Abstract
Objective
Core2 1-6-N-glucosaminyltransferase-I (C2GlcNAcT-I) modification of adhesion molecules is required for optimal binding to target ligands. The objective of this study was to determine the role of C2GlcNAcT-I in the recruitment of Ly-6Chi monocytes to atherosclerotic lesions and in lesion formation in mice.
Methods and Results
In a whole-blood binding assay, Ly-6Chi monocytes and certain lymphocytes and natural killer cells from wild-type mice bound to P- and E-selectin. C2GlcNAcT-I deficiency abrogated leukocyte binding to P- and E-selectin in this assay as well as in an in vitro flow chamber assay. Moreover, C2GlcNAcT-I deficiency decreased Ly-6Chi monocyte interactions with atherosclerotic arteries under physiological flow conditions and also inhibited monocyte recruitment to the peritoneal cavity in mice challenged with thioglycollate. In apolipoprotein E–deficient (apoE−/−) mice, lack of C2GlcNAcT-I resulted in fewer and smaller atherosclerotic lesions in mouse aortas. Atherosclerosis was also suppressed in C2GlcNAcT-I−/−/apoE−/− chimeric mice transplanted with C2GlcNAcT-I−/− bone marrow cells.
Conclusions
C2GlcNAcT-I in both leukocytes and blood vessel wall cells contributes to leukocyte recruitment to the arterial wall. C2GlcNAcT-I deficiency leads to the formation of small, macrophage-poor, and collagen-rich atherosclerotic lesions.
Adhesive interactions of leukocytes and platelets with cells of the blood vessel wall have long been known to play a crucial role in the development of atherosclerosis.1,2 Cell–cell interactions are mediated by a wide variety of adhesion molecules, including P-, E-, and L-selectins as well as P-selectin glycoprotein ligand 1 (PSGL-1), CD43, CD44, β2 integrins, and a4b1, and many others. Most adhesion molecules, such as PSGL-1, CD43, and CD44, are glycoproteins that are modified by Core2 1-6-N-glucosaminyltransferase-I (C2GlcNAcT-I). The role of these C2GlcNAcT-I–modified adhesion molecules in cell–cell interactions has been extensively studied.3–7
Ly-6Chi monocytes are key contributors to the development of atherosclerosis in mice.8 Our recent work has demonstrated that PSGL-1 is highly expressed on Ly-6Chi monocytes and is a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Deficiency of PSGL-1, a C2GlcNAcT-I–modified molecule, dramatically inhibits the formation of spontaneous atherosclerotic lesions and neointima formation after arterial injury in apoE−/− mice.9 The role of C2GlcNAcT-I deficiency in monocyte homing to the arterial wall and the formation of atherosclerotic lesions has not been studied, however.
PSGL-1 contains sialylated and fucosylated oligosaccharides (O-glycans) and has at least one sulfated tyrosine near the N terminus.10,11 Attachment of the O-glycan to PSGL-1 requires C2GlcNAcT-I, and this modification is crucial for optimal binding of PSGL-1 to selectins as demonstrated by in vitro gene transfer studies.12,13 The role of C2GlcNAcT-I in leukocyte homing varies under different pathological conditions. For example, C2GlcNAcT-I−/− mice are defective in eosinophil and neutrophil trafficking to the peritoneum but not to the lung.14 Therefore, the role of C2GlcNAcT-I in the regulation of Ly-6Chi monocyte PSGL-1 binding, monocyte recruitment, and formation of atherosclerotic lesions in vivo remains to be clarified.
Here, we used Ly-6Chi monocytes from C2GlcNAcT-I−/− mice to examine the role of C2GlcNAcT-I in the regulation of monocyte homing. We bred C2GlcNAcT-I−/− mice with apoE−/− mice to generate C2GlcNAcT-I−/−/apoE−/− double knockout mice and their controls. Using these mice, we investigated the effect of loss of C2GlcNAcT-I on atherosclerotic lesion size and on characteristics associated with the stability of atherosclerotic human lesions in apoE−/− mice. Furthermore, using chimeric mice generated through bone marrow transplantation, we determined whether C2GlcNAcT-I in bone marrow–derived cells or blood vessel wall cells plays a role in monocyte homing and formation of atherosclerotic lesions.
Methods
Mice and Atherosclerotic Models
C2GlcNAcT-I−/− mice15 were back-crossed to C57BL/6J mice more than 10 times and then bred with apoE−/− mice to generate C2GlcNAcT-I−/−/apoE−/− mice and littermate controls. Male and female mice in each group were fed either a Western diet for 3 months or a standard chow diet for 6 months, and then were euthanized for the collection of aortas. To generate chimeric mice lacking C2GlcNAcT-I in vessel wall cells or bone marrow–derived cells, mouse bone marrow transplantation was performed as described.16 To initiate rapid development of atherosclerosis in chimeric mice, these mice were fitted with perivascular carotid collars as described.17 The abnormal hemodynamics generated by collar placement led to the rapid formation of site-controlled atherosclerotic lesions in the area proximal to the collar.
All animal experiments and care were approved by the University of Minnesota Animal Care & Use Committee, in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care.
Binding of E- and P-Selectin to Ly-6Chi Monocytes
All antibodies were obtained from BD Biosciences unless otherwise specified. To measure E- and P-selectin binding to Ly-6Chi monocytes, mouse whole blood was heparinized and incubated with E- or P-selectin-IgG fusion protein, followed by incubation with allophy-cocyanin (APC)-conjugated goat antihuman IgG. After labeling with a cocktail of monoclonal antibodies (mAbs) against CD11b, Ly-6C and nonmonocyte lineage markers (CD11b, CD90, B220, CD49b, NK1.1, and Ly-6G), Ly-6Chi monocytes were gated by flow cytometry on a fractional area change (FAC)-Scan as described.8 The intensity of APC fluorescence in cells expressing Ly-6Chi was regarded as a measure of the amount of P- or E-selectin binding to mouse inflammatory monocytes. Similar procedures were also used to determine P-selectin binding to T cells or natural killer (NK) cells. HA-FL was used to determine the binding functions of monocytes and macrophages. The reagent was kindly provided by Dr Pauline Johnson at University of British Columbia (Vancouver, Canada) and Dr Mark Siegelman at the University of Texas Southwestern Medical Center.
Ly-6Chi Monocyte Interactions With a Selectin-Coated Surface Under Flow Conditions
To obtain a sufficient number of Ly-6Chi and Ly-6Clo monocytes for the monocyte-selectin interaction assay, cells were isolated from mouse spleens and incubated with a cocktail of mAbs against CD11b, Ly-6C, and nonmonocyte lineage markers. Then Ly-6Chi and Ly-6Clo monocytes were sorted from the CD11bhiCD90loB220loCD49bloNK1.1loLy-6Glo population by flow cytometry as described.8 An in vitro flow chamber assay was carried out as described.18
Ly-6Chi Monocyte Interactions With Atherosclerotic Artery Endothelium
As described, 6-week-old male apoE−/− mice were fed a Western diet for 8 weeks. Then mouse carotid arteries were isolated from these mice and used for preparation of ex vivo perfusion models.19 Spleens of 8- to 12-week-old male wild-type and C2GlcNAcT-I–deficient mice were obtained and used to prepare cell suspensions.9 Ly-6Chi and Ly-6Clo monocytes were sorted from the cell suspensions, labeled with calcein AM and then infused into the carotid arteries at a rate of 10 mL/min (1×106 cells/mL), resulting in a wall shear stress of 3.0±0.1 dyn/cm2. Cell rolling and adhesion were recorded on videotape by stroboscopic epifluoresence illumination with an intravital microscope.
Interactions of Selectin-Coated Microbeads With Atherosclerotic Arteries In Vivo
Microbeads coated with P- or L-selectin-IgG were prepared as described.5 After injection of coated microbeads via a jugular catheter, microbead interactions with atherosclerotic carotid arteries were observed by intravital microscopy with a saline immersion objective and stroboscopic epifluoresence illumination, as described.20
Preparation of Mouse Aortas and Quantification of Atherosclerosis
Aortas of atherosclerotic mice were collected, and either en face preparations of whole aortas or cross-sections of aortic sinuses were processed for oil red O staining.20 To analyze atherosclerotic lesions formed in carotid arteries in the perivascular carotid collar model, arteries proximal to the collar placement area were cross-sectioned and stained with oil red O as described.17 Images obtained from the above experiments were scanned into a Macintosh computer and analyzed with Image-ProPlus.
Histological Analysis of Atherosclerotic Lesions
Using specific antibodies, immunostaining for the expression of macrophage Mac-2, smooth muscle cell α-actin, and tissue factor (TF) was performed on paraffin sections of atherosclerotic aortic sinuses. Movat staining was performed to show the necrotic areas on cross-sections of atherosclerotic lesions. A Masson trichrome kit was used to stain collagen.
Statistical Analysis
Statistical analysis was performed with Instat software. Data are presented as means ± SEM. Atherosclerotic lesion data passed tests for normality and were analyzed with 1-way ANOVA followed by a Bonferroni correction posthoc test. Other data were evaluated with the same test or a Student t test to evaluate 2-tailed levels of significance. The null hypothesis was rejected at P<0.05.
Results
C2GlcNAcT-I–Deficient Leukocyte Binding to Selectins Under Static Conditions
To evaluate the role of C2GlcNAcT-I in binding of inflammatory monocytes to selectins under static conditions, we gated the monocyte population in whole blood using flow cytometry with a cocktail of mAbs against nonmonocyte lineage markers. Wild-type monocytes did not differ from C2GlcNAcT-I–deficient monocytes in the level of PSGL-1 expression (supplemental Figure Ia, available online at http://atvb.ahajournals.org). Almost all Ly-6Chi monocytes in the blood of wild-type mice were able to bind P- and E-selectin, whereas Ly-6Chi monocytes in C2GlcNAcT-I−/− mice could not (supplemental Figure Ib and Ic), similar to the results obtained for wild-type monocytes pretreated with EDTA or 4A10, an antibody against PSGL-1 (data not shown). The lack of C2GlcNAcT-I did not alter the expression of other important homing molecules, including L-selectin, LFA-1, VLA-4, and CCR2 (supplemental Figure IIa through IId).
In addition to monocytes, many other white blood cells, such as T cells, B cells, and NK cells, participate in atherosclerosis.21–23 In wild-type mice, about 11% of circulating CD3 lymphocytes and 32% of NK cells bound to P-selectin under static conditions. In C2GlcNAcT-I−/− mice, however, the percentage of binding dropped to 3% and 10%, respectively (supplemental Figure Id and Ie).
Homing Ability of C2GlcNAcT-I–Deficient Ly-6Chi Monocytes Under Flow Conditions
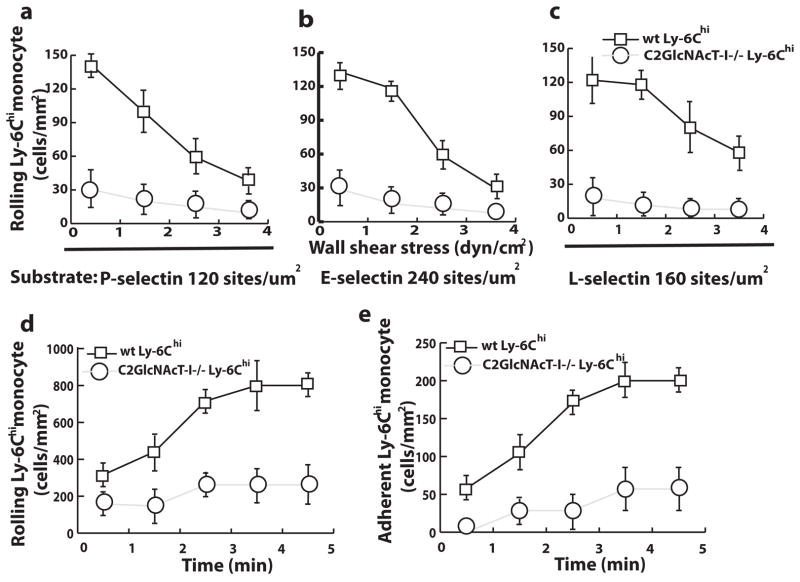
Because Ly-6Chi monocytes are major contributors to atherosclerosis, we evaluated the role of C2GlcNAcT-I in the regulation of Ly-6Chi monocyte binding to selectins under flow conditions. Sorted wild-type and C2GlcNAcT-I−/− Ly-6Chi monocytes were perfused over surfaces coated with mouse P-selectin/IgG, mouse E-selectin/IgG, or human L-selectin/IgG chimera under flow conditions. Many more wild-type than C2GlcNAcT-I–deficient Ly-6Chi monocytes rolled on the surfaces coated with P-, E-, or L-selectin at shear stresses ranging from 1 to 4 dyn/cm2 (Figure 1a through 1c). Wild-type Ly-6Chi cells rolled more stably on the selectin-coated surfaces than C2GlcNAcT-I–deficient cells. Rolling was dependent on PSGL-1 and selectin, as mAbs against PSGL-1, P-, E-, or L-selectin reduced the number of rolling cells to the basal levels observed on surfaces coated with control reagents (data not shown).
Figure 1.
C2GlcNAcT-I deficiency decreases Ly-6Chi monocyte homing under flow conditions. a, b, and c, Accumulation of rolling wild-type (wt) and C2GlcNAcT-I–deficient Ly-6Chi monocytes over selectin-coated surfaces at different wall shear stresses (P<0.01 at all tested shear stresses). d–e, Rolling and adhesion of wt and C2GlcNAcT-I–deficient Ly-6Chi monocytes on atherosclerotic endothelium in an ex vivo carotid artery model (P<0.01 at all time points).
To test whether C2GlcNAcT-I deficiency affected monocyte interactions with early atherosclerotic endothelium, we used an established ex vivo carotid artery perfusion model. Compared with wild-type Ly-6Chi monocytes, fewer C2GlcNAcT-I–deficient Ly-6Chi monocytes rolled on atherosclerotic endothelium at a shear stress of 3 dyn/cm2 (Figure 1d). Consequently, a significant decrease in C2GlcNAcT-I–deficient Ly-6Chi monocyte adhesion on atherosclerotic endothelium was found at all time points compared to wild-type Ly-6Chi monocytes (Figure 1e).
A mouse model for peritonitis allows testing of Ly-6Chi monocyte infiltration into the peritoneal cavity. Using this model, we further determined whether C2GlcNAcT-I deficiency affects Ly-6Chi monocyte recruitment to the peritoneal cavity. We found that the number of macrophages in peritoneal cavities was reduced by half in C2GlcNAcT-I–deficient mice compared to wild-type mice at different time points after peritoneal injection of thioglycollate (supplemental Figure III).
Decreased Size of Atherosclerotic Lesions in C2GlcNAcT-I–Deficient Mice
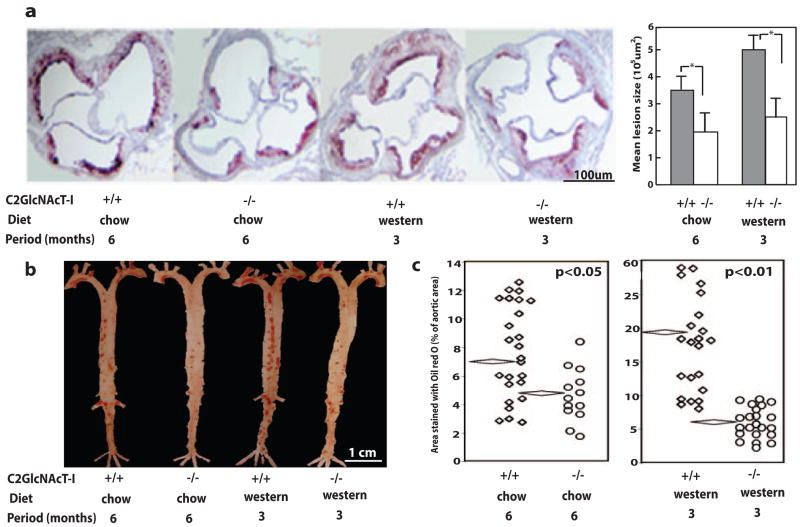
To determine the role of C2GlcNAcT-I in the formation of atherosclerotic lesions in vivo, C2GlcNAcT-I−/−/apoE−/− mice and their littermate apoE−/− mice were fed a chow diet for 6 months or a Western diet for 3 months. Neither body weight nor the number of circulating leukocytes differed between animals of the different genotypes (supplemental Table I), although there was an insignificant difference in the white blood cell counts and blood LDL-cholesterol levels between the 2 genotypes fed a Western diet. Lipid profiles of mice fed the same diet were similar regardless of genotype (supplemental Table II). Micrographs of cross-sections of the aortic sinuses showed that atherosclerotic lesions were more prevalent in apoE−/− mice than in C2GlcNAcT-I−/−/apoE−/− mice (Figure 2a). The average sizes of aortic sinus lesions were 3.4×105 and 5.2×105 μm2 for apoE−/− mice on a 6-month chow diet and 3-month Western diet, respectively, whereas average lesion sizes decreased to 1.8×105 and 2.2×105 μm2, respectively, for C2GlcNAcT-I−/−/apoE−/− mice on these diets (Figure 2a). This is a reduction of 47% and 58% in the average size of atherosclerotic lesions in C2GlcNAcT-I–deficient mice fed the 2 diets. In longitudinal sections of the aorta, lesions in apoE−/− mice fed a chow diet for 6 months or a Western diet for 3 months covered 7.6% and 19.2% of the aortic surface, respectively (Figure 2b and 2c). In contrast, only 5.2% and 5.8% of the aortic surface was covered with lesions in C2GlcNAcT-I−/−/apoE−/− mice fed a chow or Western diet, respectively (Figure 2b and 2c). Depending on the dietary regimen, therefore, C2GlcNAcT-I deficiency reduced formation of atherosclerotic lesions in aortas by 32% or 70% as assessed by en face staining (Figure 2c).
Figure 2.
C2GlcNAcT-I deficiency suppresses the formation of atherosclerotic lesions. a, Cross-sections of the aortic sinus stained with oil red O and the average lesion size in aortic sinuses. *P<0.01. b and c, Longitudinal sections of aortas en face stained with oil red O and quantitative data on lesion size in aortas. Flattened diamonds indicate mean lesion size.
Inflammatory Status of Atherosclerotic Lesions in C2GlcNAcT-I–Deficient Mice
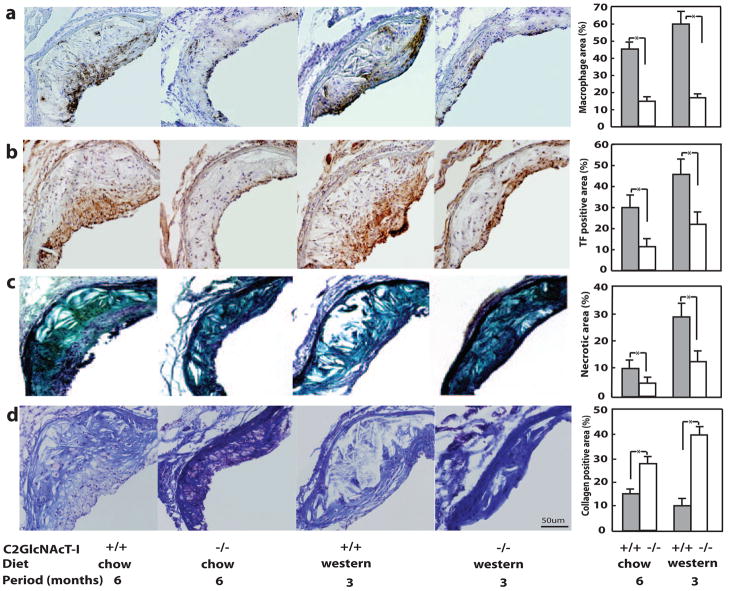
We next compared the cellular components of atherosclerotic lesions in histological cross-sections of the aortic sinus. Macrophages and foam cells were the major inflammatory cell components in lesions of apoE−/− mice, and were mainly located in the cap and shoulders of lesions (Figure 3a, first and third panels). In C2GlcNAcT-I−/−/apoE−/− mice, macrophages and foam cells remained localized in the cap and shoulders of lesions, but the total number of macrophages was greatly diminished (Figure 3a, second and fourth panels). In lesion cross-sections, the macrophage-positive area was 59.4% and 44.7% in apoE−/− mice fed the Western and chow diets, respectively, but was only 17% and 14.9% in C2GlcNAcT-I−/−/apoE−/− mice fed the same 2 diets (Figure 3a, fifth panel).
Figure 3.
C2GlcNAcT-I deficiency suppresses the inflammatory response in atherosclerotic lesions in aortic sinuses. Anti–Mac 2 staining of infiltrated macrophages (a), antimouse TF staining (b), Movat staining of necrotic areas (c), and Masson trichrome staining of collagen (d) in cross-sections of atherosclerotic lesions. Ten cross-sections from 10 mice were analyzed for each group. *P<0.01.
Cross-sections of aortic sinus lesions were also analyzed histologically to determine the role of C2GlcNAcT-I on the expression of other factors related to lesion stability. TF-positive staining colocalized with macrophage-positive areas (Figure 3b, first and third panels), indicating that TF in lesions may have been induced by macrophages and foam cells. Consistent with the effect of macrophage-induced TF expression, we observed a significant decrease in the level of TF in lesions in C2GlcNAcT-I−/−/apoE−/− mice (Figure 3b, second and fourth panels). The TF-positive areas were approximately 45% and 28.3% of the lesion areas in apoE−/− mice on a Western and chow diet, respectively; these percentages decreased to 22.7% and 10.2%, respectively, in C2GlcNAcT-I−/−/apoE−/− mice (Figure 3b, fifth panel).
C2GlcNAcT-I−/−/apoE−/− mice also had atherosclerotic lesions with fewer necrotic lipid cores. Necrotic lipid cores were often seen in the root of lesions of apoE−/− mice on a chow diet (Figure 3c, first panel). In apoE−/− mice on a Western diet, however, necrotic cores were more commonly observed in lesions, and the cores were much larger (Figure 3c, third panel). In lesions of C2GlcNAcT-I−/−/apoE−/− mice, necrotic cores were very rarely observed (Figure 3c, second and fourth panels). The necrotic core occupied 3.8% and 9.9% of the lesion areas of cross-sections of atherosclerotic arteries in C2GlcNAcT-I−/−/apoE−/− mice fed different diets, whereas these percentages in apoE−/− mice were 9.9% and 25.8%, respectively (Figure 3c, fifth panel).
We also observed increases in collagen content in lesions of C2GlcNAcT-I−/−/apoE−/− mice. Staining of lesions of apoE−/− mice with Masson trichrome revealed a very low overall collagen level in cross-sections of atherosclerotic arteries, with especially weak collagen staining of necrotic core areas and areas enriched with macrophages (Figure 3d, first and third panels). In lesions of C2GlcNAcT-I−/−/apoE−/− mice, strong collagen staining was found in both lesion and vessel wall areas (Figure 3d, second and fourth panels). Quantification showed that areas with significant levels of collagen staining in lesions of C2GlcNAcT-I−/−/apoE−/− mice were 4 times larger than areas of significant staining in lesions of apoE−/− mice (Figure 3d, fifth panel).
C2GlcNAcT-I in Blood Vessel Wall Cells Participates in Atherosclerosis
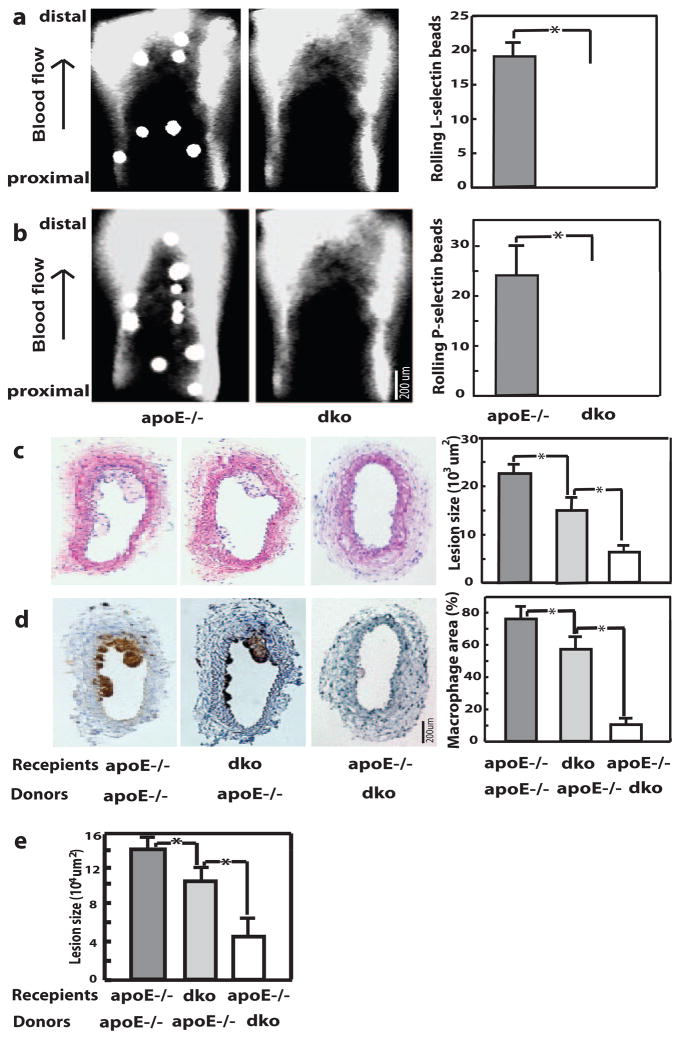
In addition to leukocytes, vascular wall cells also express C2GlcNAcT-I.24 To determine the effect of C2GlcNAcT-I expressed by vessel wall cells on the adhesiveness of the atherosclerotic arterial wall, we injected selectin-coated fluorescent microbeads into mice that had atherosclerotic carotid arteries. Epifluoresence microscopy revealed that microbeads coated with L-selectin interacted with atherosclerotic carotid arteries of apoE−/− mice but not with those of C2GlcNAcT-I−/−/apoE−/− mice (Figure 4a). Most of these interactions were characterized as tethering and rolling. Similar interactions were observed when injected beads were coated with P-selectin (Figure 4b). Control microbeads coated with bovine serum albumin flowed through atherosclerotic arteries of apoE−/− mice without visible interactions (data not shown).
Figure 4.
C2GlcNAcT-I deficiency in vessel wall cells or in bone marrow–derived cells reduces atherosclerotic lesions. Binding of fluorescent microbeads coated with L-selectin (a) or P-selectin (b) in mouse carotid arteries. c, Cross-section of atherosclerotic carotid arteries stained with hematoxylin/eosin. d, Anti–Mac 2 staining of infiltrated macrophages in cross-sections of atherosclerotic carotid arteries. e, Quantitative data of average lesion size in aortic sinuses of these chimeric mice. *P<0.01.
To dissect the role of C2GlcNAcT-I in vessel wall cells or bone marrow–derived cells in the formation of atherosclerotic lesions, we generated chimeric mice by transplanting bone marrow from C2GlcNAcT-I−/−/apoE−/− mice to apoE−/− and C2GlcNAcT-I−/−/apoE−/− mice or bone marrow from apoE−/− mice to C2GlcNAcT-I−/−/apoE−/− mice. A perivascular carotid collar model was used on these mice to rapidly initiate atherosclerosis in carotid arteries. The size of atherosclerotic lesions in chimeric apoE−/− mice without C2GlcNAcT-I in vessel wall cells or bone marrow–derived cells was reduced by 33% and 70% compared with that of control mice (Figure 4c). A similar effect on atherosclerotic lesion size was also observed in aortic sinuses of these chimeric mice (Figure 4e). Assessment of the cellular components in lesions showed fewer macrophages in the lesions of mice lacking either C2GlcNAcT-I in vessel wall cells or C2GlcNAcT-I in bone marrow–derived cells than in lesions of control mice (Figure 4d). This indicates that C2GlcNAcT-I in vessel wall cells also plays a role in monocyte recruitment and the formation of atherosclerotic lesions, although it is modest compared to that in bone marrow–derived cells.
Discussion
Monocyte recruitment to the arterial vessel wall was severely compromised in C2GlcNAcT-I–deficient mice. Consequently, atherosclerosis was dramatically inhibited. Thus, C2GlcNAcT-I may be a strong candidate for therapeutic targeting to limit the pathogenesis of atherosclerosis.
The deficiency of C2GlcNAcT-I in C2GlcNAcT-I−/− mice led to compromised PSGL-1 function and consequent decreased monocyte interaction with atherosclerotic arteries. Additionally, the lack of C2GlcNAcT-I–modified molecules on the vessel wall may also be responsible for the observed reduction in monocyte recruitment in C2GlcNAcT-I−/−/apoE−/− mice. L-selectin is an important molecule in initiating monocyte-endothelial interactions. Sperandio et al showed that the function of endothelial L-selectin ligand requires C2GlcNAcT-I activity.5 Monocyte recruitment is also facilitated by P-selectin–mediated platelet-endothelial interactions.20,25 PSGL-1 may be an endothelial ligand for platelet P-selectin on the arterial wall26,27. C2GlcNAcT-I deficiency in vessel wall cells, therefore, may compromise L-selectin–mediated monocyte homing and P-selectin–mediated platelet adhesion.
Other molecules, such as CD34, CD43, and CD44, are also modified by C2GlcNAcT-1.28 In CD34- and CD43-deficient mice, no change in recruitment of neutrophils and monocytes to inflammatory sites has been found.29,30 CD44 is an E-selectin ligand. However, its contribution to E-selectin binding is minor (supplemental Figure IVb). Hyaluronan (HA) is another major ligand for CD44 and binding of HA to CD44 mediates macrophage migration to atherosclerotic arteries.31 We found that inflammatory-activated macrophages but not monocytes were able to bind HA (supplemental Figure V). This was not affected by a deficiency of C2GlcNAcT-1 (supplemental Figure Vc), indicating that CD44/HA binding-mediated macrophage migration in C2GlcNAcT-I−/−/apoE−/− mice was not affected by lack of the C2GlcNAcT-1 modification to CD44. Therefore, PSGL-1 is the dominant molecular target of C2GlcNAcT-I in its contribution to the formation of atherosclerotic lesions.
The level of inflammation in lesions, a major factor in controlling the stability of atherosclerotic lesions,32 is suppressed in C2GlcNAcT-I−/−/apoE−/− mice. This may be attributable to inhibition of monocyte homing to lesions in C2GlcNAcT-I−/−/apoE−/− mice, as well as decreased inflammatory reactions in C2GlcNAcT-I–deficient monocytes/macrophages. Monocyte PSGL-1 activates the NF-κB pathway.33 Many cells in atherosclerotic lesions express P-selectin, such as smooth muscle cells and macrophages, in addition to endothelial cells and platelets.34 Thus, we hypothesize that P-selectin/PSGL-1–mediated inflammatory reactions may occur in atherosclerotic lesions and be important for determining the inflammatory status of these lesions.
The observed significant inhibition of atherosclerosis in C2GlcNAcT-I−/−/apoE−/− mice strongly suggests a role for C2GlcNAcT-I in the development of atherosclerotic lesions. A study is needed to determine whether inhibition of C2GlcNAcT-I activity can suppress the growth of advanced atherosclerotic lesions, as this is the stage at which most patients are diagnosed with atherosclerosis. Such a study is necessary because deficiency in the monocyte homing molecule CCR2 in bone marrow–derived cells does not stop the growth of established atherosclerotic lesions,35 although robust suppression of early atherosclerosis is observed in CCR2/apoE−/− mice.36 Furthermore, healthy and normally functioning macrophages appear to be beneficial during regression of atherosclerosis.37,38 Further study is required to clarify whether inhibition or knockout of C2GlcNAcT-I interferes with macrophage functions essential for lesion shrinkage. In addition, as shown by our present results, C2GlcNAcT-I knockout affects the PSGL-1 binding functions of T cells and NK cells. Thus, patients treated with prospective inhibitors of C2GlcNAcT-I for long periods could become immunocompromised. Clearly, this issue must be addressed before considering the use of C2GlcNAcT-I inhibitors for treatment of atherosclerosis.
Supplementary Material
Acknowledgments
Special thanks to Dr Klaus Ley for critical review.
Sources of Funding This work was supported by AHA 0430151N, NIH HL78679, and HL080569 to Y. Huo, by the Howard Hughes Medical Institute and NIH HL57345, HL78784, and GM62116 to J. Marth, and by NCI grant CA33000 to M. Fukuda.
Footnotes
Disclosures None.
References
- 1.Ross R. Atherosclerosis–an inflammatory disease [see comments] N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Borges E, Eytner R, Moll T, Steegmaier M, Campbell MA, Ley K, Mossmann H, Vestweber D. The P-selectin glycoprotein ligand-1 is important for recruitment of neutrophils into inflamed mouse peritoneum. Blood. 1997;90:1934–1942. [PubMed] [Google Scholar]
- 4.Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sperandio M, Thatte A, Foy D, Ellies LG, Marth JD, Ley K. Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood. 2001;97:3812–3819. doi: 10.1182/blood.v97.12.3812. [DOI] [PubMed] [Google Scholar]
- 6.Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 8.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norgard KE, Moore KL, Diaz S, Stults NL, Ushiyama S, McEver RP, Cummings RD, Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993;268:12764–1774. [PubMed] [Google Scholar]
- 11.McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. role of psgl-1 binding to selectins in leukocyte recruitment. [review] [70 refs] J Clin Invest. 1997;100:485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar R, Camphausen RT, Sullivan FX, Cumming DA. Core2 beta-1,6-n-acetylglucosaminyltransferase enzyme activity is critical for P-selectin Glycoprotein Ligand-1 binding to P- selectin. Blood. 1996;88:3872–3879. [PubMed] [Google Scholar]
- 13.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 14.Broide DH, Miller M, Castaneda D, Nayar J, Cho JY, Roman M, Ellies LG, Sriramarao P. Core 2 oligosaccharides mediate eosinophil and neutrophil peritoneal but not lung recruitment. Am J Physiol Lung Cell Mol Physiol. 2002;282:L259–L266. doi: 10.1152/ajplung.00214.2001. [DOI] [PubMed] [Google Scholar]
- 15.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 16.Huo Y, Zhao L, Hyman MC, Funk CD, Ley K. Critical role of macrophage 12/15-Lipoxygenase for atherosclerosis in apolipoprotein E deficient mice. J Clin Invest. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- 17.Von Der Thusen JH, Van Berkel TJ, Biessen EA. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein E-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 2001;103:1164–1170. doi: 10.1161/01.cir.103.8.1164. [DOI] [PubMed] [Google Scholar]
- 18.Xia L, Ramachandran V, McDaniel JM, Nguyen KN, Cummings RD, McEver RP. N-terminal residues in murine P-selectin glycoprotein ligand-1 required for binding to murine P-selectin. Blood. 2003;101:552–559. doi: 10.1182/blood-2001-11-0036. [DOI] [PubMed] [Google Scholar]
- 19.Huo Y, Hafezi-Moghadam A, Ley K. Role of vascular cell adhesion molecule-1 and fibronectin connecting segment-1 in monocyte rolling and adhesion on early atherosclerotic lesions. Circ Res. 2000;87:153–159. doi: 10.1161/01.res.87.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 21.Benagiano M, Azzurri A, Ciervo A, Amedei A, Tamburini C, Ferrari M, Telford JL, Baldari CT, Romagnani S, Cassone A, D’Elios MM, Del Prete G. T helper type 1 lymphocytes drive inflammation in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 2003;100:6658–6663. doi: 10.1073/pnas.1135726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 23.Major AS, Wilson MT, McCaleb JL, Ru SY, Stanic AK, Joyce S, Van KL, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- 24.Majuri ML, Pinola M, Niemelä R, Tiisala S, Natunen J, Renkonen O, Renkonen R. α2,3-sialyl and α1,3-fucosyltransferase-dependent synthesis of sialyl Lewis x, an essential oligosaccharide present on L-selectin counterreceptors, in cultured endothelial cells. Eur J Immunol. 1994;24:3205–3210. doi: 10.1002/eji.1830241244. [DOI] [PubMed] [Google Scholar]
- 25.Burger PCF, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 26.Rivera-Nieves J, Burcin TL, Olson TS, Morris MA, McDuffie M, Cominelli F, Ley K. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006;203:907–917. doi: 10.1084/jem.20052530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Costa MP, Garcia-Vallejo JJ, van Thienen JV, Fernandez-Borja M, van Gils JM, Beckers C, Horrevoets AJ, Hordijk PL, Zwaginga JJ. P-selectin glycoprotein ligand-1 is expressed on endothelial cells and mediates monocyte adhesion to activated endothelium. Arterioscler Thromb Vasc Biol. 2007;27:1023–1029. doi: 10.1161/ATVBAHA.107.140442. [DOI] [PubMed] [Google Scholar]
- 28.Barran P, Fellinger W, Warren CE, Dennis JW, Ziltener HJ. Modification of CD43 and other lymphocyte O-glycoproteins by core 2 N-acetylglucosaminyltransferase. Glycobiology. 1997;7:129–136. doi: 10.1093/glycob/7.1.129. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki A, Andrew DP, Gonzalo J-A, Fukumoto M, Spellberg J, Hashiyama M, Suda T, Takimoto H, Gerwin N, Webb J, Gutierrez-Ramos J-C, Molineux G, McNiece I, Ley K, Butcher EC, May WS, Greaves MF, Amakawa R, Tada Y, Wakcham A, Mak TW. CD34 deficient mice have reduced eosinophil accumulation after allergen exposure and reveal a novel crossreactive 90 kD protein. Blood. 1996;87:3550–3562. [PubMed] [Google Scholar]
- 30.Carlow DA, Ziltener HJ. CD43 deficiency has no impact in competitive in vivo assays of neutrophil or activated T cell recruitment efficiency. J Immunol. 2006;177:6450–6459. doi: 10.4049/jimmunol.177.9.6450. [DOI] [PubMed] [Google Scholar]
- 31.Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Pure E. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Invest. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Sanders JM, Phan ET, Ley K, Sarembock IJ. Arterial macrophages and regenerating endothelial cells express P-selectin in atherosclerosis-prone apolipoprotein E-deficient mice. Am J Pathol. 2005;167:1511–1518. doi: 10.1016/S0002-9440(10)61237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J, de WV, Van EM, Hildebrand RB, van Wanrooij EJ, Kuiper J, Maeda N, Benson GM, Groot PH, Van Berkel TJ. Repopulation of apolipoprotein E knockout mice with CCR2-deficient bone marrow progenitor cells does not inhibit ongoing atherosclerotic lesion development. Arterioscler Thromb Vasc Biol. 2005;25:1014–1019. doi: 10.1161/01.ATV.0000163181.40896.42. [DOI] [PubMed] [Google Scholar]
- 36.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2(−/−) mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 37.Daoud AS, Jarmolych J, Augustyn JM, Fritz KE. Sequential morphologic studies of regression of advanced atherosclerosis. Arch Pathol Lab Med. 1981;105:233–239. [PubMed] [Google Scholar]
- 38.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.