T. P., Finn, J. T., Peng, Y. W., McGee, T. L., Berson, E. L., and Yau, K. W. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 10177–10181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bareil, C., Hamel, C. P., Delague, V., Arnaud, B., Demaille, J., and Claustres, M. (2001) Hum. Genet. 108 328–334 [DOI] [PubMed] [Google Scholar]
- 29.Kohl, S., Marx, T., Giddings, I., Jägle, H., Jacobson, S. G., Apfelstedt-Sylla, E., Zrenner, E., Sharpe, L. T., and Wissinger, B. (1998) Nat. Genet. 19 257–259 [DOI] [PubMed] [Google Scholar]
- 30.Biel, M., Seeliger, M., Pfeifer, A., Kohler, K., Gerstner, A., Ludwig, A., Jaissle, G., Fauser, S., Zrenner, E., and Hofmann, F. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 7553–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundin, O. H., Yang, J. M., Li, Y., Zhu, D., Hurd, J. N., Mitchell, T. N., Silva, E. D., and Maumenee, I. H. (2000) Nat. Genet. 25 289–293 [DOI] [PubMed] [Google Scholar]
- 32.Zheng, J., and Zagotta, W. N. (2004) Neuron 42 411–421 [DOI] [PubMed] [Google Scholar]
- 33.Bradley, J., Reisert, J., and Frings, S. (2005) Curr. Opin. Neurobiol. 15 343–349 [DOI] [PubMed] [Google Scholar]
- 34.Boccaccio, A., Lagostena, L., Hagen, V., and Menini, A. (2006) J. Gen. Physiol. 128 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurahashi, T., and Menini, A. (1997) Nature 385 725–729 [DOI] [PubMed] [Google Scholar]
- 36.Munger, S. D., Lane, A. P., Zhong, H., Leinders-Zufall, T., Yau, K. W., Zufall, F., and Reed, R. R. (2001) Science 294 2172–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song, Y., Cygnar, K. D., Sagdullaev, B., Valley, M., Hirsh, S., Stephan, A., Reisert, J., and Zhao, H. (2008) Neuron 58 374–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pape, H. C. (1996) Annu. Rev. Physiol. 58 299–327 [DOI] [PubMed] [Google Scholar]
- 39.Baruscotti, M., Bucchi, A., and DiFrancesco, D. (2005) Pharmacol. Ther. 107 59–79 [DOI] [PubMed] [Google Scholar]
- 40.Robinson, R. B., and Siegelbaum, S. A. (2003) Annu. Rev. Physiol. 65 453–480 [DOI] [PubMed] [Google Scholar]
- 41.Frère, S. G., Kuisle, M., and Lüthi, A. (2004) Mol. Neurobiol. 30 279–305 [DOI] [PubMed] [Google Scholar]
- 42.Chen, J., Mitcheson, J. S., Tristani-Firouzi, M., Lin, M., and Sanguinetti, M. C. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 11277–11282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii, T. M., Takano, M., and Ohmori, H. (2001) J. Physiol. (Lond.) 537 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stieber, J., Thomer, A., Much, B., Schneider, A., Biel, M., and Hofmann, F. (2003) J. Biol. Chem. 278 33672–33680 [DOI] [PubMed] [Google Scholar]
- 45.Wainger, B. J., DeGennaro, M., Santoro, B., Siegelbaum, S. A., and Tibbs, G. R. (2001) Nature 411 805–810 [DOI] [PubMed] [Google Scholar]
- 46.Zong, X., Zucker, H., Hofmann, F., and Biel, M. (1998) EMBO J. 17 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou, L., Olivier, N. B., Yao, H., Young, E. C., and Siegelbaum, S. A. (2004) Neuron 44 823–834 [DOI] [PubMed] [Google Scholar]
- 48.Moosmang, S., Biel, M., Hofmann, F., and Ludwig, A. (1999) Biol. Chem. 380 975–980 [DOI] [PubMed] [Google Scholar]
- 49.Notomi, T., and Shigemoto, R. (2004) J. Comp. Neurol. 471 241–276 [DOI] [PubMed] [Google Scholar]
- 50.Nolan, M. F., Malleret, G., Lee, K. H., Gibbs, E., Dudman, J. T., Santoro, B., Yin, D., Thompson, R. F., Siegelbaum, S. A., Kandel, E. R., and Morozov, A. (2003) Cell 115 551–564 [DOI] [PubMed] [Google Scholar]
- 51.Nolan, M. F., Malleret, G., Dudman, J. T., Buhl, D. L., Santoro, B., Gibbs, E., Vronskaya, S., Buzsaki, G., Siegelbaum, S. A., Kandel, E. R., and Morozov, A. (2004) Cell 119 719–732 [DOI] [PubMed] [Google Scholar]
- 52.Ludwig, A., Budde, T., Stieber, J., Moosmang, S., Wahl, C., Holthoff, K., Langebartels, A., Wotjak, C., Munsch, T., Zong, X., Feil, S., Feil, R., Lancel, M., Chien, K. R., Konnerth, A., Pape, H. C., Biel, M., and Hofmann, F. (2003) EMBO J. 15 216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stieber, J., Herrmann, S., Feil, S., Loster, J., Feil, R., Biel, M., Hofmann, F., and Ludwig, A. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 15235–15240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herrmann, S., Stieber, J., Stöckl, G., Hofmann, F., and Ludwig, A. (2007) EMBO J. 26 4423–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulze-Bahr, E., Neu, A., Friederich, P., Kaupp, U. B., Breithardt, G., Pongs, O., and Isbrandt, D. (2003) J. Clin. Investig. 111 1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueda, K., Nakamura, K., Hayashi, T., Inagaki, N., Takahashi, M., Arimura, T., Morita, H., Higashiuesato, Y., Hirano, Y., Yasunami, M., Takishita, S., Yamashina, A., Ohe, T., Sunamori, M., Hiraoka, M., and Kimura, A. (2004) J. Biol. Chem. 279 27194–27198 [DOI] [PubMed] [Google Scholar]
- 57.Milanesi, R., Baruscotti, M., Gnecchi-Ruscone, T., and DiFrancesco, D. (2006) N. Engl. J. Med. 354 151–157 [DOI] [PubMed] [Google Scholar]
- 58.Nof, E., Luria, D., Brass, D., Marek, D., Lahat, H., Reznik-Wolf, H., Pras, E., Dascal, N., Eldar, M., and Glikson, M. (2007) Circulation 116 463–470 [DOI] [PubMed] [Google Scholar]
- 59.Sulfi, S., and Timmis, A. D. (2006) Int. J. Clin. Pract. 60 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bucchi, A., Barbuti, A., Baruscotti, M., and DiFrancesco, D. (2007) Curr. Opin. Pharmacol. 7 208–213 [DOI] [PubMed] [Google Scholar]
- 61.Knaus, A., Zong, X., Beetz, N., Jahns, R., Lohse, M. J., Biel, M., and Hein, L. (2007) Circulation 115 872–880 [DOI] [PubMed] [Google Scholar]
- 62.Vasilyev, D. V., Shan, Q., Lee, Y., Mayer, S. C., Bowlby, M. R., Strassle, B. W., Kaftan, E. J., Rogers, K. E., and Dunlop, J. (2007) J. Neurophysiol. 97 3713–3721 [DOI] [PubMed] [Google Scholar]
- 63.Kitayama, M., Miyata, H., Yano, M., Saito, N., Matsuda, Y., Yamauchi, T., and Kogure, S. (2003) Epilepsia 44 20–24 [DOI] [PubMed] [Google Scholar]
- 64.Budde, T., Coulon, P., Pawlowski, M., Meuth, P., Kanyshkova, T., Japes, A., Meuth, S. G., and Pape, H. C. (2008) Pflugers Arch. 456 1061–1073 [DOI] [PubMed] [Google Scholar]
- 65.Cacheaux, L. P., Topf, N., Tibbs, G. R., Schaefer, U. R., Levi, R., Harrison, N. L., Abbott, G. W., and Goldstein, P. A. (2005) J. Pharmacol. Exp. Ther. 315 517–525 [DOI] [PubMed] [Google Scholar]
- 66.Wohlfahrt, P., Müller, H., and Cook, N. J. (1989) J. Biol. Chem. 264 20934–20939 [PubMed] [Google Scholar]
- 67.Bönigk, W., Bradley, J., Müller, F., Sesti, F., Boekhoff, I., Ronnett, G. V., Kaupp, U. B., and Frings, S. (1999) J. Neurosci. 19 5332–5347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santoro, B., Grant, S. G., Bartsch, D., and Kandel, E. R. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 14815–14820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Much, B., Wahl-Schott, C., Zong, X., Schneider, A., Baumann, L., Moosmang, S., Ludwig, A., and Biel, M. (2003) J. Biol. Chem. 278 43781–43786 [DOI] [PubMed] [Google Scholar]
- 70.Mistrík, P., Mader, R., Michalakis, S., Weidinger, M., Pfeifer, A., and Biel, M. (2005) J. Biol. Chem. 280 27056–27061 [DOI] [PubMed] [Google Scholar]
- 71.Molday, R. S., Molday, L. L., Dosé, A., Clark-Lewis, I., Illing, M., Cook, N. J., Eismann, E., and Kaupp, U. B. (1991) J. Biol. Chem. 266 21917–21922 [PubMed] [Google Scholar]
- 72.DiFrancesco, D. (1986) Nature 324 470–473 [DOI] [PubMed] [Google Scholar]
- 73.Kole, M. H., Hallermann, S., and Stuart, G. J. (2006) J. Neurosci. 26 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michels, G., Er, F., Khan, I., Sudkamp, M., Herzig, S., and Hoppe, U. C. (2005) Circulation 111 399–404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.