Abstract
P2X receptors on dorsal root ganglion (DRG) neurons have been strongly implicated in pathological nociception after peripheral nerve injuries or inflammation. However, nothing is known of a role for purinergic receptors in neuropathic pain produced by a chronic compression of DRG (CCD) – an injury that may accompany an intraforaminal stenosis, a laterally herniated disc or other disorders of the spine leading to radicular pain. In a rat model of DRG compression, hyperexcitable neurons retain functioning axonal connections with their peripheral targets. It is unknown whether such hyperexcitability might enhance chemically mediated nociceptive stimulation of the skin. In this study, CCD facilitated the nocifensive behavior and mechanical hyperalgesia-induced by the P2X3 agonist, α,β-methylene ATP (α,β-meATP). An injection of α,β-meATP into the hind paw of CCD rats resulted in a significantly greater decrease in the mean threshold to von Frey stimuli and a greater duration of paw lifts than in sham-operated control rats. CCD also increased the levels of P2X3 receptor protein and the number of P2X3 immunoreactive, small diameter DRG neurons in the compressed ganglion. P2X3 receptors were co-labeled with the isolectin IB4, consistent with a role in nociception. In addition, a α,β-meATP induced significantly larger fast-inactivating currents in CCD- than in sham-operated acutely dissociated DRG neurons. These currents were accompanied by the generation of action potentials – but only in the CCD neurons. U0126, a specific inhibitor of the MEK1/2, greatly down-regulated the enhanced current. Taken together, these observations suggest that enhanced purinergic responses after CCD are mediated by P2X3 receptors.
Keywords: P2X receptor, Neuropathic pain, Nerve injury, Dorsal root ganglion, ERK
1. Introduction
A chronic compression of the dorsal root ganglion (CCD) in rats is an animal model of radicular pain resulting from a laterally herniated disc, an intraforaminal stenosis and other inflammatory and degenerative diseases of the spine. CCD in the rat produced spontaneous pain behavior, cutaneous hyperalgesia and tactile allodynia [26,47]. There is evidence for a role of ATP release in animal models of pain. ATP can be released from injured tissue and can excite nociceptive dorsal root ganglion (DRG) neurons [14]. In addition, ATP can be released from the axons or somata of these neurons [49,61]. Therefore, an untested possibility is that ATP might be released in the DRG or in the periphery as a consequence of the compression-induced tissue injury and may activate nociceptive neurons. Substantial evidence suggests that P2X3 receptor activation contributes to ATP-induced pain behavior. Knocking out P2X3 reduces the pain-related behavior of mice in response to ATP injection [13,48]. P2X3 receptor antisense treatment and P2X3 siRNA administration induce a down-regulation of P2X3 subunits, and prevent allodynia and hyperalgesia induced by the injection of the ATP analogue, α,β-methylene [2,18,25]. Moreover, A-37149, the specific P2X3 receptor antagonist, inhibits nociceptive responses in rats with chronic inflammation or nerve injury [27]. However, it is not known whether purinergic receptors are involved in neuropathic pain produced by a compression of the DRG.
Among the seven subunits of P2X receptor (P2X1–7) expressed in the normal DRG [31,58], the P2X3 receptor subtype is selectively expressed in small- and medium-sized DRG neurons [11,22,28,34,52]. Although P2X3 receptor subunit is strongly implicated in neuropathic pain [7–10,29,38,40], the expression levels of the P2X3 receptor subunit have varied considerably in different neuropathic pain models [4,12,41]. Hyperexcitable neurons in the chronically compressed DRG (CCD model) have intact axonal connections with their peripheral receptors. This study tested, for the first time, whether electrophysiological measurements of enhanced responses of DRG neurons to chemical stimuli, recorded in vitro, are associated with enhanced behavioral responses to the same chemicals delivered to the skin, in vivo.
The protein kinase, ERK (extracellular signal-related protein kinase), is activated (phosphorylated) in the DRG after nerve injuries [16,62]. However, the functional link between the phosphorylated ERK (pERK) and P2X3 receptor activation is poorly understood. Since the function of P2X3 receptors can be regulated by protein kinases [53,60], we investigated the role of pERK in the modulation of P2X3 receptor function in DRG neurons after CCD.
2. Materials and methods
2.1. Animal surgery
The CCD model was performed in female Sprague–Dawley rats (130–180 g) using a procedure previously described [26,47]. Briefly, under anesthesia with pentobarbital sodium (40 mg/kg, i.p.), the ipsilateral, right transverse process and intervertebral foramina of L4 and L5 were exposed and a stainless steel L-shaped rod (0.63 mm in diameter and 4 mm in length) was inserted into each foramen, one at L4 and the other at the L5 ganglion. An antibiotic, Baytril (enrofloxacin, 2.5 mg/kg IM, Bayer HealthCare LLC, Shawnee Mission, Kansas, USA), was administered immediately after surgery. The surgical procedure of sham surgery was identical to that described above but without the rod insertion. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Second Military Medical University.
2.2. Behavioral response measurement
Five days after surgery (CCD or sham-operated), two groups of 6 CCD rats and two groups of 6 sham-operated rats were used for the flinching behavior studies. Rats were placed under individual plexiglass domes and acclimatized for 20 min. The α,β-meATP (50 nmol) (Sigma–Aldrich, Shanghai, PR China) or phosphate buffer saline (PBS) was injected subcutaneously into the plantar surface of the right hind paw of each rat. The P2X receptor antagonist, trinitrophenol ATP (TNP-ATP) [51] (Invitrogen Ltd., Paisley, UK), was used to test the specificity of α,β-meATP. TNP-ATP (200 nmol) was added to the α,β-meATP solution and applied to the rat paw. All injections were given in a volume of 50 μl using a 200 μl syringe with a 30 gauge needle. After injection, rats were then immediately returned to their boxes for observation. The behavior of each rat was videotaped and subsequently analyzed. The flinching behavior was assessed by determining paw lift time per minute (PLTPM). The PLTPM was measured at different time points after injection.
CCD and sham rats were compared for their behavioral responses to mechanical stimuli after an injection of 50 μl of either α,β-meATP or vehicle. The CCD and sham groups were each subdivided into four groups of 6 rats each according to whether they received 20 or 50 nmol of α,β-meATP in PBS or just PBS alone. The same set of von Frey stimuli (5, 10, 20, 40, 60, 80, 100, and 120 mN) was given in an ascending order to designated loci on the skin. Each filament having the same tip diameter of 0.1 mm was applied for 1 s, alternately to each foot at intervals of 10–15 s, to each of the 6 sites distributed across the plantar surface of the rat hindpaw [47]. The threshold force, defined as the force corresponding to a 50% withdrawal, was determined by a Hill equation (Origin Version 6.0, Microcal Software).
2.3. Western blot analysis
For western blot analysis, rats were deeply anesthetized with diethyl ether and killed by decapitation. The L4/5 DRG was rapidly removed and lysed with 20 mM Tris–HCl buffer, pH 8.0, containing 1% NP-40, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% β-mercaptoethanol, 0.5 mM dithiothreitol, and a mixture of proteinase and phosphatase inhibitors (sigma). Protein concentration was determined by the BCA protein assay method using bovine serum albumin as standard. Sixty micrograms of protein samples from DRG were loaded per lane, separated by SDS–PAGE (8% polyacrylamide gels) and then were electrotransferred onto nitrocellulose membranes. The membranes were blocked with 10% non-fat dry milk in Tris-buffered saline with Tween 20 (50 mM Tris, 150 mM NaCl, and 0.1% Tween 20 vol/vol, pH 7.4) for 1 h and then incubated overnight at 4 °C with P2X3 antibody (Roche Palo Alto, CA, USA), and GAPDH antibody (Sigma) diluted 1:1000 in 2% BSA in TTBS. Horseradish peroxidase-conjugated anti-rabbit serum (Santa Cruz Biotechnology) was used as a secondary antibody (1:2000 dilution in 2% BSA in TBST, 1 h incubation) and the antigen–antibody complexes were visualized using an enhanced chemiluminescence detection reagent (Amersham). Bands were scanned using a densitometer (GS-700; Bio-Rad Laboratories), and quantification was performed using Multi-Analyst 1.0.2 software (Bio-Rad). The results were expressed as the ratio of P2X3 immunoreactivity to GAPDH immunoreactivity.
2.4. Immunohistochemistry protocol
The rats were anesthetized with pentobarbital sodium (40 mg/kg, i.p.) and perfused through the aorta, first with 0.9% NaCl solution followed by 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4). The DRGs were dissected out immediately and immersed in 4% paraformaldehyde in PBS (0.1 mol/L, pH 7. 2) overnight. The tissues were then transferred to 25% sucrose in PBS and kept in the solution until they sank to the bottom. Thereafter, the blocks were rapidly frozen and 10 μm sections were cut on a Leica cryostat (CM1900) and thawed onto gelatin-coated slides.
For double-immunostaining of P2X3 receptors with the isolectin IB4, the sections of the ganglia, after being pre-incubated in an antibody dilution solution for 30 min, were incubated with the P2X3 receptor antibody (1:200 dilution, Roche Palo Alto, CA, USA) and Biotin-conjugated IB4 (1:200 dilution, Sigma) overnight. The sections were subsequently incubated with Cy3-conjugated donkey–anti-rabbit IgG (1:300 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h to visualize P2X3 receptors, FITC-conjugated streptavidin (1:200 dilution) for 1 h to visualize IB4, respectively. All staining procedures were carried out at room temperature and all the incubations were separated by three washes in PBS, 5 min each. The immunoreactivity was visualized by fluorescence microscopy.
Control experiments were carried out with P2X3 receptor antiserum pre-absorbed with the P2X3 receptor peptide at a concentration of 25 μg/ml. The amino acid sequence of P2X3 383–397 (VEKQSTDSGAYSIGH) was synthesized by Roche Palo Alto. No staining was observed in those specimens where the antibody solutions had been pre-absorbed with the P2X3 receptor peptide (data not shown).
Counts were obtained of the total number of neurons and the numbers and percentages of these that were positive for both P2X3 receptors and IB4 or for either label alone. An observed neuron was considered to be ‘positive” when the measured intensity of immuno-staining was more than five times greater than the background (Computer image analysis system, Furi Company, Shanghai). The diameter of the positively labeled neuronal soma was measured using a calibrated reticule and calculated as an average of the shortest and longest axes [19]. Ten sections from each DRG were analyzed with a total of 5 CCD- and 5 sham-operated DRGs each obtained from a different rat. Neurons were identified by their large nuclei stained by Hoechst 33258 (5 μg/ml). The positive neurons with visible nuclei were counted. The total number of P2X3 receptor and IB4 positive neurons and the number of the small-sized neurons (diameter <30 μm) with P2X3 receptor immunoreactivity and/or IB4 binding were counted in one section at 20-fold magnification. The number of nuclei was counted to obtain the total number of neurons in the ten sections. The mean from each experimental group was calculated and the values obtained from each group were averaged, and the standard error of the mean was calculated. Values for both the total number and the percentage of neurons positive for a marker against the total number of neurons were obtained.
The rabbit polyclonal primary antibody for pERK (1:400; Cell Signaling Technology, Beverly, MA) and guinea pig polyclonal primary antibody for P2X3 receptors (1:600, Chemicon international) were used for double-immunostaining of P2X3 receptors with the pERK with the same preparation and protocol described above.
2.5. Neuronal dissociation and culture
Five to seven days after CCD surgery, the rats were deeply anesthetized with pentobarbital (40 mg/kg, i.p.), and the L4 and L5 lumbar DRGs were exposed. DRG neurons were dissociated and placed in short-term culture as previously described [50]. Briefly, isolated DRGs were dissected free of adherent connective tissue and placed in complete saline solution (CSS) for cleaning and mincing. The CSS contained (mM) 137 NaCl, 5.3 KCl, 1 MgCl2, 3 CaCl2, 25 Sorbitol, and 10 Hepes, adjusted to pH 7.2 with NaOH. The DRGs were then digested for 25 min with collagenase A (1 mg/ml; Boehringer Mannheim, Germany) and, for another 25 min with collagenase D (1 mg/ml; Boehringer) and papain (30 U/ml, Worthington Biochemical, Lakewood, NJ) in CSS containing 0.5 mM EDTA and 0.2 mg/ml cysteine at 37 °C. The cells were dissociated by trituration in culture medium containing 1 mg/ml bovine serum albumin and 1 mg/ml trypsin inhibitor (Boehringer) and plated on glass coverslips coated with 0.1 mg/ml polyornithine and 1 mg/ml laminin (Boehringer). The culture medium contained equal amounts of Dulbecco’s modified Eagle medium and F12 (Gibco, Grand Island, NY) with 1% Penicillin (100 U/ml)/Streptomycin (0.1 mg/ml) (Life Technologies, Rockville, MD) and 10% fetal calf serum (HyClone Laboratories, Logan, UT). The cells were incubated at 37 °C (5% CO2 balanced air) for 1 h after which culture medium was added that did not contain the trypsin inhibitor.
2.6. Whole-cell patch-clamp recording
To investigate whether P2X3 receptor function was altered after CCD-induced injuries, we measured the electrophysiological responses to α,β-meATP, an agonist for P2X1, P2X3 and P2X2/3 receptors [39], in the acutely dissociated DRG neurons, from both the sham-operated rats and CCD rats.
Whole-cell electrophysiological recordings were obtained at room temperature within 8 h of cell culture by means of an Axonclamp 200B amplifier and pClamp 9 software (Axon Instruments). Small diameter (<30 μm) neurons were selected for electrophysiological recording. Every neuron was recorded first under current clamp to identify the shape of the action potential evoked by the injection of depolarizing currents. Neurons that had an inflection on the falling phase of the action potential and a resting membrane potential equal to or more negative than −50 mV were stimulated with α,β-meATP. The data only from neurons that depolarized more than 2 mV in response to capsaicin applied at the end of the experiment were included in the analysis. Electrodes were fabricated from borosilicate glass and pulled on a PC-10 puller (Narishige, Japan). The pipette solution contained (in mM) 120 K-gluconate, 10 KCl, 5 NaCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES, 2 Mg-ATP, and 1 Li-GTP. The pH (7.2) of the solution was adjusted with Tris-base and the osmolarity (310 mOsm) adjusted with sucrose. The impedance of a typical patch pipette was 2–4 MΩ. The electrophysiological recordings were filtered at 3 kHz and digitized by A/D converter (Digidata 1322A, Axon Instruments) at 5 kHz. The recording chamber was perfused continuously at a rate of 2–3 ml/min with a bath solution containing (in mM) 145 NaCl, 3 KCl, 1 CaCl2, 2 MgCl2, 10 Hepes, and 10 glucose and adjusted to a pH of 7.4 and osmolarity of 300–310 mOsm. All command voltages were corrected for junction potentials between the internal and the external solutions.
Control solution was continuously delivered by pressure to the soma of each recorded neuron via a 100 μm diameter tip perfusion pipette controlled by a DAD-12 superfusion system and computer interface (ALA Scientific Instruments Inc., USA). A solution of α,β-meATP (10 μM) was exchanged with the bath solution and maintained for 5 s to record the current or voltage responses in neurons. U0126 (Sigma–Aldrich, Shanghai, PR China) was used to test the effects of pERK on α,β-meATP-induced currents.
2.7. Statistical analyses
For behavioral responses, either a repeated measures analyses of variance was performed followed by post hoc pairwise comparisons (Tukey’s method), or a Wilcoxon signed-rank method, to test differences in withdrawal thresholds obtained prior to surgery with those recorded on each postoperative day of testing. Student’s t tests were used to assess the differences in the relative intensity of immunostaining of P2X3 between the CCD and SHAM groups, differences in the number of P2X3- or pERK-positive neurons between the CCD and SHAM groups, and differences in α,β-meATP-induced depolarization or inward current between the CCD and SHAM groups. A paired t-test was used to assess the difference in inward currents induced by α,β-meATP before vs. during the application of an antagonist of α,β-meATP in CCD neurons. A chi-square test was used to compare the proportions of responsive neurons to α,β-meATP. For all tests, a probability of 0.05 was chosen as the criterion for significance.
3. Results
3.1. CCD increased the nociceptive behavioral responses to P2X3 agonist
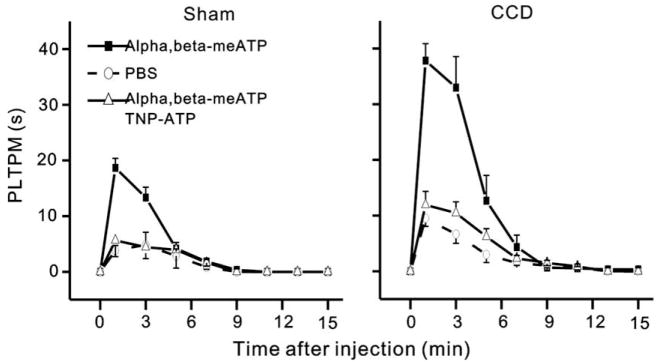
The response to α,β-meATP injection was observed in both groups of rats. The α,β-meATP-induced flinching behavior began within 10 or 20 s of injection and dissipated within 10 min. An injection of α,β-meATP at a dose of 50 nmol in 50 μl into the hind paw led to a time-dependent increase in the PLTPM in both sham-operated, control rats and CCD rats but the PLTPM was significantly longer in CCD rats than in sham-operated rats (p < 0.001, two-way repeated ANOVA, Fig. 1). The PLTPM at peak was approximately twofold longer than that of sham-operated rats (37.83 ± 3.04 vs. 18.67 ± 1.71 s p < 0.01, Tukey’s test). The α,β-meATP –induced flinching was markedly attenuated when the P2X receptor antagonist TNP–ATP (200 nmol) was co-injected (p < 0.01, two-way repeated ANOVA). The PLTPM at peak was 11.91 ± 2.45 s for CCD rats treated with TNP–ATP (p < 0.01, CCD versus CCD with TNP–ATP, Tukey’s test) and 5.63 ± 2.92 s for sham-operated rats treated with TNP–ATP (p < 0.01, SHAM versus SHAM with TNP–ATP, Tukey’s test), respectively. In contrast, α,β-meATP injection at a dose of 20 nmol in 50 μl into the hind paw did not induce flinching behavior in both sham-operated, control rats and CCD rats.
Fig. 1.
CCD increased the flinch responses evoked by intracutaneous injection of α,β-meATP. The flinching behavior was assessed by determining paw lift time per minute (PLTPM). The maximal flinch responses occurred at 1 min, and responses were completely diminished 10 min after the injection of α,β-meATP (50 nmol). The PLTPM at peak was ~twofold longer in CCD rats (right) than in sham-operated rats (left). The P2X receptor antagonist TNP–ATP (200 nmol) attenuated the flinch responses when co-injected with α,β-meATP. PBS did not produce any significant flinch responses. All rats received a single injection of 50 μl. n = 6 animals for all groups.
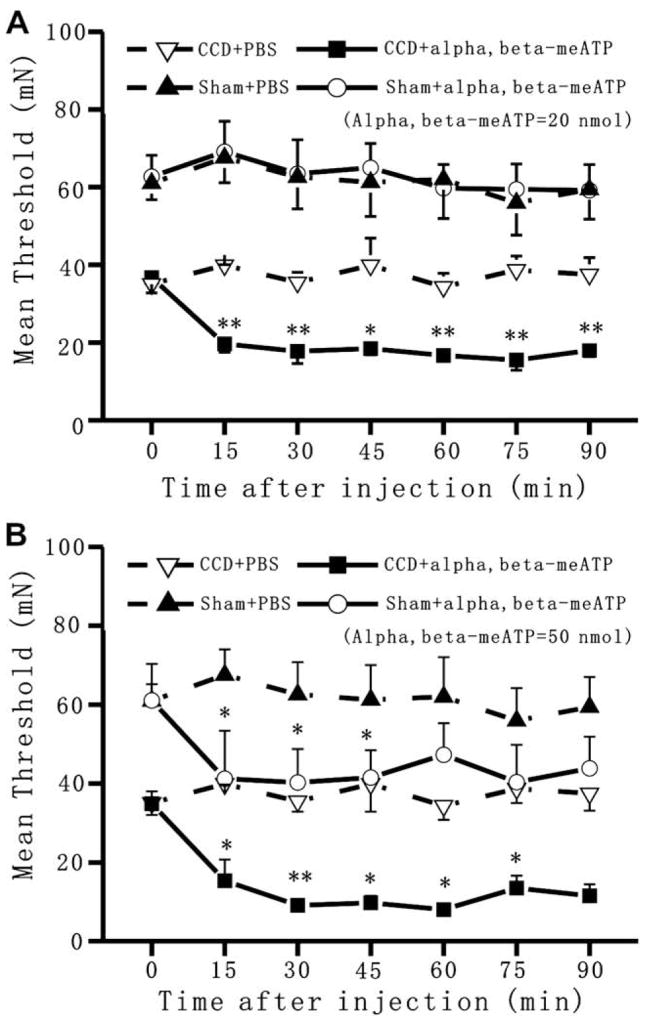
von Frey responses were then tested to examine the time course of changes in mechanical threshold after an injection of α,β-meATP in the paw (Fig. 2). Threshold values obtained in sham-operated group and CCD group were analyzed with a two-way ANOVA (experimental condition × time) with repeated measures over time followed by Tukey’s test. The mean threshold on the foot ipsilateral to the CCD surgery decreased from 62.7 ± 5.5 mN before surgery to 36.8 ± 4.0 mN on the fifth day after surgery (p < 0.05, Wilcoxon signed rank test). There were no significant effects of CCD on the contralateral mechanical threshold. Intraplantar injection of α,β-meATP at a dose of 20 nmol in 50 μl evoked a further drop of threshold force in the injected paw of CCD rats but not in the control rats (Fig. 2A). The injection of PBS had no such effects. The α,β-meATP-induced reduction of threshold forces in the CCD rats was significant (p < 0.001, vehicle versus α,β-meATP). The significant decrease in threshold forces was maintained till the end of experiments. The α,β-meATP-induced reduction of threshold forces was not observed in rats subjected to a sham operation (p = 0.99, vehicle versus α,β-meATP). In contrast, when rats were injected with 50 nmol of α,β-meATP, a significant decrease in threshold force occurred not only in the CCD rats (p < 0.01, vehicle versus α,β-meATP) but also in the sham-operated rats (p < 0.05, vehicle versus α,β-meATP) (Fig. 2B).
Fig. 2.
CCD enhanced the mechanical hyperalgesia induced by α,β-meATP injection. Mean threshold force for withdrawal to von Frey stimulation was evaluated in differently treated rats. (A) when injected at a dose of 20 nmol, α,β-meATP caused a significant decrease in mean withdrawal threshold in CCD rats but not in sham-operated rats. This mechanical hyperalgesia induced by α,β-meATP in CCD rats was maintained till the end of experiments. (B) when injected at a dose of 50 nmol, α,β-meATP caused a significant decrease in mean withdrawal threshold not only in CCD rats but also in sham-operated rats (*p < 0.05, **p < 0.01, α,β-meATP versus PBS).
3.2. CCD increased the expression of P2X3 receptors
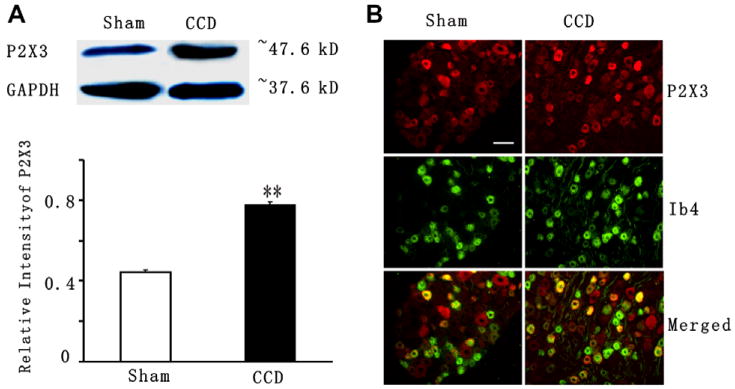
Western blot analysis was used to detect P2X3-receptor protein levels in the intact DRGs from CCD rats and sham-operated rats. Using the same antibody as that for the immunohistochemistry, a single band of approximately 50 kDa was recognized in the DRGs (Fig. 3A, top panel). As shown in Fig. 3A (bottom panel), the P2X3 protein levels increased significantly by ~1.7-fold in the ipsilateral DRG at 5–7 days after CCD (16 DRGs in 4 CCD groups with 2 rats in each group) compared with the sham control (12 DRGs in 3 sham group with 2 rats in each group) (p < 0.01, student’s t test).
Fig. 3.
Up-regulation of P2X3 receptor in the CCD-injured DRG. (A) Western blots of DRG protein extracts probed for P2X3. GADPH serves as the loading control. P2X3 levels (normalized with density of GADPH bands) increase in the DRGs ipsilateral to the CCD surgery (16 DRGs in 4 CCD groups with 2 rats in each group) compared with DRGs ipsilateral to the sham operation (12 DRGs in 3 sham group with 2 rats in each group) (**p < 0.01, student’s test). (B) P2X3 receptor (red) and IB4 (green) immunoreactivity in L4/L5 DRG in the sham-operated rats (Left) and after CCD (right). Nerve fibers as well as cell bodies were stained for IB4 after CCD (right). Note that an increased number of neurons showed colocalization of P2X3 receptors and IB4 in the CCD model (yellow, right) as compared to in the sham (yellow, left). Scale bar = 100 μm.
Immunohistochemical labeling was next performed on DRG sections to assess which sub-population of DRG cells had increased expression of P2X3 receptors as indicated by the western blot analysis. In both CCD- and sham-operated DRGs, immunostaining with the antibody to the P2X3 receptor was seen predominantly in small-sized neurons (diameter <30 μm) and a much smaller proportion of medium- sized (diameter between 30 and 50 μm) and large-sized (diameter >50 μm) neurons. The immunostaining was generally more intense for the small-sized neurons (Fig. 3B, top panels, red).
Positive labeling for IB4 was characterized by a granular staining of the cytoplasm with brightly stained plasma membrane. Binding of IB4 was restricted to small- and medium-sized neurons (Fig. 3B, middle panels, green). Most of the small-sized P2X3-positive neurons bound IB4 (Fig. 3B, bottom panels, yellow). In the DRG of CCD rats, the patterns of immunostaining for the P2X3 receptor and for IB4 were similar to those from DRGs of sham-operated rats. But, the number of neurons with P2X3 receptor-ir or IB4–ir was much more in the CCD DRG than in sham-operated DRG. When analyzed as a percentage of the total number of neurons counted, those neurons positive for P2X3 alone (Table 1) or double labeled for both markers (Table 2) were significantly greater for CCD- than for sham-operated ganglia.
Table 1.
P2X3 receptor-positive and IB4-positive DRG neurons in intact ganglia
| P2X3-ir neuron |
IB4 binding neuron |
|||
|---|---|---|---|---|
| Numbera | % | Numbera | % | |
| Sham | ||||
| Total count | 3517 ± 128 | 2973 ± 139 | ||
| Total labeled | 1203 ± 110 | 34.2 ± 0.83 | 1139 ± 146 | 38.3 ± 0.69 |
| Small-sized | 534 ± 48 | 15.18 ± 1.36 | 645 ± 62 | 21.7 ± 2.08 |
| CCD | ||||
| Total | 3809 ± 192 | 3488 ± 166 | ||
| Total labeled | 1512 ± 210 | 39.7 ± 0.75* | 1532 ± 158 | 43.9 ± 0.81* |
| Small-sized | 987 ± 67 | 25.9 ± 1.76* | 1008 ± 92 | 28.9 ± 2.64* |
ir = immunoreactive. Student’s t test was used.
p < 0.05 (comparison between CCD and sham).
Average number of neurons per DRG.
Table 2.
P2X3 receptor-positive DRG neurons that also stain for IB4 and IB4-positive DRG neurons that also stain for P2X3 receptors in intact ganglia
| Total count Numbera | P2X3 with IB4 |
Total count Numbera | IB4 with P2X3 |
|||
|---|---|---|---|---|---|---|
| Numbera | (%) | Numbera | (%) | |||
| Sham | 3517 ± 128 | 890 ± 28 | 25.3 ± 0.79 | 2973 ± 139 | 648 ± 23 | 21.80 ± 0.78 |
| CCD | 3809 ± 192 | 1279 ± 35 | 33.6 ± 0.91* | 3488 ± 166 | 971 ± 29 | 27.82 ± 0.83* |
P2X3 with IB4: P2X3 neurons with IB4-ir, IB4 with P2X3: IB4 neurons with P2XR3 receptor-ir; ir = immunoreactive. Student’s t test was used.
p < 0.05 (percentage of small-sized neuron with double labeling staining greater in CCD DRG than in sham DRG).
Average number of neurons per DRG.
3.3. CCD increased neuronal responses to P2X3 agonist
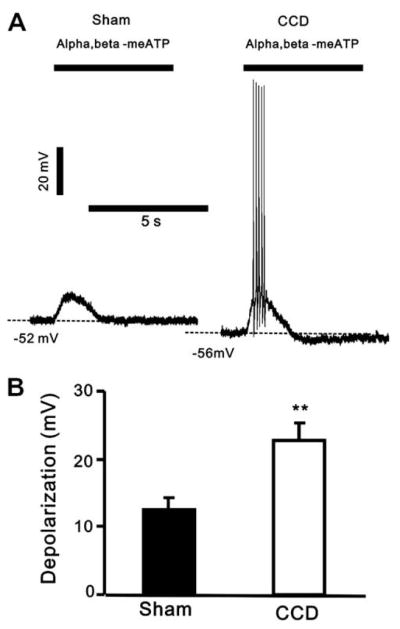
Under current-clamp recording, an application of α,β-meATP (10 μM, 5 s) evoked a rapid membrane depolarization of both CCD- and sham-operated neurons (Fig. 4A). The proportions of responsive sham-operated and CCD neurons were 15/18 and 18/25, respectively (p > 0.05, chi-square test). But the mean magnitude of depolarization to α,β-meATP was significantly greater for the CCD- than for sham-operated neurons (Fig. 4B).,β-meATP evoked discharges in a greater proportion of CCD neurons than in sham-operated neurons (12/18 versus 2/15, p < 0.01, chi square test).
Fig. 4.
α,β-meATP induced a larger depolarization in CCD neurons. (A) Representative tracings of a depolarization evoked by α,β-meATP in sham-operated DRG and CCD DRG neurons. Note that α,β-meATP triggered spike discharges in the CCD neuron. (B) The mean depolarization amplitude induced by α,β-meATP in CCD neurons was significantly increased (**p < 0.01, unpaired t-test). The whole-cell current-clamp recordings were obtained from 17 sham-operated DRG neurons and 17 CCD neurons.
Under voltage-clamp recording, at a holding potential of −60 mV, α,β-meATP (10 μM) evoked three types of inward whole-cell membrane currents in neurons from sham-operated and CCD ganglia. As described in previous studies of DRG neurons [6,36], these consisted of a fast type, with a rapidy decaying component, a slow type, with a slow decaying component, and a mixed type, with a rapidly decaying component followed by a slow decaying component. The proportions of fast, mixed and slow types were 43/57, 5/57 and 9/57, respectively, for CCD neurons and 22/29, 7/29 and 0/29, respectively, for sham-operated DRG neurons. There was no significant difference in the proportion of fast inward currents of CCD (43 of 57; 75.4%) and sham (22 of 27; 75.9%). The reason might be a result of dissociation which could cause high percentages of DRG neurons responding to ATP in vitro in normal DRGs [3,6,20,32]. Fast types of currents are thought to be mediated via P2X3 receptors [6] and the slow or mixed type of current to result from currents through the hetero-oligomeric P2X2/3 receptor [40]. Thus, the high proportion of CCD- and sham-operated neurons expressing the fast current and the low proportion exhibiting the slow or mixed current suggest that the P2X receptors in this study were primarily of the P2X3 subtype.
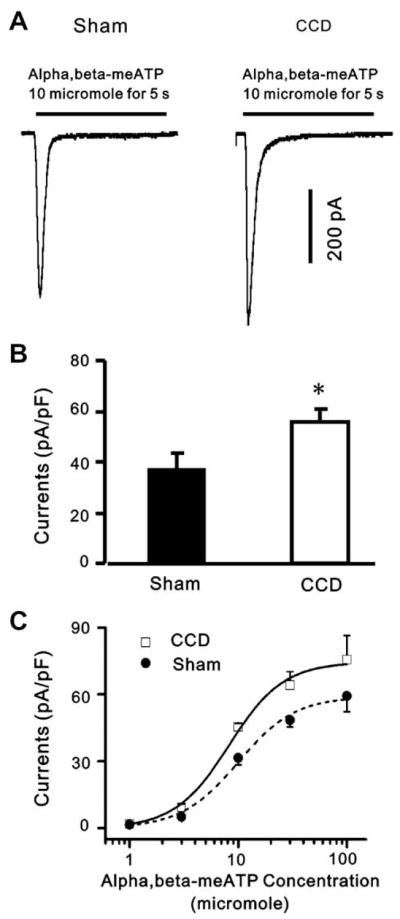
The fast type of current desensitized within less than 1 s during a maintained application for 5 s (Fig. 5A). The mean peak amplitude of fast-desensitizing inward current was significantly greater for the CCD- than for the sham-operated group (Fig. 5B).
Fig. 5.
α,β-meATP-evoked currents are potentiated in DRG neurons after CCD. (A) whole-cell voltage-clamp recordings were made to record α,β-meATP-evoked currents from DRG neurons from sham-operated and CCD groups. (B) α,β-meATP (10 μM) evoked larger inward currents in CCD neurons than in sham-operated neurons at a holding potential of −60 mV (*p < 0.05, n = 17, unpaired t-test). (C) Dose–response curves for α,β-meATP-evoked fast responses. Dose–response curves were constructed as a function of α,β-meATP concentration by averaging α,β-meATP-evoked peak amplitudes from DRG neurons from control and CCD rats, respectively. The data points were obtained from 8 to 20 neurons. The α,β-meATP EC50 values were 9.95 μM in control neurons and 8.23 μM in CCD neurons. The changes in α,β-meATP affinities for P2X receptors in CCD neurons were not significant.
To determine whether the increased neuronal responses to α,β-meATP in CCD neurons were due to an increase of α,β-meATP affinity for P2X receptors, the current density of the α,β-meATP-induced peak inward currents was plotted as a function of concentration (Fig. 5C). The Hill equation was used to fit a curve to the means to obtain a concentration–response function. In the dose-response curves constructed by averaging α,β-meATP -evoked peak amplitudes from DRG neurons from control and CCD rats, respectively, the α,β-meATP EC50 values were 9.95 μM in control neurons and 8.23 μM in CCD neurons and not significantly different (p > 0.05) Thus, the changes in α,β-meATP affinities for P2X receptors in CCD neurons were not significant.
3.4. CCD increased the role of ERK signaling in neuronal responses to P2X3 agonist
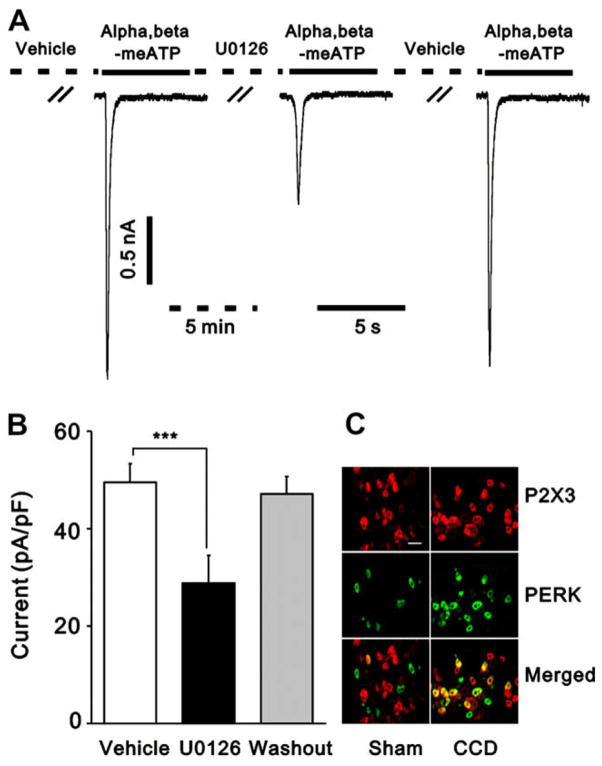
We have found that ERK is activated in DRG after CCD [62]. We presently examined the possibility that pERK might functionally interact with P2X3 receptors, contributing to the up-regulation of P2X3 receptor function in the CCD neurons. We first confirmed that P2X3-IR neurons expressed pERK-IR (Fig. 6C). Double staining of the same DRG sections revealed only a few pERK-IR neurons (2.5 ± 0.6% of total P2X3-IR neurons) in control rats. However, the percentage of pERK-IR neurons in total P2X3-IR neurons significantly increased to 54.6 ± 3.9% in CCD rats (p < 0.01, student’s t test). We then evaluated the role of ERK signaling in the up-regulation of P2X3 receptor function in CCD neurons. U0126, a specific inhibitor of mitogen-activated/ERK kinase, was used to assess the changes in α,β-meATP-induced currents. After a local, pu. application of U0126 (10 μM) to the soma of the recorded neuron for 5 min, α,β-meATP (10 μM)-induced currents reduced by 41.76% (49.6 ± 3.9 versus 28.9 ± 5.6 pA/pF, p < 0.001, paired t-test, n = 11). The inhibitory effect of U0126 on α,β-meATP-induced currents was reversible after 4 min of washout of the antagonist (Fig. 6A and B). We also detected a slight decrease of U0126 on α,β-meATP-induced currents in control neurons (29.3 ± 4.7 versus 24.2 ± 3.9 pA/pF, p < 0.01, paired t-test, n = 11), but the magnitude of inhibition was much less than that in CCD neurons (17.53% versus 41.76%, p < 0.01, student’s test).
Fig. 6.
pERK mediated the enhancement of α,β-meATP-induced currents in CCD neurons. (A) representative current traces showing an extracellular application of a specific inhibitor for the mitogen-activated/ERK kinase pathway, U0126 (10 μM), for 5 min greatly reduced α,β-meATP-induced current in a CCD neuron. Currents were recorded at a holding potential of −60 mV. The effect of U0126 was reversible. (B) The mean amplitude of α,β-meATP-induced current was significantly lower after U0126 treatment than before U0126 application (***p < 0.001, paired t-test). (C) ERK was activated in P2X3-positive DRG neurons. More P2X3 -IR (red) or pERK-IR (green) neurons were seen in CCD-injured DRG (right) than in sham-DRG (left). The merged images (yellow) of P2X3 -IR and pERK-IR from the same section show colocalization of pERK-IR and P2X3-IR in DRG neurons in a CCD rat (right) and a sham-operated rat (left). A few double-labeled neurons (yellow) were seen in DRG neurons of the CCD rat but rarely found in the sham-operated rat. Scale bar = 100 μm.
4. Discussion
In this study, we demonstrated that CCD injuries facilitated the α,β-meATP-induced mechanical hyperalgesia and nocifensive behavior. A low concentration of α,β-meATP failed to cause any change in mechanical threshold in sham-operated rats but produced a significant decrease in the mean threshold to von Frey stimulation in CCD rats. Furthermore, a higher dose of α,β-meATP induced a lower mechanical threshold and a greater duration of flinching in CCD rats than in sham-operated rats. A similar facilitation of pain behavior was observed in carrageenan-inflamed skin in rodents [24] and rats with a spared nerve injury [12]. The augmented pain behavior of α,β-meATP is attributable to the hyperexcitability of DRG neurons [23]. CCD might damage the satellite glial cells that envelope individual DRG neurons. A possible release of ATP from the injured glia of nociceptive neurons or from the hematogenous or resident macrophages might result in neuronal activation as α,β-meATP evoked depolarization and discharges in CCD neurons in vitro, thereby contributing to radicular pain, parenthesis, hyperalgesia and allodynia. The infiltration of the compressed DRG with macrophages seen in previous study [55] and present observation (data not shown) indicates an inflammatory process. Inflammatory mediators have been shown to sensitize DRG neurons [35]. Therefore, inflammatory mediators released in the CCD-inflamed ganglion might enhance neuronal excitability resulting in an enhancement of stimulus evoked responses in the periphery. Also, local inflammation increased the discharge rates of spontaneous activity CCD neurons [35,59]. If released in the inflamed ganglion, inflammatory mediators may increase ongoing spontaneous activity. The spontaneous activity in small nociceptive neurons may increase the chronic state of central sensitization which would also enhance nociceptive responses to mechanical stimuli or to ATP and other chemicals applied to or released endogenously in the periphery [33].
The involvement of P2X receptors in pain behavior was evidenced by two observations: (1) The P2X antagonist, TNP–ATP, effectively blocked α,β-meATP- induced flinching. (2) The dose required for α,β-meATP-induced mechanical hyperalgesia was lower in CCD rats than in control rats. The dose dependence of the nociceptive response is consistent with a receptor-mediated event.
In this study, we found an increased expression of P2X3 receptors in CCD-injured ganglia. It is conceivable, though not presently tested, that an up-regulation of P2X3 receptors in the soma would lead to an increase in P2X3 receptor expression at both terminals [40]. An increase in P2X3 receptor expression at peripheral terminals would result in the exaggerated pain behavior induced by an injection of α,β-meATP. The increased P2X3 receptors at central terminals would increase P2X3 receptor-mediated facilitation of the synaptic transmission in the spinal cord dorsal horn [21,37].
In addition to an increase in the P2X3 protein level or number of P2X3 receptor-labeled DRG neurons in the intact ganglia, CCD induced the potentiation of P2X3 receptor function, as evidenced by the increase in α,β-meATP-evoked current or depolarization in CCD neurons. Since the EC50 for α,β-meATP does not significantly change in CCD neurons, the potentiation cannot be attributed to an increase in the affinity of α,β-meATP for its receptors. The increased current induced by α,β-meATP gave rise to a larger depolarization, exceeding the voltage threshold of action potentials and triggering discharges in CCD-injured DRG neurons. These enhanced responses could lead to the sensitization of nociceptive afferents under pathological conditions. This possibility was supported by the present finding of larger spontaneous nocifensive flinch responses and lower threshold to mechanical stimuli occurring in CCD rats after injection of α,β-meATP into the hind paw of CCD rats.
α,β-meATP is an agonist for P2X1, P2X3 and P2X2/3 receptors [39]. Since the expression of P2X1 receptors in DRG neurons is low [58], P2X3 and P2X2/3 are likely to be the receptor subtypes that are involved in the increased behavioral responses. However, the current evoked by α,β-meATP in CCD neurons was mainly fast desensitizing, indicating that P2X3 homomeric receptors are predominant in CCD-injured neurons [6]. The nocifensive flinch responses and mechanical hyperalgesia evoked by ATP or its analogue, α,β-meATP, are thought to be mediated mainly by P2X3 in small and medium nociceptors [2,23]. In this study, P2X3 receptors are mainly localized in small nociceptive DRG neurons after CCD. Therefore, the primary reasons for the increased behavioral sensitivity to the injection of α,β-meATP in the CCD-injured state might be the up-regulation of P2X3 receptors and the enhanced α,β-meATP responses in small-sized DRG neurons, although we cannot exclude the possibility that the other P2X receptors, such as P2X2/3 heteromeric receptors, might mediate the behavioral responses.
The expression levels of the P2X3 receptor subunit are dynamically modulated in different neuropathic pain models. The decrease in P2X3 receptor levels after peripheral axotomy is prevented by treatment with GDNF, suggesting that the expression of P2X3 receptors might be GDNF dependent [4]. Furthermore, treatment with GDNF or nerve growth factor (NGF) induced the up-regulation of P2X3 receptor in DRGs [45]. NGF neutralization by anti-NGF antibody treatment decreased P2X3 receptor activity [17]. In the CCD model, it is possible that a chronic compression of the DRG could cause the release of cytokines such as MCP-1 [55] and other inflammatory mediators from the damaged tissue thereby attracting macrophages. Since the released inflammatory mediators and cytokines could stimulate the expression of NGF [1], chronic inflammation in CCD might result in an increased expression of the P2X3 subunit in the CCD neurons via NGF production. Furthermore, like substance P and bradykinin, which can potentiate ATP-gated channels through the P2X3 subunit [44], or prostaglandin E2, which potentiates P2X3 receptor-mediated currents in DRG neurons of rats [54], the released inflammatory mediators and cytokines might also contribute to the CCD-induced functional up-regulation of P2X3 receptors. In acutely dissociated small CCD neurons, we have shown that α,β-meATP-evoked current is enhanced by MCP-1 (unpublished observations). The increased electrophysiological responses to α,β-meATP in vitro are unlikely due to the released factors from macrophages because the recorded neurons were continuously superfused by bath solution.
Protein kinases are likely to play an important role in the P2X3 sensitization. For example, Ca2+- and calmodulin (CaM)-dependent protein kinase II enhances ATP responses by promoting trafficking of P2X3 receptors to the cellular membrane [60]. In addition, or alternatively, there may be an activation of protein kinase A or protein kinase C, previously shown to enhance currents through P2X3 receptors by changing the phosphorylating state of P2X3 receptors [44,53,56]. The increased ERK signaling has been implicated in the pain hypersensitivity induced in animal pain models [16,42–43] or in the hyper excitability of small CCD neurons [62]. Furthermore, the pERK localizes in DRG neurons expressing P2X3 receptors [15,46]. However, few studies were conducted to examine the functional role of pERK on the modulation of P2X3 receptors. Here we provide evidence suggesting that the currents through P2X3 receptors are modulated by ERK signaling after CCD injury. First, the immunohistochemical analysis presented here clearly reveals that ERK is activated in P2X3-positive neurons, demonstrating the possibility that ERK signaling might interact with P2X3 receptors in sensory neurons. Second, the enhanced currents through P2X3 receptors were down-regulated by a specific inhibitor for the mitogen-activated/ERK kinase pathway. In DRG neurons, some pain-related ion channels such as A-type potassium channels or TRPV1 have been shown to be regulated by ERK signaling [5,62]. GDNF and NGF regulate neuronal calcium channels via ERK-dependent signaling in DRG neurons [57]. These findings are consistent with our observations and hypothesis that the up-regulation of P2X3 receptors could be caused, at least in part, by the ERK activation, but how pERK modulates P2X3 receptor-mediated responses remains to be elucidated.
We conclude that α,β-meATP-induced mechanical hyperalgesia and nocifensive behavior were enhanced in CCD rats most likely due to the functional up-regulation of P2X3 receptors based on the following observations. First, The P2X3 receptor expression levels increased in chronically compressed DRGs. Second, a P2X3 receptor agonist, α,β-meATP evoked a larger inward current or depolarization and more discharges in small acutely dissociated CCD neurons. The fact that ERK was activated in P2X3-positive CCD neurons and the α,β-meATPinduced inward current could be down-regulated by the inhibition of MEK/ERK suggests that pERK might mediate the sensitization of P2X3 receptor in CCD neurons. These observations demonstrate that the up-regulation of P2X3 receptors might be one of the reasons for CCD-induced pain behavior, in vivo. Hence, inhibition of P2X3 receptors could be a strategy for the relief of pain [8,30], including the pain from CCD.
Acknowledgments
This work was supported by the National Natural Science Foundation of PR China (30570598 to J.H. Sun, 30670639 to Z.H. Xiang), The Shanghai Municipal Science and Technology Commission (06PJ14120 to J.H. Sun), SRF for ROCS, SEM (2006331-2 to J.H. Sun), Program for Changjiang Scholars and Innovative Research Team in University (IRT0528 to C. He) and NINDS NS14624 (R.H.L.).
Footnotes
Disclosure statement
The authors have nothing to disclose.
References
- 1.Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–42. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 2.Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, et al. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22:8139–47. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bean BP. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–68. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 5.Bron R, Klesse LJ, Shah K, Parada LF, Winter J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol Cell Neurosci. 2003;22:118–32. doi: 10.1016/s1044-7431(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 6.Burgard EC, Niforatos W, van Biesen T, Lynch KJ, Touma E, Metzger RE, et al. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophysiol. 1999;82:1590–8. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–32. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–5. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;77:428–31. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Li GW, Wang C, Gu Y, Huang LM. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain. 2005;119:38–48. doi: 10.1016/j.pain.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–5. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 14.Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–7. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- 15.Dai Y, Fukuoka T, Wang H, Yamanaka H, Obata K, Tokunaga A, et al. Contribution of sensitized P2X receptors in inflamed tissue to the mechanical hypersensitivity revealed by phosphorylated ERK in DRG neurons. Pain. 2004;108:258–66. doi: 10.1016/j.pain.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y, Iwata K, Fukuoka T, Kondo E, Tokunaga A, Yamanaka H, et al. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J Neurosci. 2002;22:7737–45. doi: 10.1523/JNEUROSCI.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, et al. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci. 2007;27:8190–201. doi: 10.1523/JNEUROSCI.0713-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, et al. SiRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–82. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 20.Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11:149–54. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- 21.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–53. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 22.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocyto-chemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–58. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton SG, McMahon SB, Lewin GR. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J Physiol. 2001;534:437–45. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol. 1999;126:326–32. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honore P, Kage K, Mikusa J, Watt AT, Johnston JF, Wyatt JR, et al. Analgesic profile of intrathecal P2X(3) antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain. 2002;99:11–9. doi: 10.1016/s0304-3959(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 26.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA. 2002;99:17179–84. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kage K, Niforatos W, Zhu CZ, Lynch KJ, Honore P, Jarvis MF. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp Brain Res. 2002;147:511–9. doi: 10.1007/s00221-002-1263-x. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy C, Assis TS, Currie AJ, Rowan EG. Crossing the pain barrier. P2 receptors as targets for noveal analgesics. J Physiol (London) 2003;3:683–94. doi: 10.1113/jphysiol.2003.049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–32. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, et al. Differential expression patterns of mRNAs for P2X receptor subunits in neurochemically characterized dorsal root ganglion neurons in the rat. J Comp Neurol. 2005;481:377–90. doi: 10.1002/cne.20393. [DOI] [PubMed] [Google Scholar]
- 32.Krishtal OA, Marchenko SM, Obukhov AG. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988;27:995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- 33.LaMotte RH. Spontaneous ectopia. In: Schmidt RF, Willis WD, editors. Encyclopedia of pain. Berlin: Springer; 2006. [Google Scholar]
- 34.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–5. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 35.Ma C, Greenquist KW, Lamotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- 36.Maruo K, Yamamoto H, Yamamoto S, Nagata T, Fujikawa H, Kanno T, et al. Modulation of P2X receptors via adrenergic pathways in rat dorsal root ganglion neurons after sciatic nerve injury. Pain. 2006;120:106–12. doi: 10.1016/j.pain.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–31. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. 2006;452:479–85. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- 39.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 40.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol (London) 2003;2:301–8. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JAM, Eflen RM, Ford APDW, et al. Immunocytochemical localization of P2X3 purinoreceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–82. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 42.Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, et al. Differential activation of extracellular signal regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003:4117–26. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004:10211–22. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paukert M, Osteroth R, Geisler HS, Brandle U, Glowatzki E, Ruppersberg JP, et al. Inflammatory mediators potentiate ATP-gated channels through the P2X3 subunit. J Biol Chem. 2001;276:21077–82. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- 45.Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem. 2001;77:864–75. doi: 10.1046/j.1471-4159.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 46.Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, et al. The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:193–203. doi: 10.1016/j.pain.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 47.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–58. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 48.Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–7. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 49.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–71. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 50.Sun JH, Yang B, Donnelly DF, Ma C, Lamotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–99. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 51.Virginio C, Robertson G, Surprenant A, North RA. Trinitrophenyl- substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol Pharmacol. 1998;53:969–73. [PubMed] [Google Scholar]
- 52.Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, et al. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–8. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 53.Wang C, Gu Y, Li GW, Huang LY. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol. 2007;584:191–203. doi: 10.1113/jphysiol.2007.135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Li GW, Huang LY. Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain. 2007;3:22. doi: 10.1186/1744-8069-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White FA, Sun JH, Waters SM, Ma C, Ren DJ, Ripsch M, et al. Excitatory MCP-1/CCR2 chemokine signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102:14092–7. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirkner K, Stanchev D, Koles L, Klebingat M, Dihazi H, Flehmig G, et al. Regulation of human recombinant P2X3 receptors by ectoprotein kinase C. J Neurosci. 2005;25:7734–42. doi: 10.1523/JNEUROSCI.2028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodall AJ, Richards MA, Turner DJ, Fitzgerald EM. Growth factors differentially regulate neuronal Ca (v) channels via ERK-dependent signalling. Cell Calcium. 2008;43:562–75. doi: 10.1016/j.ceca.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–8. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- 59.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–22. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu GY, Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA. 2004;101:11868–73. doi: 10.1073/pnas.0401490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104:9864–9. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Cai G, Ni X, Sun J. The role of ERK activation in the neuronal excitability in the chronically compressed dorsal root ganglia. Neurosci Lett. 2007;419:153–7. doi: 10.1016/j.neulet.2007.04.040. [DOI] [PubMed] [Google Scholar]