Abstract
The neonatal Fc receptor for IgG (FcRn) transfers maternal IgG to the offspring and protects IgG from degradation. The FcRn resides in an acidic intracellular compartment, allowing it to bind IgG. In this study, we found the association of FcRn and invariant chain (Ii). The interaction was initiated within the endoplasmic reticulum by Ii binding to either the FcRn H chain alone or FcRn H chain-β2-microglobulin complex and appeared to be maintained throughout the endocytic pathway. The CLIP in Ii was not required for FcRn-Ii association. The interaction was also detected in IFN-γ-treated THP-1, epithelial and endothelial cells, and immature mouse DCs. A truncated FcRn without the cytoplasmic tail was unable to traffic to early endosomes; however, its location in early endosomes was restored by Ii expression. FcRn was also detected in the late endosome/lysosome only in the presence of Ii or on exposure to IFN-γ. In immature human or mouse DCs, FcRn was barely detected in the late endosome/lysosome in the absence of Ii. Furthermore, the cytoplasmic tail of Ii conferred tailless FcRn to route to both the early endosome and late endosome/lysosome in a hybrid molecule. Because the FcRn is expressed in macrophages and DCs or epithelial and endothelial cells where Ii is induced under inflammation and infection, these results reveal the complexity of FcRn trafficking in which Ii is capable of expanding the boundary of FcRn trafficking. Taken together, the intracellular trafficking of FcRn is regulated by its intrinsic sorting information and/or an interaction with Ii chain.
The neonatal Fc receptor for IgG (FcRn)3 is first identified in the intestinal epithelial cells of a suckling rodent, where it is expressed at high levels (1). However, its functional expression has recently been acknowledged in a diverse array of cell types and tissues, including epithelial cells, endothelial cells, macrophages, dendritic cells (DC), and neutrophils of humans and rodents at all ages (2–7). FcRn is composed of a H chain (45 kDa in humans and 50 kDa in rodents) nonconvalently associated with an L chain β2m (12 kDa; Refs. 8 and 9). The H chain comprises three extracellular domains that are anchored to the cell surface by a single transmembrane segment and a C-terminal cytoplasmic tail. The association of FcRn with β2m is critical for FcRn exit to the endoplasmic reticulum (ER; Ref. 10). Although FcRn shares extensive structural homology with MHC class I, it is unable to present antigenic peptides to cognant T cells due to its narrowed Ag-binding groove (11). Instead, FcRn transfers maternal IgG across the polarized placental and/or intestinal epithelial cells (12, 13), which allows newborns to obtain humoral immunity against Ags encountered by the mother before they develop their own immune system. In addition to its function as a transporter, FcRn protects IgG and albumin by extending their life spans (14–16). Consequently, this FcR establishes IgG (11–12 mg/ml for humans) and albumin (30–50 mg/ml for human) as the most abundant proteins in the blood. This character ensures that IgG generated upon antigenic exposure or infection has a long-term protective immunity. Alternatively, FcRn could also prolong the life span of pathogenic or autoimmune IgG, which links FcRn to autoimmune diseases (17).
FcRn binds IgG isotypes in a pH-dependent manner, binding IgG at acidic pH (6.0 – 6.5) and releasing IgG at neutral or higher pH (18). In the majority of cell types, FcRn resides primarily in the early acidic endosomal vesicles with limited cell surface expression. In early endosomes, FcRn catches IgG that enters cell by pinocytosis or endocytosis (14, 19). Subsequently, FcRn recycles IgG back to the cell surface in nonpolarized cells or transcytoses IgG to the opposite surface in polarized epithelial cells. The near neutral pH of extracellular environment causes IgG release from FcRn. The IgG that does not bind to the FcRn inside cells would move to lysosomes where it undergoes degradation. The FcRn-IgG transport pathway is further elaborated by recent studies that identify early endosomes as the major sorting location for FcRn-IgG complex in endothelial cells (20, 21). In most cases, FcRn does not appear in the late endosomal/lysosomal compartment of either endothelial or epithelial cells. Two targeting signals, a tryptophanand a dileucine-based motif in the cytoplasmic tail of FcRn, have been postulated to mediate internalization of FcRn from the plasma membrane or transport of FcRn from the trans-Golgi network (TGN) to endocytic pathway (22, 23). Recently, a Ca2+-dependent calmodulin-binding motif in the cytoplasmic tail of FcRn in modulating the intracellular trafficking of FcRn was also reported (24). Despite these studies, its detailed trafficking and regulation of its entry into the endocytic pathway under physiological and pathophysiological conditions is still uncertain.
Invariant chain (CD74, Ii) is a nonpolymorphic type II integral membrane glycoprotein. Ii chains form a trimer in the ER, where each Ii noncovalently binds to a MHC class II αβ heterodimer, thereby forming a nonameric complex (αβIi)3 (25, 26). The binding of Ii chain with MHC class II in the ER stabilizes the MHC class II and protects it from binding to endogenously generated peptides. When the nonamer reaches the TGN, the complex is sorted away from the secretory pathway and routed to the endocytic pathway, ultimately to lysosome-like compartments, called MHC class II compartments. The N-terminal cytoplasmic tail of Ii chain contains two acidic dileucine-based endosomal targeting motifs (D/EXXXLL), which direct MHC class II to the endocytic pathway (26–28). On entry of the nonameric complex to endosomes, Ii chain is gradually degraded by pH-dependent cathepsin proteases (29). Consequently, a small fragment, CLIP, is left in the peptide-binding groove of MHC class II. The removal of CLIP from MHC class II, which is catalyzed by HLA-DM in humans (30), facilitates the binding of antigenic peptides derived from internalized Ags for Ag presentation (31, 32). Thus, Ii chain plays a critical role in the Ag presentation by MHC class II to CD4+ T cells by stabilizing MHC class II in the ER and directing MHC class II away from the default secretory pathway to endocytic pathway.
Although FcRn is structurally similar to MHC class I, its intracellular trafficking pathway is much more analogous to that of MHC class II. Because Ii chain plays such an important role in MHC class II trafficking to the endocytic pathway, we hypothesized that Ii molecules could play an additional role in directing FcRn trafficking within the endocytic compartments by physical association with FcRn. We were surprised to find that the Ii was indeed capable of associating with FcRn and regulating FcRn trafficking. Although dispensable under a certain circumstance, the interaction of Ii chain with FcRn offers an additional targeting signal that directs FcRn into the endosomal/lysosomal compartments, especially under immunological, inflammatory, and infectious conditions. Our results provide a novel role for the Ii chain in modulating FcRn intracellular trafficking pathway that is essentially involved for IgG transport and homeostasis.
Materials and Methods
Cell lines, Abs, and mice
Human epithelial T84, HT-29, Caco-2, HeLa, and Chinese hamster ovary (CHO) cell lines were grown in complete DMEM. Macrophage-like cell line THP-1 and melanoma FO-1 (β2m-deficient) cell lines were grown in RPMI 1640 (Invitrogen) complete medium. All complete media were supplemented with 10 mM HEPES, 10% FCS (Sigma-Aldrich), 1% L-glutamine, nonessential amino acids, and 1% penicillin-streptomycin. Cells were grown in 5% CO2 at 37°C.
Rabbit anti-FLAG epitope (DYKDDDDK, a single letter for amino acid) or mAb LN-2 for human CD74 was purchased from Sigma-Aldrich. HRP-conjugated rabbit anti-mouse or donkey anti-rabbit Ab was from Pierce. The hybridoma 12CA5, which reacts with the influenza hemagglutinin (HA) epitope, was purchased from American Type Culture Collection. Biotin-labeled mAb for Ii was from Southern Biotechnology. Anti-lysosome-associated membrane glycoprotein-1 (anti-LAMP-1; mouse IgG1, clone H4A3, rat IgG2a, clone 1D4B, developed by Drs. T. August and J. Hildreth) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa (Iowa City, IA). mAb anti-early endosomal Ag-1 (EEA1), FITC-conjugated mAb anti-LAMP-1, rat anti-mouse Ii (In-1), and rat anti-mouse transferrin receptor were obtained from BD Biosciences. Alexa Fluor 488-, Alexa Fluor 555-, and Alexa Fluor 633-conjugated secondary Abs were from Molecular Probes. Recombinant human IFN-γ, IL-4, and GM-CSF were from R&D Systems.
Homozygous BALB/c Ii−/− mice (33), originally obtained from Dr. Elizabeth Bikoff (University of Oxford, Oxford, U.K.), were bred in a specific pathogen-free facility at the National Institutes of Health animal facilities. BALB/c/J mice were purchased from The Jackson Laboratory. All mice were maintained under American Association for the Accreditation of Laboratory Animal Care-approved conditions.
Production of affinity-purified FcRn-specific Abs
The human FcRn codons corresponding to 299 –343 or mouse FcRn codons 427– 472 were amplified by PCR and subcloned into the pGEX4T-1 (Amersham Pharmacia Biotech) expression vector. Production of affinity-purified glutathione S-transferase fusion proteins was as previously described (6). For production of anti-FcRn peptide Ab, peptide CLEWKEPPSMRLKARP was synthesized by Invitrogen. The immunization of rabbits with purified fusion protein or FcRn peptides coupled with keyhole limpet hemocyanin was conducted by Rockland Immunochemicals. Anti-FcRn Abs were affinity purified from rabbit sera with affinity columns, respectively.
RT-PCR analysis
For total RNA extraction, cells were pelleted and resuspended at 106 cells/ml in Trizol reagent (Invitrogen). The human Ii gene was amplified by primers (5′-TCCCAAGCCTGTGAGCAAGATG-3′, 5′-CCAGTTCCAG TGACTCTTTCG-3′) with a one-step RT-PCR kit (Qiagen). The mRNA was also amplified with GAPDH-specific primers (5′-GAGAAGGCTGGG GCTCAT-3′, 5′-TGCTGATGATCTTGAGGCTG-3′) as an internal control to monitor the quality of the RNA purification and cDNA synthesis. The PCR products were analyzed by 1.5% agarose gel electrophoresis and stained with ethidium bromide.
Construction of FcRn wild-type, mutant, and Ii expression vector
The constructions of human β2m and FcRn expression plasmids, pCDNA β2m and pcDNA-FLAGFcRn, have been described previously (6). The pcDNA-FLAGFcRn construct fused with a preprotrypsin signal sequence, FLAG epitope, into the N terminus of the human FcRn gene (1–342 aa). PCR primer pair (5′-ATAAGAATGCGGCCGCGGCAGAAAGC CACCTCTCCCT-3′, 5′-TGCTCTAGATTACCTCATCCTTCTCCACA ACA-3′) was used to construct a plasmid encoding FLAG-tagged FcRn mutant (1–302 aa) that lacks the cytoplasmic tail. The DNA fragment was digested with NotI and XbaI and ligated into the plasmid pCDNAFLAG to generate the plasmid pcDNAFLAGFcRn-TD. To generate pcDNAFLAG-FcRn-LL/AA, FcRn cDNA in a pCDNAFLAG was used as the template for site-directed mutagenesis by an in vitro Transformer Site-directed Mutagenesis Kit (Clontech). The oligonucleotide (5′-CACCGGGGTCGCCGCGCCCACCCCA-3′) was used for mutation of leucine residues 320 –321 to alanine residues (base substitutions are underlined). To construct a pcDNAFLAGmFcRn, PCR primer pair (5′-TCACAAGCTTTCAGAGACCCGCCCCCCACTGATGTAT-3′, 5′-TGATCTAGACTAGGAAGTGGCTGGAAAGGCA) was used. The DNA fragment was digested with HindIII and XbaI (underlined in primers) for cloning. PCR primer pair (5′-CCGCTCGAGTCAGAGGCTAGCATAATCAGGAACATCATACGGATACAT GGGGACTGGGCCCAGA, 5′-ATAAGAATGCGGCCGCTTCCGAGA TGCACAGGAGGAGA-3′) was used to construct pBUDCE4Ii plasmid encoding HA-tagged Ii. The plasmids phFcRn-DMcyt and phFcRn-Iicyt were constructed by two-step PCR reactions cloning PCR fragments into pCDNAFLAG. The cytoplasmic tail codons (222–245 aa) of HLA-DM were amplified by primer pair (5′-GTAGGAGGAGCTCTGTTGTGGAGA GCTGGCCACTCTAGTTA-3′, 5′-TCTCTCTAGACTAGGAAATGTGC CATCCTT-3′). The Ii cytoplasmic tail (1– 46 aa) was synthesized by Integrated DNA Technologies. The amplified HLA-DM or synthesized Ii chain cDNA fragments contain an overhanging sequence complementary to the FcRn cDNA. In the second step, fragments of Ii or HLA-DM were annealed to FcRn plasmid and a fusion product of both cDNA fragments was synthesized by PCR primer pairs specific for FcRn and Ii or HLA-DM. In all above cloning, the primer introduced a NotI or XbaI site (underlined) to facilitate cloning. The plasmid encoding the N-glycosylation mutant (FcRn N/S) of human FcRn was generated by replacing the consensus glycosylation sequence (Asn-X-(Ser/Thr) for substitution of asparagine 102 to a serine residue (10). All constructs were sequenced to verify the fidelity of the amplification and cloning. The human Ii.Δ91–99 or Ii.M91A in pCDNA3 vectors was a gift from Dr. Norbert Koch (34). The plasmid pTFR-GFP was obtained from Dr. Gary Banker (Oregon Health and Science University, Portland, OR). The plasmid pcEXV3-mIi 31, coding for wild-type mouse Ii 31, was a gift from Dr. Ronald N. Germain (National Institutes of Health, Bethesda, MD).
Transfection and protein expression
The stable cell lines (Table I), HeLaFcRn, FO-1FcRn, and FO-1FcRn + β2m, have been described previously (6, 10). HeLa or HeLaFcRn cells were transfected with pBUDCE4-Ii vector with Effectene transfection reagent (Qiagen). Stable transfectants were selected by G418 or G418 plus Zeocin for single or double transfectants, respectively. Positive clones were tested for protein expression through Western blot using anti-FLAG or anti-HA Ab. Transfectants were maintained in medium containing G418 (500 μg/ml) and/or Zeocin (150 μg/ml). For transient transfections, cells were transfected with the 2 μg of plasmid pBUDCE4-Ii. Protein expressions were examined 48 h after transfection.
Table I.
List of cell lines used in this study
| Cell Line | FcRn and Ii Type | Expression Vector | Selection |
|---|---|---|---|
| HeLaFcRn | FLAG-tagged, full-length | pFLAGCMV + pCDNA3 | G418 |
| HeLaFcRTD | FLAG-tagged, tailless | pCDNAFLAG | G418 |
| HeLaIi | HA-tagged | pBUDCE4 | Zeocin |
| HeLaIiFcRn | FLAG, HA, full-length | pCDNAFLAG + pBUDCE4 | G418 + Zeocin |
| HeLaIiFcRnTD | FLAG, HA, tailless | pCDNAFLAG + pBUDCE4 | G418 + Zeocin |
| HeLaFcRnLL/AA | FLAG, full-length, mutant | pCDNAFLAG | G418 |
| HeLaIiFcRnLL/AA | FLAG, full-length, mutant | pCDNAFLAG + pBUDCE4 | G418 + Zeocin |
| HeLaFcRn-Iicyt | FLAG-tagged, tailless | pCDNAFLAG | G418 |
| HeLaFcRnDMcyt | FLAG-tagged, tailless | pCDNAFLAG | G418 |
| FO-1FcRn | FLAG-tagged, full-length | pFLAGCMV + pCDNA3 | G418 |
| FO-1FcRn + β2m | FLAG-tagged, full-length | pFLAGCMV + pCDNA3 | G418 |
Western blotting, immunoprecipitation, and gel electrophoresis
Cell lines and transfectants were lysed in 0.5% CHAPS in PBS with a mixture of protease inhibitor (Roche). Protein concentrations were determined by Bradford assay (Bio-Rad Laboratories). The lysates were resolved on a 12% SDS-PAGE gel under reducing conditions. Proteins were electrotransferred onto a nitrocellulose membrane (Schleicher & Schuell). The membranes were blocked with 5% nonfat milk, probed separately with anti-FcRn or Ii Ab for 1 h, and followed by incubation with HRP-conjugated rabbit anti-mouse or donkey anti-rabbit Ab. All blocking, incubation, and washing were performed in PBST solution (PBS and 0.05% Tween 20). Proteins were visualized by ECL (Pierce). Immunoprecipitation was as described previously (35). Protein was precipitated with anti-FLAG or anti-HA mAb. The immunoreactive products were eluted from the protein G complex with gel-loading buffer at 95°C.
Deglycosylation
Endo-β-N-acetylglucosaminidase H (endo-H) (New England Biolabs) was digested as described previously (10, 35). For FcRn digestion in immunoprecipitates, proteins were resuspended in 0.5 ml of endo-H digestion buffer (100 mM sodium acetate, pH 5; 150 mM NaCl; 1% Triton X-100; 0.2% SDS; and 0.5 mM PMSF). Beads containing AbAg complexes were pelleted and then eluted with reducing sample buffer. Mock digestion without enzymes was performed for each digestion. All digestions were performed for 18 h at 37°C. Proteins were analyzed on a 12% SDS-PAGE gel under reducing conditions and immunoblotted with anti-FLAG Ab.
IgG binding assay
An IgG binding assay was performed as previously described (2, 6). Cells were lysed in PBS (pH 6.0 or 7.5) with 0.5% CHAPS (Sigma-Aldrich) and protease inhibitor mixture on ice for 1 h. Postnuclear supernatants containing 0.5–1 mg of soluble proteins were incubated with human IgG-Sepharose (Amersham Pharmacia Biotech) at 4°C overnight. The unbound proteins were removed with PBS (pH 6.0 or 7.5) containing 0.1% CHAPS. The adsorbed proteins were boiled with reducing electrophoresis sample buffer at 95°C for 5 min. The eluted fractions were subjected to Western blot analysis as above in Western blotting, immunoprecipitation, and gel electrophoresis.
Isolation of human monocyte-derived DCs, knockdown of CD74 in DCs by small interfering RNA (siRNA), and generation of bone marrow-derived DC (BMDC) from mice
Human immature DCs were obtained by culturing the adherent fraction of normal human PBMCs (Institute of Human Virology) in the presence of GM-CSF and IL-4 as previously described (6). Briefly, monocytes were isolated from PBMCs by adherence to plastic petri dish for 2 h and then cultured for 6 days in RPMI 1640 complete medium containing human GM-CSF (50 ng/ml) and 100 ng/ml IL-4 (R&D Systems). The medium was replaced every 3 days. The 1 × 106 immature DC cells were transfected with 2.5 μg of short hairpin RNA plasmid for human CD74 (Sigma-Aldrich) in 100 μl of nucleofactor solution using a DC nucleofection kit (Amaxa) according to the instructions of the manufacturer. Cells were then resuspended with RPMI 1640 and cultured on a coverslip. After 24 h, cells were fixed, permebilized, and stained for confocal microscope analysis.
BMDCs from wild-type BALB/c and Ii−/− mice were generated as previously described (36). Immature DCs were purified from CD11c+ magnetic microbead-containing columns (MACS) and characterized by flow cytometry. The purified immature DCs were then used for experiments.
Confocal immunofluorescence
Immunofluorescence was performed based on methods previously described (2, 10). Briefly, cells were cultivated on coverslips for 24 h. The cells were rinsed in PBS, cold fixed in 3.7% paraformaldehyde (Sigma-Aldrich) in PBS for 30 min at 4°C, and quenched with glycine for 10 min. Subsequent procedures were done at room temperature. After two washings with PBS, the coverslips were permeabilized in solution (PBS containing 0.2% Triton X-100) for 20 min and then blocked with blocking buffer containing 3% normal goat serum. Abs diluted in blocking buffer were added onto the coverslips and incubated for 30 min. Cells were incubated with affinity-purified primary Ab in PBST with 3% BSA for 1 h. Cells were then incubated with Alexa Fluor 555-conjugated goat anti-mouse IgG and Alexa Fluor 488-conjugated goat anti-rabbit IgG in blocking buffer. After each step, cells were washed at least three times with 0.1% Tween 20 in PBS. Coverslips were mounted on slides with ProLong antifade kit (Molecular Probes) and examined using a Zeiss LSM 510 confocal fluorescence microscopy. The images were processed using the LSM Image Examiner software (Zeiss). Quantitative colocalization measurement was performed using Zeiss LSM 510 Examiner Software. Pearson’s correlation coefficient was calculated for describing the colocalization correlation of the intensity distributions between two channels as previously described (37). In each quantitative experiment with transfected HeLa cells, 15 cells in total were analyzed. For immature DCs, 10 cells in total were analyzed. A value of p < 0.05 was considered significant.
Results
Association of FcRn with Ii
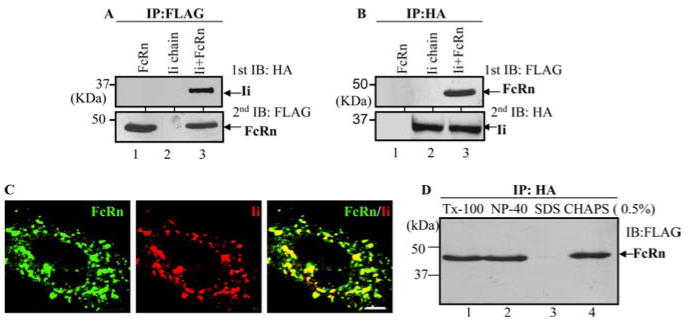
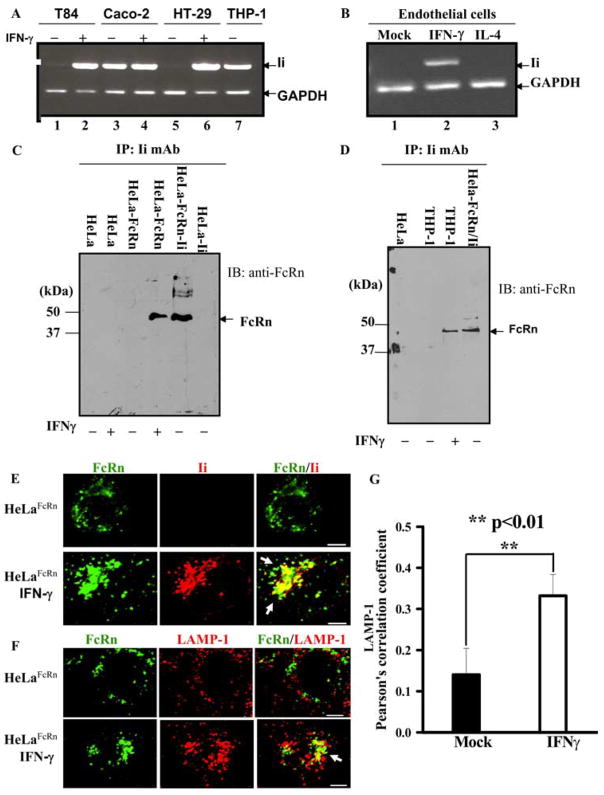
HeLa is an Ii- and FcRn-double-negative cell line (Table I. To test whether Ii chain interacts with FcRn molecule, HeLaFcRn or HeLamock cells (Table I) were transfected with plasmids encoding HA-tagged Ii cDNA. Cells were lysed with a low-stringency CHAPS buffer and subjected to immunoprecipitation with either an anti-FLAG (for FcRn) or anti-HA (for Ii chain) Ab. The immunoprecipitate from either an anti-FLAG-FcRn (Fig. 1A) or anti-HA-Ii Ab (Fig. 1B) was analyzed by blotting with an anti-HA (Fig. 1A) or anti-FLAG Ab (Fig. 1B). As shown in Fig. 1, anti-FLAG Ab coimmunoprecipitated Ii (Fig. 1A, lane 3). The anti-HA mAb coimmunoprecipitated FcRn H chain (Fig. 1B, lane 1). Interaction between FcRn and Ii was further analyzed by confocal microscopy (Fig. 1C). Consistent with data reported previously (12), both FcRn and Ii appeared as punctuate and vesicular staining. They were colocalized with each other (Fig. 1C, right). In addition, the association of FcRn and Ii could be maintained in the presence of other detergents such as 0.5% Triton X-100 (Fig. 1D, lane 1) and Nonidet P-40 (Fig. 1D, lane 2); however, the association was disrupted by 0.5% SDS (Fig. 1E, lane 3). To verify that the association was not occurring nonspecifically after cells lysed, HeLaFcRn and HeLaIi cell lysates were mixed and then subjected to coimmunoprecipitation. The lysate from the same number of HeLaFcRn + Ii cells was extracted as a positive control. Coimmunoprecipitation of FcRn with Ii molecules was detected only in the HeLaFcRn + Ii extract, but not in the mixed cell lysates (data not shown). Additionally, the calnexin is shown to associate with FcRn (35) and Ii (38) molecules; it is possible that calnexin bridges the FcRn-Ii association. To examine this, Ii was expressed in calnexin-negative CEM-NKRFcRn cells (35). FcRn and Ii association was detected in CEM-NKRFcRn + Ii cells (data not shown), indicating that FcRn molecules can interact with Ii independent of calnexin. Therefore, these complementary methods show that Ii specifically associates with FcRn.
FIGURE 1.
FcRn interacts with the Ii chain. Cell lysates were immunoprecipitated (IP) with Abs, and the immunoprecipitates were subjected to 12% SDS-PAGE electrophoresis under reducing conditions and then transferred to a nitrocellulose membrane for Western blotting. Immunoblots (IB) were developed with ECL. Each experiment was performed at least twice. A and B, The cell lysates from HeLaFcRn (lane 1), HeLaIi (lane 2), and HeLaFcRn + Ii (lane 3) were immunoprecipitated by anti-FLAG M2 mAb or anti-HA mAb. The immunoprecipitates were subjected to Western blotting with anti-HA or FLAG mAb as indicated. C, Colocalization of FcRn and Ii in HeLaFcRn + Ii. Cells grown on glass coverslips were fixed with 3.7% paraformaldehyde and permeabilized in 0.2% Triton X-100 (Tx-100). Subsequently, the cells were incubated with affinity-purified rabbit anti-FLAG (FcRn, left)- or anti-HA (Ii, middle)-specific Ab, followed by Alexa Fluor 488- or 555-conjugated IgG. Puncta that appear yellow in the merged images (right) indicate colocalization of FcRn with the Ii chain. Bar, 5 μm. D, HeLaFcRn + Ii cell lysates were generated by 0.5% Triton X-100 (lane 1), Nonidet P-40 (lane 2), SDS (lane 3), and CHAPS (lane 4) buffers and immunoprecipitated by HA-specific Ab. The immunoprecipitates were subjected to Western blot analysis. Arrow, Location of the FcRn H chain.
Ii chain interacts with both FcRn H chain alone and FcRn-β2m complex
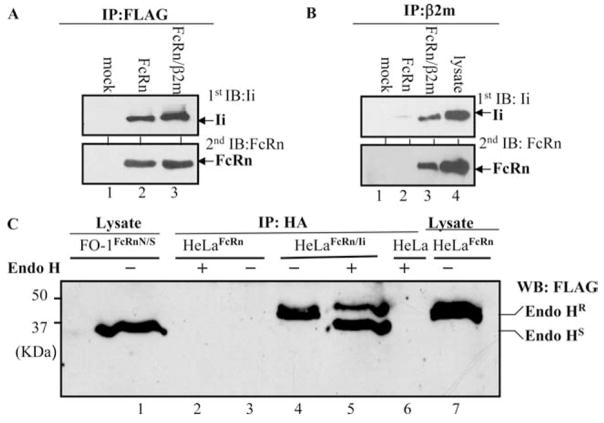
The functional FcRn molecule consists of an FcRn H chain and β2m. We have previously expressed FcRn, alone or with β2m together in FO-1 cells (10). FO-1 is a melanoma cell line that lacks β2m expression. To test whether Ii chain interacts with FcRn H chain alone or in an FcRn-β2m complex, the cell lysates were generated from FO-1mock, FO-1FcRn, and FO-1FcRn + β2m cells (Table I) that were transiently transfected with HA-Ii, respectively. The cell lysates were immunoprecipitated with an anti-FLAG Ab for FcRn or anti-β2m mAb, BBM1. The immunoprecipitates were sequentially blotted with anti-HA for Ii chain (Fig. 2A, lanes 2 and 3) and FLAG Ab for FcRn (Fig. 2B, lane 3). The results showed that Ii was detected in either FLAG immunoprecipitates from FO-1FcRn or anti-β2m immunoprecipitates from FO-1FcRn + βm. We were unable to coimmunoprecipitate the FcRn H chain and Ii chain from the lysates of untransfected FO-1 cells by anti-β2m Ab, suggesting the specificity. Therefore, we conclude that Ii interacts with both FcRn H chain alone and FcRn-β2m complex. Furthermore, addition of purified β2m to the immunoprecipitated FcRn-Ii complex from FO-1FcRn + Ii resulted in the association of FcRn H chain with β2m (data not shown), suggesting that the association of FcRn H chain with Ii did not affect the assembly of FcRn H chain and β2m molecules. To further confirm the presence of FcRn-Ii complex in the ER and Golgi apparatus, HeLaFcRn + Ii cells were lysed in CHAPS buffer; and anti-H immunoprecipitates were subjected to endo-H digestion. Human FcRn possesses one N-linked glycosylation site. As shown in Fig. 2C, FcRn H chain in an Ii immunoprecipitate from HeLaFcRn + Ii cells exhibited a mixture of sensitivities to endo-H digestion (Fig. 2C, lane 5) as compared with mock digestion (Fig. 2C, lane 4). Endo-H-sensitive FcRn H chain had a mobility similar to that of FcRn N/S (Fig. 2C, lane 1), an N-glycan mutant of FcRn (10). These data show that Ii associated with both endo-H-sensitive and endo-H-resistant forms of FcRn, corresponding to post-ER modification of the glycan structure, therefore supporting the initiation of the FcRn-Ii interaction in the ER and maintenance during transport through the Golgi stack.
FIGURE 2.
Ii chain interacts with both FcRn H chain alone and FcRn H-β2m complex. All immunoprecipitates (IP) were subjected to Western blotting analysis. Blots were developed with ECL. Each experiment was performed at least three times. Only the region of interest in the gel is shown. A and B, The FO-1mock (lane 1), FO-1FcRn (lane 2), and FO-1FcRn + β2m (lane 3) cells were transiently transfected with pBUDCE4Ii-HA. After 48 h, cell lysates were immunoprecipitated by anti-FLAG M2 mAb (A) or anti-β2m mAb BBM1 (B). The immunoprecipitates were blotted with anti-HA (Ii, first blot) or rabbit anti-FLAG (FcRn, second blot) Ab. The cell lysates from HelaFcRn + Ii were used as a positive control for immunoblotting (IB; B, lane 4). C, Sensitivity of Ii-associated FcRn H chain to endo-H digestion. The lysates (500 μg) from HeLaFcRn (lanes 2 and 3), HeLaFcRn + Ii (lanes 4 and 5), and HeLa (lane 6) were immunoprecipitated by HA mAb. The immunoprecipitates were digested by mock (lanes 3 and 4) and endo-H (lanes 2, 5, and 6) for 18 h, respectively. Lane 5 represented a mixture of endo-H-sensitive (HS) and -resistant (HR) FcRn proteins. The cell lysates from glycan mutant FO-1FcRnN/S (lane 1) and HeLaFcRn (lane 7) were used as controls.
FcRn associating with Ii chain can bind to IgG in acidic pH
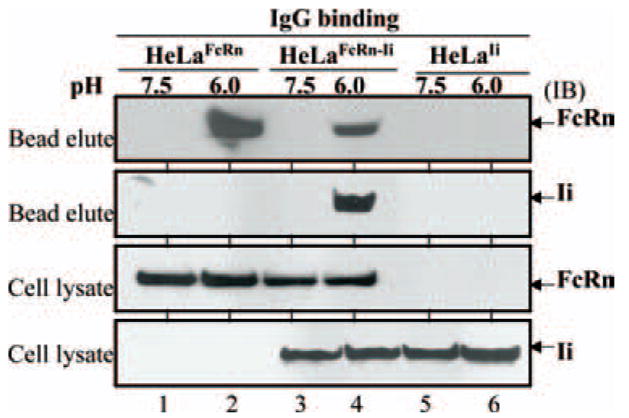
FcRn binds IgG at acidic pH 6.0 and releases IgG at neutral pH (14, 18, 35). We tested whether the association of FcRn with Ii affects its ability to bind to its natural ligand IgG. We incubated cell lysates from HeLa cells expressing FcRn and/or Ii with IgG-Sepharose at either pH 6.0 or pH 7.5. Cell lysates from HeLaFcRn cells were used as positive controls. The binding elutes and cell lysates were subjected to Western blot analysis. As expected, FcRn from HeLaFcRn cells bound to IgG at pH 6.0 (Fig. 3, lane 2), but not at pH 7.5 (Fig. 3, lane 1). Similarly, FcRn from HeLaFcRn + Ii cells bound to IgG at pH 6.0 (Fig. 3, lane 4), but not at pH 7.5 (lane 3). Both Ii and FcRn were detected in the binding elutes of IgG beads, suggesting the presence of Ii in FcRn-IgG complexes. Hence, it is very likely that Ii association does not interfere with FcRn function in the IgG binding assay.
FIGURE 3.
FcRn/Ii chain complex in IgG binding. HeLa transfectants as indicated were lysed in sodium phosphate buffer (pH 6.0 or 7.5) with 0.5% CHAPS and proteinase inhibitors. Approximately 0.5 mg of the soluble proteins was incubated with human IgG-Sepharose at 4°C. The eluted proteins or cell lysates were subjected to Western blotting analysis. Proteins were probed with rabbit anti-FLAG Ab or anti-HA mAb and developed with HRP-conjugated Abs and ECL. Arrows, Locations of the human FcRn H chain and Ii chain. IB, Immunoblot.
CLIP is not required for the interaction of Ii with FcRn
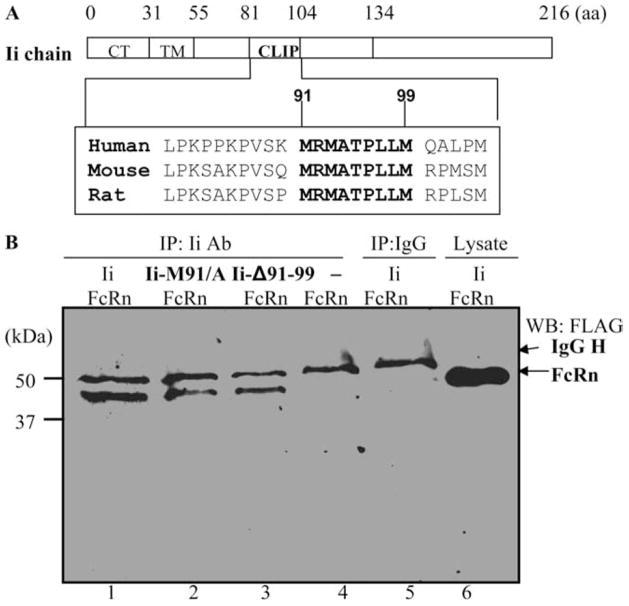
Human Ii has a short, 30-aa, N-terminal cytoplasmic tail, followed by a single 24-aa transmembrane region and an ~150-aa long luminal domain (Fig. 4A). A segment of residues 81–104 is called CLIP. The CLIP region contains a central 9-aa sequence, MRMATPLLM (91–99 aa), which is conserved among humans, mice, and rats (Fig. 4A). Analysis of MHC class II supermotifs (39) and the crystal structures of CLIP complexed with MHC class II molecules (40) indicate the importance of methionine residues at positions 91 and 99 in its binding to MHC class II. In addition, different MHC class I alleles display different dependencies upon the sequence of CLIP (41). Therefore, it is pertinent to assess whether the CLIP or its methionine 91 is important for the interactions of Ii with FcRn. To do so, the CLIP region of Ii chain was deleted, or methionine 91 was replaced with alanine (construct Ii.M91A). The ability of these Ii mutants to associate with FcRn molecule is tested using HeLaFcRn cells transiently transfected with these Ii constructs. Deletion of CLIP (Fig. 4B, lane 3) or substitution of methionine at position 91 (Fig. 4B, lane 2) did not appreciably alter the ability of Ii association with FcRn, as shown by the immunoprecipitation-Western blot analysis. HelaFcRn (Fig. 4B, lane 1) or isotype-matched IgG (Fig. 4B, lane 5) was used as a positive or negative control in this assay. Taken together, these data suggest that the CLIP91–99 is not required for binding to the FcRn.
FIGURE 4.
Function of CLIP in the Ii association with FcRn. A, Schematic representation of the full-length Ii chains. TM, Transmembrane domain; CT, cytoplasmic tail. Ii chain sequence alignment of human (accession no. 10835070), mouse (accession no. 13097485), and rat (accession no. 37589621) indicates conservation of the CLIP91–99 regions. B, CLIP91–99 is not important for interaction with FcRn. HeLaFcRn cells were transiently transfected by plasmid pCDNA3 encoding the full-length Ii chain (Ii), Ii.Δ91–99, and Ii.M91A, respectively. After 48 h, the cell lysates were immunoprecipitated (IP) by anti-Ii mAb (lanes 1–3) and an isotype-matched IgG control (lane 5). The cell lysates from HeLa FcRn (lane 4) or HeLaFcRn + Ii (lane 6) were used as a negative or positive control. The immunoprecipitates were subjected to Western blotting (WB) with FLAG-specific Ab and HRP-conjugated goat anti-rabbit Ab and finally visualized with ECL.
Both Ii and the cytoplasmic tail of FcRn target FcRn into the early endosomes
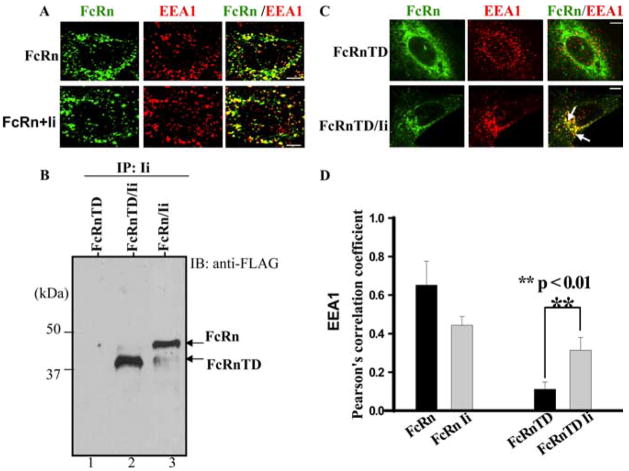
Immunofluorescence microscopy studies showed that in HeLaFcRn transfectant, a high proportion of the FcRn colocalized with the early endosomal marker, EEA1 (Fig. 5A, top panel), indicating a preferential distribution of FcRn to the early endosomes. The expression of Ii in HeLaFcRn cells did not significantly change the steady-state distribution of FcRn and EEA1 (Fig. 5A, bottom panel). An FcRn mutant that lacks its cytoplasmic tail (FcRn-TD) was immunoprecipitated with Ii (Fig. 5B, lane 2), indicating that the cytoplasmic tail of FcRn is not necessary for its association with Ii chain. Immunofluorescence studies showed that the tail deletion mutation profoundly altered the cellular distribution pattern of FcRn appearing as a honeycomb with rare colocalization with EEA1 (Fig. 5C, top panel). However, HeLaFcRn-TD cells transfected with Ii chain resulted in a significant redistribution of FcRn-TD into an EEA1-positive intracellular compartment (Fig. 5C, bottom panel; Fig. 5D). This result suggests that the association of Ii and FcRn is functional and has the ability to drive associated FcRn into the early endosomes independently of the FcRn cytoplasmic tail.
FIGURE 5.
The Ii chain redirects tailless FcRn-TD to the early endosome. Bars, 5 μm. A, Endocytic trafficking of FcRn in HeLaFcRn and HeLaFcRn + Ii transfectants. HeLaFcRn cells were transiently transfected with pBUDCE4 or pBUDCE4-Ii vector. Cells were fixed, permeabilized, and costained for FcRn (green) or early endosomal marker EEA1 (red). Puncta that appear yellow in the merged images (right) indicate colocalization of FcRn with the EEA1. B, Association of tailless FcRnTD with the Ii chain. The cell lysates from HeLaFcRnTD (lane 1), HeLaFcRnTD/Ii (lane 2), and HeLaFcRn/Ii (lane 3) were immunoprecipitated by anti-HA mAb. The immunoprecipitates were subjected to Western blot. Immunoblots (IB) were blotted with rabbit anti-FLAG Ab and HRP-conjugated goat anti-rabbit Ab. The blot was developed with ECL. C, Immunofluorescence analyses of HeLa cells expressing either tailless FcRn-TD alone (top panel) or FcRn-TD and Ii (bottom panel). Transfected cells were fixed, permeabilized, and stained with an Ab to FLAG (green) or EEA1 (red). Arrows, Colocalization (yellow) of the proteins. D, Averages of the colocalization coefficients in A and C. Pearson’s correlation coefficient were calculated. For each experiment, 15 cells were analyzed in 3 different optical regions.
Ii directs FcRn to the late endosomes/lysosomes
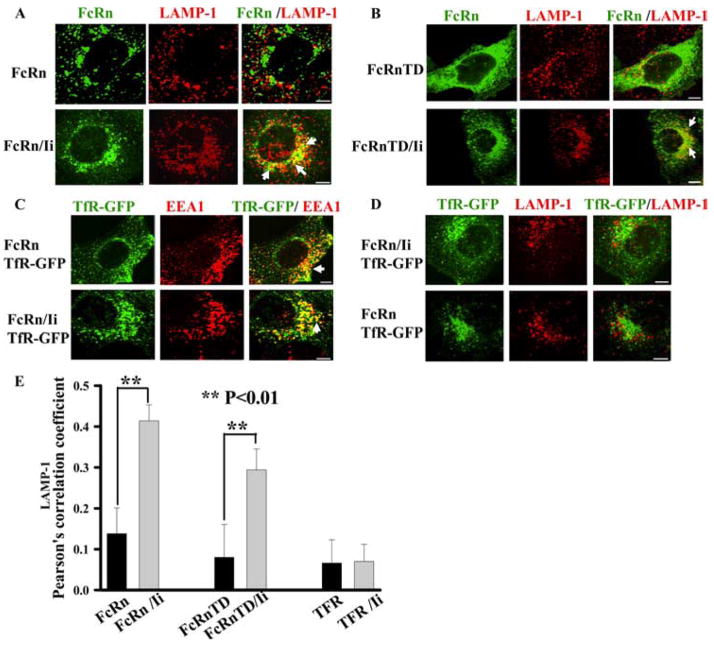
In HeLaFcRn cells, the majority of FcRn appeared in the early endosomal compartment (Fig. 5A, top panel) and did not colocalize well with LAMP-1 (Fig. 6A, top panel), a marker for late endosomal/lysosomal compartments. To determine the effect of Ii expression on the cellular distribution of FcRn, we examined the intracellular FcRn distribution by immunofluorescence staining in HeLaFcRn + Ii cells. In comparison with HeLaFcRn, coexpression of Ii greatly increased the colocalization of FcRn with LAMP-1 (Fig. 6A, bottom panel; Fig. 6E). As expected, FcRn-TD, when expressed alone, exhibited a honeycomb appearance and had limited colocalization with LAMP-1 (Fig. 6B, top panel). Cotransfection of HeLa cells with both FcRn-TD and Ii chain resulted in a redistribution of FcRn-TD into an intracellular compartment that largely overlapped with the LAMP-1 (Fig. 6B, bottom panel; Fig. 6E).
FIGURE 6.
Ii chain directs FcRn to the late endosome/lysosome compartment. Right panels, Merged images. The yellow in the merged images indicates colocalization of FcRn with EEA1 or LAMP-1 marker. Similar observations were seen from three independent experiments. The arrows indicate the colocalization of the proteins. Scale bars represent 5 μm. A and B, Lysosomal sorting of full-length FcRn (A) or tailless FcRn-TD (B) in the presence of Ii chain expression. HeLaFcRn or HeLaFcRn + Ii cells were fixed, permeabilized, and immunostained for LAMP-1 (red) or FcRn (green), followed by Alexa Fluor 555- or Alexa Fluor 488-conjugated IgG. C and D, Localization of transferrin receptor (TfR) in HeLaFcRn or HeLaFcRn + Ii cells. HeLaFcRn or HeLaFcRn + Ii cells were transiently transfected with pcDNA-TfR-GFP, fixed, permeabilized, and immunostained for EEA1 (C) or LAMP-1 (D) marker. TfR was directly visualized by GFP fluorescence (green); EEA1 or LAMP-1 is shown in red. Merged images are represented in the third panel of each row. E, Averages of the LAMP-1 colocalization coefficients in A, B, and D. Pearson’s correlation coefficient was measured. The fifteen cells were analyzed.
It is possible that overexpression of Ii nonspecifically alters the endocytic system (42), consequently resulting in a redistribution of tailless or full-length FcRn into the LAMP-1+ compartment. Hence, we followed the effect of overexpression of Ii on the cellular distribution of GFP fusion of transferrin receptor (TfR-GFP), an endosome marker. TfR-GFP was predominantly accumulated in early endosomes in either the presence (Fig. 6C, top panel) or absence (Fig. 6C, bottom panel) of Ii expression and did not significantly route to the LAMP-1+ compartment in HeLaFcRn (Fig. 6D, top panel) or HeLaFcRn + Ii cells (Fig. 6D, bottom panel, and Fig. 6E). This suggests that Ii overexpression did not result in the redistribution of TfR from early endosome to the LAMP-1+ compartment. These results indicate that the Ii specifically targets FcRn from the early endosome to the late endosomes/lysosomes without significantly modifying the endocytic pathway.
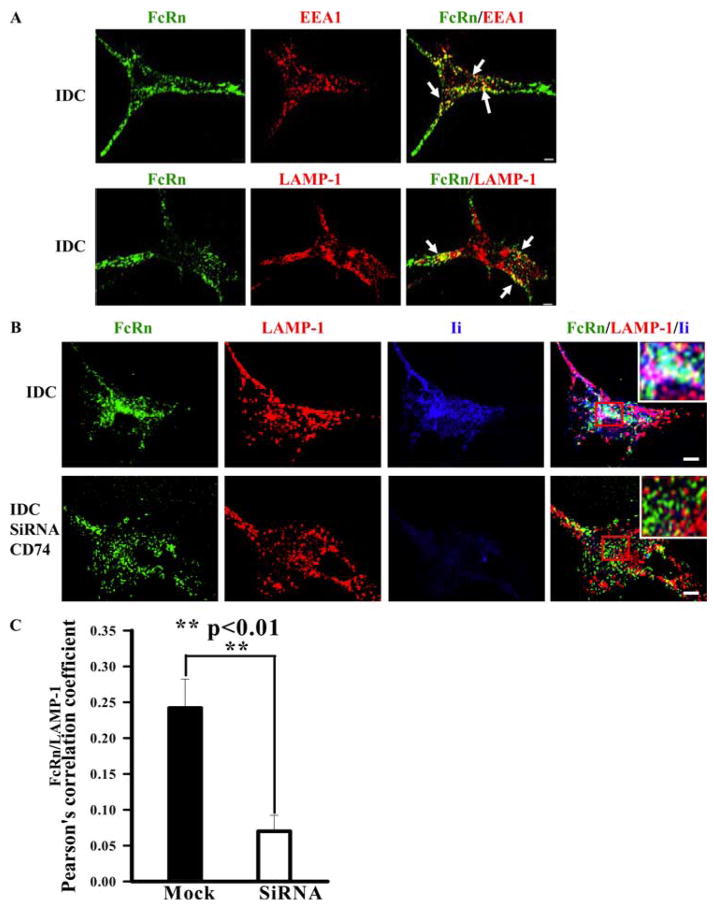
To further study the effect of Ii on FcRn trafficking in cells naturally expressing both FcRn and Ii molecules, we examined the intracellular distribution of FcRn in PBMC-derived DCs using immunofluorescence with affinity-purified FcRn-specific Ab. In immature DCs, FcRn colocalized with both EEA1 (Fig. 7A, top panel) and LAMP-1 markers (Fig. 7A, bottom panel), verifying the cellular location of FcRn in both early and late endosomes/lysosomes. The colocalization of FcRn (green) and Ii (blue) with LAMP-1 (red) was observed in immature DCs, which appeared white (Fig. 7B, bottom panel). To test the role of Ii in targeting of FcRn to the late endosomal/lysosomal compartment in human immature DCs, we knocked down Ii expression using siRNA. In Ii-knockdown immature DCs, the colocalization of FcRn with LAMP-1 staining was significantly decreased (Fig. 7B, top panel, and Fig. 7C), suggesting that FcRn failed to colocalize with LAMP-1 marker when Ii was knocked down.
FIGURE 7.
FcRn appearance in the late endosome/lysosome of human immature DCs (IDC) is dependent on the Ii chain expression. Human immature DCs were obtained by culturing human PBMCs in the presence of GM-CSF and IL-4 for 6 days. Cells grown on glass coverslips were fixed and permeabilized before staining. Bars, 5 μm. A, Colocalization of FcRn (green) and EEA1 or LAMP-1 (red) in DC cells. The human immature DCs were incubated with affinity-purified rabbit anti-FcRn, mAb anti-EEA1, or LAMP1 Ab, followed by Alexa Fluor 555- or Alexa Fluor 488-conjugated IgG of the corresponding species. Punctuate staining that appears in yellow in the merged images indicates (right panels) the colocalization of FcRn with the endosomal or lysosomal marker. Arrows, Colocalization of the proteins. B, FcRn trafficking to the late endosome/lysosome becomes less in Ii-depleted human immature DCs. Human immature DCs were transfected with Ii siRNA plasmid (bottom panel) or vehicle (top panel). Cells were immunostained for FcRn (green), LAMP-1 (red), and Ii (blue). Colocalization of all three molecules appears in white (bottom panel, inset). Similar results were observed from three independent experiments. C, Averages of the LAMP-1 and FcRn colocalization coefficients in IDC transfected with vehicle (mock) or Ii siRNA plasmid in B. Pearson’s correlation coefficient was measured. The 10 cells were analyzed in 3 different optical regions in each experiment.
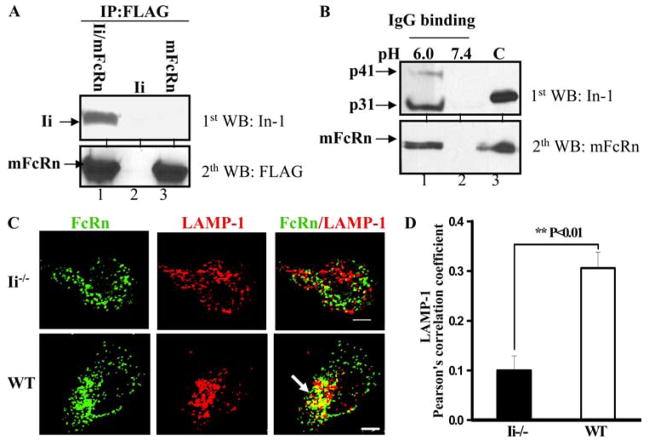
To examine the role of mouse Ii chain in mouse FcRn (mFcRn) trafficking, we first determined the interaction of mFcRn and Ii using coimmunoprecipitation. The anti-FLAG Ab for mFcRn immunoprecipitate was analyzed by immunoblotting for Ii chain (Fig. 8A). Anti-FLAG Ab coimmunoprecipitated mouse Ii chain (Fig. 8A, lane 1) in CHO cells expressing both mFcRn and murine Ii chain, but not in CHO cells expressing Ii (Fig. 8A, lane 2) or mFcRn (Fig. 8A, lane 3) alone, further confirming the association of mFcRn with Ii. To test the IgG-binding ability of mouse FcRn-Ii complexes, we incubated cell lysates from mouse BMDCs at either pH 6.0 or pH 7.4 with IgG-Sepharose. Cell lysates from CHOmFcRn cells were used as a positive control. The binding elutes or cell lysates were analyzed using Western blot for either Ii or mFcRn. Both Ii and mFcRn were detected in the eluate from IgG beads (Fig. 8B, lane 1). As expected, mFcRn-Ii from BMDC cells bound to IgG at pH 6.0 (Fig. 8B, lane 1), but not at pH 7.4 (Fig. 8B, lane 2). These data confirm that like the human Ii chain, the murine Ii chain also interacts with the mFcRn-IgG complexes. To examine the role of mouse Ii in the routing of mFcRn to the late endosomes/lysosomes, we compared the cellular distribution of mFcRn in BMDCs from wild-type and Ii−/− mice. In immature BMDC from Ii−/− mice, the colocalization between mFcRn and LAMP-1 staining was significantly decreased (Fig. 8C, top panel, and Fig. 8D) in comparison with the colocalization level in BMDC from wild-type mice (Fig. 8C, bottom panel, and Fig. 8D), suggesting that mFcRn fails to colocalize with LAMP-1 marker in the major population of Ii−/− DCs. Furthermore, the mFcRn colocalized well with the early endosomal marker TfR in BMDC from both wild-type and Ii−/− mice (data not shown). These data confirm that the targeting of endogenous FcRn in human immature DCs (Fig. 7C) or mouse BMDC (Fig. 8D) to the late endosomes lysosomes is significantly dependent on Ii.
FIGURE 8.
The mFcRn appearance in the late endosome/lysosome of BMDCs is significantly dependent on the Ii expression. A, mFcRn associates with mouse Ii chain. The cell lysates from CHO cells transiently transfected with plasmids encoding murine Ii and mFcRn (lane 1), Ii (lane 2), and mFcRn cDNA (lane 3) were immunoprecipitated (IP) by anti-FLAG M2 mAb. The immunoprecipitates were subjected to Western blotting (WB) with anti-Ii (In-1) or FLAG mAb as indicated. Immunoblots were developed with ECL. B, mFcRn/Ii complex in IgG binding. BMDCs were lysed in PBS (pH 6.0 or 7.5) with 0.5% CHAPS and proteinase inhibitors. Approximately 0.5 mg of the soluble proteins was incubated with IgG-Sepharose at 4°C. The eluted proteins (lanes 1 and 2) or cell lysates (lane 3) were subjected to Western blotting. Proteins were probed with anti-Ii (In-1) or rabbit anti-FLAG Ab and developed with HRP-conjugated secondary Abs and ECL. Arrows, Locations of mFcRn H chain and Ii (p31 and p41). C, Control. C, Colocalization of FcRn (green) and LAMP-1 (red) in BMDC cells. The BMDC from Ii−/− (top panel) or wild-type (WT; bottom panel) mice was incubated with affinity-purified rabbit anti-FcRn or mAb anti-LAMP-1 Ab, followed by Alexa Fluor 555- or Alexa Fluor 488-conjugated IgG of the corresponding species. The yellow and arrows indicate the colocalization of FcRn with LAMP-1. Bars, 5 μm. D, Averages of the LAMP-1 and FcRn colocalization coefficients in BMDC. Pearson’s correlation coefficient was measured for the colocalization correlation of the intensity distributions between two channels. The 10 cells were analyzed in 3 different optical regions in each experiment.
Ii chain interacts with FcRn and directs FcRn into the late endosomes/lysosomes under inflammatory conditions
In addition to the expression in macrophages and DCs, FcRn is also expressed in epithelial and endothelial cells. Under physiological conditions, most epithelial and endothelial cells are Ii negative. However, the Ii chain can be induced for expression in these types of cells by proinflammatory conditions (43, 44), such as IFN-γ or bacterial infections (45). Hence, we verified the induction of Ii in the intestinal epithelial HT-29 and T84 (Fig. 9A), primary endothelial (Fig. 9B), and HeLa (data not shown) cells on exposure to IFN-γ. Caco-2 cells showed Ii chain expression by PCR even in absence of induction by IFN-γ (Fig. 9B). Coimmunoprecipitation of FcRn with anti-Ii Ab from the lysates of HeLaFcRn or THP-1 cells treated with IFN-γ showed the Ii-FcRn association in IFN-γ-stimulated HelaFcRn (Fig. 9C) or THP-1 cells (Fig. 9D). In IFN-γ-stimulated HeLaFcRn cells, approximately 49.1% of FcRn was associated with Ii chain (data not shown). However, we were unable to coimmunoprecipitate the FcRn H chain from the lysates of HeLaFcRn cells that were not treated with IFN-γ by anti-Ii mAb, which served as a negative control (Fig. 9C). Furthermore, immunofluorescence staining showed that the colocalization of FcRn with Ii and LAMP-1were observed in HelaFcRn cells upon exposure to IFN-γ stimulation (Fig. 9, E and F, bottom panels), but not in mock-stimulated cells (Fig. 9, E and F, top panels). This was further confirmed by Pearson’s colocalization coefficients of FcRn, Ii, and LAMP-1 staining (Fig. 9G). Statistical data showed the significance of FcRn and LAMP-1 colocalization (Fig. 9G). Therefore, we conclude that the intracellular trafficking of FcRn can be regulated by inflammatory cytokine through induction of Ii expression.
FIGURE 9.
Ii chain can interact with FcRn under IFN-γ stimulation. A and B, The Ii chain was expressed in IFN-γ-stimulated epithelial and endothelial cells. Intestinal epithelial (A) and endothelial (B) cells were incubated with IFN-γ (50 ng/ml) for 48 h. THP-1 cells were used as a positive control for the expression of Ii. Total RNA was extracted. RT-PCR was performed for amplification of FcRn. GAPDH was used as an internal control. C and D, Association of Ii chain and FcRn in IFN-γ-treated HeLaFcRn and THP-1 cells. HeLa, HeLaFcRn (C), and THP-1 (D) cells were stimulated with or without IFN-γ for 24 h. The cell lysates were immunoprecipitated by anti-Ii mAb. Immunoprecipitates (IP) were subjected to Western blotting with affinity-purified rabbit anti-FcRn Ab. Immunoblots (IB) were developed with ECL. E and F, Colocalization of FcRn and Ii (E) or LAMP-1 (F) in HeLaFcRn cells without (top panel) or with (bottom panel) IFN-γ treatments. Cells were stimulated with or without IFN-γ for 24 h. HeLaFcRn cells were incubated with anti-FLAG (green), anti-Ii, or LAMP-1 (red) Ab, followed by Alexa Fluor 555- or Alexa Fluor 488-conjugated IgG of the corresponding species. Yellow and arrows (right), Colocalization of FcRn with the Ii or LAMP-1 marker. Bar, 5 μm. G, Averages of FcRn and LAMP-1 colocalization coefficients in F. Pearson’s correlation coefficient were calculated. For each experiment, 15 cells were analyzed.
The cytoplasmic tail of Ii is responsible for regulating FcRn trafficking to the endosomal and lysosomal compartments
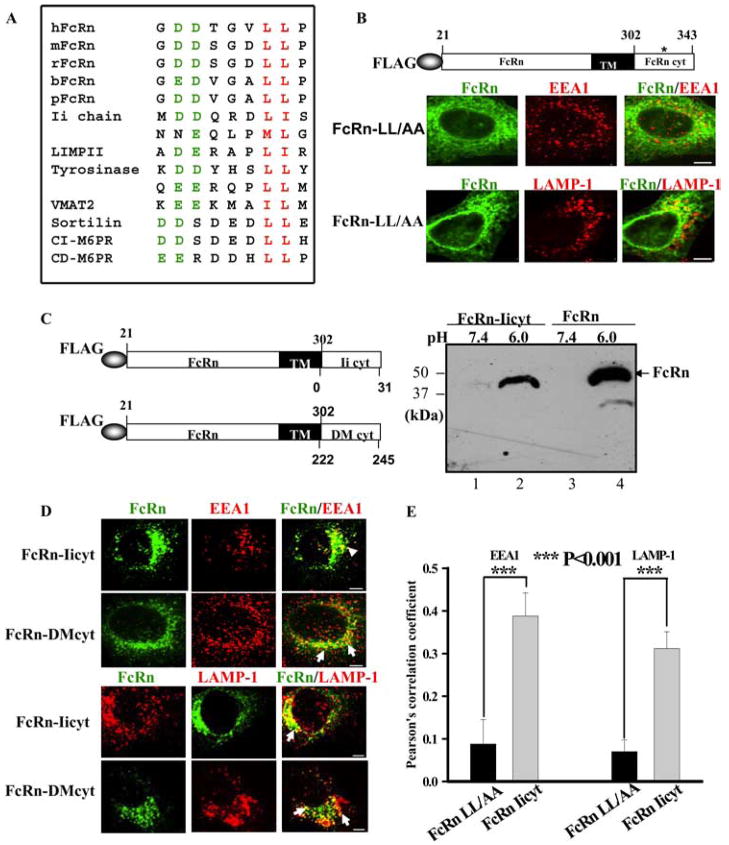
We further examined how Ii chain regulates FcRn trafficking inside cells. The key signals determining the subcellular location of most membrane proteins are in the cytoplasmic tail. The cytoplasmic tail of both human and murine FcRn contains a dileucine-based motif (Fig. 10A and Refs. 22 and 23), which exists in a number of endosomal/lysosomal resident proteins (Fig. 10A). To examine whether the dileucine-based motif of human FcRn is the early endosome targeting signal of FcRn, the two leucine residues were mutated into alanine (Fig. 10B). The FcRn-LL/AA mutant showed a honeycomb distribution with little colocalization of the EEA1 (Fig. 10B, top panel) or LAMP-1 (Fig. 10B, bottom panel) marker in HeLaFcRnLL/AA cells. This distribution pattern was similar to that of tailless FcRn (Fig. 10B, top panel). Therefore, the dileucine motif in the FcRn is responsible for targeting of FcRn to the early endosome.
FIGURE 10.
The cytoplasmic tail of Ii chain can direct FcRn trafficking to both the early endosomal and late endosomal/lysosomal compartments. Arrows, Colocalization of the proteins. Bars, 5 μm. A, Dileucine-based motifs involved in intracellular sorting and/or cell surface endocytosis that present upstream acidic residues. The critical dileucine (Met) pairs are shown in red, and acidic residues at positions upstream of the dileucine are in green. Sequences were taken from the indicated proteins: human, mouse, rat, bovine, and swine FcRn, Ii chain, LIMPII (lysosomal integral membrane protein II), tyrosines, sportily VMAT2 (vesicular monoamine transporter 2), CI-M6PR (action independent mannose 6-phosphate receptor), and CD-M6PR (action-dependent mannose 6-phosphate receptor). B, Immunofluorescence analyses of HeLa cells expressing FcRn-LL/AA. The dileucine motif was replaced by alanine residues (*) in the FcRn cytoplasmic tail (top panel). Transfected cells were immunostained with a mAb to FLAG, EEA1, or LAMP-1, followed by Alexa Fluor 555- or Alexa Fluor 488-conjugated IgG. C, Schematic representation of the chimeric FcRn-Iicyt or FcRn-HLA-DMcyt (DM). Left, The extracellular domain of FcRn (21–302 aa) was in-frame fused to the cytoplasmic tail of Ii (0 –31 aa) or HLA-DMβ (222–245 aa) in pCDNAFLAG. Right, The chimeric FcRn-Iicyt bound IgG. HeLa cells expressing FLAG-tagged FcRn-Iicyt (lanes 1 and 2) or HeLaFcRn cells (lanes 3 and 4) were lysed at both pH 6.0 and pH 7.4. The IgG binding assay was performed. The eluted proteins were subjected to Western blotting with anti-FLAG Ab. The blot was visualized by ECL. D, Immunofluorescence analyses of HeLa cells expressing FcRn-Iicyt and HLA-DMcyt. Transfected cells were fixed, permeabilized, and immunostained with a mAb to FLAG, EEA1, or LAMP-1, followed by Alexa Fluor 555- or Alexa Fluor 488-conjugated IgG. E, Averages of endosomal or lysosomal colocalization coefficients between FcRn LL/AA and FcRn FcRn-Iicyt in B and D. Pearson’s correlation coefficient were calculated. For each experiment, 15 cells were analyzed in 3 different optical regions.
The two dileucine motifs in the cytoplasmic tail of the Ii molecule are required for targeting MHC class II molecules to the endocytic pathway directly from the TGN (26–28). This suggests that the cytoplasmic tail of the Ii molecule is also responsible for targeting FcRn to the endocytic pathway. To test this hypothesis, we expressed a chimeric protein fusing the cytoplasmic tail of Ii to the extracellular domain of FcRn (FcRn-Iicyt) (Fig. 10C). Because the Ii chain is a type II glycoprotein, we also generated a similar chimeric protein to replace the cytoplasmic tail of FcRn with that of HLA-DMβ as a control (Fig. 10C). A tyrosine-based motif in the cytoplasmic tail of HLA-DMβ chain has been shown to target HLA-DM to the endocytic compartments (46). Similar to full-length FcRn in HeLaFcRn cells, the chimeric protein FcRn-Iicyt bound IgG at pH 6.0 (Fig. 10C, lane 2) but not at pH 7.4 (lane 1) in an IgG binding assay, suggesting that the fusion of the Ii cytoplasmic tail did not affect the integrity of FcRn structure. As shown in Fig. 10D, the cytoplasmic tail of Ii chain targeted FcRn into an intracellular compartment that largely overlapped with either EEA1+ (Fig. 10D, left) or the LAMP-1+ (Fig. 10D, right) marker. As a control, the cytoplasmic tail of HLA-DMβ chain directed FcRn into the LAMP-1+ late endosomal/lysosomal compartment (Fig. 10D), although a relatively small portion of FcRn appeared in the EEA1+ compartment in HeLa cells transfected with FcRn-DMcyt. Pearson’s correlation coefficient analysis shows the significance of colocalization of hybrid FcRn-Iicyt molecule with endosomal or lysosomal marker (Fig. 10E). Taken together, these data suggest that the cytoplasmic tail of the Ii chain, likely two dileucine-based motifs, is responsible for directing FcRn trafficking into the endocytic pathway.
Discussion
The Ii chain was thought to mainly function as an MHC class II chaperone, which prevents the binding of endogenous peptides to MHC class II in the ER and directs MHC class II to endocytic compartments, where they are loaded with antigenic peptides generated from endocytosed proteins (47, 48). However, Ii has recently been shown to have additional functions by interacting with other molecules, such as with CD44 (49), MHC class I (50), and CD1d molecules (51) during T or NK T cell-mediated responses, the macrophage migration-inhibitory factor to induce the phosphorylation of the ERK-1/2 (52), Helicobacter pylori urease B subunit to stimulate IL-8 production (45) and HIV-2 Vpx (53). In this study, we showed a novel role of Ii chain in FcRn-mediated IgG transport and catabolism by regulating intracellular trafficking of FcRn.
This study provides several lines of evidence to demonstrate the interaction of Ii chain with FcRn. First, Ii was coimmunoprecipitated with FcRn in HeLa transfectant (Fig. 1). Furthermore, FcRn colocalization with Ii in HeLa transfectant suggests their association in vivo. Second, although the Ii is not constitutively expressed in certain types of epithelial and endothelial cells, FcRn/Ii complexes were coimmunoprecipitated from these cells when Ii chain expression was induced by IFN-γ (Fig. 9), suggesting the interaction of FcRn and Ii in epithelial or endothelial cells can be caused under inflammatory conditions. Third, the FcRn/Ii complexes were also present in either the endo-H-sensitive or -resistant form. This biochemical evidence suggests that the FcRn/Ii complexes were formed as early as in the ER and remained as a complex when passing through the Golgi stack. Fourth, Ii chain interacted with the nascent FcRn H chain alone or with FcRn-β2m complex (Fig. 2), suggesting that the Ii chain may play a role in retaining FcRn H chain in the ER until assembly of the complex of H chain with β2m. This is in agreement with the studies showing that Ii chain retains incompletely folded CD1d H chain in the ER (51), but in disagreement with studies showing that the Ii-MHC class I association in the ER appears to require essentially fully folded MHC class I because free H chain does not associate efficiently with Ii chain (41, 54).
It is possible that Ii does not directly interact with FcRn but associates with FcRn indirectly through MHC class II or clanexin. Our recent finding implicates the involvement of calnexin in the assembly of FcRn H chain and β2m (35). However, FcRn assembly and binding to IgG appears normal in a calnexin-deficient mutant cell line, CEM-NKR, suggesting that alternative chaperones or mechanisms for FcRn assembly in the ER might exist in CEM-NKR cells. The association of Ii chain with FcRn suggests that Ii serves as a chaperone FcRn assembly in the ER. Ii is a chaperone for both MHC class II (47, 48) and cathepsin L (55). This may help explain the structural and functional normality of FcRn in calnexin-deficient cells. Because FcRn associates with Ii in MHC II-negative HeLa cells or calnexin-deficient cells (CEM-NKRFcRn; Ref. 35 and data not shown), these exclude an indirect association of FcRn with the Ii via MHC II (56) or calnexin. In addition, the formation of Ii trimer is essential for exiting of MHC II-Ii complex (MHC II (αβ)3Ii3; Ref. 25) and targeting signals contained in the cytoplasmic tails of multimerized Ii are required for targeting the MHC class II-Ii complexes (57). This suggests that FcRn also associates with a trimer of Ii chain.
The CLIP fragment of Ii binds to the Ag-binding groove of MHC class II or I (41). In contrast to the interaction of Ii with MHC class II, the CLIP of Ii is not required for interaction of Ii with FcRn due to a narrowed Ag-binding groove of FcRn. This raises an interesting question whether CLIP is important for the association of Ii with FcRn (11). Our finding that mutation or deletion of the CLIP did not disrupt FcRn-Ii interaction suggests that the CLIP fragment is not directly involved in the FcRn-Ii association (Fig. 4). It is still possible that the CLIP positions itself above the binding groove of FcRn by the flanking sequences. This would result in the engagement of Ii to FcRn at multiple sites other than the peptide-binding groove (58–60). For example, the transmembrane segment of Ii chain can interact with MHC class II in a CLIP-independent manner (32). The multiple binding sites between Ii and FcRn may enhance the interaction. Indeed, the FcRn-Ii complex resisted multiple detergents (Fig. 1E). Further study is required for mapping FcRn-Ii interaction as described for MHC class II and Ii (61).
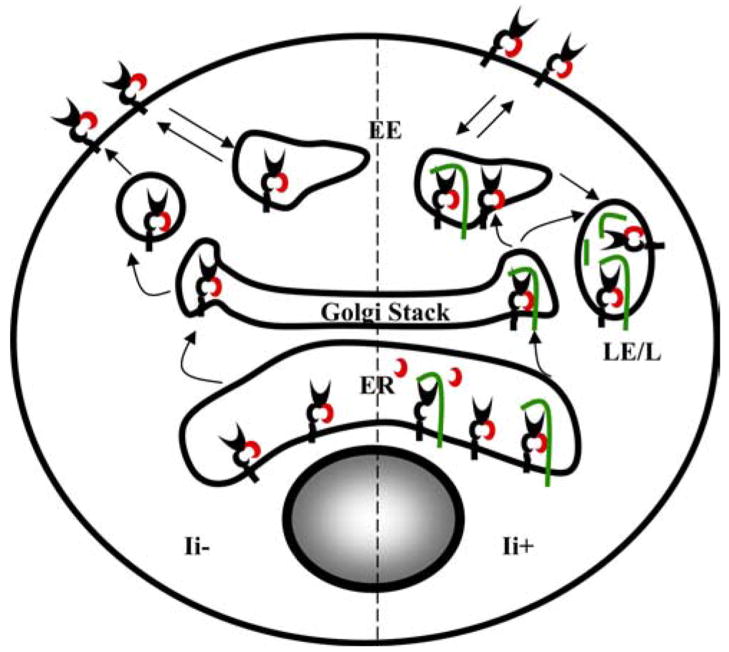
The association of Ii chain with FcRn clearly expands the distribution of FcRn within the endocytic pathway. FcRn has been predominantly detected in the early endosomes (20, 21, 62). The deletion of the FcRn cytoplasmic tail alters the distribution of FcRn to the cell surface and cytoplasm. The cellular location of tailless FcRn was partially restored by the expression of Ii chain (Fig. 5). Furthermore, Ii directs both tailless and intact FcRn to LAMP-1+ late endosomes/lysosomes (Fig. 5). This observation was further verified by the fact that FcRn appeared in the late endosomes/lysosomes in all of wild-type immature DCs (Figs. 7 and 8) or IFN-γ-treated HeLaFcRn cells (Fig. 9F), but not in the majority of Ii-deficient DCs (Figs. 7 and 8). In this aspect, the role of Ii in FcRn intracellular trafficking mirrors its role in directing the MHC class II intracellular trafficking. Furthermore, our data showed the transplantation of Ii cytoplasmic tail conferred tailless FcRn to appear in both early endosome and late endosome/lysosome (Fig. 10D), suggesting the dileucine-based motif in the Ii molecule would compensate for the sorting function absent in the tailless FcRn. Mutations of the dileucine-based motif in the cytoplasmic tail of FcRn associated with a profound loss of its early endosomal location (Fig. 10B), suggesting that this motif is sufficient to direct FcRn to the endosomes at the steady state. Dileucine signals have been reported to bind adaptor protein complexes that are essential for intracellular protein sorting (63). The additional two dileucine-based motifs in Ii would further enhance the interaction of FcRn-Ii complex with adaptor protein complexes, leading FcRn trafficking from the early endosome to the late endosome/lysosome. We propose that the intracellular locations of FcRn may be controlled by two different mechanisms, which lead to either early endosomal or late endosomal/lysosomal targeting of FcRn. This provides flexibility for FcRn traffic in Ii-positive or Ii-negative cell types. In Ii-negative cell types, FcRn probably traffics directly to the cell surface from the TGN and is subsequently internalized into the early endosomes. In Ii-expressing cell types, Ii-associated FcRn is likely to be segregated from the secretory pathway at the TGN and directly targeted to the endocytic pathway without access to the plasma membrane (Fig. 11), although further studies are needed to test this hypothesis.
FIGURE 11.
Two pathways for intracellular trafficking of FcRn. In cells without Ii expression, FcRn may reach the cell surface through the secretory pathway and recycle between the plasma membrane and endosomes via endocytosis. In the presence of Ii expression, a portion of FcRn molecules is associated with the Ii in the ER and is targeted to the endosomes and lysosomes via Golgi stack. In the endosome/lysosome, Ii was released from FcRn, presumably, by proteolytic cleavage. Green, Ii; black, FcRn H chain; red, β2m. EE, early endosome; LE, late endosome; L, lysosome.
What would be the biological consequences of Ii-FcRn association and the expanded intracellular trafficking of FcRn as conferred by the Ii chain, particularly in heightened inflammatory circumstances? Several speculations can be made. FcRn has two known biological functions: transcytosis of IgG across the polarized epithelial cells; and maintenance of IgG or albumin homeostasis. Under physiological conditions, the cytoplasmic tail of FcRn clearly predominates its trafficking in the majority of Ii-negative epithelial or endothelial cells to early endosomes. However, this pattern of trafficking may be significantly altered in inflammatory conditions because Ii expression is induced in epithelial and endothelial cells by inflammatory cytokines or during bacterial and viral infections (44, 45). FcRn transports normal or pathogen-specific neutralizing IgG across polarized epithelial cells, potentially seeding mucosal immunity. It remains to be determined precisely how the altered trafficking to late endosomal/lysosomal compartments in Ii-expressing epithelial and endothelial cells would influence the IgG transport and homeostasis and the local immune response. Nevertheless, from the aspect of IgG protection, one can envision that due to the association of FcRn with Ii, FcRn might bind IgG not only in the early endosome but also in the late endosomal/lysosomal compartment, thus extending its boundary for sampling IgG. The evidence that FcRn was capable of binding IgG under lysosomal (pH 5.0) conditions (X. Zhu et al., unpublished data) makes this event highly likely. Hence, the appearance of FcRn in the late endosome/lysosome may function as a second line that salvages the IgG from degradation. Second, because MHC class II are most efficiently targeted into proximity with antigenic peptides by Ii chain in APCs, it is interesting to bring into perspective the known functions of Ii in conventional APCs to understand the role of FcRn/Ii association in influencing Ag presentation. Our previous findings show that the FcRn is expressed in macrophages and DCs (6). Because FcRn appears to associate with the Ii chain, FcRn and MHC class II might occupy the same acidic compartments. In macrophages and DCs, FcγRs can promote the internalization of immune complexes into the endosomes and lysosomes to increase the efficiency of MHC class II presentation to CD4+ T cells (64). FcRn, alternatively, may mediate the Ag presentation by binding the immune complexes in these Ag-processing compartments. The evidences that FcRn is able to bind immune complexes (65) and IgG in the pH range of endosomes and lysosomes (X. Zhu et al., unpublished data) supports this probability. Therefore, the association of Ii chain and FcRn could further influence the Ag presentations. Third, FcRn was involved in IgG-mediated phagocytosis with its expression in the phagolysosomes in human neutrophils (7). It would be interesting to know whether FcRn-Ii association in the neutrophils mediates the translocation of FcRn into the phagolysosomes. Fourth, an N-terminal product, liberated from Ii chain via regulated intramembrane proteolysis, has been recently shown to function as a transcription factor in activating the NF-κB-dependent transcription program, at least in B lymphocytes (66, 67). Our recent finding revealed that the activation of NF-κB signaling up-regulates the level of FcRn expression (68). As a result, coexpression of Ii chain and FcRn might regulate the FcRn expression.
In conclusion, this study delineated a novel intracellular destinations for FcRn trafficking and demonstrated a novel, unexpected function of Ii chain. Our study suggests that the intracellular trafficking pathway and functions of FcRn may be altered by the association of Ii chain during physiological and inflammatory conditions and points to a potential effect of Ii in modifying IgG functions of FcRn. Thus, the association of FcRn with Ii chain is physiologically relevant, and appreciation of this process is important to the understanding of how IgG is transported and how IgG levels are maintained throughout the body. Given that the Ii chain could function at different conditions, it is of interest to examine how these various effects will ultimately manifest in terms of IgG transcytosis and protection.
Acknowledgments
We are grateful to Dr. Elizabeth Bikoff for supplying us with breeder pairs for the mutant BALB/c Ii−/− mice. We acknowledge the receipt of protein expression plasmids from Dr. Norbert Koch, Dr. Gary Banker, and Dr. Ronald N. Germain. We are most grateful for technical help from Amy Beaven and Guozhen Gao. We also acknowledge the editing of the manuscript by Ireen Dryburgh-Barry.
Footnotes
This work was supported in part by National Institutes of Health Grants AI65892, AI67965, AI73139 (to X.Z.), N01-AO-6009 (to S.K.S.), and AI59617 (to W.S.); the faculty start-up package and Maryland Agricultural Experimental Station competitive grants from the University of Maryland (to X.Z.); and the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this paper: FcRn, neonatal FcR; EEA1, early endosomal Ag-1; endo H, endo-N-acetylglucosaminidase; ER, endoplasmic reticulum; LAMP-1, lysosome-associated membrane glycoprotein-1; β2m, β2-microglobulin; TGN, trans-Golgi network; Ii, invariant chain; CHO, Chinese hamster ovary; HA, hemagglutinin; siRNA, small interfering RNA; BMDC, bone marrow-derived DC; TfR, transferrin receptor; TfR-GFP, GFP fusion of TfR; mFcRn, mouse FcRn; FcRn-Iicyt, a chimeric protein fusing the cytoplasmic tail of Ii to the extracellular domain of FcRn.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Wallace KH, Rees AR. Studies on the immunoglobulin-G Fc-fragment receptor from neonatal rat small intestine. Biochem J. 1980;188:9–16. doi: 10.1042/bj1880009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye L, Tuo W, Liu X, Simister NE, Zhu X. Identification and characterization of an alternatively spliced variant of the MHC class I-related porcine neonatal Fc receptor for IgG. Dev Comp Immunol. 2008;32:966–979. doi: 10.1016/j.dci.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumberg RS, Koss T, Story CM, Barisani D, Polischuk J, Lipin A, Pablo L, Green R, Simister NE. A major histocompatibility complex class I-related Fc receptor for IgG on rat hepatocytes. J Clin Invest. 1995;95:2397–2402. doi: 10.1172/JCI117934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10:1289–1298. doi: 10.1093/intimm/10.9.1289. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, et al. MHC class I-related Fc Receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108:3573–3579. doi: 10.1182/blood-2006-05-024539. [DOI] [PubMed] [Google Scholar]
- 8.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 9.Burmeister WP, Huber AH, Bjorkman PJ. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372:379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X, Peng J, Raychowdhury R, Nakajima A, Lencer WI, Blumberg RS. The heavy chain of neonatal Fc receptor for IgG is sequestered in the endoplasmic reticulum by forming oligomers in the absence of β2m association. Biochem J. 2002;367:703–714. doi: 10.1042/BJ20020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firan M, Bawdon R, Radu C, Ober RJ, Eaken D, Antohe F, Ghetie V, Ward ES. The MHC class I related receptor, FcRn, plays an essential role in the maternofetal transfer of γ-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 14.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 15.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–322. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian DC. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest. 2004;113:1328–1333. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghavan M, V, Bonagura R, Morrison SL, Bjorkman PJ. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry. 1995;34:14649–14657. doi: 10.1021/bi00045a005. [DOI] [PubMed] [Google Scholar]
- 19.Brambell FW, Hemmings WA, Morris IG. A theoretical model of γ-globulin catabolism. Nature. 1964;203:1352–1354. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- 20.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172:2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 21.Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, Ober RJ, Ward ES. Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc Natl Acad Sci USA. 2007;104:5889–5894. doi: 10.1073/pnas.0700337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Simister NE. Tryptophan- and dileucine-based endocytosis signals in the neonatal Fc receptor. J Biol Chem. 2001;276:5240–5247. doi: 10.1074/jbc.M006684200. [DOI] [PubMed] [Google Scholar]
- 23.Wernick NL, Haucke V, Simister NE. Recognition of the tryptophan-based endocytosis signal in the neonatal Fc receptor by the mu subunit of adaptor protein-2. J Biol Chem. 2005;280:7309–7316. doi: 10.1074/jbc.M410752200. [DOI] [PubMed] [Google Scholar]
- 24.Dickinson BL, Claypool SM, D’Angelo JA, Aiken ML, Venu N, Yen EH, Wagner JS, Borawski JA, Pierce AT, Hershberg R, et al. Ca2+-dependent calmodulin-binding to FcRn affects IgG transport in the transcytotic pathway. Mol Biol Cell. 2008;19:414– 423. doi: 10.1091/mbc.E07-07-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–394. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 26.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 27.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, Peterson PA. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600– 605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 28.Pieters J, Bakke O, Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci. 1993;106:831– 846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- 29.Riese RJ, Wolf PR, Bromme D, Natkin LR, Villadangos JA, Ploegh HL, Chapman HA. Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 30.Denzin LK, Cresswell P. HLA-DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 31.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellino F, Germain RN. Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity. 1995;2:73– 88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 33.Kenty G, Bikoff EK. BALB/c invariant chain mutant mice display relatively efficient maturation of CD4+ T cells in the periphery and secondary proliferative responses elicited upon peptide challenge. J Immunol. 1999;163:232–241. [PubMed] [Google Scholar]
- 34.Neumann J, Koch N. Assembly of major histocompatibility complex class II subunits with invariant chain. FEBS Lett. 2005;579:6055– 6059. doi: 10.1016/j.febslet.2005.09.070. [DOI] [PubMed] [Google Scholar]
- 35.Zhu X, Peng J, Chen D, Liu X, Ye L, Iijima H, Kadavil K, Lencer WI, Blumberg RS. Calnexin and ERp57 facilitate the assembly of the neonatal Fc receptor for IgG with β2-microglobulin in the endoplasmic reticulum. J Immunol. 2005;175:967–976. doi: 10.4049/jimmunol.175.2.967. [DOI] [PubMed] [Google Scholar]
- 36.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 37.Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem. 2007;40:101–111. doi: 10.1267/ahc.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romagnoli P, Germain RN. Inhibition of invariant chain (Ii)-calnexin interaction results in enhanced degradation of Ii but does not prevent the assembly of α β Ii complexes. J Exp Med. 1995;182:2027–2036. doi: 10.1084/jem.182.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malcherek G, Gnau V, Jung G, Rammensee HG, Melms A. Supermotifs enable natural invariant chain-derived peptides to interact with many major histocompatibility complex-class II molecules. J Exp Med. 1995;181:527–536. doi: 10.1084/jem.181.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh P, Amaya M, Mellins E, Wiley DC. The structure of an intermediate in class II MHC maturation: CLIP bound to HLA-DR3. Nature. 1995;378:457– 462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 41.Powis SJ. CLIP-region mediated interaction of Invariant chain with MHC class I molecules. FEBS Lett. 2006;580:3112–3116. doi: 10.1016/j.febslet.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 42.Lagaudriere-Gesbert C, Newmyer SL, Gregers TF, Bakke O, Ploegh HL. Uncoating ATPase Hsc70 is recruited by invariant chain and controls the size of endocytic compartments. Proc Natl Acad Sci USA. 2002;99:1515–1520. doi: 10.1073/pnas.042688099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins T, Korman AJ, Wake CT, Boss JM, Kappes DJ, Fiers W, Ault KA, Gimbrone MA, Strominger JL, Pober JS. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci USA. 1984;81:4917– 4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hershberg RM, Framson PE, Cho DH, Lee LY, Kovats S, Beitz J, Blum JS, Nepom GT. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–215. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beswick EJ, Das S, Pinchuk IV, Adegboyega P, Suarez G, Yamaoka Y, Reyes VE. Helicobacter pylori-induced IL-8 production by gastric epithelial cells up-regulates CD74 expression. J Immunol. 2005;175:171–176. doi: 10.4049/jimmunol.175.1.171. [DOI] [PubMed] [Google Scholar]
- 46.Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 48.Matza D, Kerem A, Shachar I. Invariant chain, a chain of command. Trends Immunol. 2003;24:264–268. doi: 10.1016/s1471-4906(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 49.Naujokas MF, Morin M, Anderson MS, Peterson M, Miller J. The chondroitin sulfate form of invariant chain can enhance stimulation of T cell responses through interaction with CD44. Cell. 1993;74:257–268. doi: 10.1016/0092-8674(93)90417-o. [DOI] [PubMed] [Google Scholar]
- 50.Sugita M, Brenner MB. Association of the invariant chain with major histocompatibility complex class I molecules directs trafficking to endocytic compartments. J Biol Chem. 1995;270:1443–1448. doi: 10.1074/jbc.270.3.1443. [DOI] [PubMed] [Google Scholar]
- 51.Jayawardena-Wolf J, Benlagha K, Chiu YH, Mehr R, Bendelac A. Two pathways of CD1d endosomal trafficking are independently regulated by an intrinsic CD1d-encoded tyrosine motif and by the invariant chain. Immunity. 2001;15:897–908. doi: 10.1016/s1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- 52.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pancio HA, Vander Heyden N, Kosuri K, Cresswell P, Ratner L. Interaction of human immunodeficiency virus type 2 Vpx and invariant chain. J Virol. 2000;74:6168– 6172. doi: 10.1128/jvi.74.13.6168-6172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viga JL, Smith KD, Lutz CT. Invariant chain association with MHC class I: preference for HLA class I/b2-microglobulin heterodimers, specificity, and influence of the MHC peptide-binding groove. J Immunol. 1996;157:4503– 4510. [PubMed] [Google Scholar]
- 55.Lennon-Dumenil AM, Roberts RA, Valentijn K, Driessen C, Overkleeft HS, Erickson A, Peters PJ, Bikoff E, Ploegh HL, Wolf Bryant P. The p41 isoform of invariant chain is a chaperone for cathepsin L. EMBO J. 2001;20:4055– 4064. doi: 10.1093/emboj/20.15.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang SJ, Cresswell P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 2002;21:1650–1660. doi: 10.1093/emboj/21.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arneson LS, Miller J. Efficient endosomal localization of major histocompatibility complex class II-invariant chain complexes requires multimerization of the invariant chain targeting sequence. J Cell Biol. 1995;129:1217–1228. doi: 10.1083/jcb.129.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stumptner P, Benaroch P. Interaction of MHC class II molecules with the invariant chain: role of the invariant chain (81–90) region. EMBO J. 1997;16:5807–5818. doi: 10.1038/sj.emboj.7590555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson NA, Wolf P, Ploegh H, Ignatowicz L, Kappler J, Marrack P. Invariant chain can bind MHC class II at a site other than the peptide binding groove. J Immunol. 1998;161:4777– 4784. [PubMed] [Google Scholar]
- 60.Castellino F, Han R, Germain RN. The transmembrane segment of invariant chain mediates binding to MHC class II molecules in a CLIP-independent manner. Eur J Immunol. 2001;31:841– 850. doi: 10.1002/1521-4141(200103)31:3<841::aid-immu841>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 61.Bijlmakers MJ, Benaroch P, Ploegh HL. Mapping functional regions in the lumenal domain of the class II-associated invariant chain. J Exp Med. 1994;180:623– 629. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tesar DB, Tiangco NE, Bjorkman PJ. Ligand valency affects transcytosis, recycling and intracellular trafficking mediated by the neonatal Fc receptor. Traffic. 2006;7:1127–1142. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395– 447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Goldsteine J, Graziano RF, He J, O’Shea JK, Deo Y, Guyre PM. FcγRI-targeted fusion proteins result in efficient presentation by human monocytes of antigenic and antagonist T cell epitopes. J Clin Invest. 1996;98:2001–2007. doi: 10.1172/JCI119004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abrahamson DR, Powers A, Rodewald R. Intestinal absorption of immune complexes by neonatal rats: a route of antigen transfer from mother to young. Science. 1979;206:567–569. doi: 10.1126/science.493961. [DOI] [PubMed] [Google Scholar]
- 66.Matza D, Wolstein O, Dikstein R, Shachar I. Invariant chain induces B cell maturation by activating TAFII105-NF-κB dependent transcription program. J Biol Chem. 2001;276:27203–27206. doi: 10.1074/jbc.M104684200. [DOI] [PubMed] [Google Scholar]
- 67.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I. CD74 is a member of the regulated intramembrane proteolysis (RIP)-processed protein family. Mol Biol Cell. 2005;16:5061–5069. doi: 10.1091/mbc.E05-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu X, Ye L, Christianson G, Yang J, Roopenian DC, Zhu X. NF-κB signaling regulates the functional expression of MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J Immunol. 2007;179:2999–3011. doi: 10.4049/jimmunol.179.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]