Abstract
Human decidual CD14+ macrophages and CD56+ NK cells were isolated from material obtained after first-trimester pregnancy terminations. Each cell type expressed a specific surface receptor for histocompatibility leukocyte antigen (HLA)-G (an MHC class Ib protein that is expressed on extravillous trophoblasts), LILRB1 on CD14+ macrophages and KIR2DL4 on CD56+ NK cells. Cross-linking with anti-LILRB1 or anti-KIR2DL4 resulted in up-regulation of a small subset of mRNAs including those for IL-6, IL-8, and TNFα detected using a microarray representing 114 cytokines. Incubation with transfectants expressing the HLA-G homodimer (but not with transfectants expressing the HLA-G monomer) resulted in secretion of the same cytokine proteins from both leukocyte sets. Moreover, cytokine secretion from both leukocyte sets was blocked by both the appropriate anti-receptor mAb and by anti-HLA-G. The amount of these cytokines secreted by decidual macrophages was substantially greater than that secreted by decidual NK cells. VEGF was constitutively secreted by both cell types. LILRB1, which contains an immunoreceptor tyrosine-based switch motif, functions here as an activating receptor, although it has been known as an inhibitory receptor. KIR2DL4 also functions as an activating receptor, although it also has the potential to function as an inhibitory receptor. Secretion of proinflammatory and proangiogenic proteins supports a role for these leukocytes in important processes that are essential for successful pregnancy, but they may represent only a portion of the proteins that are secreted.
Keywords: IL-6, IL-8, pregnancy, trophoblast, VEGF
The human fetus expresses paternally encoded and maternally encoded molecules. As such, it is a hemiallogeneic graft with respect to the mother but is not rejected, an immunological paradox first pointed out by Medawar in 1953 (1). Twenty-five years later it was discovered that the highly polymorphic class I MHC molecules histocompatibility leukocyte antigen (HLA)-A and -B, potentially the primary immunological obstacle to successful pregnancy, are not expressed on the extraembryonic tissues in direct contact with maternal tissues (2). Villous cytotrophoblasts and syncytiotrophoblasts, which are in direct contact with maternal blood in the intervillous space, express neither class I nor class II MHC molecules. Although the extravillous trophoblasts (EVT), which migrate deep into the maternal decidua where they encounter maternal leukocytes, are also HLA-A- and -B-negative, they do express the classical class I MHC molecule, HLA-C, and the nonclassical class I molecules HLA-E, -F, and G (3–7). All of the latter three are nonpolymorphic and are encoded telomeric of the HLA-A locus. HLA-C and HLA-E function to stimulate the inhibitory KIR and CD94/NKGA receptors found on NK cells, respectively (8). The function of HLA-F is unknown.
Although HLA-G has long been known to be uniquely expressed on EVT (9–11), its role in the placenta is still unclear. HLA-G is unique in two ways: (i) It has only a “stub” for a cytoplasmic tail. As a result, it does not undergo endocytosis and is unusually stable at the cell surface (12). (ii) It has two free cysteine residues (Cys-42 and Cys-147) unlike other class I MHC proteins. We reported earlier that the bacterial recombinant soluble form of HLA-G formed a disulfide-linked homodimer with the intermolecular Cys-42–Cys-42 disulfide bond (13). In addition, surface-expressed HLA-G on transfectants also formed a disulfide-linked dimer, but the dimer was not present on the low-expressing HLA-G+ Jeg3 choriocarcinoma cell line that is derived from EVT (13, 14). However, the membrane-bound HLA-G homodimer, sometimes called G1, was recently shown to be the major form expressed by isolated EVT (14). It is unlikely that any of the other forms G2–G7 that have been postulated to result from alternative gene splicing occur as physiologically relevant proteins, with the possible exception of G5, soluble HLA-G (15–17). Recent observations from several groups (18–21) suggest that at least one function of HLA-G could be to modulate cytokine secretion from decidual leukocytes to induce immune tolerance, control EVT invasion, and/or contribute to vascular remodeling of spiral arteries to allow for successful embryo implantation and pregnancy maintenance.
Leukocytes, consisting primarily of NK cells and macrophages, infiltrate the uterine mucosa and populate the luteal/secretory phase endometrium. They are maintained in the decidua after implantation. Decidual NK cells exhibit a distinct phenotype (CD56brightCD16−CD3−) that differs from the majority of peripheral blood NK cells (CD56dimCD16+CD3−). The unusual phenotype and relatively large number of these CD56brightCD16− NK cells set them apart from normal peripheral blood NK cells. They represent ≈70% of the leukocytes found in decidua, whereas the number of NK cells with this unusual CD56bright phenotype among peripheral lymphocytes is ≈5%. Most of the remaining populations of leukocytes in the decidua are CD14+ macrophages (20–30%) and CD3+ T cells (5–15%). They have not been studied in as much detail as decidual NK cells.
The HLA-G receptors reported on these leukocytes to date are the leukocyte Ig-like receptors (LILR) B1 and B2 [formerly known as Ig-like transcripts (ILT) 2 and 4] expressed on macrophages, killer cell Ig-like receptor (KIR) 2DL4 expressed on NK cells, and possibly CD160 (22–24). Previous reports clearly demonstrated that LILRB1 and LILRB2 preferentially bind to HLA-G relative to other classical class I MHC proteins such as HLA-A2 (25). HLA-G has a much higher affinity for LILRB1 than LILRB2 (26, 27). A recent structural study showed that the HLA-G dimer binds with greater avidity to LILRB1/2 and signals more strongly through LILRB than monomeric HLA-G and other class I MHC proteins (25). The disulfide-linked dimer exhibits an oblique configuration exposing two upward facing binding sites on the α3 domain of the HLA-G heavy chain. CD8 also binds to HLA-G and competes with LILRB for binding to this site. These sites are readily accessible to receptors, explaining the increased avidity (26). However, no crystal structure of an HLA-G homodimer complexed with a LILRB protein has been published, although one for the HLA-G monomer–LILRB2 complex was reported (28). The HLA-G dimer modulates the function of LILRB1-expressing antigen-presenting cells (macrophages) by principally binding to LILRB1. Therefore, the HLA-G dimer presumably has a pivotal role in vivo at the maternal–fetal interface compared with the monomeric form. In the periphery, LILRBs are also expressed by peripheral T and B lymphocytes and by NK cells and monocytes (29).
KIR are a second group of Ig-like receptors encoded in the same chromosomal region as LILR receptors. KIR2DL4 has an unusual structure relative to the other members of this group, having a D0–D2 configuration of its Ig domains and a transmembrane region encoding an arginine residue and a long cytoplasmic region encoding an ITIM (23, 30). Remarkably, it is also a specific receptor for HLA-G (23). No structural study of its interaction with HLA-G has been reported.
The purpose of the present work was to investigate the expression of LILRB1 and KIR2DL4 on decidual macrophages and NK cells and to assess the possibility that the HLA-G monomer and/or dimer might induce cytokine secretion through these receptors.
Results
Surface Expression of LILRB1 and KIR2DL4 on Decidual and Peripheral Macrophages and NK Cells.
Decidual leukocytes were prepared by using material obtained from first-trimester pregnancy terminations (31). Briefly, a Ficoll gradient was used to separate leukocytes. After incubation with anti-CD14 mAb for 30 min the decidual CD14+ macrophage population was isolated by sorting on a MoFlo (see Materials and Methods). Approximately 0.3–1 × 106 cells were obtained from one decidual preparation. Decidual CD56+ NK cells were prepared similarly by using anti-CD56 mAb with a yield of ≈2–4 × 106 cells per preparation. Peripheral CD14+ monocytes and CD56+ NK cells were obtained from a fresh Leukopak by using a similar procedure.
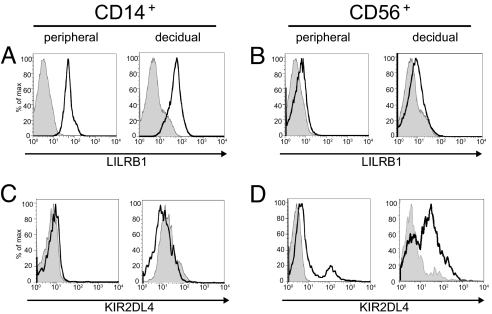
FACS analysis was carried out by using antibodies to the two receptors, LILRB1 (mAb GHI75, anti-ILT2) and KIR2DL4 (mAb 33) (32), that have been reported to be important in recognizing HLA-G. LILRB1 was expressed at a moderate level on CD14+ decidual macrophages and at a comparable level on CD14+ peripheral monocytes (Fig. 1). Low-level expression was found on both decidual and peripheral CD56+ NK cells. Similarly, a moderate level of KIR2DL4 expression was found on decidual NK cells. Very low-level expression was found on peripheral NK cells, as reported (18). No surface expression of KIR2DL4 could be detected on either decidual CD14+ macrophages or peripheral CD14+ monocytes (Fig. 1).
Fig. 1.
LILRB1 and KIR2DL4 surface expression on peripheral and decidual CD14+ and CD56+ cells assessed by flow cytometry. (A) LILRB1 expression on peripheral and decidual CD14+ cells. (B) LILRB1 expression on peripheral and decidual CD56+ cells. (C) KIR2DL4 expression on peripheral and decidual CD14+ cells. (D) KIR2DL4 expression on peripheral and decidual CD56+ cells. Staining relative to an isotype control antibody (shaded) is shown.
Cytokine mRNA Expression by Decidual CD14+ Macrophages and CD56+ NK Cells after Cross-Linking with Anti-LILRB1 or Anti-KIR2DL4 mAb or with HLA-G Homodimer.
CD14+ Decidual macrophages, 3 × 105 cells, were incubated with anti-LILRB1 (mAb GHI75) for 30 min followed by the addition of goat anti-mouse IgG and further incubation for 5 h. Total RNA was extracted, transcribed into cDNA, and then into cRNA by using biotinylated UTP for detection. cRNA was hybridized to the common cytokine array containing 60-mer DNA probes on a small chip representing 114 cytokines (Fig. S1C) (Superarray; the complete list may be found at www.sabiosciences.com/gene_array_product/html/ohs-021.html). Up-regulation of mRNA was observed for 10 cytokines [supporting information (SI) Fig. S1]. Stronger signals for IL-8 were obtained with peripheral CD14+ cells (Fig. S2) than with decidual CD14+ cells. Notably, many of the stronger up-regulated signals were proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, and TNFα).
Next, the same procedure was carried out, but the CD14+ cells were stimulated by coincubation with three different cells for 5 h: 721.221 cells (a B lymphblastoid cell line that lacks expression of all MHC proteins) as control, the same cells transfected with a mutant HLA-G (C42S) in which the cysteine required for homodimerization was mutated to serine, and finally with the unmutated HLA-G cDNA (4C4) in which 90% of the surface expressed HLA-G is the homodimer and 10% the monomer (13). Similar results were obtained as with antibody cross-linking with up-regulation of mRNA for proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, and TNFα) seen with the HLA-G homodimer (Figs. S1 and S2). In addition to the proinflammatory cytokines, IL-14 was a prominently expressed cytokine. However, the variability observed after incubation with 721.221 cells and its transfectants was much greater and the signal somewhat weaker than that seen on cross-linking with mAb, and statistical significance compared with controls was not reached for any cytokine with four microarrays.
CD56+ decidual NK cells, 3 × 105 cells, were incubated with anti-KIR2DL4 mAb 33 for 30 min followed by addition of goat anti-mouse IgG and further incubation for 5 h. cRNA was obtained as described above and hybridized with the same cytokine array.
The pattern of cytokine mRNA expression by decidual CD56+ cells was remarkably similar to that found with decidual CD14+ cells. Cross-linking with anti-KIR2DL4 antibody resulted in expression of relatively large amounts of IL-1β, IL-6, IL-8, and TNFα with lower-level expression of granulocyte colony-stimulating factor (G-CSF) and IL-14 (Fig. S3). The HLA-G homodimer similarly induced up-regulation of expression of G-CSF, IL-1β, IL-6, and IL-8 mRNA (Fig. S3), but again the large variability observed prevented obtaining statistical significance relative to controls for any cytokine with the four microarrays used.
VEGF-B mRNA expression was relatively high in both decidual CD14+ macrophages and CD56+ NK cells and similar whether the cells were stimulated with IgG, with anti-LILRB1, or with anti-KIR2DL4 or were incubated with control 721.221 cells or with these cells transfected with HLA-G monomer or with the HLA-G homodimer [Fig. S1C (116; 15th row, 4th column) and Figs. S2 and S3]. This mRNA was constitutively expressed. A number of additional mRNAs are expressed constitutively in decidual macrophages and in decidual NK cells (Table S1).
Cytokine Protein Secretion by Decidual and Peripheral CD14+ Macrophages and CD56+ NK Cells.
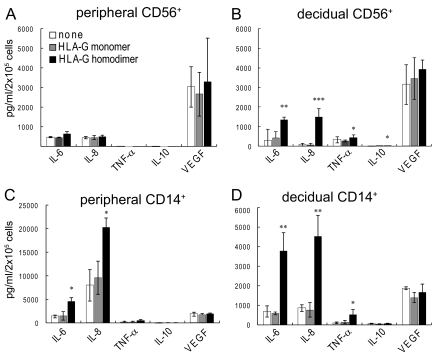
The experiments described above established that only a limited number of the 114 common cytokines represented in the microarray were up-regulated as mRNA under the conditions used and allowed us to focus on this small group of cytokines. Because of the variability in the microarray data, next secretion of cytokine protein by decidual CD14+ macrophages and CD56+ NK cells was measured by using the Luminex bead system (Bio-Rad) after a 24- to 48-h incubation with 721.221 cells or the same cells expressing the HLA-G monomer or HLA-G homodimer; comparable preparations of CD14+ and CD56+ peripheral cells obtained from fresh Leukopaks were examined for comparison (Fig. 2). Five fluorescent beads coupled to specific antibodies of interest were available for this assay: IL-6, IL-8, TNFα, VEGF, and IL-10. IL-6 and IL-8 were both secreted in significant amounts from all cell types in the presence of the HLA-G homodimer, but little secretion occurred in the presence of the monomer or untransfected 721.221 cells, particularly in the case of decidual cells. In contrast to the microarray data, statistical significance was obtained in all cases by using three or four experiments with cells from different individuals. However, the amount of IL-6 and IL-8 produced by decidual CD14+ cells was much larger than that produced by decidual CD56+ NK cells, particularly in the case of IL-8. Despite the relatively high level of message for TNFα, very little TNFα was secreted by either cell type at the time point and under the conditions used. Similarly, the HLA-G homodimer induced secretion of a relatively low amount of IFNγ from both cell types (Fig. 3), even though no differential expression of its mRNA was observed [Fig. S1C (44; 6th row, 4th column) and Fig. S3]. VEGF was produced by all of the cell populations used, but no specific effect of either of the forms of HLA-G on its secretion was observed. A very low level of IL-10 production was observed with each cell type. (IL-10 was examined because of its importance in immunosuppression and as a negative control.)
Fig. 2.
HLA-G homodimer induced cytokine protein secretion by human peripheral and decidual CD14+ macrophages and CD56+ NK cells. Peripheral CD56+ (A), decidual CD56+ cells (B), peripheral CD14+ cells (C), or decidual CD14+ cells (D) were cocultured in a 1:1 ratio with HLA class I-negative 721.221 cells (white bars), 721.221 cells transfected to express HLA-G monomer (gray bars), or 721.221cells transfected to express HLA-G homodimer (black bars). Supernatants were collected after 24 h for CD14+ macrophages or after 48 h for CD56+ NK cells. The concentration of cytokines (IL-6, IL-8, IL-10, TNFα, or VEGF) in each supernatant was measured by a multiplex cytokine assay. The SD was calculated from the mean of 4 experiments, each with an individual donor. Statistical significance: *, P < 0.05; **, P < 0.01; and ***, P < 0.001. Note that the scale of C is compressed ≈4-fold relative to D because the secretion of IL-8 by peripheral CD14+ cells is so much larger than that of decidual CD14+ cells.
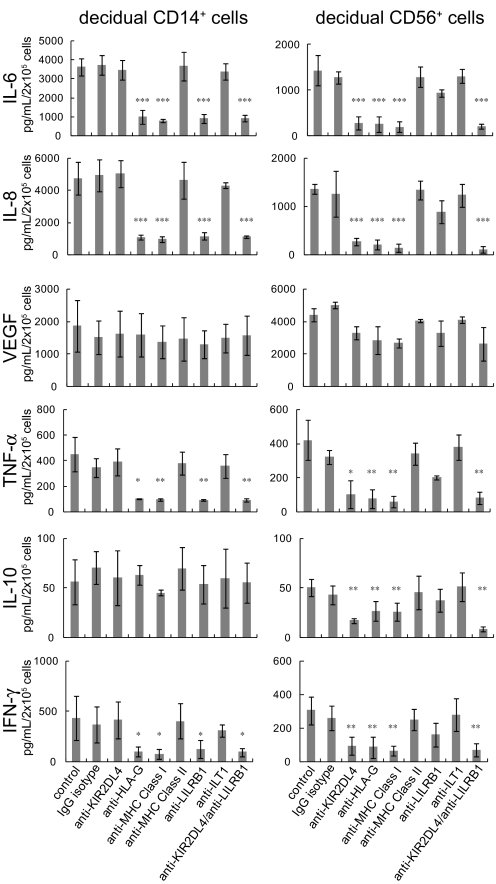
Fig. 3.
Cytokine secretion induced by the HLA-G homodimer was blocked in the presence of specific antibodies against HLA-G or its receptors. Decidual CD14+ cells (Left) or decidual CD56+ cells (Right) were cocultured in a 1:1 ratio with 721.221 cells transfected with HLA-G homodimer in the presence of various mAb as shown. Supernatants were collected after 24 h for CD14+ macrophages or after 48 h for CD56+ NK cells. The concentration of cytokines (IL-6, IL-8, IL-10, IFNγ, TNFα, or VEGF) in each supernatant was measured by a multiplex cytokine assay. The SD was calculated from the mean of 3 experiments for decidual CD56+ cells and 4 experiments for decidual CD14+ cells, each with an individual donor. Statistical significance: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Antibody Blocking of Protein Secretion.
Addition of anti-HLA-G mAb (G9), anti-LILRB1 (anti-ILT2, GHI75) or anti- MHC class I antibody (W6/32) effectively blocked IL-6, IL-8, TNFα, and IFNγ secretion induced by the HLA-G homodimer by decidual CD14+ cells but had little or no effect on VEGF secretion by these cells (Fig. 3). No blocking by the control mAb, anti-KIR2DL4 (mAb 33), anti-class II MHC (anti-HLA-DR), or anti-ILT1 was observed.
Similarly, cytokine secretion by decidual CD56+ NK cells induced by the HLA-G homodimer was appropriately blocked by anti-KIR2DL4, anti-HLA-G, and anti-MHC class I, but not by anti-MHC class II, anti-LILRB1, or anti-ILT1.
Discussion
These data show that the HLA-G homodimer, but not the monomer, induces secretion of the proinflammatory cytokines IL-6 and IL-8 and a small amount of TNFα (and probably also IL-1α, IL-1β, and IFNγ) from both decidual macrophages and NK cells. VEGF is secreted constitutively. A few other cytokines were found in smaller amounts, but no up-regulation of expression of the vast majority of the 114 cytokine mRNAs found in the microarray used was evident.
The amount of the proinflammatory cytokines secreted by decidual macrophages was much larger than that secreted by decidual NK cells. Much attention has been paid to the secretion of the proinflammatory cytokines described above as well as chemokines and VEGF by decidual NK cells and their possible roles in implantation that involves invasion of EVT into the decidua and in vascular remodeling of the uterine spiral arteries that insures an adequate blood supply to the developing fetus (14, 18–21,). IL-6 has received particular attention because of its possible role in preeclampsia (33). However, decidual macrophages that may make equal or more extensive contributions to these and other pregnancy-related phenomenon have been little studied.
Only a little information is available about the signaling mechanisms in decidual and peripheral macrophages and NK cells that lead to cytokine secretion. KIR2DL4 on peripheral NK cells has been shown to be endocytosed together with its reported ligand sHLA-G into an early endosomal compartment from which it signals up-regulation of both proinflammatory and proangiogenic molecules (18). Whether or not this phenomenon is related to the signaling that occurs in decidual NK cells, which express a much larger amount of surface KIR2DL4, remains to be studied. Moreover, the findings reported here may represent only a fraction of protein whose secretion is induced in these cells by the HLA-G homodimer because other biologically active materials, particularly galectin-1, pregnancy-specific glycoprotein 11, progestagen protein 14 and others (31) have been shown to be differentially expressed at high levels as mRNAs in human decidual NK cells. Protein secretion was also confirmed in the case of galectin-1. The secreted galectin-1 is involved in T cell apoptosis at the maternal–fetal interface in early human pregnancy (34) and appears to play an important role in maternal–fetal tolerance (35). Other secreted proteins may also have important functions in maternal–fetal tolerance or another aspect of maternal–fetal physiology.
LILRB1 and B2 on macrophages have both been known as inhibitory receptors and as having four immunoreceptor tyrosine-based inhibitory motifs (ITIMs; V/LX XXV/L) in their cytoplasmic domains (22, 36). The enhanced production of cytokine mRNA and protein on stimulation of LILRB1 on decidual macrophages by cross-linking with mAb GHI75 or with HLA-G homodimer was therefore unexpected. However, inspection of the sequence of the cytoplasmic region of LILRB1 revealed that one of the reported ITIMs is an immunoreceptor tyrosine-based Switch Motif (ITSM; SXVXXV) located 3 aa before the C terminus. The occurrence of ITSMs in this location in other receptors has been reported, and that in LILR family members has been mentioned briefly but without further investigation (37). The adaptors SAP and EAT bind to an ITSM and can convert inhibitory to activating receptors (38, 39). EAT is expressed in all peripheral macrophages and may be responsible for the activating phenotype of decidual macrophages. KIR2DL4 on decidual NK cells also encodes both an ITIM in its cytoplasmic region and an activating module, in this case an arginine residue in its transmembrane region that associates with the signaling module FcεRIγ (40). Further studies of control of the signaling potential of these two types of decidual cells are needed.
In addition to membrane-bound HLA-G (G1) a second form of this protein has been described, soluble HLA-G (sHLA-G, G5). This material has been reported to be produced in the fetal placenta and to be secreted into maternal serum (7). However, no biochemical evidence of its occurrence has been reported that might also shed light on the question of whether it is formed by shedding or by alternative gene splicing. Its detection has almost entirely been carried out by ELISA, and questions have been raised about the specificity of this immunological test (15, 16). In addition, a Western blot was recently reported that showed immunoreactive material of the correct size for full-length HLA-G (≈40 kDa) (17), mainly present in EVT with far smaller amounts at the basal plate and chorion. The same study used RT-PCR to examine the presence of different isoforms and in particular the amount of G5 message was extremely low, ≈1.5% of G1 message. However, this area of research has engendered considerable controversy.
Nevertheless, the possibility that sHLA-G might stimulate peripheral NK cells to produce important factors related to pregnancy has been an important research focus (18). sHLA-G was shown to induce internalization of the small amount of KIR2DL4 on the surface of peripheral NK cells together with sHLA-G to an early endosomal compartment where signaling was initiated (18). A profile of up-regulated genes very similar to those observed upon stimulation of human decidual NK cells by 721.221 cells expressing the HLA-G homodimer (but not by those expressing the monomer) was described.
Whether these two systems to induce cytokine secretion are related to each other or not remains to be clarified. Notably, none of the available studies of sHLA-G has reported what fraction of the recombinant sHLA-G produced in bacteria or of the naturally occurring sHLA-G reportedly found in fetal placenta and maternal serum is monomer or dimer. Previously full-length recombinant HLA-G (G1) produced in bacteria has been shown to form the dimer (13) as has sHLA-G (G5) reported to be produced by villous trophoblast (41).
Two other recent studies have addressed the question of the interaction of EVT with decidual leukocytes. In one of these (14), 721.221 cells transfected with HLA-G were used as a surrogate for EVT together with peripheral monocyte-derived dendritic cells as responders. Thus, in this work, both cells were surrogates, and the phenotype of DC stimulated by LPS is very different from that of decidual macrophages. A very small increase in secretion of IL-6 and IL-10 was observed. In the other study (19), decidual NK cells were used that were stimulated for 24 h in the presence of IL-15. Freshly isolated decidual NK cells do not polarize the microtubule organizing center and cytotoxic granules to the synaptic region and are not cytotoxic (42). However, on treatment with IL-15, polarization occurs, and these cells become cytotoxic, a different phenotype from that found in the decidua. An important future goal would be to use freshly isolated decidual NK cells or macrophages together with EVT, the natural cell that may present the HLA-G dimer to its receptors, together with cell-specific costimulatory molecules that may elicit a similar or a distinct pattern of protein secretion.
Materials and Methods
Antibodies.
Anti-human isotype controls, anti-LILRB1-PE, anti-CD14-FITC, anti-CD56-PE-Cy5, and anti-MHC class I and anti-MHC class II mAb were all purchased from BD Biosciences. The anti-human monoclonal antibodies, anti-KIR2DL4 33, and anti-ILT1 were kindly provided by Sumati Rajagopalan and Eric Long (National Institute of Allergy and Infectious Diseases/National Institutes of Health) and Marco Colonna (Washington University School of Medicine, St. Louis, MO), respectively. The anti-HLA-G (G9) mAb was described in ref. 13.
Human Cells and Cell Lines.
First-trimester decidual samples were obtained from patients undergoing elective pregnancy termination at a women's health center in Boston, MA. Lymphocytes from decidual tissue were processed as described in ref. 31. Briefly, the tissue was minced and digested with 0.1% collagenase type IV and 0.01% DNase I (Sigma–Aldrich) in RPMI medium 1640 for 45 min at 37 °C. Released lymphocytes were subsequently purified by using a Ficoll–Hypaque gradient (Amersham Biosciences). Similarly, peripheral blood lymphocytes were isolated from Leukopaks obtained from anonymous donors at Massachusetts General Hospital, Boston, MA. The EBV-transformed B-cell line 721.221 transfected with either the wild-type HLA-G cDNA that forms protein homodimer or a mutant HLA-G cDNA (C42S) that can only form the protein monomer was described in ref. 13. The monomeric HLA-G molecule was produced by the cysteine to serine mutation at position 42, thereby preventing disulfide bond formation. The wild-type and mutant transfectants have nearly the same HLA-G expression level (13). Approximately 90% of surface HLA-G in the wild-type transfectant is the dimer. These cells have remained stable since their creation 6–7 years ago. Approximately 30% of the cells in the mutant transfectant lose expression in 3 months whereas the remaining 70% retain the original expression level. As a consequence, standard G418 selection is used every 3 months to restore the original phenotype of the culture.
Cell Culture.
Peripheral and decidual CD14+ and CD56+ cells were cultured in RPMI medium 1640 with 10% FBS. For total RNA isolation, CD14+ cells were incubated for 5 h in the presence of control or anti-LILRB1 mAb and secondary antibody or cocultured with 721.221 or its HLA-G transfectants. Similarly, CD56+ cells were incubated for 5 h in the presence of control or anti-KIR2DL4 mAb and secondary antibody or cocultured with 721.221 or its HLA-G transfectants. For cytokine detection and blocking assays, decidual CD14+ and CD56+ cells were cocultured with 721.221 transfectants expressing dimeric HLA-G in the presence or absence of blocking mAb (10 μg/mL), i.e., without adding secondary cross-linking antibodies. All supernatants were collected and stored immediately at −80 °C until cytokines were measured. Each experiment shown represents an average of at least 3 experiments each with an individual donor.
Flow Cytometry.
LILRB1 surface expression was assessed by staining peripheral or decidual leukocytes with phycoerythrin (PE)-conjugated anti-human LILRB1 mAb and either FITC-conjugated anti-human CD14 or anti-CD56 mAb. KIR2DL4 surface expression was assessed by incubating peripheral or decidual leukocytes with unconjugated anti-KIR2DL4 mAb for 20 min followed by incubation with PE-conjugated goat anti-mouse IgG. Cells were then incubated with PBS containing 20% mouse serum for 20 min and stained with either FITC-conjugated anti-human CD14 or FITC-conjugated anti-human CD56 antibody. Stained cells were analyzed on a FACSCalibur (Becton Dickinson).
Superarray Cytokine Analysis.
Total cellular RNA was isolated by using the RNeasy mini kit (Qiagen). Human common cytokine array (SABiosciences Corporation) was used to evaluate mRNA expression of 114 human cytokine genes (see Fig. S1C). Approximately 3 μg of total RNA was used for generating the biotin-11–UTP-labeled cRNA (PerkinElmer) by the TrueLabeling-AMP 2.0 kit (SABiosciences Corporation). According to the manufacturer's protocol, the array membrane was prehybridized for 2 h at 60 °C in GEAhyb hybridization solution. The denatured cRNA probe was hybridized with specific DNA probes on the membrane at 60 °C for >24 h. After the washing and blocking steps, membranes were incubated for 10 min at room temperature in 2 mL of binding buffer with alkaline phosphatase-conjugated streptavidin at a 1:8,000 dilution. The signals were developed in CDP-Star chemiluminescent substrate for 5 min. The arrays were exposed to an X-ray film. The images were then analyzed by using GEArray Expression Analysis Suite (SABiosciences Corporation), in which the results were normalized to GAPDH gene expression. Each of the microarray images shown represents one donor and is representative of 3 or 4 experiments.
Multiplex Cytokine Assay.
Supernatants collected from coculture experiments were thawed and analyzed by using the Bio-Plex multiple cytokine assay kit (Bio-Rad). A predesigned 6-plex kit that was used to determine the concentration of IL-6, IL-8, IL-10, IFNγ, TNFα, or VEGF for each supernatant collected. All reagents needed for the assays were provided in the kit, as were the necessary protocols for data acquisition. Data were obtained by using the Bio-Plex manager software program (Bio-Rad) followed by conversion to Excel (Microsoft) for further analysis. Data shown are representative of 3 experiments for CD56+ cells and 4 for CD14+ cells, each with an individual donor.
Data Analysis and Statistics.
All data are presented as means of 3 or 4 independent experiments performed ± SD. Statistical analysis was done by using the unpaired Student's t test, with a 2-tailed P value <0.05, < 0.01, or <0.001 taken to indicate statistical significance.
Supplementary Material
Acknowledgments.
We thank Drs. Sumati Rajagopalan and Eric Long for providing anti-KIR2DL4 mAb 33 and Dr. Marco Colonna for anti-ILT1. This work was supported by National Institutes of Health Research Grant AI053330.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901173106/DCSupplemental.
References
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–338. [Google Scholar]
- 2.Faulk WP, Temple A. Distribution of β2-microglobulin and HLA in chorionic villi of human placentae. Nature. 1976;262:799–802. doi: 10.1038/262799a0. [DOI] [PubMed] [Google Scholar]
- 3.Orr HT, et al. Use of HLA loss mutants to analyze the structure of the human major histocompatibility complex. Nature. 1982;296:454–456. doi: 10.1038/296454a0. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci USA. 1987;84:9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King A, et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol. 2000;30:1623–1631. doi: 10.1002/1521-4141(200006)30:6<1623::AID-IMMU1623>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.King A, et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta. 2000;21:376–387. doi: 10.1053/plac.1999.0496. [DOI] [PubMed] [Google Scholar]
- 7.Shobu T, et al. The surface expression of HLA-F on decidual trophoblasts increases from mid to term gestation. J Reprod Immunol. 2006;72:18–32. doi: 10.1016/j.jri.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Botet M, et al. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol. 2000;61:7–17. doi: 10.1016/s0198-8859(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 9.Kovats S, et al. Class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–224. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 10.McMaster MT, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154:3771–3778. [PubMed] [Google Scholar]
- 11.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis DM, et al. Impaired spontaneous endocytosis of HLA-G. Eur J Immunol. 1997;27:2714–2719. doi: 10.1002/eji.1830271035. [DOI] [PubMed] [Google Scholar]
- 13.Boyson JE, et al. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc Natl Acad Sci USA. 2002;99:16180–16185. doi: 10.1073/pnas.212643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–1937. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sageshima N, et al. Soluble HLA-G is absent from human embryo cultures: A reassessment of sHLA-G detection methods. J Reprod Immunol. 2007;75:11–22. doi: 10.1016/j.jri.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Blaschitz A, et al. The soluble pool of HLA-G produced by human trophoblasts does not include detectable levels of the intron 4-containing HLA-G5 and HLA-G6 isoforms. Mol Hum Reprod. 2005;11:699–710. doi: 10.1093/molehr/gah185. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 20.van der Meer A, et al. Soluble HLA-G promotes Th1-type cytokine production by cytokine-activated uterine and peripheral natural killer cells. Mol Hum Reprod. 2007;13:123–133. doi: 10.1093/molehr/gal100. [DOI] [PubMed] [Google Scholar]
- 21.Miah SM, Hughes TL, Campbell KS. KIR2DL4 differentially signals downstream functions in human NK cells through distinct structural modules. J Immunol. 2008;180:2922–2932. doi: 10.4049/jimmunol.180.5.2922. [DOI] [PubMed] [Google Scholar]
- 22.Colonna M, Nakajima H, Cella M. Inhibitory and activating receptors involved in immune surveillance by human NK and myeloid cells. J Leukocyte Biol. 1999;66:718–722. doi: 10.1002/jlb.66.5.718. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189:1093–1100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Callaghan CA, Bell JI. Structure and function of the human MHC class Ib molecules HLA-E, HLA-F, and HLA-G. Immunol Rev. 1998;163:129–138. doi: 10.1111/j.1600-065x.1998.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 25.Shiroishi M, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiroishi M, et al. Efficient leukocyte Ig-like receptor signaling and crystal structure of disulfide-linked HLA-G dimer. J Biol Chem. 2006;281:10439–10447. doi: 10.1074/jbc.M512305200. [DOI] [PubMed] [Google Scholar]
- 27.Gonen-Gross T, et al. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and β2-microglobulin-free HLA-G molecules. J Immunol. 2005;175:4866–4874. doi: 10.4049/jimmunol.175.8.4866. [DOI] [PubMed] [Google Scholar]
- 28.Shiroishi M, et al. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci USA. 2006;103:16412–16417. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allan DS, McMichael AJ, Braud VM. The ILT family of leukocyte receptors. Immunobiology. 2000;202:34–41. doi: 10.1016/S0171-2985(00)80050-9. [DOI] [PubMed] [Google Scholar]
- 30.Selvakumar A, Steffens U, Dupont B. NK cell receptor gene of the KIR family with two IG domains but highest homology to KIR receptors with three IG domains. Tissue Antigens. 1996;48:285–294. doi: 10.1111/j.1399-0039.1996.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 31.Koopman LA, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajagopalan S, Fu J, Long EO. Cutting edge: Induction of IFN-γ production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol. 2001;167:1877–1881. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 33.Lockwood CJ, et al. Preeclampsia-related inflammatory cytokines regulate interleukin-6 expression in human decidual cells. Am J Pathol. 2008;172:1571–1579. doi: 10.2353/ajpath.2008.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopcow HD, et al. T cell apoptosis at the maternal–fetal interface in early human pregnancy: Involvement of galectin-1. Proc Natl Acad Sci USA. 2008;105:18472–18477. doi: 10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blois SM, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 36.Bellón T, Kitzig F, Sayós J, López-Botet M. Mutational analysis of immunoreceptor tyrosine-based inhibition motifs of the Ig-like transcript 2 (CD85j) leukocyte receptor. J Immunol. 2002;168:3351–3359. doi: 10.4049/jimmunol.168.7.3351. [DOI] [PubMed] [Google Scholar]
- 37.Shlapatska LM, et al. CD150 association with either the SH2- containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 38.Calpe S, et al. The SLAM and SAP gene families control innate and adaptive immune responses. Adv Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 39.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 40.Kikuchi-Maki A, Catina TL, Campbell KS. Cutting edge: KIR2DL4 transduces signals into human NK cells through association with the Fc receptor γ protein. J Immunol. 2005;174:3859–3863. doi: 10.4049/jimmunol.174.7.3859. [DOI] [PubMed] [Google Scholar]
- 41.Morales PJ, Pace JL, Platt JS, Langat DK, Hunt JS. Synthesis of β2-microglobulin-free, disulfide-linked HLA-G5 homodimers in human placental villous cytotrophoblast cells. Immunology. 2007;122:179–188. doi: 10.1111/j.1365-2567.2007.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopcow HD, et al. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci USA. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.