Abstract
Dentin matrix protein 1 (DMP1) has been identified in the extracellular matrix (ECM) of dentin and bone as the processed NH2-terminal and COOH-terminal fragment. However, the full-length form of DMP1 has not been identified in these tissues. The focus of this investigation was to search for the intact full-length DMP1 in dentin and bone. We used two types of anti-DMP1 antibodies to identify DMP1: one type specifically recognizes the NH2-terminal region and the other type is only reactive to the COOH-terminal region of the DMP1 amino acid sequence. An ~105-kDa protein, extracted from the ECM of rat dentin and bone, was recognized by both types of antibodies; and the migration rate of this protein was identical to the recombinant mouse full-length DMP1 made in eukaryotic cells. We concluded that this ~105-kDa protein is the full-length form of DMP1, which is considerably less abundant than its processed fragments in the ECM of dentin and bone. We also detected the full-length form of DMP1 and its processed fragments in the extract of dental pulp/odontoblast complex dissected from rat teeth. In addition, immunofluorescence analysis showed that in MC3T3-E1 cells the NH2-terminal and COOH-terminal fragments of DMP1 are distributed differently. Our findings indicate that the majority of DMP1 must be cleaved within the cells that synthesize it and that minor amounts of uncleaved DMP1 molecules are secreted into the ECM of dentin and bone.
Keywords: Dentin matrix protein 1, Posttranslational modification, Extracellular matrix, Dentin, Bone
Dentin matrix protein 1 (DMP1), discovered by cDNA cloning, was originally postulated to be dentin-specific [1]. Later on, its expression was observed in bone [2, 3]. The distinctive feature of DMP1 is the presence of a large number of acidic domains, a property that implicates it as a possible participant in regulating matrix mineralization. This purported biological function is supported by observations that MC3T3-E1 cells overexpressing DMP1 demonstrate an earlier onset of mineralization and the formation of a significantly larger size of the induced mineralized nodules compared to nontransfected control cells [4]. Findings from Dmp1 knockout mouse experiments and gene mutation studies on human osteomalacia strengthen the conclusion that DMP1 plays an important role in bone and dentin mineralization [5–7]. In addition to its direct role in biomineralization, studies indicated that DMP1 may regulate osteoblast-specific and/or odontoblast-specific genes [8, 9]. More recent studies indicated that DMP1 may also be involved in the regulation of phosphate homeostasis through fibroblast growth factor 23 (FGF23), a newly identified hormone that is released from bone and targeted in the kidneys; deletion of the Dmp1 gene leads to a dramatic increase of FGF23 mRNA in osteocytes [7].
Full-length DMP1 cDNA from a number of species has been cloned and sequenced [1, 2, 3, 10, 11], but the corresponding full-length form of the protein has not been identified. In searching for naturally occurring DMP1, we discovered that the extracellular matrix (ECM) of bone and dentin contains fragments originating from intact DMP1, namely, a 37-kDa fragment from the NH2-terminal region and a 57-kDa fragment from the COOH-terminal region of the DMP1 amino acid sequence [12]. More recently, we discovered that the NH2-terminal fragment of DMP1 in the ECM of bone and dentin also occurs as a proteoglycan [13]. The proteoglycan variant, referred to as DMP1-PG, possesses a single glycosaminoglycan side chain linked to the core protein via Ser74 in the rat DMP1 amino acid sequence.
Thus, in the ECM of bone and dentin, three forms of DMP1 have been identified: (1) the NH2-terminal fragment, (2) the COOH-terminal fragment, and (3) DMP1-PG. Based on the obvious differences in their biochemical features, it is logical to hypothesize that these variants may have distinct functions and play different roles in biomineralization. In vitro mineralization studies have demonstrated that the COOH-terminal fragment promotes mineralization by acting as a nucleator for hydroxyapatite formation [14–16]. Information regarding the biological functions of the NH2-terminal fragment and DMP1-PG is lacking.
In this investigation, we detected the processed fragments of DMP1 in the extract of dental pulp/odontoblast complex dissected from rat teeth. Even though not found in earlier studies, we were able to identify the full-length form of DMP1 in the ECM of rat dentin and bone; full-length DMP1 is present in the ECM at a concentration considerably less than that of its processed fragments.
Materials and Methods
Extraction and Separation of Noncollagenous Proteins from the ECM of Rat Dentin and Bone
The procedures for protein extraction from the ECM of dentin and bone were similar to those described previously [17, 18]. Briefly, after removal of the soft tissues, the incisor dentin and long bone from rats 10–12 weeks old were placed in 4 M guanidium-HCl (Gdm-HC) solution (pH 7.2) containing proteinase inhibitors overnight, and the solution was discarded. The dentin and bone tissues were ground to particles of 1–2 mm in diameter. Then, the dentin and bone particles were extracted with 4 M Gdm-HC solution containing proteinase inhibitors; this procedure mainly extracts noncollagenous proteins (NCPs) present in the unmineralized collagen matrices (predentin and osteoid). The extract from this step was not used in the present study. Subsequently, the NCPs in the dentin and bone powders were extracted in 4 M Gdm-HCl solution containing 0.5 M EDTA and proteinase inhibitors; this second step extracted proteins that were embedded in the mineralized phase (mineralized ECM), and the extract from the second step was used as the source of NCPs in the present investigation. The Gdm-HCl/EDTA extracts were first subjected to gel chromatography on a Sephacryl S-200 (Amersham Biosciences, Piscataway, NJ) column. The Sephacryl S-200 column separated the extracts into four major fractions: an earlier fraction known as ES1 [17, 18] contained groups of proteins of higher molecular weight, which included bone sialoprotein, osteopontin, the processed fragments of DMP1, and dentin sialophosphoprotein (DSPP). The latter three fractions contained molecules of lower molecular weight such as osteocalcin, proteinase inhibitors, and EDTA. Next, the ES1 fraction was applied to Q-Sepharose (Amersham Biosciences) ion-exchange chromatography with a gradient ranging 0–0.7 M NaCl in 6 M urea solution of pH 7.2. NCPs from ES1 were eluted into 120 fractions, each containing 0.5 mL of the 6 M urea solution. Each chromatographic fraction in the 6 M urea solution was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Stains-All staining, and Western immunoblotting, to test for the presence or absence of the full-length DMP1 and its processed fragments. During this ion-exchange chromatography, DMP1-PG eluted in later fractions (i.e., at a higher Cl− concentration), and thus the proteoglycan form of DMP1 did not interfere with the detection of full-length DMP1 or its processed fragments, which eluted in earlier fractions.
Extraction and Separation of NCPs in the Extract of Dental Pulp from Rat Teeth
For extraction of NCPs from the dental pulp, the pulp/odontoblast complex was dissected from rat teeth and then extracted with 4 M Gdm-HC containing proteinase inhibitors (without EDTA). The NCPs from the 4 M Gdm-HC extract were separated as described above, and the corresponding chromatographic fractions were assayed for the full-length DMP1 and its processed fragments.
SDS-PAGE and Western Immunoblotting
For SDS-PAGE, 5–15% gradient gels were used in all of the experiments. For Western immunoblotting, two types of anti-DMP1 antibodies (Table 1) were used. One was a monoclonal antibody generated using the NH2-terminal fragment of rat bone DMP1 as the antigen [13]. This monoclonal antibody, designated anti-DMP1-N-9B6.3, reacts specifically with the NH2-terminal fragment of DMP1 in Western immunoblotting but shows a very weak reaction to DMP1 in immunohistochemical analysis. A second type of antibody was an affinity-purified rabbit polyclonal antibody made to the synthetic peptide AYNHKPIGDQDDNDC (mouse DMP1 residues 471–485, very highly conserved among species), which only recognizes the COOH-terminal region of DMP1 and is referred to as anti-DMP1-C-785 [19]. This polyclonal antibody recognizes the COOH-terminal (57 kDa) fragment of DMP1 in both Western immunoblotting and immunohistochemical analyses; for the latter application, the immunoreaction of this polyclonal antibody is not as strong as that of the monoclonal antibody anti-DMP1-C-8G10.3 (see later). Both types of antibodies have been thoroughly characterized and previously used in Western immunoblotting for detection of the NH2-terminal or COOH-terminal fragment of DMP1, respectively [13, 19]. Western immunoblotting was performed as previously described [18].
Table 1.
Antibodies used in this study
| Antibody | Antibody type | Immunizing antigen | Immunoreactivity in Western immunoblotting | Immunoreactivity in immunohistochemistry |
|---|---|---|---|---|
| Anti-DMP1-N-9B6.3a | Monoclonal | 37 kDa (N-terminal) | Yes | Very weak |
| Anti-DMP1-N-859b | Polyclonal | Oligopeptide (residues 101–121) | Yes | Yes |
| Anti-DMP1-C-785c | Polyclonal | Oligopeptide (residues 471–485) | Yes | Yes |
| Anti-DMP1-C-8G10.3d | Monoclonal | 57 kDa (C-terminal) | No | Yes |
This monoclonal antibody was used to detect the NH2-terminal fragment of DMP1 by Western immunoblotting analysis. The characterization of this antibody has been described previously [13]
This polyclonal antibody was used to detect the NH2-terminal fragment of DMP1 by immunofluorescence (primary utilization) and Western immunoblotting (secondary utilization) in this study. This antibody was recently generated in our laboratory (see “Materials and Methods”)
This polyclonal antibody was used to detect the COOH-terminal fragment of DMP1 by Western immunoblotting analysis in this study. A detailed description of the characteristics of this antibody was reported previously [19]
This monoclonal antibody was used to detect the COOH-terminal fragment of DMP1 by immunofluorescence analysis. The characterization of this antibody has been reported [20]
Generation of Mouse DMP1 cDNA Construct
A full-length mouse DMP1 cDNA was obtained by digesting the DMP1-pBC-KS+ construct [9] with EcoRI restriction enzyme, and the released DMP1 cDNA fragment was subcloned into a mammalian expression vector, pcDNA3.1. The DMP1–pcDNA3.1 construct was used to transfect a human embryonic kidney cell line, HEK-293, or a murine preosteoblastic cell line, MC3T3-E1.
Transfection of the DMP1–pcDNA3.1 Construct into HEK-293 and MC3T3-E1 Cells
HEK-293 cells were cultured in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum. MC3T3-E1 cells were cultured with α-minimal essential medium (Invitrogen) supplemented with 10% fetal bovine serum, 5 mM β-glycerophosphate, and 100 μg/mL ascorbic acid. After these cells grew to 80–90% confluence, the DMP1–pcDNA3.1 construct premixed with Lipofectamine 2000 (Invitrogen) was added to the cultures. After transfection, the cells were cultured for 48 hours and then either harvested for cell lysate analysis or fixed with 4% paraformaldehyde for immunofluorescence microscopic analysis.
For cell lysate analysis, the cell layer was first washed with phosphate-buffered saline (PBS). Then, the cells were lysed with the lysis buffer containing protease inhibitors. The cell lysate samples were centrifuged to remove cell debris, and the supernatant containing proteins extracted from the cells was immediately lyophilized. The lyophilized cell lysate sample was later resuspended in 6 M urea solution and used for Western immunoblotting analysis.
For immunofluorescence, MC3T3-E1 or HEK-293 cells, nontransfected or transfected with the DMP1–pcDNA3.1 construct, were fixed with 4% paraformaldehyde for 15 minutes on ice. After washing with PBS, cells were incubated with blocking buffer containing 2% bovine serum albumin and 10% normal goat serum for 1 hour. Two types of primary antibodies (Table 1) were used for immunofluorescence. One was a polyclonal antibody recently generated (Sigma Genosys, Woodlands, TX) using an oligopeptide with the sequence of GLGPEEGQWGGP SKLDSDEDS (mouse DMP1 residues 101–121, highly conserved among species) as the antigen; this antibody, which recognizes the amino terminal fragment of DMP1, was designated anti-DMP1-N-859 and used for identifying the NH2-terminal fragment of DMP1 by immunofluorescence microscopy. The specificity of the polyclonal antibody anti-DMP1-N-859 was confirmed by enzyme-linked immunosorbent, dot blot, and Western immunoblotting analyses using the above synthetic oligopeptide as well as the NH2-terminal (37 kDa) fragment of DMP1 isolated from rat bone; this antiserum did not show immunoreaction to the COOH-terminal (57 kDa) fragment of DMP1 isolated from rat bone. In addition to its application for detecting DMP1 NH2-terminal fragment in immunofluorescence analysis (as a standard antibody), this polyclonal anti-DMP1-N-859 antibody was used in Western immunoblotting to identify the full-length form of DMP1 as well as the NH2-terminal (37 kDa) fragment. A monoclonal antibody generated using the COOH-terminal fragment of DMP1 isolated from rat bone as the antigen [20] was designated as anti-DMP1-C-8G10.3. The anti-DMP1-C-8G10.3 antibody was used for identifying the COOH-terminal fragment of DMP1 in immunofluorescence analysis. This monoclonal anti-DMP1-C-8G10.3 antibody does not react with DMP1 in Western immunoblotting analysis. The above primary antibodies were diluted at 1:200 in the blocking solution and applied to fixed MC3T3-E1 or HEK-293 cells for 1 hour. After washing with PBS, the goat anti-rabbit F(ab′) 2 fragment conjugated with Alexa 488 (for green color) and the goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546 (for red color, both from Invitrogen) were added at a dilution of 1:600 and incubated with the cells for 1 hour. After washing with PBS, cells were treated with TO-PRO-3 (Invitrogen) at a dilution of 1:500 for 5 minutes to stain the nuclei. Immunofluorescent staining was assessed under a Leica (Heidelberg, Germany) SP2 scanning laser confocal microscope. All of the immunohistochemical procedures were performed at room temperature.
Immunofluorescence Analysis of Rat Bone
Five-week-old Sprague-Dawley rats (Harlan, Indianapolis, IN) were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer. The humerus was dissected and further fixed in the same fixative for 2 days at 4°C, followed by decalcification in 8% EDTA (pH 7.4) at 4°C for approximately 4 weeks. Tissues were processed for paraffin embedding, and serial 8 μm sections were prepared. Immunofluorescence analysis was performed as described above. The animal protocol was approved by the Animal Welfare Committee of Baylor College of Dentistry of the Texas A & M University System Health Science Center.
Results
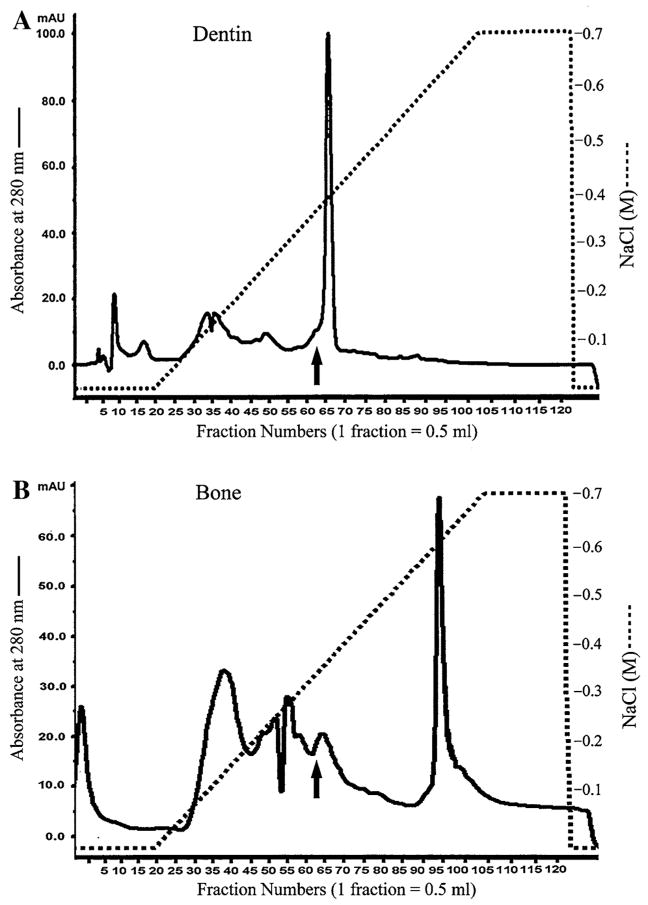
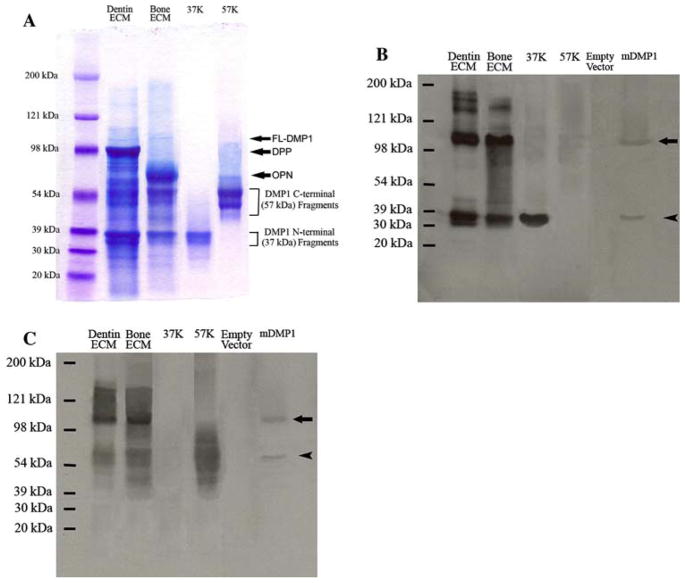
The Q-Sepharose ion-exchange chromatography separated ES1 of the Gdm-HCl/EDTA extracts of dentin and bone into 120 fractions (Fig. 1). We analyzed each of the Q-Sepharose ion-exchange chromatographic fractions with the aim of detecting the full-length form of DMP1. During chromatography for the dentin ECM extract, the full-length form of DMP1 co-eluted with the NH2-terminal (37 kDa) and COOH-terminal (57 kDa) fragments of DMP1 in fractions immediately before the majority of dentin phosphoprotein (DPP, a COOH-terminal fragment of DSPP) eluted. Due to its abundance, certain amounts of DPP co-eluted with DMP1 and its processed fragments. The full-length form of DMP1 was primarily detected in fractions 60–66. In some of these fractions (e.g., fraction 63), Stains-All staining (Fig. 2A) revealed a thin blue band between the 98 and 121 kDa molecular weight markers, estimated at approximately 105 kDa, in the same position as a band recognized by both the anti-DMP1-N-9B6.3 (Fig. 2B) and anti-DMP1-C-785 (Fig. 2C) antibodies. The migration rate of this protein band at approximately 105 kDa was identical to that of the recombinant full-length mouse DMP1 (Fig. 2B, C) made in HEK-293 cells transfected with the DMP1-pcDNA3.1 construct. Taken together, we concluded that this sharp blue band at approximately 105 kDa is the full-length form of DMP1. In Stains-All staining, the processed fragments of DMP1 appeared to be much more abundant than the full-length form.
Fig. 1.
Separation of NCPs from the ECM of rat dentin and bone. (A) The Q-Sepharose ion-exchange chromatogram of ES1 of the dentin extract. Arrow indicates the fraction that contained DMP1 and its fragments and was used for Stains-All staining and Western immunoblotting in Fig. 2. (B) The Q-Sepharose ion-exchange chromatogram of ES1 of the bone extract. Arrow indicates the fraction used for Stains-All staining and Western immunoblotting in Fig. 2
Fig. 2.
Detection of the full-length form of DMP1 and its processed fragments in the ECM extracts of rat dentin and bone. (A) Stains-All staining for Q-Sepharose chromatographic fractions of the ECM extracts of dentin and bone. Dentin ECM, The sample was from a chromatographic fraction (fraction 63, vertical arrow in Fig. 1A) of the Gdm-HCl/EDTA extract of dentin ECM. Sixty microliters of sample in 6 M urea solution was loaded. The thin, blue band migrating between 98 and 121 kDa represents the full-length form of DMP1 (FL-DMP1, estimated to be ~105 kDa). In this chromatographic fraction, the DMP1 fragments co-elute with the full-length form of DMP1 as well as DPP (approximate migrating position of DPP marked by arrow). Note that the DMP1 fragments are much more abundant than the full-length form in the dentin ECM. Bone ECM, The sample was from a chromatographic fraction (fraction 63, vertical arrow in Fig. 1B) of the Gdm-HCl/EDTA extract of rat bone ECM. Sixty microliters of sample in 6 M urea solution was loaded. The approximate migrating position of osteopontin (OPN) is indicated by arrow. 37K, Four micrograms of pure 37-kDa (NH2-terminal) fragment isolated from rat bone. 57K, Six micrograms of pure 57-kDa (COOH-terminal) fragment isolated from rat bone. Please note that both the 37- and 57-kDa fragments are present as clusters of bands, not a single protein band. The identification of these two clusters of bands as the NH2-terminal and COOH-terminal fragments of DMP1 was confirmed previously [12]. (B) Western immunoblotting using monoclonal anti-DMP1-N-9B6.3 antibody. Samples for the lanes of dentin ECM, bone ECM, 37K, and 57K are the same as in (A). Empty vector, Protein extract from cell lysates of HEK-293 cells transfected with a pcDNA3.1 vector that does not carry DMP1 cDNA; the total protein extract from one well of the HEK-293 cells of a six-well culture plate was loaded. mDMP1, protein extract from the cell lysates of the HEK-293 cells transfected with the mouse DMP1-DNA3.1 construct; the total protein extract from one well of the 293 cells of a six-well culture plate was loaded. Note the size of the recombinant full-length mouse DMP1 (~105 kDa, arrow) is identical to that of the full-length DMP1 detected in the ECM of rat dentin and bone. This monoclonal antibody recognizes both the full-length form (~105 kDa) and the NH2-terminal fragment (arrowhead) of DMP1. Note that the cleavage pattern in HEK-293 cells (a single cleavage) appears different from that occurring in the dentin and bone. (C) Western immunoblotting using polyclonal anti-DMP1-C-785 antibody. Loaded samples are the same as in (B). This polyclonal antibody recognizes both the full-length form (arrow) and the COOH-terminal fragment (arrowhead) of DMP1
During the chromatographic separation of bone ECM proteins, the full-length form of DMP1 was mainly detected in fractions 60–66 and co-eluted with its processed fragments and osteopontin. Similar to that observed in dentin ECM, a thin blue band at about 105 kDa, representing full-length DMP1, was observed by Stains-All staining (Fig. 2A); and this protein band was recognized by both the anti-DMP1-N-9B6.3 (Fig. 2B) and anti-DMP1-C-785 (Fig. 2C) antibodies in Western immunoblotting analysis. In Stains-All staining, the amount of full-length DMP1 appeared to be much less than that of its processed fragments.
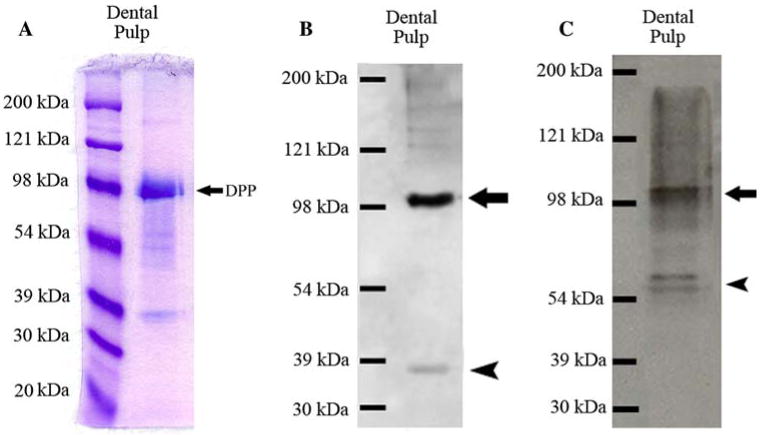
The full-length form of DMP1 and its processed fragments were observed in the Gdm-HC extract from the pulp/odontoblast complex dissected from rat teeth (Fig. 3). Due to the limited amounts of NCPs from the dental pulp extract, we did not observe a blue-staining ~105-kDa band representing the full-length form of DMP1 by Stains-All staining (Fig. 3A), but we detected the full-length form of DMP1 as well as its processed fragments by Western immunoblotting using both the anti-DMP1-N-9B6.3 and anti-DMP1-C-785 antibodies (Fig. 3B, C). Again, full-length DMP1 appeared to be more abundant using Western blotting than Stains-All staining.
Fig. 3.
Detection of the full-length form of DMP1 and its processed fragments in the extract of rat dental pulp. (A) Stains-All staining for a Q-Sepharose chromatographic fraction of dental pulp extract. The sample was from a chromatographic fraction of the Gdm-HCl extract of the dental pulp (corresponding to fraction 63 of the Q-Sepharose chromatograms of dentin or bone extracts). Sixty microliters of sample in 6 M urea solution was loaded. The strong blue band migrating just below 98 kDa represents DPP (arrow). DMP1 fragments are visible below the DPP band. The protein band representing the full-length form of DMP1 cannot be observed between 98 and 121 kDa, due to the relatively small amounts of sample loaded. (B) Western immunoblotting using monoclonal anti-DMP1-N-9B6.3 antibody. Note that both the full-length form of DMP1 (arrow) and its processed NH2-terminal fragment (arrowhead) are present in the extract of the pulp/odontoblast complex. (C) Western immunoblotting using polyclonal anti-DMP1-C-785 antibody. Both the full-length form of DMP1 (arrow) and its processed COOH-terminal fragment (arrowhead) are present in the extract of pulp/odontoblast complex
The ~105-kDa protein band in the ECM of dentin and bone was also detected by the polyclonal anti-DMP1-N-859 antibody, which was raised against a synthetic peptide matching the amino acid sequence from the NH2-terminal region of mouse/rat DMP1. Similar to the results obtained using the monoclonal antibody anti-DMP1-N-9B6.3 (used as a standard antibody for detecting the NH2-terminal fragment of DMP1 in Western immunoblotting), this polyclonal antibody detected both the NH2-terminal (37 kDa) fragment and the full-length form of DMP1 in Western immunoblotting analysis for dentin and bone extracts. These findings further strengthened our conclusion that the ~105-kDa protein band is the full-length form of DMP1.
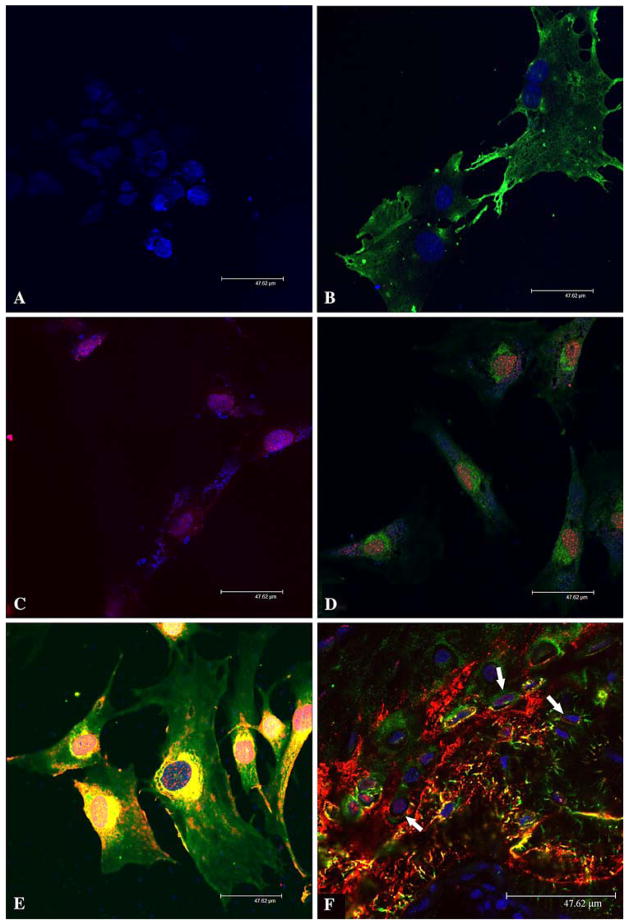
We also analyzed DMP1 and its processed fragments in MC3T3-E1 cells, either nontransfected or transfected with the mouse DMP1–pcDNA3.1 construct. Western immunoblotting failed to detect any DMP1-related molecules in the cell lysates from MC3T3-E1 cells transfected with the DMP1–pcDNA3.1 construct (data not shown). This failure to detect DMP1 was most likely attributed to the lower transfection efficiency of MC3T3-E1 cells. The immunofluorescence using the anti-DMP1-N-859 and anti-DMP1-C-8G10.3 antibodies showed that within MC3T3-E1 cells the location of the NH2-terminal fragment differed from that of the COOH-terminal fragment; the former (Fig. 4B, D, E, stained green) was primarily observed in the cytoplasm surrounding the nuclei, whereas the latter (Fig. 4C–E, stained red) was mainly present in the nuclei. In cells transfected with the DMP1–pcDNA3.1 construct (Fig. 4E), we observed greater amounts of green and yellow in the cytoplasm, indicating greater amounts of DMP1 and/or its processed fragments in the transfected cells.
Fig. 4.
Immunofluorescence analysis of DMP1 in MC3T3-E1 cells and rat bone. Nuclei were stained by TO-PRO-3 fluorescent dye (blue) in all figures. Fluorescent immunostaining was assessed under a Leica SP2 scanning laser confocal microscope. Bar = 47.62 μm. (A) Immunofluorescence staining of nontransfected MC3T3-E1 cells (negative control). The preimmune serum was used in place of the primary anti-DMP1 antibodies. The secondary antibodies were a mixture of the goat anti-rabbit F(ab′)2 fragment conjugated with Alexa 488 (for green color) and goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546 (for red color). Note that only the nuclei show a positive fluorescent signal (TO-PRO-3 staining, blue). (B) Immunofluorescence staining of nontransfected MC3T3-E1 cells. The primary antibody was the anti-DMP1-N-859 polyclonal antibody (made in rabbit). The secondary antibody was the goat anti-rabbit F(ab′)2 fragment conjugated with Alexa 488 (for green color). The signal for the NH2-terminal fragment of DMP1 (green) is observed in the cytoplasm but not in the nuclei. (C) Immunofluorescence staining of nontransfected MC3T3-E1 cells. The primary antibody was anti-DMP1-C-8G10.3 monoclonal antibody (made in mouse). The secondary antibody was the goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546 (for red color). The signal for the COOH-terminal fragment (red) is observed in the nuclei. (D) Immunofluorescence staining of nontransfected MC3T3-E1 cells. The primary antibodies were a mixture of the anti-DMP1-N-859 polyclonal antibody and anti-DMP1-C-8G10.3 monoclonal antibody. The secondary antibodies were a mixture of the goat anti-rabbit F(ab′)2 fragment conjugated with Alexa 488 and goat anti-mouse F(ab′)2 fragment conjugated with Alexa 546. The signal for the NH2-terminal fragment of DMP1 (green color) is observed primarily in the cytoplasm, whereas that for the COOH-terminal fragment (red color) is mainly detected in the nuclei. (E) Immunofluorescence staining of MC3T3-E1 cells transfected with the DMP1-pcDNA3.1 construct. The primary and secondary antibodies are the same as in (D). Note the distribution difference between DMP1 fragments. (F) Immunofluorescence staining of osteocytes. The tissue section was from the ossified metaphysis region of the humerus of a 5-week-old rat. The primary and secondary antibodies were the same as in (D) and (E). Note that within the osteocytes (arrows) some signal for the COOH-terminal fragment of DMP1 (red) is observed in the nuclei. Also note that the NH2-terminal fragment is mainly found in the cytoplasm. In the cytoplasm, the NH2-terminal fragment of DMP1 appears more abundant along the processes of osteocytes
In HEK-293 cells transfected with the DMP1–pcDNA3.1 construct, we observed a similar distribution pattern for DMP1 fragments: the NH2-terminal fragment was primarily localized in the cytoplasm, while the signal for the COOH-terminal fragment was mainly observed in the nuclei (data not shown). In osteocytes of the rat humerus (Fig. 4F), the NH2-terminal fragment of DMP1 was present in the cytoplasm, while the signal for the COOH-terminal fragment was found in the nuclei.
Discussion
Previously, we had isolated and characterized three forms of DMP1 from the ECM of dentin and bone: the NH2-terminal fragment, the COOH-terminal fragment, and a proteoglycan form of the NH2-terminal fragment. In this study, we report the detection of a fourth one, the full-length form of DMP1 in the ECM of dentin and bone. The full-length form was more easily identified using Western immunoblotting than with Stains-All staining. We also showed that full-length DMP1 can be expressed intracellularly with transient transfection of eukaryotic cells. Although DMP1, a member of the SIBLING family [21], has been shown to be critical for the mineralization of bone and dentin, the exact mechanisms by which DMP1 functions and participates in the biomineralization process remain ill-defined. With the identification of four forms of DMP1 in the ECM, the function of DMP1 in its various forms can be more easily elucidated.
In the ECM of either dentin or bone, Stains-All staining showed that there are more DMP1 fragments than its full-length form, whereas in Western immunoblotting the area and density of the band representing the full-length form of DMP1 appear similar in amount to that of the processed fragments. To investigate this discrepancy, we loaded pure DMP1 fragments (37 and 57 kDa) isolated from rat long bone onto the same SDS-PAGE gels in which samples from the chromatographic fractions of the dentin and bone ECM extracts were run. Using protein purification and sequencing analysis, we confirmed that these DMP1 fragments isolated from rat bone are highly pure, containing very little or no contaminants of other proteins [12]. Loading of a known quantity of such highly pure DMP1 fragments can be used to assess the relative amounts of these fragments in the ECM of dentin and bone. In order to detect DMP1 fragments by Western immunoblotting using the anti-DMP1 antibodies, relatively large amounts of purified DMP1 fragments (at least 3 μg of either the 37- or 57-kDa fragment) were needed for the detection of a clear band. In contrast, we observed that Stains-All staining is more sensitive than Western immunoblotting using the two antibodies for the detection of DMP1 fragments; Stains-All staining could detect as little as 1 μg of the 57-kDa fragment. The exact opposite was observed for the full-length form of DMP1; Western immunoblotting using the same two antibodies was more sensitive than Stains-All staining. This observation was further confirmed by Western immunoblotting using pure recombinant full-length bovine DMP1 made in bone marrow stromal fibroblasts (a gift from Dr. Larry Fisher of the National Institute of Dental and Craniofacial Research, Bethesda, MD) as the sample; as little as 0. 2 μg of the recombinant full-length bovine DMP1 (migrating slower than that of mouse DMP1) can be clearly detected by the anti-DMP1-N-9B6.3 or anti-DMP1-C-785 antibody (data not shown). Based on these observations, we conclude that in Western immunoblotting analysis the full-length form of DMP1 is considerably more immunoreactive to the anti-DMP1-N-9B6.3 or anti-DMP1-C-785 antibody. Therefore, Western immunblotting using these antibodies is not ideal for estimating the relative ratio of the full-length DMP1 to its processed fragments.
Although at this point we are unable to accurately calculate the quantity of full-length DMP1 in the ECM, we have concluded that the full-length form is considerably less than its processed fragments in the ECM of dentin and bone. Based on the area and intensity of Stains-All staining, it appears that the amounts of full-length DMP1 in the ECM of dentin are less than 1% of its processed fragments.
In this investigation, we detected the full-length form of DMP1 and its processed NH2-terminal and COOH-terminal fragments in the corresponding ion-exchange chromatographic fractions of the Gdm-HCl extract of rat pulp/odontoblast complex that contains NCPs from the cellular compartments (i.e., dental pulp cells and odontoblasts). Using Western immunoblotting, we also detected DMP1 fragments in the cell lysates of HEK-293 cells transfected with the DMP1–pcDNA3.1 construct, indicating that these cells contain DMP1 fragments. The presence of DMP1 fragments in HEK-293 cells and in the Gdm-HCl extract of rat pulp/odontoblast complex, along with the presence of trace amounts of full-length DMP1 in the ECM, indicates that the majority of DMP1 molecules must be cleaved within the cells (odontoblasts/osteoblasts/osteocytes). Detection of the full-length form of DMP1 in the ECM of dentin and bone suggests that trace amounts of uncleaved DMP1 molecules are secreted into the ECM. At present, it is unclear whether the trace amounts of full-length DMP1 secreted into the ECM have a function(s) different from that of its processed fragments. Previous in vitro mineralization studies showed that full-length bovine DMP1 made by bone marrow stromal fibroblasts inhibits hydroxyapatite formation whereas the COOH-terminal fragment isolated from rat bone promotes formation of the apatite crystals [14]. Future in vivo studies are warranted to test the biological functions of the full-length form of DMP1 in comparison with those of its processed fragments.
Immunofluorescence analysis showed that in MC3T3-E1 cells, HEK-293 cells, and osteocytes of the rat humerus, the NH2-terminal fragment of DMP1 is primarily located in the cytoplasm surrounding the nuclei and the signal for the COOH-terminal fragment is observed in the nuclei. These findings not only strengthen our conclusion that the majority of DMP1 must be cleaved within the cells that synthesize it but also indicate that the NH2-terminal and COOH-terminal fragments of DMP1 must play different biological roles. It has been reported that in addition to its direct role in the formation and/or growth of hydroxyapatite crystals, DMP1, acting as a transcription factor, may be involved in regulating other genes associated with dentinogenesis and osteogenesis [8, 22]. The presence of the COOH-terminal fragment of DMP1 in the nuclei suggests that it might be the COOH-terminal fragment rather than the full-length form that has transcriptional activity. It should be noted that the nuclear localization sequence is present at the COOH-terminal fragment of DMP1 [8].
Bone morphogenetic protein-1/tolloid-like proteinases, which are expressed in the bone and dentin as well as a variety of other tissues/cells [23], have been shown to cleave bacteria-derived recombinant DMP1 in vitro [24]. However, the in vitro cleavage pattern of DMP1 resulting from incubation with bone morphogenetic protein-1/tol-loid-like proteinases appeared different from that found in bone and dentin [12]. Further investigations are needed to provide more convincing evidence regarding the proteolytic activity of bone morphogenetic protein-1/tolloid-like proteinases upon DMP1.
Acknowledgments
This work was supported by National Institutes of Health grant DE 005092 (to C. Q.). I. M. is on sabbatical leave from the Medical University of Gdansk, Poland.
References
- 1.George A, Sabsay B, Simonian PAL, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein: implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 2.Hirst KL, Ibaraki-O’Connor K, Young MF, Dixon MJ. Cloning and expression analysis of the bovine dentin matrix acidic phosphoprotein gene. J Dent Res. 1997;76:754–760. doi: 10.1177/00220345970760030701. [DOI] [PubMed] [Google Scholar]
- 3.MacDougall M, Gu T, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci USA. 2001;98:4516–4521. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, Lu Y, Mishina Y, Feng JQ. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 6.Ye L, Mishina Y, Chen D, Huang H, Dallas SL, Dallas MR, Sivakumar P, Kunieda T, Tsutsui TW, Boskey A, Bonewald LF, Feng JQ. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1230–1235. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, George A. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, Li YC, Kong J, Eick JD, Dallas SL, Feng JQ. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Dev Biol. 2007;303:191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyosawa S, Sato A, O’hUigin C, Tichy H, Klein J. Expression of the dentin matrix protein 1 gene in birds. J Mol Evol. 2000;50:31–38. doi: 10.1007/s002399910004. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Yamakoshi Y, Iwata T, Hu YY, Zhang H, Hu JC, Simmer JP. Eur J Oral Sci. Vol. 114. 1 University of Michigan Dental Research Laboratory; Ann Arbor, MI, USA: 2006. Porcine dentin matrix protein 1: gene structure, cDNA sequence, and expression in teeth; pp. 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C, Brunn JC, Cook RG, Orkiszewski RS, Malone JP, Veis A, Butler WT. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- 13.Qin C, Huang B, Wygant JN, McIntyre BW, McDonald CH, Cook RG, Butler WT. A chondroitin sulfate chain attached to the bone dentin matrix protein 1 NH2-terminal fragment. J Biol Chem. 2006;281:8034–8040. doi: 10.1074/jbc.M512964200. [DOI] [PubMed] [Google Scholar]
- 14.Tartaix PH, Doulaverakis M, George A, Fisher LW, Butler WT, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey AL. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 15.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, Hao J, George A. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 17.Butler WT, Bhown M, Dimuzio MT, Linde A. Nonocollagenous proteins of dentin: isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1981;1:187–199. doi: 10.1016/s0174-173x(81)80019-2. [DOI] [PubMed] [Google Scholar]
- 18.Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 19.Qin C, Baba O, Brunn JC, McKee MD, Bonewald L, Butler WT. Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP) share unique properties including tissue localization, proteolytic processing, and high molecular weight forms. In: Sodek J, Landis W, editors. Proceedings of the 8th ICCBMT. University of Toronto Press; Toronto: 2005. pp. 174–177. [Google Scholar]
- 20.Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004;23:371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- 22.Narayanan K, Gajjeraman S, Ramachandran A, Hao J, George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J Biol Chem. 2006;281:19064–19071. doi: 10.1074/jbc.M600714200. [DOI] [PubMed] [Google Scholar]
- 23.Takahara K, Lyons GE, Greenspan DS. Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem. 1994;269:32572–32578. [PubMed] [Google Scholar]
- 24.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]