Abstract
Reports from the United States have demonstrated that elevated markers of microbial translocation from the gut may be found in chronic and advanced HIV-1 infection and are associated with an increase in immune activation. However, this phenomenon's role in HIV-1 disease in Africa is unknown. This study examined the longitudinal relationship between microbial translocation and circulating inflammatory cytokine responses in a cohort of people with varying rates of HIV-1 disease progression in Rakai, Uganda. Multiple markers for microbial translocation (lipopolysaccharide, endotoxin antibody, and sCD14) did not change significantly during HIV-1 disease progression. Moreover, circulating immunoreactive cytokine levels either decreased or remained virtually unchanged throughout disease progression. These data suggest that microbial translocation and its subsequent inflammatory immune response do not have a causal relationship with HIV-1 disease progression in Africa.
Keywords: HIV, microbial translocation
HIV type 1 (HIV-1) disease progression is believed to be caused in part by constant immune activation that detrimentally affects the immune system through a combination of direct loss of infected CD4+ T lymphocytes and bystander cell killing, causing the slow decline of CD4+ T cells, which eventually leads to acquired immunodeficiency syndrome (AIDS) (1–3). Studies examining simian immunodeficiency virus (SIV) infection in primates and HIV-1–infected humans have demonstrated a significant loss of CD4+ cells early in infection at mucosal barriers in the gastrointestinal tract, possibly leading to increased migration of bacteria and bacterial byproducts from the lumen of the gut into the portal bloodstream (4–7). This phenomenon, termed “microbial translocation,” results in increased levels of these bacterial byproducts in the bloodstream without evident bacteremia and has been reported in graft-versus-host disease and inflammatory bowel disease (8–10). This increase in bacterial byproducts, particularly lipopolysaccharide (LPS), was found to be associated with systemic immune activation in these ailments (8–10). This finding led to several cross-sectional studies carried out in patients in the United States, which demonstrated that different stages of HIV-1 disease were associated with changes in markers for microbial translocation, including higher circulating LPS levels, decreased endotoxin core antibody (EndoCAb) levels, and higher levels of monocyte activation (10, 11). These markers also were found to be associated with increased levels of cellular and innate immune activation (10). Based on these findings, it was theorized that the gastrointestinal-associated CD4 cell loss increased rates of microbial translocation across the gut, causing a systemic inflammatory immune response, which in turn is associated with increased HIV-1 disease progression (10, 12). To further explore the relationship of microbial translocation, immune activation, and HIV-1 disease progression, we measured markers of microbial translocation and levels of circulating inflammatory cytokines longitudinally in a cohort of HIV-infected Africans with different rates of disease progression from preinfection to death.
Results
Baseline Levels of Microbial Translocation Markers Differ Between African and U.S. HIV-Uninfected Subjects.
To directly examine the relationship of microbial translocation, inflammatory cytokine response, and HIV-1 disease progression, 107 African individuals with known years of HIV-1 seroconversion between 1994 and 2000 were identified in a community cohort under annual surveillance in Rakai, Uganda. These individuals were stratified into 3 progression groups according to known disease outcomes: long-term nonprogressors (LTNPs; n = 27), standard progressors (SPs; n = 41), and rapid progressors (RPs; n = 39) (Table 1) (13). Seroconverters were identified as individuals who had a negative HIV-1 serologic test followed by a subsequent positive test the next year. Archived serum samples from the last HIV-1– seronegative time point (baseline/year 0) and all later positive time points (years 1, 2, 3, etc.) were used in subsequent assays. The median age at infection, sex, and subtype distribution did not differ significantly among the progression groups. As expected, the HIV-1 viral load setpoint was lower in the LTNP group compared with the other 2 progression groups (P < .001) (14).
Table 1.
African subject and disease progression group parameters
| LTNPs (n = 27) | Standard progressors (n = 41) | Rapid progressors (n = 39) | |
|---|---|---|---|
| Median age at infection | 26.7 (23.0–31.0) | 31.1 (25.2–38.5) | 31.5 (25.4–43.3) |
| Percent female | 51.9% | 46.3% | 51.9% |
| Median viral load setpoint (log) | 3.54* (2.60–4.26) | 4.86* (4.38–5.40) | 5.06* (4.30–5.57) |
| Subtype A (%) | 21.7% | 8.8% | 15.2% |
| Subtype D (%) | 52.2% | 67.6% | 78.8% |
| Recombinant (%) | 26.1% | 23.5% | 6.1% |
Group dynamics, HIV-1 subtype distribution, and viral setpoint for LTNPs (CD4 > 600 at 7+ years postinfection), standard progressors (death < 9 and > 5 years), and rapid progressors (death < 4 years) are shown. Subtype percentages were calculated from patients with known subtypes. Median values (25%–75%) for viral load setpoint and age are shown.
* Statistical significance is shown (P < .001, Kruskal–Wallis ANOVA on ranks).
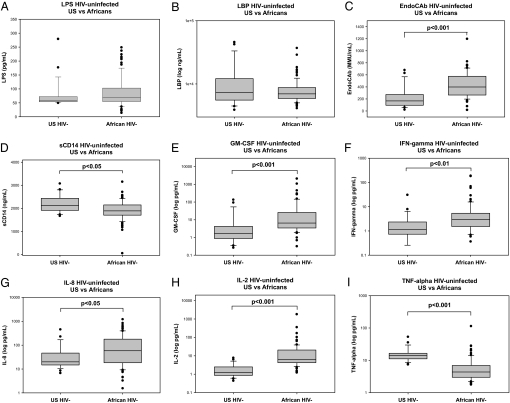
Evidence of microbial translocation was measured by examining circulating levels of LPS, EndoCAb, LPS binding protein (LBP), and soluble CD14 (sCD14) in all longitudinal samples from the African subjects and also in a comparison group of U.S. HIV-negative individuals, included to examine any underlying population differences in the immune and microbial translocation markers tested (10). The comparison group was 62.5% African-American and 50% female, with a median age of 53 years. Levels of circulating LPS, a component of Gram-negative bacterial cell walls, and baseline LBP levels, which increase in response to higher LPS levels, did not differ between the African HIV-1–negative baseline samples and U.S. HIV-1–negative comparison group (Fig. 1A and B); however, baseline levels of EndoCAb were significantly higher in the African subjects (P < .001) (Fig. 1C). In addition, levels of sCD14, which binds LPS and is an indicator of monocyte activation, were significantly lower in the African subjects at baseline (P < .05) (Fig. 1D). These data indicate that the underlying levels of EndoCAb were elevated in the African individuals, but that this elevation was restricted to antibody levels and was not indicative of a more activated LPS-induced immune response.
Fig. 1.
Baseline microbial markers and circulating cytokine levels of uninfected U.S. and African subjects. Differences between HIV-negative U.S. subjects (n = 24) and uninfected African subjects in baseline serum levels (n = 86) of LPS (A), LBP (B), EndoCAb (C), sCD14 (D), GM-CSF (E), IFN-γ (F), IL-8 (G), IL-2 (H), and TNF-α (I) were examined using the Mann-Whitney rank-sum test. The results are shown with significance indicated where applicable (P < .05).
Levels of LPS, EndoCAb, and sCD14 in HIV-Infected Africans Remain Relatively Stable Throughout Disease Progression.
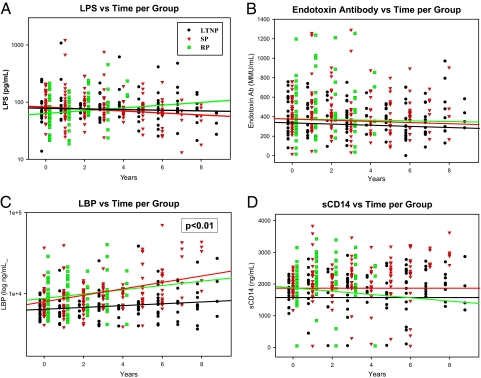
To assess whether longitudinal changes in the microbial translocation markers differed among the disease progression groups, a linear multilevel regression model was fitted for each marker, and the slopes were compared to assess differences in the progressor groups (Fig. 2; individual subject analysis is shown in Fig. 3). There were no significant differences in LPS levels among the HIV-1 progression groups over time (Fig. 2A). There was also no significant change in LPS levels in any of the groups even at the later disease stages (Fig. 3A–C). LPS is quickly removed from the circulation in healthy individuals and can be difficult to measure accurately, especially at low levels; therefore, other markers for longitudinal changes in microbial translocation were examined.
Fig. 2.
Longitudinal changes of microbial translocation markers according to African disease progression groups. The last HIV-negative time point for each patient (year 0) and all subsequent years postseroconversion are shown for each progression group [LTNP (circles), SP (inverted triangles), and RP (squares)] for LPS (A), EndoCAb (B), LBP (C), and sCD14 (D). Longitudinal variation in patient levels was determined using linear multilevel modeling fit with slopes in the log scale for each group. The resulting regression line for each group is shown in the respective color. Significant inequality in slopes across groups is indicated where applicable (P < .05).
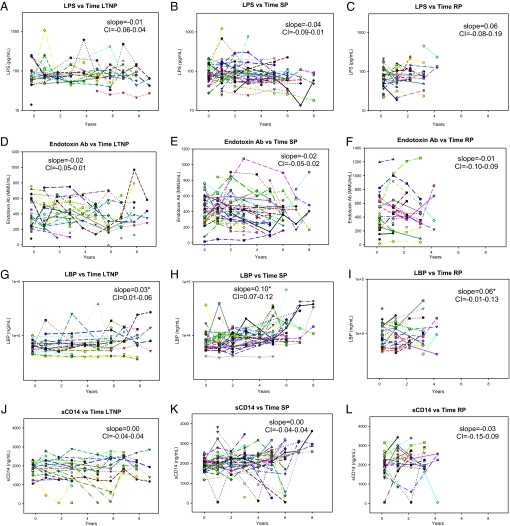
Fig. 3.
African subject-specific microbial translocation markers according to progression group. Levels of microbial translocation marker levels are shown for the LTNP, SP, and RP groups for LPS (A–C), EndoCAb (D–F), LBP (G–I), and sCD14 (J–L). Linear multilevel modeling fit slopes in the log scale of each group are shown with the 95% CI on each graph, with significance noted by an asterisk (P < .05). Patient-specific color-coding is consistent throughout multiple assays within the denoted progression group (Fig. S2).
Cross-sectional studies from the United States have found lower EndoCAb levels in individuals in the acute/early phase of HIV-1 infection compared with uninfected controls (10). These levels are even lower at later disease stages, dependent on the individual's progression status (10, 11). In the African subjects, EndoCAb levels remained relatively constant over time (Fig. 3D–F), and there were no significant differences in the slopes of the regressions among the 3 disease progression groups (Fig. 2B).
Cross-sectional U.S. studies also have demonstrated increased LBP and sCD14 levels at later stages of HIV-1 infection (10, 11). The longitudinal analysis of LBP levels in the standard and rapid progression groups found elevated LBP levels in the African subjects as well (Figs. 2C and 3H and I). This increase was significantly lower in the LTNP progression group compared with the standard and rapid progression groups, in agreement with previous U.S.-based data that demonstrated virtually no increase in LBP levels among HIV controllers (Figs. 2C and 3G) (10). However, sCD14 levels did not consistently increase in the African subjects (Fig. 3 J–L), and there were no differences in the slopes of the regressions among the 3 progression groups (Fig. 2D). There also was no difference when these data were analyzed according to gender. These data suggest that the increased LBP levels observed at later disease stages in the standard and rapid progression groups may not be directly associated with higher levels of circulating LPS.
Baseline Levels of Circulating Cytokine Levels Differed Between the African and U.S. Comparison Groups.
A primary effect of increased microbial translocation is an enhanced proinflammatory cytokine response, which can differ between Western and African subjects (2, 10, 12, 15–19). This increase in inflammatory cytokines has been hypothesized to contribute to the heightened immune state found in HIV-infected individuals in the United States (2, 12). Consequently, levels of 9 proinflammatory cytokines were examined to explore the association of cytokine induced-immune activation with LPS translocation and HIV-1 disease progression as a whole in the African population. At baseline, significantly higher levels of GM-CSF, IFN-γ, IL-8, and IL-2 were detected in the African subjects compared with the U.S. subjects (Fig. 1E–H); however, TNF-α levels were lower in the African subjects (Fig. 1I). No differences in baseline levels of IL-1β, IL-6, IL-10, or IL-12p70 were found [supporting information (SI) Fig. S1].
Levels of Circulating Inflammatory Cytokines Decrease During HIV-1 Disease Progression in African Patients.
Linear multilevel regression analysis was used to assess whether longitudinal changes in specific cytokine levels differed among the disease progression groups (Table 2; individual subject analysis is shown in Fig. S2). IFN-γ levels differed among the 3 groups, with the SPs demonstrating increasing levels over time (Table 2). The SPs also displayed an increase in IL-6 levels compared with the other 2 groups. TNF-α levels were significantly increased in both the standard and rapid progression groups compared with the LTNP group; however, the differences in the slopes of TNF-α, IFN-γ, and IL-6 often were small, and the possible effects of these changes on activity levels is unknown (Table 2).
Table 2.
Longitudinal changes in circulating cytokine levels according to African disease progression groups
| Cytokine | LTNP regression slope (95% CI) | SP regression slope (95% CI) | RP regression slope (95% CI) | P value |
|---|---|---|---|---|
| IL-1b | −0.12 (−0.18–−0.06) | −0.08 (−0.13–−0.02) | −0.25 (−0.40–−0.10) | .083 |
| IL-2 | −0.12 (−0.18–−0.06) | −0.10 (−0.16–−0.04) | −0.20 (−0.35–−0.04) | .519 |
| IL-6 | −0.09 (−0.14–−0.04) | 0.07 (0.02–0.12) | −0.13 (−0.27–0.01) | <.001 |
| IL-8 | −0.11 (−0.19–−0.02) | −0.21 (−0.30–−0.11) | −0.21 (−0.45–0.02) | .271 |
| IL-10 | −0.12 (−0.18–−0.07) | −0.07 (−0.12–−0.02) | −0.23 (−0.38–−0.08) | .099 |
| IL-12p70 | −0.10 (−0.16–−0.04) | −0.13 (−0.19–−0.06) | −0.20 (−0.38–−0.02) | .568 |
| GM-CSF | −0.12 (−0.19–−0.06) | −0.19 (−0.26–−0.12) | −0.23 (−0.42–−0.04) | .315 |
| IFN-g | −0.09 (−0.15–−0.03) | 0.04 (−0.02–0.10) | −0.01 (−0.17–0.15) | .007 |
| TNF-a | −0.03 (−0.06–0.01) | 0.10 (0.06–0.14) | 0.05 (−0.06–0.16) | <.001 |
Longitudinal variation in cytokine levels per group was determined using linear multilevel modeling fit in the log scale for each group with slopes shown (95% CIs). Significant inequality in slopes across groups is indicated in bold (P < .05). Individual subject analysis is shown in Fig. S2.
Limited Associations Exist Between Markers for Microbial Translocation and Circulating Cytokine Responses.
Previous reports from the United States noted multiple correlations between markers for microbial translocation and the inflammatory cytokine response. To assess whether this was the case in our African study population as well, the cytokine levels and markers for microbial translocation were examined for significant associations (Table S1). As expected, GM-CSF, IFN-γ, IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, and TNF-α were found to be significantly correlated with one another to varying degrees (Table S1). In addition, weak positive correlations were observed between TNF-α and IL-6 with LBP and sCD14, and weak negative correlations were found between IL-8 and LBP and between IL-12p70 and sCD14 (Fig. S3 and Table S1). LBP and sCD14 levels were significantly correlated (r = 0.424, P < .001), as were sCD14 and EndoCAb levels (r = 0.112, P < .05), but both sCD14 and LBP levels were negatively correlated with LPS levels (Fig. S3 and Table S1). EndoCAb levels were not correlated with LPS levels, differing from previous U.S.-based findings (Table S1) (10, 11). These data further highlight the limited effect of microbial translocation on HIV-1 disease in this African population.
Discussion
In the longitudinal cohort study of HIV-1–infected African subjects, no differences in the levels of circulating LPS were observed among the 3 HIV-1 disease progression groups over time. This finding suggests that LPS levels had little effect on the rate of HIV-1 disease progression in this population. It should be noted that previous studies examining this phenomenon in the United States and Europe were cross-sectional and do not necessarily reflect changes within individuals over time; therefore, it is impossible to directly compare the present results with those reported previously (10, 11, 20). However, the present study did assess individual and group changes, and found no evidence of a systematic increase in LPS level over time in this population. It also has been previously reported that EndoCAb level is lower in patients in later disease stages (10, 11). EndoCAb is produced by B cells in a T cell–dependent manner, which could be detrimentally affected during chronic HIV-1 infection (21, 22). But no systemic changes in EndoCAb level were found in our African subjects or among the 3 disease progression groups over time. This finding could be due to the observation of significantly higher EndoCAb levels in the African subjects before seroconversion, most likely related to differences in sanitation between the U.S. and African environments, and the endemic parasitic and bacterial disease load in the African population. It also is possible that genetic differences between the 2 uninfected populations affected the differences observed; however, the U.S. comparison group was predominantly African-American, which should limit this effect (23). EndoCAb levels in the African subjects remained consistently high throughout infection regardless of progression status, which may explain in part why circulating LPS levels did not increase in the African subjects. This would further support the hypothesis that elevated LPS level does not directly affect HIV-1 disease progression in Africa.
LBP level increased in the standard and rapid progression groups at significantly higher rates compared with the LTNP group. This increase did not result in a consistent increase in sCD14 level over time, however, even though the 2 levels were closely correlated. This discrepancy suggests that an increase in LBP level may not affect activation of the monocyte population without an increase in LPS level, regardless of HIV-1 disease progression status. The sCD14 levels were significantly higher in the uninfected U.S. individuals compared with the African subjects at baseline. This could indicate heightened monocyte activation in the U.S. population; however, a more detailed cellular analysis is needed to determine whether this is the case. It should be noted that peripheral blood mononuclear cell samples were not available for the African subjects, limiting the ability of this study to fully characterize the state of the cellular immune response in these subjects.
The differences in increased baseline levels of IL-2, IFN-γ, IL-8, TNF-α, and GM-CSF in the African subjects indicates an underlying difference in the immune activation level of Africans, consistent with previous findings (2, 18, 24). The subsequent decreasing levels of GM-CSF, IL-1β, IL-2, IL-8, IL-10, and IL-12p70 found in all 3 African progression groups was surprising and differed from previous reports (2, 15, 17, 18, 25). One possible explanation for this apparent discrepancy is that many of the previous reports of increased cytokine production were based on increased cellular cytokine production ex vivo, whereas the present study examined total circulating cytokine levels. The lower circulating cytokine levels observed here suggests that the increased production by cytokine-producing cells observed ex vivo cannot compensate for the decreased number of total immune cells found in HIV-infected individuals (16). Therefore, the heightened cellular immune state that is believed to play an important role in HIV disease progression is most likely a directed response centered in areas where increased numbers of immune cells are found, such as lymph nodes, but may not be reflected in the bloodstream.
In conclusion, this longitudinal study found no evidence of consistent changes in markers of microbial translocation or immune activation associated with microbial translocation over time in HIV-1–infected African subjects. It also demonstrated no differences in these markers among African subjects with differing rates of HIV-1 disease progression. These findings seemingly disagree with results from previous cross-sectional studies performed in the United States (10, 11). One possible explanation for this difference may be that the present study was longitudinal, allowing for within-subject evaluation from preinfection through AIDS in the context of different rates of disease progression, whereas the U.S. cross-sectional studies evaluated groups of uninfected, acute/early infection, chronic infection, and AIDS subjects. Although the African subjects were evaluated within the first year of HIV-1 infection, the present study did not directly examine acute infection (10). Studies examining microbial translocation in the United States and Europe have consisted of predominantly male populations who most likely contracted the disease through homosexual sex or intravenous drug use, whereas the HIV-infected African population studied here is exclusively heterosexual (11, 26, 27). Therefore, mode of transmission may play a role in the differences observed between these populations. Another potential explanation for the different findings is that Africans have higher preinfection levels of endotoxin antibody as well as differences in certain cytokine levels due to environmental exposure to intestinal infections, which could alter their immunologic responses during subsequent HIV infection. It also is possible that the different cytokine levels and microbial translocation markers observed in HIV-1–infected individuals in the present study and previous studies might be a symptom, not a direct cause of HIV-1 disease progression, but further research is needed to explore this possibility. Although exact explanations for these results are unclear, it seems evident from these data that microbial translocation and its subsequent immune effects do not appear to have a causal relationship with HIV-1 disease progression in the African population.
Materials and Methods
Study Population.
African individuals were identified from known HIV-1 seroconverters who were part of a community cohort under annual surveillance in Rakai, Uganda from 1994 to 2000 (28). Seroconverters were identified as individuals who had a negative serologic test for HIV followed by a subsequent positive test a year later. Archived serum samples from the last HIV seronegative time point (year 0) and all subsequent HIV seropositive time points (years 1, 2, 3, etc.) were used in subsequent assays. All African and U.S. comparison group samples were stored continuously at or below −70 °C, which should stabilize the cytokines and proteins tested in this study. Due to temporary absences and outmigration, not all subjects had complete sequences of follow-up samples. Sample availability of all subjects per time point ranged from 94% to 35%, with the later time points having lower percentages of samples available. The seroconverters were classified into 3 groups according to known disease outcome parameters: LTNPs (CD4 lymphocyte count >600 cells/μL at 7+ years after seroconversion), SPs (death >5 years but <9 years after seroconversion), and RPs (death <4 years after seroconversion). All subjects were antiretroviral treatment (ART)-naïve throughout the study. ART became available in Rakai only after June 2004, and persons initiating ART after that time were not included in this study.
Serum samples also were obtained from 24 HIV-negative U.S. patients from the Johns Hopkins Emergency Department to serve as a comparison group for any differences in baseline levels between the African and U.S. subjects.
Institutional Review Board approvals were obtained from the Uganda Virus Research Institute's Scientific and Ethics Committee, the Uganda National Council for Science and Technology, and the Institutional Review Boards of collaborating U.S. institutions (Walter Reed Army Institute of Research, Columbia University, and Johns Hopkins University).
Serum LPS, EndoCAb, LBP, and sCD14 Levels.
LPS level was determined according to the modified version of the limulus amebocyte lysate assay described by Brenchley et al. (10) (Cambrex). EndoCAb (Hycult Biotechnology), LBP (Hycult Biotechnology), and sCD14 (R&D Systems) were quantified with ELISA assays according to the manufacturer's recommendations.
Proinflammatory Serum Cytokine Levels.
Levels of IFN-γ, TNF-α, GM-CSF, IL-1β, IL-2, IL-6, IL-8, IL-10, and IL-12p70 were quantified using a multiplex electrochemiluminescence detection system (Meso-Scale Discoveries), according to the manufacturer's instructions. Cytokine level detection ranges were determined from analysis of standard curves for each run and averaged ≈1–10,000 pg/mL.
Viral Characteristics.
Serum HIV-1 RNA concentrations (viral loads) were determined using an Amplicor v1.5 (Roche Diagnostics) with setpoints determined as described previously (14). Viral subtype was determined by either direct sequencing of multiple regions or multiregion hybridization assay and designated as subtype A, D, or recombinant (26, 28).
Statistical Analysis.
Baseline HIV-uninfected levels of LPS markers and cytokines and HIV-positive viral loads were compared using a Mann–Whitney Rank sum test. LPS markers and cytokine levels over time were assessed for each disease progression group by comparing slopes of the log-transformed measurements for each marker using linear multilevel modeling fit, which estimates the slope for the group allowing for varying numbers of data points per subject. Nonlinear relationships also were examined for the longitudinal analysis and were found to be not applicable. The slope of the regression for each group along with the 95% confidence interval (CI) are shown on each graph, with significant P values (< .05) noted for inequality across groups.
Supplementary Material
Acknowledgments.
This study was supported in part by funding from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, HIV Prevention Trials Network (HPTN), and Office of AIDS Research, National Institutes of Health (Grant U01AI068613); Department of the Army (U.S. Army Medical Research and Material Command Cooperative Agreement DAMD17–98-2–8007); the National Institute of Allergy and Infectious Diseases (Grants R01 A134826 and R01 A134265); the National Institute of Child Health and Human Development (Grant 5P30HD06826); the World Bank STI Project, Uganda; the Henry M. Jackson Foundation; the Fogarty Foundation (Grant 5D43TW00010); and the Bill and Melinda Gates Institute for Population and Reproductive Health at Johns Hopkins University. We thank all of the participants of the Rakai cohort and the staff of the Rakai health science program. We also thank A. Asher, J. Brenchley, and D. Douek for assistance with technical training; A. Fauci for assistance with manuscript preparation; M. Chen and I. Boaz for sample identification; the Johns Hopkins Hospital Emergency Department for providing U.S. samples, and the Becton Dickinson Immune Function Laboratory at Johns Hopkins University.
Footnotes
The authors declare no conflicts of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901983106/DCSupplemental.
References
- 1.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: How CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 2.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 3.Douek DC. Disrupting T-cell homeostasis: How HIV-1 infection causes disease. AIDS Rev. 2003;5:172–177. [PubMed] [Google Scholar]
- 4.Mattapallil JJ, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 5.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 7.Arthos J, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 8.Caradonna L, et al. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: Biological and clinical significance. J Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- 9.Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft-versus-host disease. J Endotoxin Res. 2002;8:441–448. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- 10.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 11.Ancuta P, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenchley JM, Price DA, Douek DC. HIV disease: Fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo G, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 14.Kiwanuka N, et al. Effect of Human Immunodeficiency Virus Type 1 (HIV-1) on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197:707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, et al. The effect of acute infectious illnesses on plasma human immunodeficiency virus (HIV) type 1 load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis. 1998;178:1642–1648. doi: 10.1086/314491. [DOI] [PubMed] [Google Scholar]
- 16.Chatt JA, et al. Peripheral blood cell–specific cytokines in persons with untreated HIV infection in Malawi, Africa. AIDS Res Hum Retroviruses. 2002;18:1367–1377. doi: 10.1089/088922202320935447. [DOI] [PubMed] [Google Scholar]
- 17.Graziosi C, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzardini G, et al. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS. 1996;10:1535–1542. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 20.Gori A, et al. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008;46:757–758. doi: 10.1128/JCM.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen IR, Norins LC. Natural human antibodies to gram-negative bacteria: Immunoglobulins G, A, and M. Science. 1966;152:1257–1259. doi: 10.1126/science.152.3726.1257. [DOI] [PubMed] [Google Scholar]
- 22.Titanji K, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 23.Laeyendecker O, et al. The effect of viral suppression on cross-sectional incidence testing in the Johns Hopkins Hospital emergency department. J Acquired Immune Defic Syndr. 2008;48:211–215. doi: 10.1097/QAI.0b013e3181743980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn TC, et al. Serologic and immunologic studies in patients with AIDS in North America and Africa: The potential role of infectious agents as cofactors in human immunodeficiency virus infection. JAMA. 1987;257:2617–2621. [PubMed] [Google Scholar]
- 25.Lee BN, et al. Type 1 and type 2 cytokine profiles in children exposed to or infected with vertically transmitted human immunodeficiency virus. Clin Diagn Lab Immunol. 1996;3:493–499. doi: 10.1128/cdli.3.5.493-499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wawer MJ, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 27.Wawer MJ, et al. Incidence of HIV-1 infection in a rural region of Uganda. BMJ. 1994;308:171–173. doi: 10.1136/bmj.308.6922.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutalo T, et al. Survival of HIV-infected treatment-naive individuals with documented dates of seroconversion in Rakai, Uganda. AIDS. 2007;21(Suppl 6):S15–S19. doi: 10.1097/01.aids.0000299406.44775.de. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.