Abstract
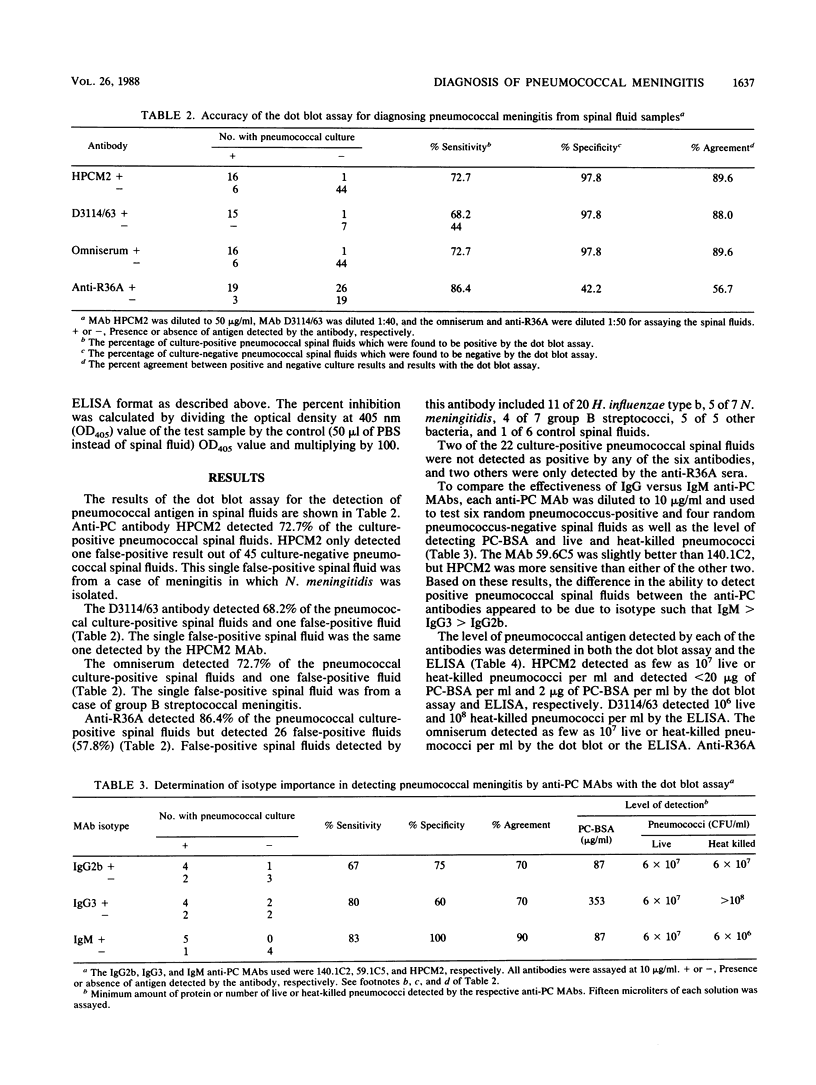
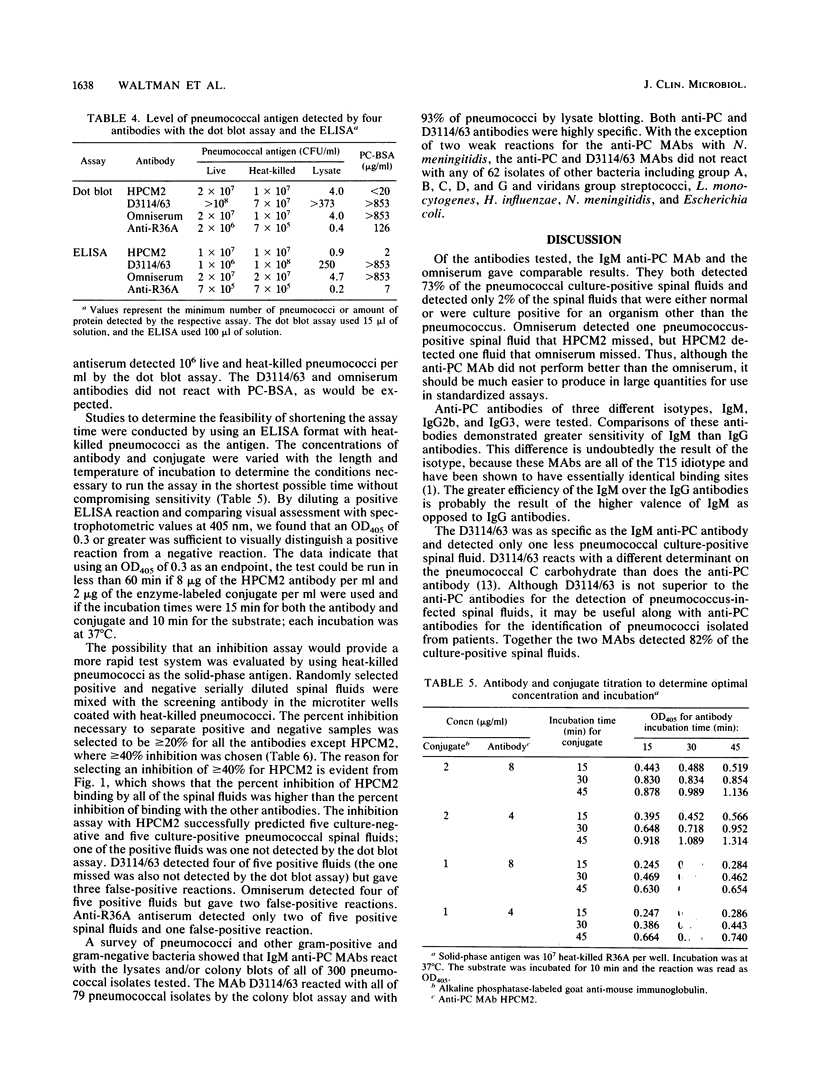
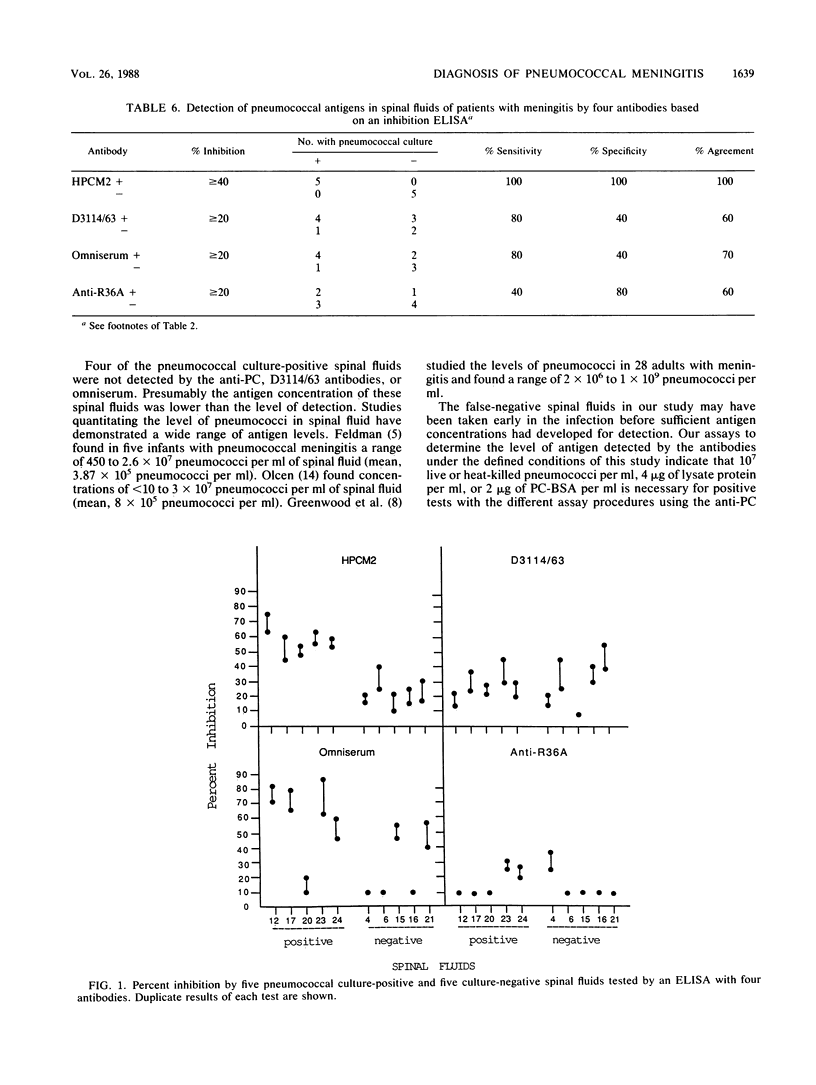
A diagnostic test for the detection of Streptococcus pneumoniae meningitis was developed using monoclonal antibodies (MAbs) to phosphocholine (PC) and non-PC determinants of pneumococcal teichoic acids. These MAbs do not recognize other bacteria that commonly cause meningitis. By using a dot blot assay, these MAbs were compared with a polyvalent pneumococcal capsular omniserum and an antiserum made to whole cells for their ability to detect pneumococci in infected spinal fluids. An immunoglobulin M (IgM) anti-PC antibody gave a positive reaction with 16 of 22 (73%) pneumococcal culture-positive spinal fluids. One false-positive result out of 45 pneumococcal culture-negative spinal fluids was also observed. D3114/63, an IgM MAb to non-PC determinants of teichoic acids, detected 15 of 22 of the pneumococcal culture-positive spinal fluids with one false-positive result. IgG2b and IgG3 anti-PC MAbs were less efficient than the IgM anti-PC MAb at detecting pneumococci in spinal fluids. Like the IgM anti-PC MAb, omniserum detected 73% of the culture-positive pneumococcal spinal fluids, with one false-positive result. The use of anti-PC or D3114/63 MAbs instead of a pooled serum such as omniserum has several advantages: (i) use of a single cross-reactive antibody rather than 83 pooled antibodies; (ii) possibility of a higher concentration of reactive antibody, which may increase the sensitivity of the test; (iii) a standardized antibody preparation; (iv) ease of preparation of the antibody; and (v) less expense.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres C. M., Maddalena A., Hudak S., Young N. M., Claflin J. L. Anti-phosphocholine hybridoma antibodies. II. Functional analysis of binding sites within three antibody families. J Exp Med. 1981 Nov 1;154(5):1584–1598. doi: 10.1084/jem.154.5.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J Mol Cell Immunol. 1984;1(5):305–309. [PubMed] [Google Scholar]
- Colding H., Lind I. Counterimmunoelectrophoresis in the diagnosis of bacterial meningitis. J Clin Microbiol. 1977 Apr;5(4):405–409. doi: 10.1128/jcm.5.4.405-409.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drow D. L., Welch D. F., Hensel D., Eisenach K., Long E., Slifkin M. Evaluation of the Phadebact CSF test for detection of the four most common causes of bacterial meningitis. J Clin Microbiol. 1983 Dec;18(6):1358–1361. doi: 10.1128/jcm.18.6.1358-1361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman W. E. Relation of concentrations of bacteria and bacterial antigen in cerebrospinal fluid to prognosis in patients with bacterial meningitis. N Engl J Med. 1977 Feb 24;296(8):433–435. doi: 10.1056/NEJM197702242960806. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr Clinical and epidemiologic studies of pneumococcal infection in children. Pediatr Infect Dis. 1986 Mar-Apr;5(2):201–207. doi: 10.1097/00006454-198603000-00009. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Hassan-King M., Cleland P. G., Macfarlane J. T., Yahaya H. N. Sequential bacteriological findings in the cerebrospinal fluid of Nigerian patients with pneumococcal meningitis. J Infect. 1986 Jan;12(1):49–56. doi: 10.1016/s0163-4453(86)94891-7. [DOI] [PubMed] [Google Scholar]
- Holmberg H., Danielsson D., Hardie J., Krook A., Whiley R. Cross-reactions between alpha-streptococci and Omniserum, a polyvalent pneumococcal serum, demonstrated by direct immunofluorescence, immunoelectroosmophoresis, and latex agglutination. J Clin Microbiol. 1985 May;21(5):745–748. doi: 10.1128/jcm.21.5.745-748.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1981 Mar-Apr;3(2):246–253. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- Lund E., Munksgaard A., Stewart S. M. A new pneumococcus type. Type 47 A. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):497–500. [PubMed] [Google Scholar]
- Lund E., Rasmussen P. Omni-serum. A diagnostic Pneumococcus serum, reacting with the 82 known types of Pneumococcus. Acta Pathol Microbiol Scand. 1966;68(3):458–460. doi: 10.1111/apm.1966.68.3.458. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Waltman W. D., 2nd, Gray B., Briles D. E. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987 Oct;3(4):249–260. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- Olcén P. Serological methods for rapid diagnosis of haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumoniae in cerebrospinal fluid: a comparison of Co-agglutination, immunofluorescence and immunoelectroosmophoresis. Scand J Infect Dis. 1978;10(4):283–289. doi: 10.3109/inf.1978.10.issue-4.05. [DOI] [PubMed] [Google Scholar]
- Sørensen U. B. Monoclonal phosphorylcholine antibody binds to beta-lipoprotein from different animal species. Infect Immun. 1986 Aug;53(2):264–266. doi: 10.1128/iai.53.2.264-266.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton R. C., Dias F., Ryan R. W. Comparative evaluation of three commercial products and counterimmunoelectrophoresis for the detection of antigens in cerebrospinal fluid. J Clin Microbiol. 1984 Aug;20(2):231–234. doi: 10.1128/jcm.20.2.231-234.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


