How does evolution produce a complex brain? What are the factors that contribute to aspects of cortical expansion and organization in different mammals? How did human brains become so large and complex, and are they fundamentally different than the brains of other mammals? While these questions are inherently interesting, generating theories about brain evolution is a tricky business because one must strike a balance between known experimental data from extant mammals and inferences that can be made from these data regarding the unknown form of ancestral brains. For this reason, studies of brain evolution have not been at the forefront of neuroscience research until quite recently. Without question, it can be more rewarding to directly record from a neuron and characterize its response properties, or identify cells on a microscope than to assemble data from diverse disciplines to weave a cohesive story about a process that has been occurring for over 4 billion years.
However, within the last five years one can find numerous research papers, books and popular press articles about brain evolution, and this resurgence of interest is due in large part to the development of new techniques. For example, recent advances in genetics have allowed us to sequence the genome of a number of animals including humans (Venter et al., 2001; also see, Gregory et al., 2002; Waterston et al., 2002; The Chimpanzee Sequencing and Analysis Consortium, 2005; Mikkelsen et al., 2007). By comparing the genomes from these different species we are beginning to appreciate the genetic similarities that species share as well as the unique genetic alterations that have evolved in different species, especially in humans (e.g. Enard et al., 2002a; b; Hill and Walsh, 2005; Mekel-Bobrov and Lahn, 2006; Vallender and Lahn, 2006). Another contributing factor to the resurgence of interest in brain evolution is the advancement of molecular techniques that have expanded our knowledge of the genetic and molecular mechanisms involved in neural development. Results from these studies enable us to make inferences about how developmental mechanisms may have been altered during evolution to produce variant phenotypes (see below). Finally, the use of non-invasive brain imaging techniques has allowed us to directly study the human brain and appreciate its stunning complexity in terms of sheer size, number of cortical fields, space allocation and cognitive processing capacity. Our increased understanding of the brain and its development garnered from the techniques mentioned above has sparked a new interest, or at least revived a sleeping dinosaur in the field of neuroscience, and permits us to move out of the realm of speculation and into the realm of testable hypotheses.
What is a cortical field and why study it?
Despite disagreement about how complex brains may have evolved and the relative contribution of genetic versus activity dependent mechanisms to cortical development (see Krubitzer and Hunt, 2007; Larsen and Krubitzer, 2008 for review), most would agree that for mammals, the major structure of the brain associated with higher level processes such as perception, cognition and voluntary motor control is the neocortex. Support for this is particularly compelling in comparative studies of both primates and non-primate mammals that demonstrate that the neocortex has changed dramatically in different lineages in terms of size and organization compared to other structures. In addition, increases in size and complexity of organization are particularly pronounced in species that are considered “sentient” or those that exhibit complex behavior such as dolphins, elephants and humans.
The neocortex is subdivided into basic units of processing that we term cortical fields. Cortical fields are identified and segregated from each other based on a number of criteria including a unique set of cortical and subcortical connections, neural response properties, a distinct appearance in histologically processed tissue, and sometimes a unique molecular signature. These criteria provide an important and useful means of subdividing the neocortex into distinct cortical fields, and allow us to appreciate how cortical fields function collectively to generate particular types of perceptual, cognitive, and motor behaviors. However, theories of cortical evolution and studies of developmental and adult plasticity suggest that a cortical field is not a static structure, but rather a dynamic process or an event that changes throughout the life of an individual, either dramatically during development, or in a more limited fashion in adulthood. Because of this, it is important to keep in mind that how we define a cortical field should be relative to the time during development or adulthood at which it is studied. Further, this view of a cortical field as a dynamic process or event underscores the difficulty inherent in the study of brain evolution.
How do we study cortical evolution?
One of the largest problems confronting the study of brain evolution is that it cannot be studied directly. For example, while a great deal can be learned about evolutionary trends and the genetic underpinning of mechanisms that generate specific phenotypes in a variety of different animals, it is much more difficult, and probably impossible to directly study how complex circuits in mammalian brains evolve. Fortunately, there are two approaches, the comparative approach and the developmental approach, that can be used to uncover the types of alterations that have occurred in the neocortex throughout the course of mammalian evolution, and how those alterations were achieved.
The comparative approach
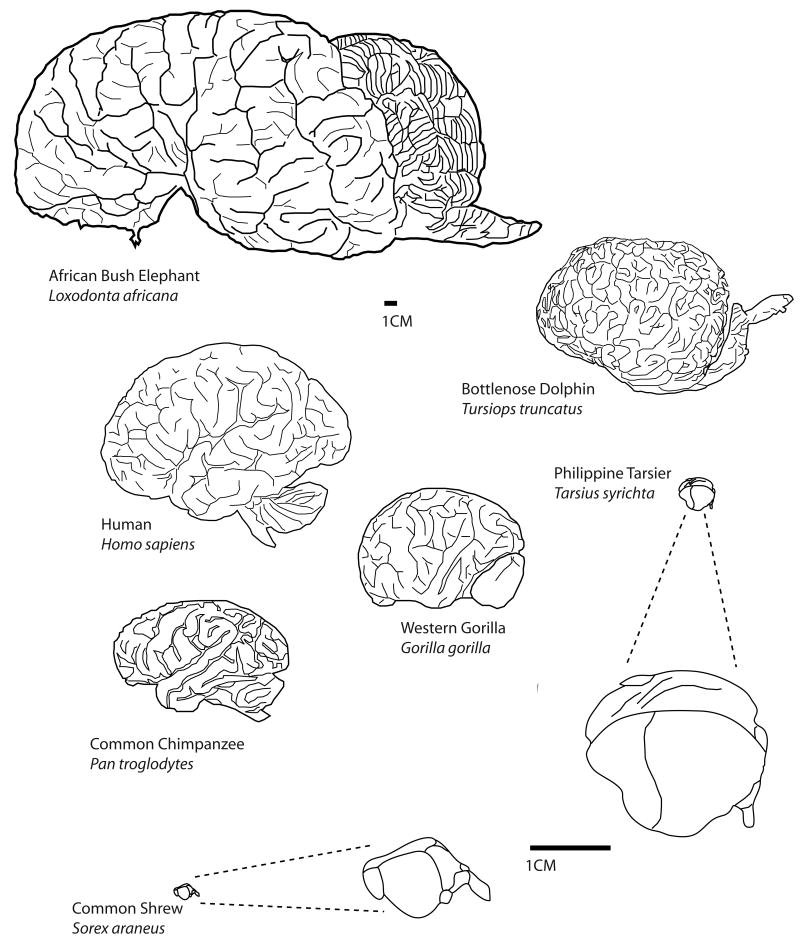
The comparative approach is a compelling method for examining similar features that all brains share as well as derivations or specializations that have arisen in different mammalian brains as a consequence of adaptation to a unique lifestyle and environment (Kaas, 2007; Krubitzer, 2007). This approach involves comparing distinct features of the neocortex of select mammals that ideally represent a number of phylogenetic branches of evolution, rather than just a few species such as monkeys, cats and mice (Fig. 1). There are a number of techniques that have been used to study the neocortex in different mammalian brains so that comparisons in the number, location and connections of cortical fields can be made across different brains (Kaas, 1982).
Figure 1.
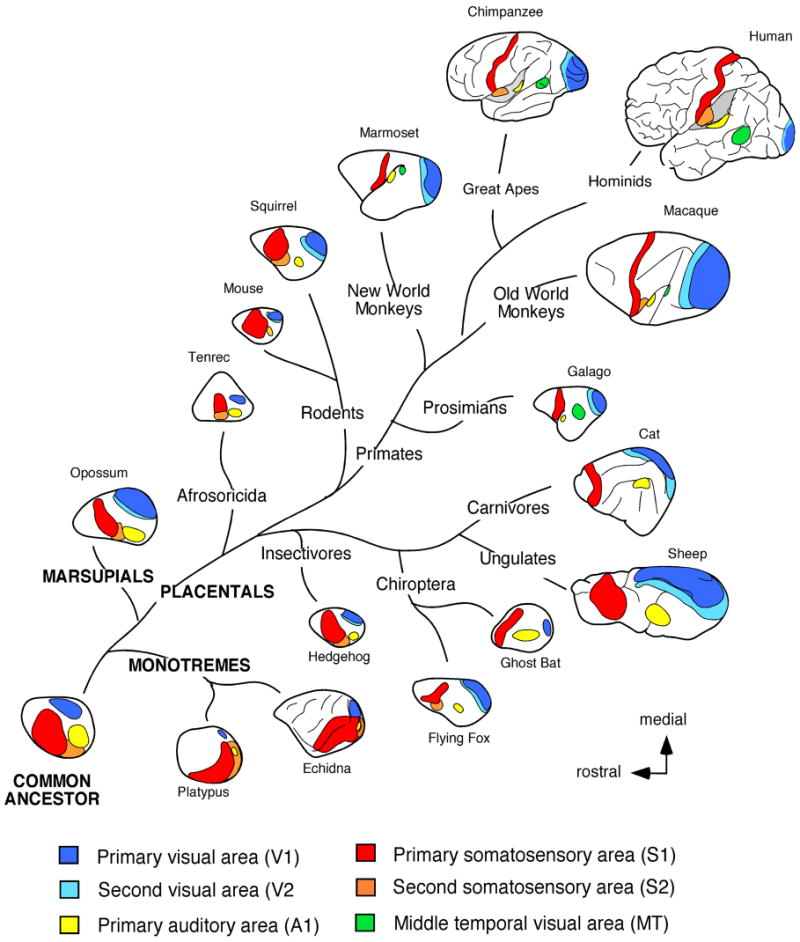
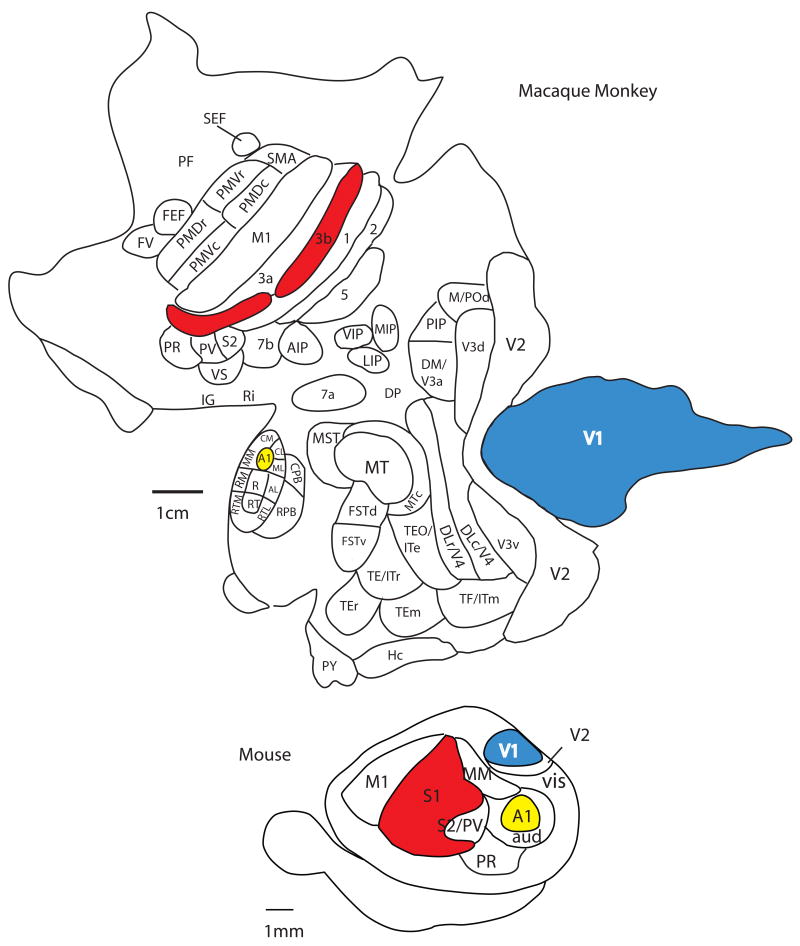
The traditional method used to make comparisons of the neocortex of different mammals is the architectonic method. This includes the use of cytoarchitecture, myeloarchitecture, chemoarchitecture and a variety of other histological stains in different animals to examine various characteristics of a cortical field such as laminar organization, neurotransmitters utilized and types of receptors present. Another set of techniques that are utilized to subdivide the neocortex are electrophysiological recording techniques for sensory cortex and intracortical microstimulation for motor cortex. Multi unit and single unit electrophysiological recording techniques allow us to determine the topographic organization of cortical fields, or cortical maps, as well as the response properties of neurons when stimulated with a unique type of sensory stimulus. Using this type of data we can segregate cortical fields based on modality to which neurons respond, as well as the features of a stimulus (e.g. direction, orientation) that neurons within a particular cortical field or cortical module prefer. Comparisons across brains can be quite compelling when electrophysiological recording data are combined with architectonic and histochemical distinctions. Studies that have combined these techniques have revealed an underlying organization of sensory and motor areas of the neocortex that all species share. This organization consists of a constellation of cortical fields including a primary visual area (V1), somatosensory area (S1; 3b in primates), and auditory area (A1), second sensory areas (e.g. V2, AAF or R, S2 and/or PV), a small posterior parietal region, and in eutherian mammals a primary motor area (Fig. 1; Krubitzer and Hunt, 2007 for review).
In addition to electrophysiological and architectonic techniques, neuroanatomical tracing techniques that reveal the connections of a cortical field are also used to make comparisons between species. These comparisons reveal that there are basic patterns of thalamocortical and cortico-cortical connections which are present in different species, but that elaborations in connectivity have been made with the addition of new cortical fields in some lineages (Fig. 2). Similarities in cortical field organization, connectivity, and architectonic appearance that are ubiquitous across species are considered to be inherited from a common ancestor and thus homologous. On the other hand, there are aspects of organization, connectivity and architecture that can be remarkably similar in one or two groups of mammals, but are found to be independently evolved through a comparative analysis. Such similarities are homoplaseous and indicate that there are serious constraints imposed on evolving nervous systems (see below). Taken together, the comparative approach allows us to appreciate similarities in brains that may be homologous or homoplaseous. This approach also allows us to identify the differences that have evolved in different lineages, and to determine if the ways in which brains have been modified is restricted (Krubitzer and Kaas, 2005).
Figure 2.
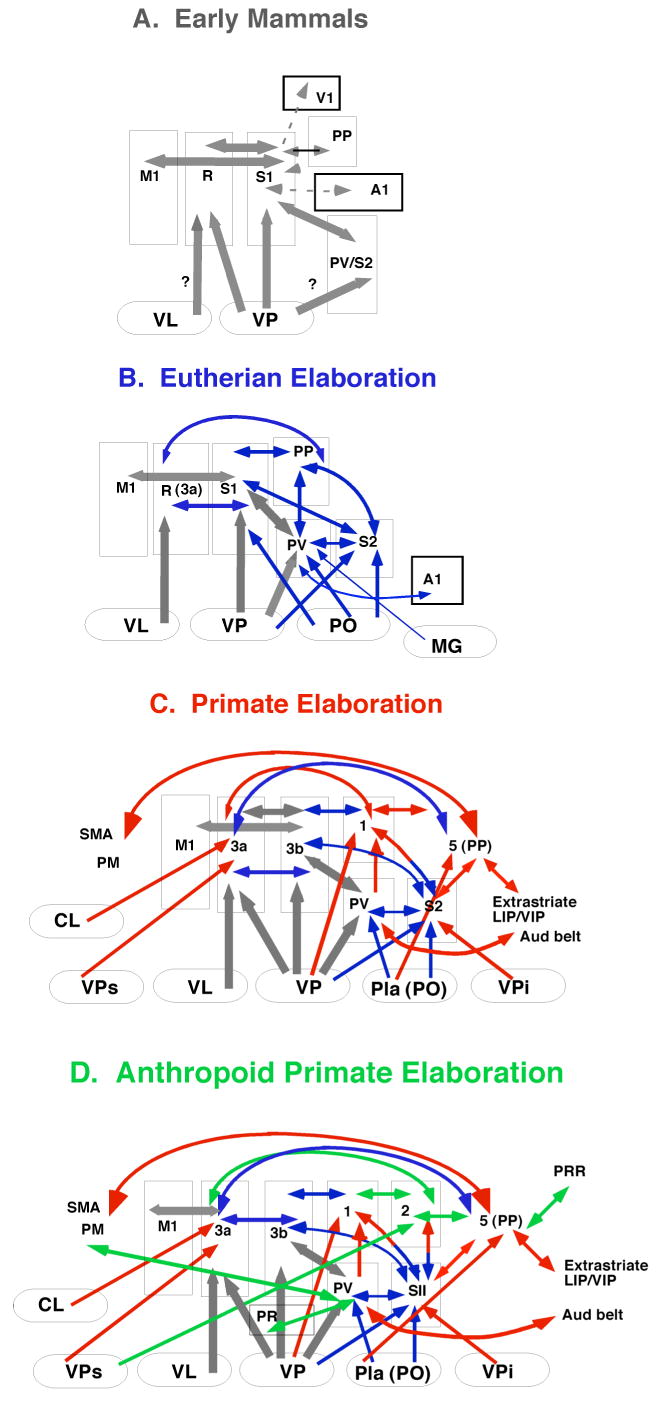
Because the cortical field is a dynamic event rather than a concrete immutable structure, any type of analysis we perform on the brain of an extant (living) mammal only provides a snapshot in the process of evolution. While the comparative approach has provided a lens with which to view these snapshots, it tells us little about the time dependent mechanisms that drive these alterations, or how these alterations are produced.
The developmental approach
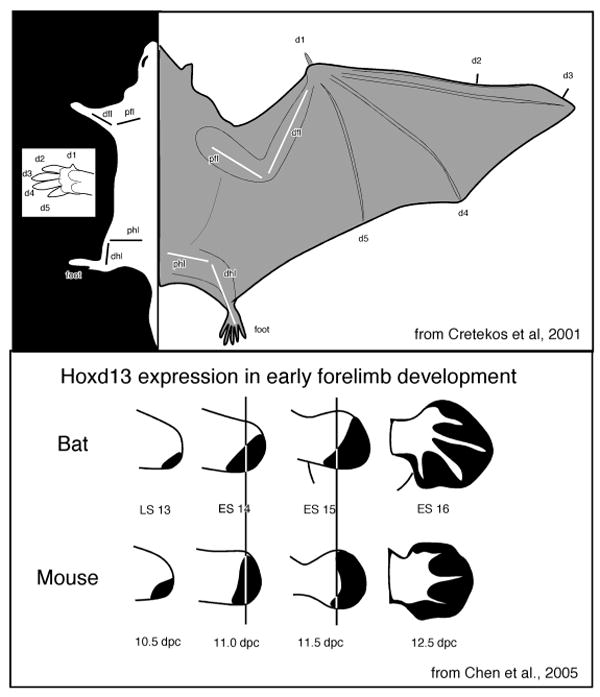
The study of the evolution of a cortical field or any other portion of the brain is actually the study of the evolution of developmental mechanisms that give rise to an adult phenotype. Presumably developmental mechanisms are ‘tweaked’ during evolution and these changes in developmental regimes produce variant adult phenotypes. Studies of development have revealed that both intrinsic genetic and activity dependent mechanisms contribute to the formation of the body and the brain, and that alterations in both produce species differences. In terms of the former, there is a large body of work that demonstrates that the expression patterns for genes involved in the patterning of the body plan and basic brain morphology are highly conserved (e.g. Tallafub and Baily-Cuif, 2002; Hirth and Reichert, 2007 for review). For example, homeobox genes from the Hox family are involved in forelimb development in all mammals that have been studied, and in mammals with specialized forelimb morphology, such as bats, the expression pattern of these genes is slightly altered during forelimb development (Chen et al., 2005; Fig. 3). These “tweaks” are thought to be involved in the transformation of the forelimb/hand morphology into a wing (Cretekos et al., 2001; Sears, 2008; Sears et al., 2006). Specifically, retention of the webbing is accomplished through a combination of increased FGF and reduced BMP signaling, elongation of wing skeletal elements is thought to arise from increased BMP during chondrocyte maturation, and reduction of wing skeletal elements appears to arise from a posteriorization in the expression of Hoxd13 in comparison to the expression of these genes in mice. Like portions of the body, the emergence of the vertebrate forebrain and its major subdivisions is also accomplished by highly conserved homeobox genes from the Hox and Otx families, and similar combinatorial patterns of expression of these genes that generate basic forebrain organization are observed in both mammalian and non-mammalian vertebrates (Boncinelli et al., 1995; 2000; Hirth and Reichert, 2007).
Figure 3.
In terms of the mammalian neocortex, there has been tremendous progress in identifying the early patterning genes that specify the location and size of cortical fields (e.g. Shimamura and Rubenstein, 1997; Monuki et al., 2001; Grove, and Fukuchi-Shimogori, 2003; see O'Leary et al., 2007 for review). For example, early in development signaling molecules are expressed in patterning centers in the commissural plate (Fgfs), the cortical hem (Bmps and Wnts), and the ventral telencephalon (Shh). These signaling molecules define the major axes of the cortex (e.g. anterior/posterior, dorsal/ventral), and direct the graded expression of transcriptional factors such as Pax6, Emx2, and COUP-TFI. These transcriptional factors provide positional identities to cortical progenitors and their progeny in the cortical plate (e.g. Bishop et al., 2000; Fukuchi-Shimogori and Grove, 2001; Armentano et. al., 2007), and in turn guide the expression of other genes (e.g. cadherins and ephrins) that are localized to regions of the developing cortex and particular layers of the cortex. The genes expressed later in development are involved in a variety of functions that establish the identities of individual cortical fields. (see O'Leary et al., 2007 for review). In addition to specifying the axis and location of cortical fields, a number of genes, including the ephrins, slits, and semaphorins through a process of attraction and repulsion are involved in the axon guidance and fasciculation, and synaptic development (Strochlic et al., 2007 for review). Studies in which the signaling molecules and transcription factors expressed early in development have been disrupted demonstrate that aspects of cortical organization including size and position of cortical fields can be altered dramatically (e.g. Bishop et al., 2000; Hamasaki et al., 2004; Armentano et. al., 2007), but are never lost (Fig. 4).
Figure 4.
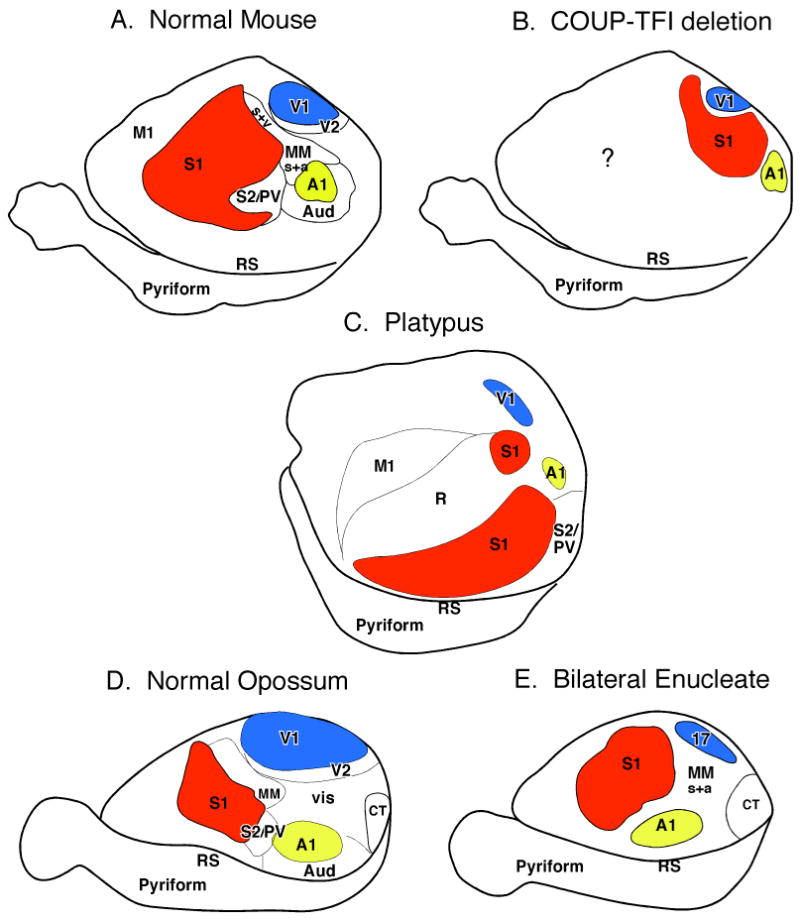
In addition to intrinsic genetic mechanisms, activity dependent processes also play a major role in development. There is a plethora of studies which demonstrate that both spontaneous and sensory driven activity in development play a role in the emergence of a number of aspects of cortical and subcortical organization including cortical field size, map formation, and connectivity (e.g. Feller and Scanziani, 2005; Smith and Trachtenberg, 2007; White and Fitzpatrick, 2007 for review). The contributions of sensory input and sensory driven activity to cortical organization are well demonstrated in studies that have removed sensory inputs or have modified sensory driven neural activity. For example, both classic and modern studies in the visual system in cats and monkeys in which one or both eyes were sutured or removed early in development demonstrate that features of the organization of V1 and the response of neurons in V1, such as ocular dominance, orientation selectivity and direction preference are altered (White and Fitzpatrick, 2007 for review). At a systems level, bilateral enucleations which results in complete loss of visual input to subcortical structures result in a reduced size of V1 in both monkeys (Dehay et al., 1991; Rakic et a., 1991) and opossums (Kahn and Krubitzer; 2002; Karlen and Krubitzer, 2008). Further, the internal organization of V1, and both cortical and subcortical connections are altered (Karlen et al., 2006). Specifically, neurons in V1 respond to stimulation of other sensory modalities (Fig. 5), and V1 receives cortical and subcortical inputs from both visual and non-visual structures. Major re-routing of subcortical connections has also been observed in congenitally blind and bilaterally enucleated mice in that ascending somatosensory projections from the dorsal column nucleus innervate the lateral geniculate nucleus (LGd; Asanuma and Stanfield, 1990). In bilaterally enucleated hamsters and blind mole rats, the inferior colliculus, normally associated with auditory processing, innervates the LGd (Izraeli et al., 2002; Piche et al., 2004; Doron and Wollberg, 1994).
Figure 5.
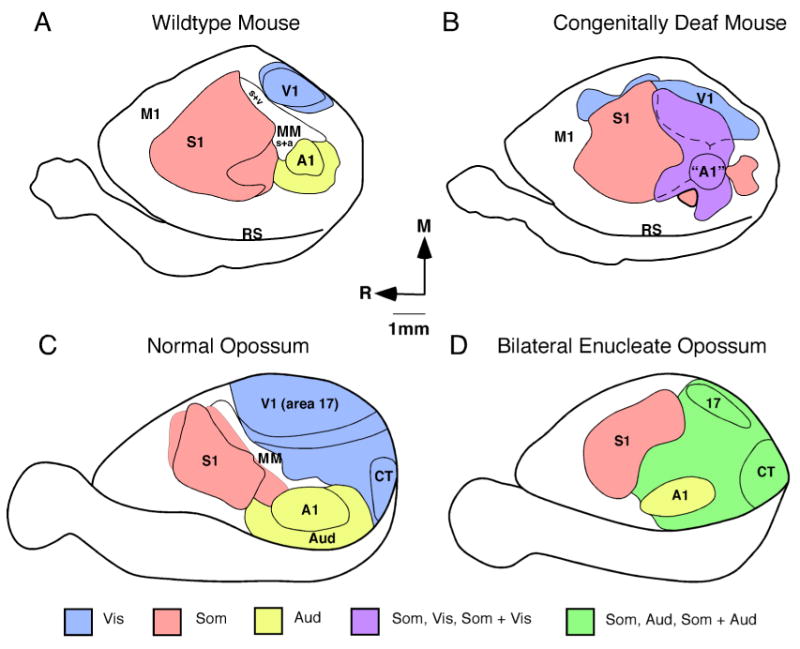
Experiments in the auditory system in mice in which the cochlea was still present but functionally impaired (Hunt et al., 2006) demonstrate similar alterations in the organization and connections of the primary auditory area (A1). In these mice, A1 is reduced in size and neurons in A1 respond to somatosensory and visual stimulation (Fig. 5). Studies in rats in which the cochlea was intact and fully functioning but acoustic stimulation was greatly modified early in development demonstrate that cortical maps of A1 were dramatically altered, as were neural response properties (Viller-Sidani et al., 2008; Zhou et al., 2008; Zhou and Merzenich, 2008). These types of changes in map organization, cortical field size and neural response properties have also been demonstrated in adults when sensory driven activity is altered with loss of input or with skilled learning (e.g. Kaas, 2000; Recanzone, 2000). This cortical plasticity that occurs during development to construct cortical fields, and the persistence of cortical plasticity throughout adulthood to more subtlety alter cortical maps, neural response properties and connections is a defining and extraordinary feature the mammalian neocortex.
The detailed molecular changes that allow for this plasticity in cortical organization are often driven by changes in gene expression during development (Hooks and Chen, 2007 for review). However, the resulting phenotype produced by such molecular based changes is not heritable, and thus only persist when the environment in which the individual develops is relatively static.
While much of the work described above was done in mice, it is likely that the early genetic patterning of cortex involving the same genes exists in all species. This would explain the persistence of a common plan of organization in every mammal examined (Fig. 1), and the existence of vestigial sensory apparatus and cortical areas in mammals that do not appear to use a particular sensory system (see below). Many of the alterations to the cortical phenotype in terms of cortical field size, number and connectivity, occur via alterations in transcription factors and the genes they regulate, but can also be achieved through activity dependent mechanisms (Fig. 4 and 5). However, unlike the known mechanisms for the formation of the wing of a bat, we do not know how these early genetic cascades are modified in the cortex of different species to produce variable phenotypes. Alterations in gross morphology of the brain, including the proportion of neural tissue devoted to the telencephalon, diencephalon and midbrain structures, for example, are likely due to alterations in the expression patterns of early homeobox genes. But again, how these genes are altered in different species of mammals with different gross brain morphologies has only been studied in a limited fashion. Developmental studies identifying the genes and genetic cascades involved in cortical development provide an important foundation for future investigations of brain evolution. Specifically they have introduced the “major players” we can examine across multiple species with markedly different cortical organization (Fig. 4). This type of comparative gene expression analysis would allow us to determine if alterations in both spatial and temporal patterns of expression have actually occurred in the past to produce the plethora of different cortical phenotypes observed in living mammals.
Levels of organization and complexity
Two important factors to consider in any discussion of the evolution of complex nervous systems is how we define and measure complexity (Bullock, 2007) and what level of organization we are interested in. The Oxford English dictionary defines complex as: “consisting of parts”, “formed by combination”, “intricate, not easily analyzed or disentangled”, and I think a good definition from a biological perspective is to consider brains with many parts as complex, and a complex neocortex as one with multiple cortical fields. When we examine the brain we can look at many levels of organization from the macro level that includes social systems, individuals and cortical networks to the micro level that includes individual neurons, synapses and molecules.
At a macro level of organization, groups of individuals (brains) interact to form social systems, and this level of organization has been observed in a variety of mammals from prairie voles to primates to dolphins. Some social systems are relatively simple such as that of the prairie vole in which alloparenting and pair bonding occur (Carter and Getz, 1993), while others are more complex. For example, a number of primate species have been observed to form cooperative alliances, engage in alloparenting, and disseminate culture and tradition throughout the group (e.g. Whiten et al., 1999; Perry, 2008). It is interesting that other species such as dolphins (e.g. Marino, 2002) and elephants (Shoshani et al., 2006), also engage in some of these behaviors, and like many primates, have advanced cognitive abilities (Connor, 2007). All of these species that have complex social interactions also have an extremely large cortical sheet (Fig. 6).
Figure 6.
While this increase in the size of the cortical sheet was independently evolved in elephants, cetaceans and primates, developmental studies suggest that increases in the size of the cortical sheet can be accomplished by altering cell cycle kinetics during neurogenesis in a variety of ways (e.g. Kriegstein et al., 2006; Chenn and Walsh, 2002; see Fish et al., 2008). For example, the period of neurogenesis may be lengthened, the rate at which cells divide may be increased, and/or the number of cells that re-enter the cell cycle and divide may be increased in mammals with a large cortical sheet compared to animals that have a small cortical sheet (e.g. Kornack and Rakic, 1998; Kornack, 2000). Regardless of the mechanism employed during evolution to produce changes in the size of the neocortex, it appears that increasing the size of the cortical sheet is necessary, although it may not be sufficient, to generate the type of cortical complexity necessary to support sophisticated social systems.
Not only is the overall size of the cortical sheet variable in different mammals, the number of cortical fields is variable as well. Some mammals such as mice have about 10 cortical fields; other mammals such as macaque monkeys have more than 50 cortical fields (Fig. 7), and it is likely that humans have over a hundred cortical fields. While different areas are often associated with a particular function, in large brains with multiple cortical fields processing is distributed in networks so that a single cortical field often participates in multiple tasks or behaviors, and a given behavior is generated by multiple cortical fields. Further, larger brains with multiple cortical areas have increased the numbers of connections and this increased connectivity endows the brain with both tremendous processing power and flexibility in processing (Fig. 2).
Figure 7.
In addition to and increase in the number of cortical fields and their connections, there is a change in the type of connectional network that has evolved, and this may fundamentally alter how bigger brains process information compared to smaller brains. For example, larger brains appear to have relatively fewer long range connections which connect distant areas of the cortex or the cerebral hemispheres, and more short, local connections necessary for time critical tasks (Ringo et al., 1991; 1994). These connections have optimal layouts based on the number, size and location of cortical areas (e.g. Cherniak et al., 2004), and it has been proposed that such “small-world” networks within the cortex have evolved to maximize complexity of processing with minimal costs (Bassett and Bullmore, 2006). Selection for minimizing wiring costs such as decreasing the length of axons, decreasing brain size, and reducing conduction delays is exemplified in a number of aspects of cortical organization including the segregation of gray and white matter (Wen and Chklovskii, 2005), the dimensions of axonal and dendritic arbors, and the existence of topographic maps to name a few (see Chklovskii et al., 2002; Bassett and Bullmore, 2006 for review).
Micro levels of organization, like the macro levels discussed above also vary in terms of their complexity in different mammals. At a cellular level, one can count the number of neurons in larger brains with more cortical areas and ask if neurons become more plentiful, if they become more densely packed, or if neurons get larger. Using a technique called isotropic fractionation, Herculano-Houzel and colleagues (2007; 2008) found that for primates, larger brains have more neurons (and more glia) and that the number of neurons scale linearly with the increased size of the brain. Thus, the average size of neurons does not increase in bigger brains, neurons are just more plentiful, although there are some exceptions. This conclusion is supported by comparative studies of basic cellular morphology in large-brained macaque monkeys and small brained dunnarts (Tyler et al., 1998). These studies demonstrate that despite the differences in the size of the primary visual area (V1) and the thickness of the cortex, the size of pyramidal neurons and their branching patterns is constant, as are the patterns of intrinsic connections (Tyler et al., 1998). In general, other features of cellular organization including the types of cortical neurons present and the percentage, length and density of synapses appear to be constant in mammals (DeFelipe et al., 2007). Thus, certain features of cellular morphology are relatively stable across species.
However, alterations to these conserved features of cellular morphology have been observed. For example, in primates, specialized dendritic branching patterns and increases in spine density have been observed in pyramidal cells in sensory, motor and prefrontal cortex (e.g. Elston et al., 2005a; b), and some of these specializations appear to be linear (e.g. more spines) as brains increase in size. Comparative studies of cell morphology demonstrate that humans and some great apes have distinct cellular subtypes such as spindle-shaped cells in anterior cingulate, frontoinsular and primary motor cortex, and that only humans have a distinct type of pyramidal cell (CR-ir) in anterior paracingulate cortex (Sherwood and Hof, 2007). Further, humans appear to have more interneurons and more types of interneurons (DeFilipe et al., 2007). Finally, the basic electrochemical properties of neurons are highly conserved across the animal kingdom. The presence of sodium and potassium channels, aspects of membrane permeability and synaptic transmission, for example, are observed universally. However, the addition of pre-and post-synaptic elements, such as the evolution of new receptor types or the cooperative function of receptors (e.g. NMDA and AMPA) in some groups of animals change the conditions under which neurons fire, and have important implications for learning and plasticity.
The observations that, in general, neurons do not change in size and basic synaptic structure, and that basic electrochemical properties of neurons are highly conserved suggests that there are large constraints on cellular evolution. Recent computational studies provide some insight into this issue and suggest that dendritic branching patterns of neurons have evolved to minimize costs while optimizing information processing of synaptic inputs that combine nonlinearly (Wen and Chklovskii; 2008). These investigators demonstrate that the optimal dendritic arbor for the Purkinje cell for example, which reduces the cost of plasma membrane (associated with energy to maintain the resting potential) would be planar, compact and centripetal. Thus, although subject to a number of constraints, neurons tend to optimize their morphology based on a few simple requirements.
There appears to be a loose correlation between the emergence of complexity at the different levels of organization; complex social systems are associated with a larger and complex neocortex, and a large complexly organized neocortex has more neurons that have morphological specializations associated with increased processing capacity. What is not clear is whether similar principles or rules for generating more parts are applied to all levels of organization, and how changes that have evolved at one level affect the organization of other levels. It is likely that these interactions are complex and non-linear. Further the resultant phenotype at any given level may be functionally optimal, but not necessarily the best independent solution to a particular challenge.
Finally, it is important to note that while brains have generally increased in size from that of the common ancestor, this is not always the case for all lineages (Kaas, 2002). From an evolutionary standpoint, a neocortex that is more complex does not necessarily provide a selective advantage. For example, rodents are a highly successful order composed of species occupying terrestrial, arboreal, aquatic and fossorial habitats as well as diurnal and nocturnal lifestyles, and with few exception, rodents have relatively small brains. In fact, most mammals have relatively small brains that are functionally optimized for cortical processing in their respective environment (Catania, 2007).
Convergent evolution and constraints imposed on developing and evolving nervous systems
Before we discuss the factors that constrain evolution, it is important to establish that such constraints exist. Examples of both homologous and homplastic features of cortical organization are compelling evidence for these constraints. For instance, the ubiquity of a common mammalian plan of cortical organization (homology) with a consistent spatial layout is remarkable. This is particularly compelling when one considers that some animals may not use the visual system for activities other than circadian rhythms such as blind mole rats, but still maintain a primary visual area (V1; Heil et al., 1991; Bronchti et al., 2002). Further, in experimental paradigms in opossums in which both eyes were removed very early in development (Kahn and Krubitzer, 2002) V1 is still present (Fig. 5). In both blind mole rats and opossums, V1 is reduced in size and some aspects of connectivity are maintained while others are altered (e.g. Bronchti et al., 1989; Karlen et al., 2006), suggesting that while some features of a primary cortical field can be altered, primary cortical fields cannot be eliminated. This persistence of highly conserved features of structure and function occurs at cellular and molecular levels as well (see above).
Another indication that very large constraints are imposed on evolving nervous systems is that structures or aspects of organization look remarkably similar despite distant phylogenetic relationships (Homoplasy). This convergent evolution is observed at all levels of organization. Anthropoid primates and cetaceans (dolphins, whales and porpoises) have differences in body and brain morphology and cortical organization compared to other mammals. Despite these differences and the differences in the environments they inhabit (aquatic versus terrestrial/arboreal) both groups have similar social structure (fission-fusion), both groups form cooperative alliances or coalitions, and both engage in alloparenting, tradition, and culture (see Marino, 2002; 2007; Marino et al., 2007 for review). In addition, both groups have evolved complex communication systems, and self-recognition ability. Finally, as noted in the previous section of this review, both groups have independently undergone extreme cortical expansion. This convergence of complex, high-level social and cognitive abilities in primates and cetaceans is remarkable given that the divergence between the ancestral mammal groups that eventually led to cetaceans and primates occurred around 90-95 million years ago (MYA; Kumar and Blair Hedges, 1998).
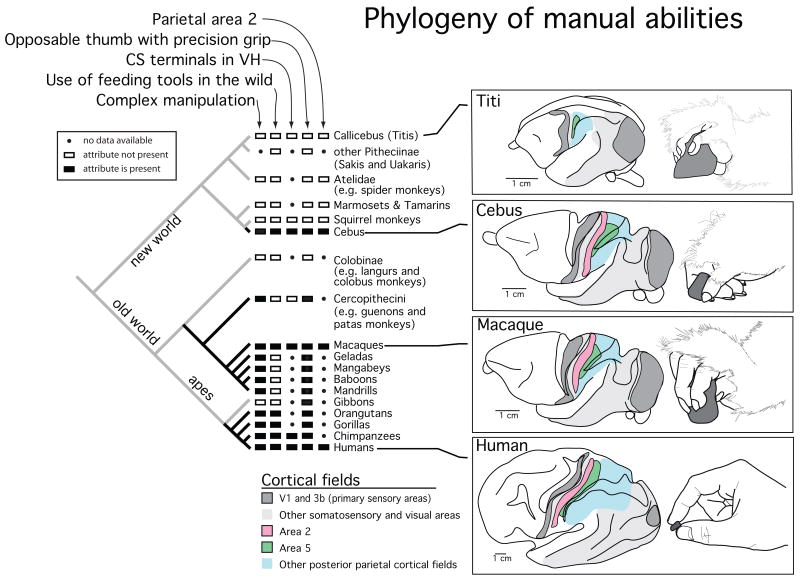
At the level of the cortex, convergent layouts of visual areas and their interconnections have emerged independently in squirrels and tree shrews (see Kaas, 2002). In the somatosensory cortex, individual cortical areas that are similar in location, organization and connectivity have emerged in New World cebus monkeys and anthropoid primates (Fig. 8; Padberg at al, 2007). Cebus monkeys have an opposable thumb that has evolved independently from that of anthropoid primates (Napier and Napier, 1967; Fleagel and Simons, 1995; Rose, 1996). In addition, they have the highest encephalization quotient of all New World monkeys (Rilling and Insel, 1999), and are considered to be socially complex because of sophisticated tool use and social transmission of culture and tradition (Fernandes, 1991; Frazaszi et al., 2004; Waga et al., 2006; Perry, 2008). Despite sixty million years of independent evolution, like anthropoid primates but unlike other New World monkeys, cebus monkeys have a cortical proprioceptive area, termed area 2 and an expanded posterior parietal cortical area 5 (Fig. 8), and both of these areas are involved in intentional reaching and grasping.
Figure 8.
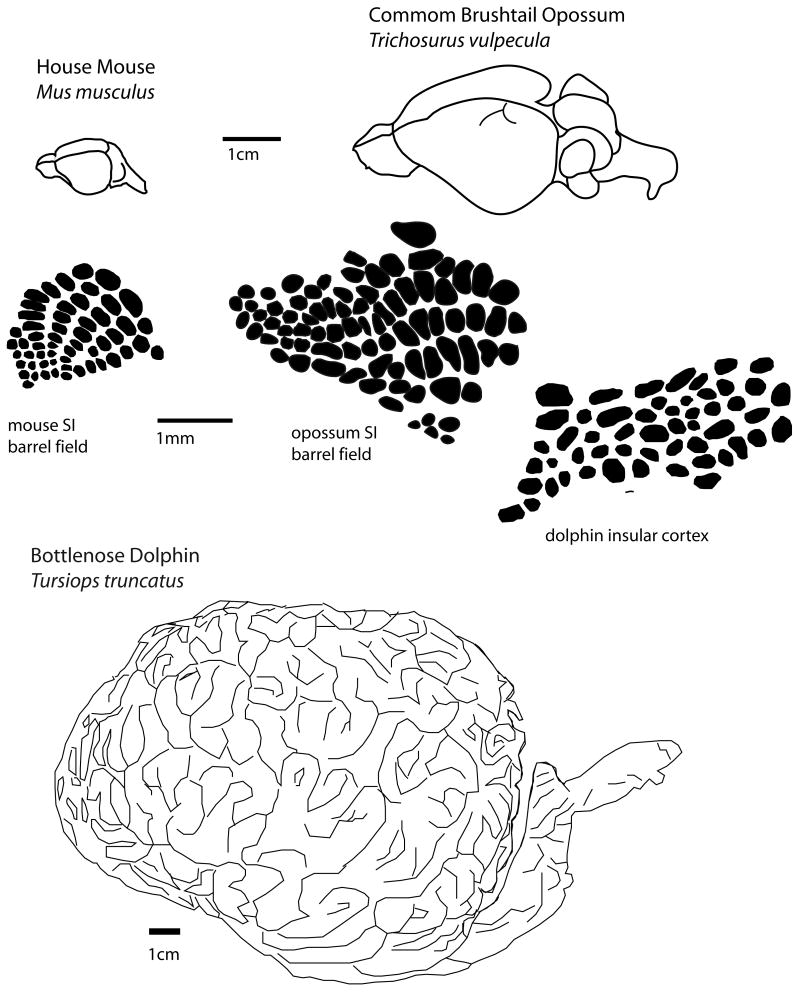
Convergence has also been observed at the level of modules within cortical fields in that many rodents and at least one species of marsupial (Weller, 1993; see Karlen and Krubitzer, 2007 for review) have evolved a barrel field in S1 associated with the mystacial vibrissae. The ancestral mammals that ultimately gave rise to rodents and marsupials diverged about 180 MYA. Similar barrel-like arrangements of cells have been observed in insular cortex in dolphins (Manger et al., 1998). It is interesting that although the dolphin brain is about 3000 times larger than the brains of most rodents and marsupials, the size of the “barrels” observed in each is similar. In fact, a comparative analysis of module size in different cortical areas in a variety of mammals indicates that the range of module size is limited, regardless of the overall size of the brain or cortical field (Manger et al., 1998). It has been suggested that selection for minimal wiring length drives the size of modules in all mammals (Manger et al., 1998). Finally, convergence has been observed at the level of individual neurons. Electrophysiological recordings in V1 of the diurnal wallaby indicate that a number of response properties including orientation selectivity and spatiotemporal tuning are similar to that of primates, but not other marsupials (Ibbotson and Mark, 2003), most of which are nocturnal.
The studies cited above represent only a few of the many examples of convergent evolution in mammals from the level of social systems and cognition to the level of neural response properties. These examples of independent emergence of behaviors, brains, and cortical fields indicate that the evolution, and thus the development of the nervous system are highly constrained. Although genes are the cornerstones of evolution, natural selection acts on individuals and behaviors, rather than on larger or smaller levels of organization. Because the constraints imposed on developing nervous systems are the same for all animals, it is not surprising that cortical phenotypes are highly predictable. In fact, it has been argued that homologous developmental programs that are subject to similar selective pressures and the same intrinsic and extrinsic constraints generate inescapable outcomes at all levels of organization (e.g. Padberg et al., 2007).
The persistence of a common plan of organization and the independent evolution of similar types of modifications to this basic plan are due to two major constraints imposed on developing and evolving nervous systems, the genetic code and the laws of physics. There are several ways in which genes constrain the evolution of the nervous system. First, several highly conserved genes expressed early in development in animals ranging from arthropods to mammals generate immutable aspects of body plan development including establishing the anterior/posterior axis, segmentation of limbs, and midline designation (See Hirth and Reichert, 2007 for review). Second, highly conserved homeobox genes such as those from the Otx and Hox families, Emx genes and Pax families and columnar genes (e.g. Msx, Dsh, Nkx) regulate the major axes of the brain including the head/trunk boundary (Tallafub and Balley-Cuif, 2002), the dorsoventral axis (see Urbach and Technau, 2008) and anterior/posterior axis (see Lichtneckert and Reichert, 2008). While the amount of territory devoted to different major brain subdivisions varies across species, these subdivisions are never lost, the axes of organization is maintained, and their spatial relationship is highly consistent. Third, recent evidence has shown that there are very old, highly conserved non-coding elements of genes in both vertebrates and invertebrates and these elements (cis-regulatory) regulate genes involved in major developmental processes such as body patterning and morphogenesis (McEwen et al., 2006; Vavouri et al., 2007). Thus, not only are genes that regulate major aspects of the brain and body highly conserved, but also how they are coordinated appears to predate vertebrates.
A final way in which genes constrain cortical evolution is the way in which they are deployed during development. As noted in the above section entitled: The developmental approach, there is a cascade of genetic events, possibly coordinated by duplication of highly conserved, cis-regulatory elements (see above) that set up contingencies that effectively constrain how development proceeds. If we consider cortical development as an “if/then” proposition, then the removal of one event will halt or disrupt the subsequent cascade of events necessary for individual and brain viability. The earlier in development that some change to this genetic cascade occurs, the greater the probability for significant change to the phenotype, but also for decreased viability. Thus, while evolution is the continuing process of modifying contingencies, selection acts to reduce change that results in early death or decreased neural viability.
The second major constraint on evolving nervous systems is the shared physical environment that includes but is not limited to light, sound, temperature, heat and gravity. The nervous system must interpret the patterns and intensity of these stimuli as they occur in different combinations and different media. There is a large body of evidence that these factors directly affect the developing body and behavior, which in turn affects brain organization, and further evidence indicates that these factors can also affect the developing brain directly. For example, different gravitational forces can alter body morphology by affecting bone density (Singh et al., 2005), and the extracellular matrix of tissue such as muscle via changes in gene expression (Silver and Siperko, 2003). Thus, different gravitational stress conditions (e.g. terrestrial versus aquatic) produce different and optimal morphological phenotypes, and these types of alterations to the body can affect the organization of both sensory and motor cortical areas. Gravity also has a direct effect on the development of descending pathways of the spinal cord, motor neuron development (Brocard et al., 2003), the levels of nerve growth factor and brain-derived nerve growth factor produced, and even behavior (Santucci et al., 2008). Salinity and humidity can affect body morphology (Johnston and Gottleib, 1990), and temperature can affect body mass, body length, and ear and tail length (e.g. Villarreal et al., 2007). Temperature can also affect the genes associated with neural development whose pattern of expression is thermosensitive (Tsai et al., 2007). In some amniotes such as lizards, temperature can determine the sex of the offspring, and different incubation temperatures differentially affect male versus female the fitness (Warner and Shine, 2008).
At a more refined level, there is a wealth of data that demonstrates the important role of sensory driven activity during early development. For example, alterations in the acoustic environment can result in changes in the size and internal organization of the primary auditory area (A1), and the neural response properties of A1 (deVillers-Sidani et al., 2008; Zhou et al., 2008; Zhou and Merzenich, 2008). Complete loss of input or lack of sensory driven activity from one sensory system can dramatically alter cortical field size, functional organization and connectivity (Fig. 5; Kahn and Krubitzer, 2002; Karlen et al., 2006; Karlen and Krubitzer, 2008; see Krubitzer and Hunt, 2007 for review). I use only a few examples of the systems level changes that can occur when sensory driven activity is altered or lost during development to underscore that the amount and types of physical stimulation present during development are crucial for determining the phenotype that emerges. These stimuli, even when they take the form of a mother's voice, screaming siblings, or a television droning, are nothing more than complex packages of physical stimuli present in any given environment.
Given that the laws of physics are invariant, it is not surprising that sensory driven activity not only plays a major role in shaping the cortex to function optimally in a given environment, but that the physical parameters of any form of energy place formidable constraints on brain evolution (see Krubitzer, 2007, for review).
Questions posed
If I return to the questions posed at the beginning of this review I think we can begin to answer some of them. We have some understanding about how brains become larger; we also know that genes provide the basic framework of body plan and brain organization. On the other hand, the ultimate adult phenotype that is produced owes as much to the specific parameters of the physical environment in which it develops as it does to genes. Because all mammals are enslaved by the same rules of construction during development and by the laws of physics, there are far more similarities between species than there are differences.
But what about humans; are they fundamentally different? Human brains have undergone enormous expansion during evolution, cortical asymmetries have emerged which allow particular tasks to be processed intrahemispherically (Corbalis, 2007). Human brains have more cortical fields, cortical fields have more neurons and specific cortical fields are associated with unique behavioral specializations such as language. Humans have unique types of neurons and more and varied interneurons, and several features of the genome that have been characterized as unique to humans are involved in brain development. So, of course humans have specializations that make them unique, but so do all other species. The difficult part is to figure out how these varied specializations observed at all levels of organization make us uniquely human, rather than uniquely cat or uniquely platypus.
Probably the most remarkable feature of the human neocortex is one that is difficult to measure and cannot be reduced to a cell type or a particular cortical field. That is the ability to loosen the constraints imposed on the development and evolution of our own nervous system; the ability to be plastic; the ability of our neocortex, and in turn our behavior to change dramatically throughout a lifetime. This plasticity is present in all mammals, but appears to be exaggerated in humans. Thus the dynamic alterations to the physical environment that include changes in voice cadence, light levels, and even global temperature that humans so notoriously produce, allow us to directly and often knowingly influence the development and evolution of our own brains and the brains of other mammals.
Is there a unifying theory for brain evolution?
Yes, Darwin provided the most important unifying theory in biology with his discovery that evolution is driven through natural selection of variant phenotypes. While his theory is still the foundation of all biological science, at the time it was proposed it was incomplete because there was no information about molecules or genes, and our understanding of brains was in its infancy. Since that time, there has been tremendous progress on all fronts of biology as well as in physics. Today, science is becoming more multidisciplinary, and we have more information from more disciplines on brain evolution and development then we have ever had in the history of science. However, despite our progress, we are restricted by our own capacity to understand the larger levels of organization and their boundary conditions, and the interplay between different levels of organization to produce complex biological systems (Polyani, 1968; Streidter, 2007).
What we know is that the brain and particularly the neocortex is not a static entity, but a dynamic process that is continuously shifting and changing within the life of an individual and in species over time. When we consider larger levels of organization in which groups of individuals within and across species interact within a common physical environment, we have a collective biomass or an emergent entity in which the behavior of individuals and populations substantially alters the physical environment in which an individual develops, and thus the brain itself. Because of this, we need to put the neurons that we study back into the brain, the brain back into the body, and the body back into a physical environment if we hope to achieve an understanding of the larger story of the evolution of complex nervous systems.
Figure 9.
Literature Cited
- Armentano M, Chou SJ, Srubek Tomassy G, Leingartner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Stanfield BB. Induction of somatic sensory inputs to the lateral geniuclate nucleus in congenitally blind mice and in phenotypically normal mice. Neuroscience. 1990;39:533–569. doi: 10.1016/0306-4522(90)90241-u. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bishop K, Goudreau G, O'Leary D. Science. New York, NY: 2000. Emx2 and Pax6 regulate area identity in the mammalian neocortex. in press. [DOI] [PubMed] [Google Scholar]
- Boncinelli E, Gulisano M, Spada F, Broccoli V. Emx and Otx gene expression in the developing mouse brain. Ciba Foundation Symposium. 1995;193:100–116. doi: 10.1002/9780470514795.ch6. [DOI] [PubMed] [Google Scholar]
- Boncinelli E, Mallamaci A, Muzio L. Evolutionary Developmental Biology of the Cerebral Cortex. Chichester: John Wiley and Sons, LTD; 2000. Genetic control of regional identity in the developing vertebrate forebrain; pp. 53–61. [DOI] [PubMed] [Google Scholar]
- Brocard F, Clarac F, Vinay L. Gravity influences the development of inputs from the brain to lumbar motoneurons in the rat. Neuroreport. 2003;14:1697–1700. doi: 10.1097/00001756-200309150-00008. [DOI] [PubMed] [Google Scholar]
- Bronchti G, Heil P, Sadka R, Hess A, Scheich H, Wollberg Z. Auditory activation of “visual” cortical areas in the blind mole rat (Spalax ehrenbergi) Eur J Neurosci. 2002;16:311–329. doi: 10.1046/j.1460-9568.2002.02063.x. [DOI] [PubMed] [Google Scholar]
- Bronchti G, Heil P, Scheich H, Wollberg Z. Auditory pathway and auditory activation of primary visual targets in the blind mole rat (Spalax ehrenbergi): I. 2-Deoxyglucose study of subcortical centers. Journal of Comparative Neurology. 1989;284:253–274. doi: 10.1002/cne.902840209. [DOI] [PubMed] [Google Scholar]
- Bullock T. Relevance of understanding brain evolution. In: Striedter G, Rubenstein J, editors. The Evolution of Nervous Systems, Volume 1: Theories, Development, Invertebrates. Oxford: Academic Press; 2007. pp. 283–287. [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Scientific American. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Catania K. Epigenetic responses to a changing periphery – Wagging the dog. In: Striedter G, Rubenstein J, editors. The Evolution of Nervous Systems, Volume 1: Theories, Development, Invertebrates. Oxford: Academic Press; 2007. pp. 143–151. [Google Scholar]
- Chen CH, Cretekos CJ, Rasweiler JJt, Behringer RR. Hoxd13 expression in the developing limbs of the short-tailed fruit bat, Carollia perspicillata. Evol Dev. 2005;7:130–141. doi: 10.1111/j.1525-142X.2005.05015.x. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Science. Vol. 297. New York, NY: 2002. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors; pp. 365–369. [DOI] [PubMed] [Google Scholar]
- Cherniak C, Mokhtarzada Z, Rodriguez-Esteban R, Changizi K. Global optimization of cerebral cortex layout. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1081–1086. doi: 10.1073/pnas.0305212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Schikorski T, Stevens CF. Wiring optimization in cortical circuits. Neuron. 2002;34:341–347. doi: 10.1016/s0896-6273(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Connor RC. Dolphin social intelligence: complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Philosophical transactions of the Royal Society of London. 2007;362:587–602. doi: 10.1098/rstb.2006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium TCSaA. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The evolution of hemispheric specializations of the human brain. In: Kaas JH, Preuss TM, editors. The Evolution of Nervous Systems, Volume 4: Primates. Oxford: Academic Press; 2007. pp. 379–394. [Google Scholar]
- Cretekos CJ, Rasweiler JJ, Behringer RR. Comparative studies on limb morphogenesis in mice and bats: a functional genetic approach towards a molecular understanding of diversity in organ formation. Reprod Fertil Dev. 2001;13:691–695. doi: 10.1071/rd01115. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–965. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, J A, Ballesteros-Yanez I, Benavides-Piccione R, Munoz A. Specializations of the cortial microstructure of humans. In: Kaas J, Preuss T, editors. The Evolution of Nervous Systems , Volume 4: Primates. Oxford: Academic Press; 2007. pp. 167–190. [Google Scholar]
- Dehay C, Horsburgh G, Berland M, Killackey H, Kennedy H. The effects of bilateral enucleation in the primate fetus on the parcellation of visual cortex. Developmental Brain Research. 1991;62:137–141. doi: 10.1016/0165-3806(91)90199-s. [DOI] [PubMed] [Google Scholar]
- Doron N, Wolberg Z. Cross-modal neuroplasticity in the blind mole rat Spalax ehrenbergi: a WGA-HRP tracing study. Neuroreport. 1994;5:2697–2701. doi: 10.1097/00001756-199412000-00072. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Elston A, Manger PR, Defelipe J. Specialization in pyramidal cell structure in the sensory-motor cortex of the Chacma baboon (Papio ursinus) with comparative notes on macaque and vervet monkeys. Anat Rec A Discov Mol Cell Evol Biol. 2005a;286:854–865. doi: 10.1002/ar.a.20217. [DOI] [PubMed] [Google Scholar]
- Elston GN, Elston A, Casagrande V, Kaas JH. Pyramidal neurons of granular prefrontal cortex of the galago: complexity in evolution of the psychic cell in primates. Anat Rec A Discov Mol Cell Evol Biol. 2005b;285:610–618. doi: 10.1002/ar.a.20198. [DOI] [PubMed] [Google Scholar]
- Enard W, Khaitovich P, Klose J, Zollner S, Heissig F, Giavalisco P, Nieselt-Struwe K, Muchmore E, Varki A, Ravid R, Doxiadis GM, Bontrop RE, Paabo S. Science. Vol. 296. New York, NY: 2002a. Intra- and interspecific variation in primate gene expression patterns; pp. 340–343. [DOI] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Paabo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002b;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- Feller M, Scaziani M. A precritical period for plasticity in visual cortex. Current Opinion in Neurobiology. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Fernandes M. Tool use and predation of oysters (Crassostrea rhizophorae) by the tufted capuchin, Cebus apella apella, in brackish water mangrove swamp. Primates. 1991;32:529–531. [Google Scholar]
- Fish J, Dehay C, Kennedy H, Huttner W. Making bigger brains- the evolution of neural-porgenitor-cell division. Journal of Cell Science. 2008;121:2783–2793. doi: 10.1242/jcs.023465. [DOI] [PubMed] [Google Scholar]
- Fleagle J, Simons E. Limb skeleton and locomotor adaptations of Apidium phiomens, and Oligocene anthropoid from Egypt. American Journal of Physical Anthropology. 1995;97:235–289. doi: 10.1002/ajpa.1330970303. [DOI] [PubMed] [Google Scholar]
- Fragaszy D, Izar P, Visalberghi E, Ottoni E, de Oliveira M. Wild capuchin monkeys (Cebus libidinosus) use anvils and stone pounding tools. American Journal of Primatology. 2004;64:359–366. doi: 10.1002/ajp.20085. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Science. Vol. 294. New York, NY: 2001. Neocortex patterning by the secreted signaling molecule FGF8; pp. 1071–1074. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Sekhon M, Schein J, Zhao S, Osoegawa K, Scott CE, Evans RS, Burridge PW, Cox TV, Fox CA, Hutton RD, Mullenger IR, Phillips KJ, Smith J, Stalker J, Threadgold GJ, Birney E, Wylie K, Chinwalla A, Wallis J, Hillier L, Carter J, Gaige T, Jaeger S, Kremitzki C, Layman D, Maas J, McGrane R, Mead K, Walker R, Jones S, Smith M, Asano J, Bosdet I, Chan S, Chittaranjan S, Chiu R, Fjell C, Fuhrmann D, Girn N, Gray C, Guin R, Hsiao L, Krzywinski M, Kutsche R, Lee SS, Mathewson C, McLeavy C, Messervier S, Ness S, Pandoh P, Prabhu AL, Saeedi P, Smailus D, Spence L, Stott J, Taylor S, Terpstra W, Tsai M, Vardy J, Wye N, Yang G, Shatsman S, Ayodeji B, Geer K, Tsegaye G, Shvartsbeyn A, Gebregeorgis E, Krol M, Russell D, Overton L, Malek JA, Holmes M, Heaney M, Shetty J, Feldblyum T, Nierman WC, Catanese JJ, Hubbard T, Waterston RH, Rogers J, de Jong PJ, Fraser CM, Marra M, McPherson JD, Bentley DR. A physical map of the mouse genome. Nature. 2002;418:743–750. doi: 10.1038/nature00957. [DOI] [PubMed] [Google Scholar]
- Grove E, Fukuchi-Shimogori T. Generating the cerebral cortical map. Annual Review of Neuroscience. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Hackett TA. Organization and correspondence of the auditory cortex of humans and nonhuman primates. In: Kaas JH, Preuss TM, editors. The Evolution of Nervous Systems, Volume 4: Primates. Oxford: Academic Press; 2007. pp. 109–119. [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O'Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Heil P, Bronchti G, Wollberg Z, Scheich H. Invasion of visual cortex by the auditory system in the naturally blind mole rat. NeuroReport. 1991;2:735–738. doi: 10.1097/00001756-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, Kaas JH, Lent R. The basic nonuniformity of the cerebral cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12593–12598. doi: 10.1073/pnas.0805417105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- Hirth F, Reichert H. Basic nervous system types: one or many? In: Striedter G, Rubenstein J, editors. The Evolution of Nervous Systems, Volume 1: Theories, Development, Invertebrates. Oxford: Academic Press; 2007. pp. 55–72. [Google Scholar]
- Hof PR, Chanis R, Marino L. Cortical complexity in cetacean brains. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1142–1152. doi: 10.1002/ar.a.20258. [DOI] [PubMed] [Google Scholar]
- Hooks B, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Hunt DL, Yamoah EN, Krubitzer L. Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience. 2006;139:1507–1524. doi: 10.1016/j.neuroscience.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Ibbotson MR, Mark RF. Orientation and spatiotemporal tuning of cells in the primary visual cortex of an Australian marsupial, the wallaby Macropus eugenii. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:115–123. doi: 10.1007/s00359-002-0379-6. [DOI] [PubMed] [Google Scholar]
- Izraeli R, Koay G, Lamish M, Heicklen-Klein AJ, Heffner HE, Heffner RS, Wollberg Z. Cross-modal neuroplasticity in neonatally enucleated hamsters: structure, electrophysiology and behavior. European Journal of Neuroscience. 2002;15:693–712. doi: 10.1046/j.1460-9568.2002.01902.x. [DOI] [PubMed] [Google Scholar]
- Johnston TD, Gottlieb G. Neophenogenesis: a developmental theory of phenotypic evolution. Joural of Theoretical Biology. 1990;147:471–495. doi: 10.1016/s0022-5193(05)80260-7. [DOI] [PubMed] [Google Scholar]
- Kaas J. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The segregation of function in the nervous system: Why do the sensory systems have so many subdivisions? Contributions to Sensory Physiology. 1982;7:201–240. [Google Scholar]
- Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Progress in Brain Research. 2000;128:173–179. doi: 10.1016/S0079-6123(00)28015-1. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Reconstructing the organization of neocortex of the first mammals and subsequent modifications. In: Kaas J, Krubitzer L, editors. The Evolution of Nervous Systems, Volume 3: Mammals. Oxford: Academic Press; 2007. pp. 27–48. [Google Scholar]
- Kahn DM, Krubitzer L. Massive cross-modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11429–11434. doi: 10.1073/pnas.162342799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen S, Krubitzer L. Effects of bilateral enucleation on the size of visual and nonvisual areas of the brain. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn176. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen SJ, Kahn DM, Krubitzer L. Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience. 2006;142:843–858. doi: 10.1016/j.neuroscience.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Karlen SJ, Krubitzer L. The functional and anatomical organization of marsupial neocortex: evidence for parallel evolution across mammals. Progress in neurobiology. 2007;82:122–141. doi: 10.1016/j.pneurobio.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR. Neurogenesis and the evolution of cortical diversity: Mode, tempo, and partitioning during development and persistence in adulthood. Brain Behavior and Evolution. 2000;55:336–344. doi: 10.1159/000006668. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proceedings of the National Academy of Science, USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nature reviews. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. The magnificent compromise: cortical field evolution in mammals. Neuron. 2007;56:201–208. doi: 10.1016/j.neuron.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Disbrow E. The evolution of parietal areas involved in hand use in primates. In: Kaas Jon, Gardner Ester., editors. The Senses: A Comprehensive Reference. Volume 6, Somatosensation. Elsevier; London: 2008. pp. 183–214. [Google Scholar]
- Krubitzer L, Hunt DL. Captured in the Net of Space and Time: Understanding Cortical Field Evolution. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous systems, Volume 3: Mammals. San Diego: Elsevier; 2007. pp. 49–52. [Google Scholar]
- Krubitzer L, Kaas J. The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr Opin Neurobiol. 2005;15:444–453. doi: 10.1016/j.conb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Manger P, Pettigrew JD, Calford MB. The organization of neocortex in monotremes: In search of the prototypical plan. J Comp Neurol. 1995;348:1–45. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- Kumar S, Blair Hedges S. A molecular time scale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Larsen DD, Krubitzer L. Genetic and epigenetic contributions to the cortical phenotype in mammals. Brain Res Bull. 2008;75:391–397. doi: 10.1016/j.brainresbull.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtneckert R, Reichert H. Anteroposterior regionalization of the brain: genetic and comparative aspects. Adv Exp Med Biol. 2008;628:32–41. doi: 10.1007/978-0-387-78261-4_2. [DOI] [PubMed] [Google Scholar]
- Lyon DC. The evolution of visual cortex nad visual systems. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems, Volume 3: Mammals. Oxford: Academic Press; 2007. pp. 267–306. [Google Scholar]
- Manger P, Sum M, Szymanski M, Ridgway S, Krubitzer L. Modular subdivisions of dolphin insular cortex: Does evolutionary history repeat itself. Journal of Cognitive Neuroscience. 1998;10:153–156. doi: 10.1162/089892998562627. [DOI] [PubMed] [Google Scholar]
- Marino L. Convergence of complex cognitive abilities in cetaceans and primates. Brain Behav Evol. 2002;59:21–32. doi: 10.1159/000063731. [DOI] [PubMed] [Google Scholar]
- Marino L. Cetacean Brain Evolution. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems, Volume 3: Mammals. Oxford: Academic Press; 2007. pp. 261–266. [Google Scholar]
- Marino L, Connor R, Fordyce R, Herman L, Hof P, Lefebvre L, Lusseau D, McCowan B, Ninchinsky E, Pack A, Rendell L, Reidenberg J, Reiss D, Uhen M, Van der Gucht E, Whitehead H. Cetations have complex brains for complex cognition. Public Library of Science. 2007;5:966–972. doi: 10.1371/journal.pbio.0050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen GK, Woolfe A, Goode D, Vavouri T, Callaway H, Elgar G. Ancient duplicated conserved noncoding elements in vertebrates: a genomic and functional analysis. Genome Res. 2006;16:451–465. doi: 10.1101/gr.4143406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekel-Bobrov N, Lahn B. What makes us human: Revisiting the age-old question in the genomic era. Journal of Biomedical Discovery and Collaboration. 2006;I:18. doi: 10.1186/1747-5333-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S, Garber M, Gentles AJ, Goodstadt L, Heger A, Jurka J, Kamal M, Mauceli E, Searle SM, Sharpe T, Baker ML, Batzer MA, Benos PV, Belov K, Clamp M, Cook A, Cuff J, Das R, Davidow L, Deakin JE, Fazzari MJ, Glass JL, Grabherr M, Greally JM, Gu W, Hore TA, Huttley GA, Kleber M, Jirtle RL, Koina E, Lee JT, Mahony S, Marra MA, Miller RD, Nicholls RD, Oda M, Papenfuss AT, Parra ZE, Pollock DD, Ray DA, Schein JE, Speed TP, Thompson K, VandeBerg JL, Wade CM, Walker JA, Waters PD, Webber C, Weidman JR, Xie X, Zody MC, Graves JA, Ponting CP, Breen M, Samollow PB, Lander ES, Lindblad-Toh K. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature. 2007;447:167–177. doi: 10.1038/nature05805. [DOI] [PubMed] [Google Scholar]
- Monuki E, Porter F, Walsh C. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Napier J, Napier P. Morphology, ecoloty and behaviour of nonhuman primates. London: Academic; 1967. A handbook of linving primates. [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Padberg J, Franca J, Cooke D, Soares J, Rosa M, Fiorani M, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. Journal of Neuroscience. 2007;27:10106–10115. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. Manipulative Monkeys: The Capuchins of Lomas Barbudal. Boston: Harvard University Press; 2008. [Google Scholar]
- Piche M, Robert S, Miceli D, Bronchti G. Environmental enrichment enhances auditory takeover of the occipital cortex in anophthalmic mice. European Journal of Neuroscience. 2004;20:3463–3472. doi: 10.1111/j.1460-9568.2004.03823.x. [DOI] [PubMed] [Google Scholar]
- Polanyi M. Science. Vol. 160. New York, NY: 1968. Life's irreducible structure; pp. 1308–1312. [DOI] [PubMed] [Google Scholar]
- Preuss T. Who's afraid of Homo sapiens? Journal of Biomedical Discovery and Collaboration. 2006;I:17. doi: 10.1186/1747-5333-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Suner I, Williams RW. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proceedings of the National Acadamy of Science. 1991;88:2083–2087. doi: 10.1073/pnas.88.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone G. Cerebral cortical plasticity: Perception and skill acquisition. In: Gazzaniga M, editor. The New Cognitive Neurosciences. Cambridge: MIT Press; 2000. pp. 237–247. [Google Scholar]
- Rilling J, Insel T. The primate neocortex in comparative perspective using magnetic resonance imaging. Journal of Human Evolution. 1999;37:191–223. doi: 10.1006/jhev.1999.0313. [DOI] [PubMed] [Google Scholar]
- Ringo JL. Neuronal interconnectin as a function of brain size. Brain Behavior and Evolution. 1991;38:1–6. doi: 10.1159/000114375. [DOI] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. Time is of the essence: A conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cerebral Cortex. 1994;4:331–343. doi: 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Santucci D, Francia N, Trincia V, Chiarotti F, Aloe L, Alleva E. A mouse model of neurobehavioural response to altered gravity conditions: An ontogenetical study. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Sears KE. Molecular determinants of bat wing development. Cells, tissues, organs. 2008;187:6–12. doi: 10.1159/000109959. [DOI] [PubMed] [Google Scholar]
- Sears KE, Behringer RR, Rasweiler JJt, Niswander LA. Development of bat flight: morphologic and molecular evolution of bat wing digits. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6581–6586. doi: 10.1073/pnas.0509716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Hof PR. The evolution of neuron types and cortical histology in apes and humans. In: Kaas JH, Preuss TM, editors. The Evolution of Nervous Systems, Volume 4: Primates. Oxford: Academic Press; 2007. pp. 355–378. [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Shoshani J, Kupsky WJ, Marchant GH. Elephant brain. Part I: gross morphology, functions, comparative anatomy, and evolution. Brain Res Bull. 2006;70:124–157. doi: 10.1016/j.brainresbull.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Silver FH, Siperko LM. Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix? Crit Rev Biomed Eng. 2003;31:255–331. doi: 10.1615/critrevbiomedeng.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- Smith S, Trachtenberg J. Experience-dependent binocular competition begins at eye opining. Nature Neuroscience. 2007;10:370–375. doi: 10.1038/nn1844. [DOI] [PubMed] [Google Scholar]
- Striedter G. A history of ideas in evolutionary neuroscience. In: Striedter G, Rubenstein J, editors. The Evolution of Nervous Systems, Volume 1: Theories, Development, Invertebrates. Oxford: Academic Press; 2007. pp. 1–15. [Google Scholar]
- Strochlic L, Weinl C, Piper M, Holt C. Axon Pathfinding. In: Striedter G, Rubenstein J, editors. The Evolution of Nervous Systems in Mammals. Oxford: Academic Press; 2007. pp. 187–209. [Google Scholar]
- Tallafuss A, Bally-Cuif L. Formation of the head-trunk boundary in the animal body plan: an evolutionary perspective. Gene. 2002;287:23–32. doi: 10.1016/s0378-1119(01)00829-0. [DOI] [PubMed] [Google Scholar]
- Tsai CL, Wang LH, Shiue YL, Chao TY. Influence of temperature on the ontogenetic expression of neural development-related genes from developing tilapia brain expressed sequence tags. Mar Biotechnol (NY) 2007;9:243–261. doi: 10.1007/s10126-006-6089-2. [DOI] [PubMed] [Google Scholar]
- Tyler CJ, Dunlop SA, Lund RD, Harman AM, Dann JF, Beazley LD, Lund JS. Anatomical comparison of the macaque and marsupial visual cortex: common features that may reflect retention of essential cortical elements. J Comp Neurol. 1998;400:449–468. doi: 10.1002/(sici)1096-9861(19981102)400:4<449::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Urbach R, Technau GM. Dorsoventral patterning of the brain: a comparative approach. Adv Exp Med Biol. 2008;628:42–56. doi: 10.1007/978-0-387-78261-4_3. [DOI] [PubMed] [Google Scholar]
- Vallender E, Lahn B. A primate-specific acceleration in the evolution of the caspase-dependent apoptosis pathway. Human Molecular Genetics. 2006;15:3034–3040. doi: 10.1093/hmg/ddl245. [DOI] [PubMed] [Google Scholar]
- Vavouri T, Walter K, Gilks WR, Lehner B, Elgar G. Parallel evolution of conserved non-coding elements that target a common set of developmental regulatory genes from worms to humans. Genome Biol. 2007;8:R15. doi: 10.1186/gb-2007-8-2-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. Science. Vol. 291. New York, NY: 2001. The sequence of the human genome; pp. 1304–1351. [DOI] [PubMed] [Google Scholar]
- Villarreal JA, Schlegel WM, Prange HD. Thermal enviornment affects morphological and behavioral development of Rattus norvegicus. Physiology & Behavior. 2007;91:26–35. doi: 10.1016/j.physbeh.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Waga I, Dacier A, Pinha P, Tavares M. Spontaneous tool use by wild capuchin monkeys (Cebus libidnosus) in Cerrado. Folia Primatol. 2006;77:337–344. doi: 10.1159/000093698. [DOI] [PubMed] [Google Scholar]
- Warner DA, Shine R. The adaptive significance of temperature-dependent sex determination in a reptile. Nature. 2008;41:566–568. doi: 10.1038/nature06519. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Weller WL. SmI cortical barrels in an Australian marsupial, Trichosurus vulpecula (brush-tailed possum): Structural organization, patterned distribution, and somatotopic relationships. The Journal of Comparative Neurology. 1993;337:471–492. doi: 10.1002/cne.903370310. [DOI] [PubMed] [Google Scholar]
- Wen Q, Chklovskii DB. Segregation of the brain into gray and white matter: a design minimizing conduction delays. PLoS Comput Biol. 2005;1:e78. doi: 10.1371/journal.pcbi.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, Chklovskii DB. A cost-benefit analysis of neuronal morphology. J Neurophysiol. 2008;99:2320–2328. doi: 10.1152/jn.00280.2007. [DOI] [PubMed] [Google Scholar]
- White L, Fitzpatrick D. Vision and cortical map development. Neuron. 2007;56:327–338. doi: 10.1016/j.neuron.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CE, Wrangham RW, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Wise SP. Evolution of ventral premotor cortex and the primate way of reaching. In: Kaas JH, Preuss TM, editors. The Evolution of Nervous Systems, Volume 4: Primates. Oxford: Academic Press; 2007. pp. 157–165. [Google Scholar]
- Zhou X, Merzenich MM. Enduring effects of early structured noise exposure on temporal modulation in the primary auditory cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4423–4428. doi: 10.1073/pnas.0800009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience. 2008;154:390–396. doi: 10.1016/j.neuroscience.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]