Abstract
Previous studies have suggested that 1α,25-dihydroxyvitamin D3[1,25(OH)2D3] has a role in reproductive function. Gonadal insufficiencies were observed as a result of 1,25(OH)2D3 deficiency and in 1,25(OH)2D3 receptor (VDR) null mutant mice. To study human sperm anatomy at the molecular level, we first evaluated the ultrastructural localization of VDR by immunogold electron microscopy using a monoclonal antibody against amino acids 344–424 of human VDR, in normozoospermic samples. Intriguingly, VDR was associated predominantly with the cell nucleus. In fact, it is known that VDR is a transcription factor, and that in vitamin-D-depleted animals, VDR is largely localized in the cell nucleus. To assess the significance of VDR in the male gamete, we investigated the role of 1,25(OH)2D3/VDR in sperm survival and capacitation. Our results revealed that the action of 1,25(OH)2D3 depended on its concentration because although lower doses induced cholesterol efflux, protein phosphorylation and sperm survival, a higher concentration seemed to be ineffective or did not show an increased effect. These results increase our knowledge of human sperm anatomy at the molecular level and suggest that 1,25(OH)2D3/VDR may have an important role in sperm survival and the acquisition of fertilizing ability.
Keywords: 1,25(OH)2D3 receptor (VDR); 1a,25-dihydroxyvitamin D3. sperm; male reproduction; nuclear receptors
Introduction
Vitamin D3 is synthesized in the epidermis. The hormone is then metabolized in the liver and afterwards in the kidney to give 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), which is the most biologically active metabolite of vitamin D (Kuritzky et al. 2008). The functional activities of 1,25(OH)2D3 require a high-affinity receptor, the 1,25(OH)2D3 receptor (VDR), which is a member of the superfamily of nuclear steroid hormone receptors. In addition to its well-known effects on calcium and phosphate homeostasis, 1,25(OH)2D3, which is also known as calcitriol, acts on a variety of tissues (Johnson & DeLuca, 2001). This may be due to the presence of VDR in more than 30 tissues, which include the brain, pancreas, pituitary, skin, muscle, placenta, immune cells, parathyroid, ovary, prostate, and testis (Habib et al. 1990; Johnson et al. 1996; Chatterjee, 2001).
In the male genital tract of rodents, VDR has been found in the smooth muscle of the epididymis, spermatogonium and Sertoli cells. The extensive presence of binding sites for 1,25(OH)2D3 detected in Sertoli cells and the caput epididymis at the time of spermiogenesis suggests that 1,25(OH)2D3 is involved in spermatogenesis and in sperm maturation in rats (Johnson et al. 1996). VDR null mutant mice demonstrate significant gonadal insufficiency, with decreased sperm count and motility, and histological abnormalities of the testis (Kinuta et al. 2000). The testes of 1,25(OH)2D3-depleted animals show incomplete spermatogenesis, impaired development, and degenerative changes in the seminiferous tubules. These results suggest that 1,25(OH)2D3 plays a very important role in the maturation of germ cells. Therefore, it can be concluded that 1,25(OH)2D3 is involved in male reproduction, presumably at the molecular level.
The VDR mediates 1,25(OH)2D3-dependent responses through both genomic and non-genomic pathways (Norman et al. 1997, 2004). With regard to the latter, 1,25(OH)2D3 can rapidly stimulate phosphoinositide metabolism, protein kinase C (PKC), and mitogen-activated protein (MAP) kinases, as well as increase cytosolic calcium and cGMP levels (Barsony & Marx, 1991; Massheimer et al. 1994; Buitrago et al. 2002; Rebsamen et al. 2002). Several nuclear receptors (El-Hefnawy et al. 2000; Aquila et al. 2004) have been reported to be expressed in ejaculated human spermatozoa, and to regulate cellular processes through non-genomic mechanisms. Recently, it has been shown by Western blotting and immunohistochemistry that human sperm express the VDR (Corbett et al. 2006). However, the significance of 1,25(OH)2D3 and its receptor in male reproduction needs to be investigated further, and the ultrastructural localization of VDR in the male gamete has not yet been reported in the literature.
The subcellular localization of VDR in the sperm nucleus is reported here. We employed immunogold electron microscopy to gain an improved understanding of sperm anatomy at the molecular level. In addition, we studied the possible involvement of 1,25(OH)2D3/VDR in important biological functions of human sperm, such as capacitation and survival.
Materials and methods
Chemicals
Percoll (PVP-coated colloidal silica particles for cell separation), sodium bicarbonate, sodium lactate, sodium pyruvate, dimethyl sulfoxide (DMSO), Earle's Balanced Salt Solution (EBSS), 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), and all other chemicals were purchased from Sigma Chemical (Milan, Italy). Acrylamide/bisacrylamide was purchased from Labtek Eurobio (Milan, Italy). Triton X-100 and Eosin Y were purchased from Farmitalia Carlo Erba (Milan, Italy). The ECL Plus Western blotting detection system, Hybond™ ECL™, and HEPES sodium salt were purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). The cholesterol-oxidase (CHOD)-peroxidase (POD) enzymatic colorimetric kit was from Inter-Medical (Biogemina Italia Srl, Catania, Italy). The colloidal gold-conjugated goat anti-mouse IgG secondary antibody (Ab) was purchased from Sigma-Aldrich (Milan, Italy). The goat polyclonal actin Ab (1–19), mouse monoclonal anti-human VDR (D-6) Ab, mouse monoclonal anti-phosphotyrosine PY99 Ab, peroxidase-coupled anti-mouse and anti-goat IgG secondary Abs were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). Monoclonal anti-phosphothreonine Ab was purchased from Calbiochem (Nottingham, UK).
Semen samples and spermatozoa preparations
Human semen was collected according to the recommended procedure of the World Health Organization (WHO) by masturbation from healthy volunteer donors of proven fertility, who were undergoing semen analysis in our laboratory. Spermatozoa preparations were made as described previously (Aquila et al. 2005). Briefly, semen samples with normal measurements for volume, sperm count, motility, vitality and morphology, according to the WHO Laboratory Manual (WHO, 1999), were included in this study. For each experiment, three normozoospermic samples were pooled. Pooled sperm were washed, subjected to the indicated treatments, and incubated at 37 °C and 5% CO2. Prior to centrifugation to collect sperm, several aliquots were used to analyse sperm viability. The study had been approved by the local medical ethics committee and all participants gave their informed consent.
Processing of ejaculated sperm
After liquefaction, normal semen samples were pooled and subjected to centrifugation for 7 min at 800 g on a discontinuous Percoll density gradient (80 : 40% v : v) (WHO, 1999). The 80% Percoll fraction was examined using an optical microscope that was equipped with a ×100 oil immersion objective to ensure that a pure sample of sperm was obtained. An independent observer inspected the cells and examined several fields for each slide. Percoll-purified sperm were washed with non-supplemented EBSS (incapacitating medium) and were incubated for 30 min at 37 °C and 5% CO2, either with no additional treatment (control, NC) or with treatment (experimental). The treatments were as follows: increasing doses of 1,25(OH)2D3 (0.01, 0.1 and 1 nm), or anti-VDR Ab (1 µg mL−1) combined with 0.1 nm 1,25(OH)2D3. When the cells were treated with the anti-VDR Ab, a pretreatment of 15 min was performed.
Immunogold labelling of VDR
Sperm that had been fixed overnight in 4% paraformaldehyde were washed in phosphate-buffered saline (PBS) to remove excess fixative, and dehydrated in a graded series of alcohol. They were then infiltrated with LR white resin, which was polymerized in a vacuum oven at 45 °C for 48 h. Ultrathin 60-nm sections were cut and placed on coated nickel grids for post-embedding immunogold labelling with a mouse anti-human VDR monoclonal Ab VDR (D-6), which was raised against amino acids 344–424 of human VDR. Potential non-specific labelling was blocked by incubating the sections in PBS containing 5% normal goat serum, 5% bovine serum albumin and 0.1% cold water fish gelatine at room temperature for 1 h. Sections were then incubated overnight at 4 ºC with the mouse monoclonal anti-VDR primary Ab at a dilution of 1 : 500 in PBS. This was followed by incubation with 10 nm colloidal gold-conjugated goat anti-mouse IgG secondary Ab at a dilution of 1 : 50 for 2 h at room temperature. The sections were subsequently washed in PBS, fixed in glutaraldehyde, counterstained with uranyl acetate and lead acetate, and examined using a Zeiss EM 900 transmission electron microscope. To assess the specificity of the immunolabelling, a negative control was performed in which sperm sections were incubated with the colloidal gold-conjugated secondary Ab but not the primary Ab.
Evaluation of sperm viability
Sperm viability was analysed by red-eosin exclusion, using Eosin Y, to evaluate the potentially toxic effects of the treatments. An aliquot of each sperm sample, which had been incubated in the absence (NC) or presence of increasing concentrations of 1,25(OH)2D3(0.01–1 nm) or 1 µg mL−1 anti-VDR Ab combined with 0.1 nm 1,25(OH)2D3, was examined by optical microscopy after incubation with Eosin Y.
An independent observer scored 200 cells for stain uptake (dead cells) or exclusion (live cells), and sperm viability was expressed as the percentage of total live sperm.
Measurement of cholesterol in sperm culture medium
Cholesterol levels in the medium in which the sperm had been incubated were measured in duplicate by a CHOD-POD enzymatic colorimetric method according to the manufacturer's instructions (Aquila et al. 2006). Sperm samples that had been washed twice with incapacitating medium were incubated in the same medium with or without the above-described treatments for 30 min at 37 °C and 5% CO2. At the end of the incubation period, the culture media were recovered by centrifugation and lyophilized. The samples were subsequently dissolved in 1 mL reaction buffer, and incubated for 10 min at room temperature. The cholesterol content was measured by spectrophotometry at 505 nm. A cholesterol standard of 200 mg dL−1 was used. The limit of sensitivity for the assay was 0.05 mg dL−1. Inter- and intra-assay variations were 0.04 and 0.03%, respectively. The results were presented as mg cholesterol per 1 × 107 spermatozoa.
Western blot analysis of sperm proteins
Sperm samples that had been washed twice with EBSS were incubated in the absence (NC) or presence of the indicated treatments, and then centrifuged for 5 min at 5000 g. The pellets were resuspended in lysis buffer as described previously (Aquila et al. 2002). Equal amounts of sperm protein (80 µg) were boiled for 5 min, separated by electrophoresis on a 10% polyacrylamide gel, transferred to nitrocellulose sheets, and probed with an appropriate dilution of Ab. Binding of the secondary Ab was revealed using the ECL Plus Western blotting detection system (GE Healthcare), according to the manufacturer's instructions. For an internal control, all the membranes were stripped (0.2 m glycine, pH 2.6 for 30 min at room temperature) and re-probed with an anti-β-actin Ab.
Statistical analysis
The immunogold labelling was repeated on at least three independent occasions, whereas Western blot analysis was performed in a further six independent experiments. The data that were obtained from the CHOD-POD enzymatic colorimetric analysis (six replicate experiments using duplicate determinations) and the viability analysis (six replicate experiments using duplicate determinations) were presented as the mean ± SE. The differences in mean values were calculated using analysis of variance (anova) with a significance level of P ≤ 0.05.
Results
Immunogold localization of VDR in human sperm
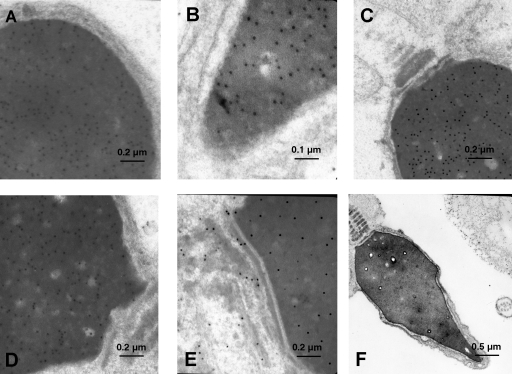
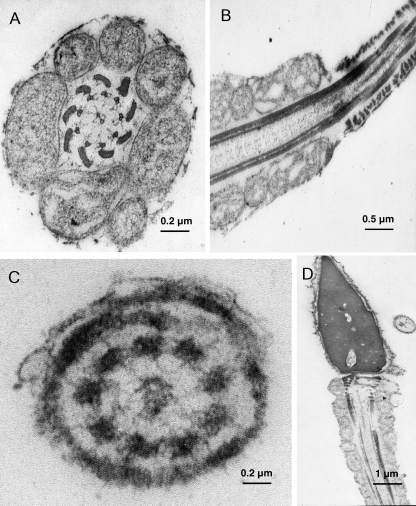
The general appearance of the human whole sperm mount revealed good structural preservation (Fig. 1): the cytoskeletal elements, the neck (segmented columns), and the outer dense fibres were all well defined. The majority of the sperm showed normal morphological features and the general cell ultrastructure corresponded exactly to that described previously (Friedlaender, 1952). Ultrastructural immunogold analysis of 60-nm ultrathin sections revealed that the VDR was associated particularly with the sperm head. In fact, the gold particles appeared to be clustered in the sperm nucleus (Fig. 1A–D) and were not present in the cytoplasm or the membranes in the head. The chromatin was homogeneous and showed no remarkable changes; there were some clear areas as described previously (Lung, 1972). All the samples showed an identical staining intensity and localization of the immunoreactive components. Some particles also decorated the neck (Fig. 1E). In all the samples, neither the axoneme and outer dense fibres of the mid-piece, which are covered by the mitochondria, nor the tail of the sperm were labelled (Figs 1C and 2A–C). This was observed in transverse and sagittal sections throughout the end-piece. Simultaneous negative control experiments with the secondary Ab alone did not show any labelling (Figs 1F and 2D).
Fig. 1.
Ultrastructural localization of VDR in human ejaculated spermatozoa. Subcellular localization of VDR in human ejaculated spermatozoa by immunoelectron microscopy. Electron micrographs of sperm allowed to react with Ab directed against VDR. (A) Region of the head (×50 000, scale bar = 0.2 µm). (B) Region of the apical head (×81 000, scale bar = 0.1 µm). (C and D) Region of the neck (×29 000–50 000, scale bar = 0.2 µm). (E) Region of the neck in which few isolated gold particles are present (×50 000, scale bar = 0.2 µm). (F) Electron micrograph of control section incubated without the primary Ab, in which the sperm are totally without reaction product (×12 000, scale bar = 0.5 µm).
Fig. 2.
VDR is absent in the midpiece and along the tail in human spermatozoa. Subcellular localization of VDR in human ejaculated spermatozoa by immunoelectron microscopy. Electron micrographs of sperm allowed to react with Ab directed against VDR. (A) Electron micrograph of a cross-section of the tail probed with anti-VDR Ab, which shows a region of the midpiece (×30 000, scale bar = 0.2 µm). (B) Sagittal section through the tail probed with anti-VDR Ab, which shows the transition between the middle piece with its mitochondria and the principal piece with its fibrous sheath (×18 000, scale bar = 0.5 µm). (C) Electron micrograph of a cross-section of the tail probed with anti-VDR Ab, which shows a region of the end piece (×65 000, scale bar = 0.2 µm). (D) In the negative control section, no immunoreaction was present (×4000, scale bar = 1 µm).
1,25(OH)2D3modulates cholesterol efflux in human sperm
Due to the fact that it had never been investigated previously, we evaluated whether 1,25(OH)2D3 could influence extratesticular sperm maturation by examining its effect on capacitation.
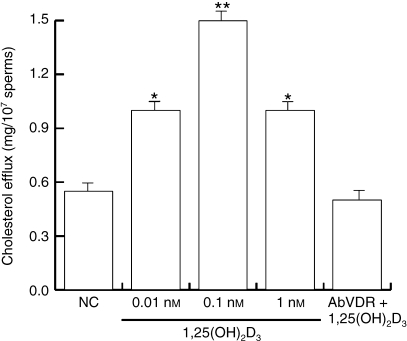
Capacitation is a post-ejaculation process that is associated with the acquisition of the ability to fertilize an egg, and encompasses different features. Cholesterol efflux across the sperm membrane contributes to one signalling mechanism that controls sperm capacitation (Aquila et al. 2006 and references therein). To evaluate cholesterol efflux, the sperm were collected by centrifugation after they had been treated as described above. Cholesterol levels in the incubation medium were measured, and the sperm were lysed to evaluate protein phosphorylation. Our results showed a significant increase in cholesterol efflux (Fig. 3) upon treatment with 0.01 and 0.1 nm 1,25(OH)2D3, but 1 nm 1,25(OH)2D3did not induce a further increase. This effect was reversed by co-treatment with the anti-VDR Ab, which suggested that VDR was involved in the induction of sperm cholesterol efflux.
Fig. 3.
1,25(OH)2D3 modulates cholesterol efflux in human sperm. Washed spermatozoa were incubated in non-supplemented Earle's medium for 30 min at 37 °C and 5% CO2, in the absence (NC) or presence of increasing 1,25(OH)2D3 concentrations (0.01, 0.1 and 1 nm) or with 0.1 nm 1,25(OH)2D3 combined with anti-VDR Ab. Cholesterol in culture medium from human ejaculated spermatozoa was measured by enzymatic colorimetric assay. Columns are mean ± SE of six independent experiments performed in duplicate. Data are expressed in mg/107 sperms. *P < 0.05 vs. control, **P < 0.01 vs. control.
1,25(OH)2D3 influences the phosphorylation of proteins on tyrosine and threonine residues in human sperm
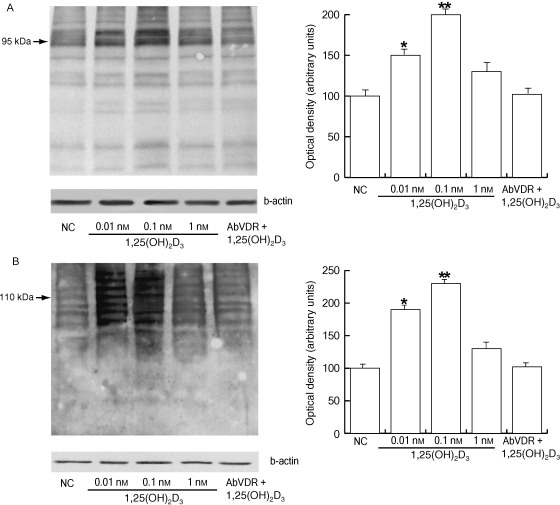
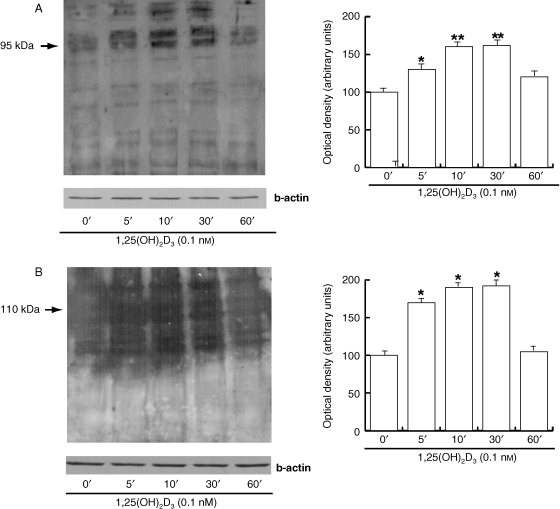
Cholesterol efflux across the sperm membrane is a key priming event in the capacitation process because it initiates signalling events that lead to the phosphorylation of sperm proteins (Visconti et al. 1995; Osheroff et al. 1999). Therefore, we investigated whether 1,25(OH)2D3 was also able to influence this important feature of the capacitation process. A significant increase in both tyrosine (Fig. 4A) and threonine phosphorylation (Fig. 4B) was observed with increasing concentrations of 1,25(OH)2D3 (0.01–0.1 nm), whereas 1 nm 1,25(OH)2D3 did not induce a significant increase. In both cases, the anti-VDR Ab abolished the 0.1 nm 1,25(OH)2D3-induced effect. It is important to point out that, under our experimental conditions, the anti-phosphoprotein Abs that were used recognized protein bands that were predominantly within the same size ranges as those that have been documented previously (Naz, 1999). For tyrosine phosphorylation we measured the density of the 95-kDa band; for threonine phosphorylation we used the 110-kDa band.
Fig. 4.
1,25(OH)2D3 affects tyrosine and threonine phosphorylation of the human sperm proteins. Washed spermatozoa were incubated in non-supplemented Earle's medium for 30 min at 37 °C and 5% CO2, in the absence (NC) or presence of increasing 1,25(OH)2D3 concentrations (0.01, 0.1 and 1 nm) or with 0.1 nm 1,25(OH)2D3 combined with anti-VDR Ab. Sperm lysates of 80 µg were used for Western blot analysis. Actin was used as a loading control. (A) Protein tyrosine phosphorylation. On the right, quantitative representation after densitometric evaluation of the 95-kDa band. (B) Protein threonine phosphorylation. On the right, quantitative representation after densitometric evaluation of the 110-kDa band. Autoradiographs presented are representative examples of experiments that were performed at least six times with repetitive results. *P < 0.05 vs. control, **P < 0.01 vs. control.
To investigate whether the protein phosphorylation in sperm that was induced by 0.1 nm 1,25(OH)2D3 represented an early event, we performed a time-course study (0, 5, 10, 30 and 60 min). This experiment revealed that both types of phosphorylation followed a similar pattern: they were observed at 5 min, increased at 10 min and were sustained until 30 min, and then dropped significantly at 1 h (Fig. 5A,B).
Fig. 5.
Time course of the effect of 1,25(OH)2D3 on tyrosine and threonine phosphorylation of sperm proteins. Time-course study (0, 5, 10, 30 and 60 min) in sperm treated with 0.1 nm 1,25(OH)2D3. Actin was used as a loading control. On the right side are reported the quantitative representations after densitometric evaluation as indicated above. Autoradiographs presented are representative examples of experiments that were performed at least six times with repetitive results. *P < 0.05 vs. control, **P < 0.01 vs. control.
Effect of 1,25(OH)2D3 on sperm survival
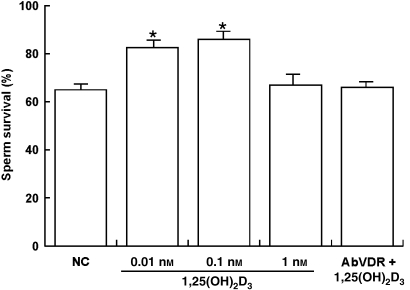
As shown in Fig. 6, sperm viability was significantly increased in the presence of 0.01 and 0.1 nm 1,25(OH)2D3, whereas 1 nm 1,25(OH)2D3 did not affect sperm viability as compared to the controls. Interestingly, the effect that was induced by 0.1 nm 1,25(OH)2D3was reversed in the presence of the anti-VDR Ab, which suggested that the effect was mediated by VDR.
Fig. 6.
1,25(OH)2D3 modulates human sperm survival. Washed spermatozoa were incubated in non-supplemented Earle's medium for 30 min at 37 °C and 5% CO2, in the absence (NC) or presence of increasing 1,25(OH)2D3 concentrations (0.01, 0.1 and 1 nm) or with 0.1 nm 1,25(OH)2D3 combined with anti-VDR Ab. Columns are mean ± SE of six independent experiments performed in duplicate. *P < 0.05 vs. control.
Discussion
The archetypal target tissues of 1,25(OH)2D3 include bone, intestine and kidney. Recently, it has become clear that 1,25(OH)2D3 has many additional functions that are not closely linked to its classical role as a skeletal regulator. The importance of this secosteroid in reproduction has emerged from studies that have demonstrated that 1,25(OH)2D3 deficiency results in reduced fertility in rats (Kinuta et al. 2000). However, the function of 1,25(OH)2D3 in the genitourinary organs is unknown, even though this has become a widely researched area. Here, with the aim of studying human sperm anatomy at the molecular level, we investigated for the first time the ultrastructural compartmentalization of VDR in the human male gamete. In addition, we evaluated the molecular mechanisms through which 1,25(OH)2D3 may affect important sperm functions such as the acquisition of fertilizing ability and viability.
The intracellular localization of VDR in somatic cells has been an issue of some controversy because there is not complete agreement about whether VDR is translocated to the cell nucleus in response to 1,25(OH)2D3, or whether it shuttles continuously between the cytosol and nucleus (Shaffer et al. 2005). In addition, it appears that the classic VDR is also associated with the caveolae that are present in the plasma membrane in some cell types, as has been reported for other steroid receptors (Levin, 2002; Huhtakangas et al. 2004). The structure of human spermatozoa has proved rather difficult to elucidate because of the opacity of the head to electron penetration. Therefore, in spite of active interest, our understanding of the ultrastructural organization of ejaculated human sperm is still incomplete, and our knowledge of the molecular anatomy of sperm is very limited.
In our study, immunogold analysis showed that VDR was localized uniformly in the sperm nucleus, although some particles also decorated the neck of the sperm. In somatic cells, the receptor–hormone complex becomes localized to the nucleus and then interacts with the 1,25(OH)2D3-responsive element. This binding modifies transcription of the target genes. In addition to their classic genomic action, nuclear receptors regulate cellular processes through a non-genomic mechanism (Losel et al. 2003; Norman et al. 2004). It is generally accepted that the sperm nucleus is transcriptionally inactive due to the highly condensed architecture of its chromatin (Grunewald et al. 2005). In this study, we investigated the rapid effects of the VDR, which we have also observed previously for other nuclear receptors in human sperm (Aquila et al. 2004, 2006, 2007). Indeed, this mode of action seems to be particularly appropriate in the male gamete because sperm functions need to be activated rapidly to accommodate dynamic changes in the surrounding milieu. In addition, sperm have a highly differentiated architecture, and their peculiar anatomy compartmentalizes proteins and other molecules within the areas in which they are needed.
An interesting finding in somatic cells may provide a possible explanation for the significance of the presence of VDR in the sperm nucleus. It has been reported that the VDR is closely related to the nuclear matrix, or part of it, and has a role in ensuring genomic stability (Nangia et al. 1998). DNA is generally considered to be the most critical cellular target for the lethal mutagenic effects of drugs, radiation and environmental chemicals. As Chatterjee has reported, vitamin D stabilizes chromosomal structure and prevents the induction of DNA double-strand breaks by endogenous or exogenous factors (Chatterjee, 2001). Recently, it has been suggested that the sperm nuclear matrix plays a crucial role in the regulation of DNA fragmentation and degradation, both before and after fertilization (Shaman et al. 2007). The accurate transferral of genetic information to progeny is a prerequisite for the conservation and evolution of a species. Therefore, proper control of the integrity of sperm DNA is critical for the maintenance of genome stability, and the speculation that VDR in the sperm nucleus acts as a protective genomic factor is very intriguing.
Successful sperm maturation depends on sequential steps in both the male and female reproductive tracts. After ejaculation, the male gamete must undergo capacitation to be capable of fertilizing an egg; this is a prerequisite for fertilization by mammalian spermatozoa. Capacitation, which in vivo occurs within the female reproductive tract, and which can also be accomplished in defined media in vitro, confers on the sperm the ability to undergo the acrosome reaction (Yanagimachi, 1994; Visconti et al. 1995). Capacitation has been associated with modifications in cholesterol efflux, plasma membrane fluidity, intracellular ion concentrations, metabolism, and motility, and with the increased phosphorylation of a number of proteins. It has been demonstrated that cholesterol efflux is a priming event that induces changes in the fluidity of the plasma membrane, and sequentially stimulates the activation of sperm adenylyl cyclase and phosphorylation of sperm proteins at tyrosine and threonine residues (Visconti et al. 1995; Visconti & Kopf, 1998; Osheroff et al. 1999; Naz, 1999). Recently, treatment with 1,25(OH)2D3 has been shown to elevate intracellular cAMP levels within 10 min in human syncytiotrophoblast cells (Avila et al. 2007). In sperm, 1,25(OH)2D3might play a role in capacitation by inducing firstly an increase in cAMP levels and then phosphorylation of certain proteins. It is known that phosphorylation and cholesterol efflux are early phases of the capacitation process; however, the molecular mechanisms and signal transduction pathways that are involved have only been defined partially.
In our study, certain concentrations of 1,25(OH)2D3were able to modulate both cholesterol efflux and tyrosine and threonine phosphorylation. The effects of 1,25(OH)2D3 appeared to be very dependent on the concentration of the hormone. Intriguingly, the 1,25(OH)2D3-mediated biphasic rapid response that was obtained for cholesterol efflux in our study was reminiscent of the 1,25(OH)2D3-mediated stimulation of the rapid transport of Ca2+ (transcaltachia) in the perfused chick intestine, which also follows a biphasic dose–response curve (De Boland & Norman, 1990; Norman, 2006). The outcome of signalling activation can depend on differences in ligand concentration, and this effect has also been recently demonstrated in human sperm (Aquila et al. 2004, 2006, 2007; Andò & Aquila, 2005).
In different mammalian species, sperm may utilize distinct survival strategies, which are probably related to the differing management of energy metabolism. To date, two distinctive phenotypes have been observed, which are characterized by sperm survival under laboratory and in vivo conditions. These can be represented by the boar sperm phenotype, which corresponds to a short survival time inside the female vaginal tract (~48 h) (Viring & Eirnasson, 1981), and by the human sperm phenotype, which corresponds to a long survival time inside the female vaginal tract (~ 1 week) (Feldman & Nelson, 1987). Long-term sperm viability in humans may be an important evolutionary adaptation because it is necessary for the sperm to reach the oocyte whilst it occupies the correct position in the fallopian tubes. As the oocyte may not be in this specific location when the sperm reach it, the sperm may have to wait until this event occurs. It emerged from our study that VDR has the ability to modulate sperm survival, which suggests that it could play an important role in this aspect of the physiology of the human male gamete We observed that low doses of 1,25(OH)2D3 stimulated the effect, whereas a higher concentration was ineffective.
Most of the classical physiological actions of 1,25(OH)2D3 have been known since the early part of this century, when dietary vitamin D deficiency was first demonstrated. However, non-classical roles have emerged from studies that have probed the mechanism of 1,25(OH)2D3 action at the molecular level, and from studies of the VDR knockout mouse. Furthermore, the ubiquitous distribution of VDR opens the possibility of unforeseen biological functions of 1,25(OH)2D3. From our results, it emerged that VDR was involved both in the early phases of the functional maturation of ejaculated sperm and in sperm survival. Interestingly, these effects of the hormone were observed at lower doses of 1,25(OH)2D3, whereas a higher concentration was ineffective or did not have a greater effect. It is important to note that human physiological serum levels of 1,25(OH)2D3 are between 37.5 and 150 pM (15–60 pg mL−1) (Masuda & Jones, 2006), which correlates well with our results.
As concerns the rapid effects that we observed, it is possible that sperm might respond to 1,25(OH)2D3 by a VDR-independent mechanism. Schwartz et al. (2002) have observed, in the growth zone of chondrocytes, that 1,25(OH)2D3 activates PKCα by activating phospholipase C-β1 and -β3 through the G-protein Gq, and this activation is not reduced in cells from VDR null mice. A similar mode of action of 1,25(OH)2D3 is also proposed in sperm, on the basis of the predominantly nuclear anatomic location of the VDR.
The discovery that 1,25(OH)2D3 influences sperm function may be useful for the development of novel therapeutic approaches to the treatment of male reproductive disorders. However, the physiological significance and the specific roles of 1,25(OH)2D3/VDR in the human male gamete require further investigation.
Acknowledgments
Our special thanks to Dr Vincenzo Cunsulo (Biogemina Italia Srl, Catania, Italy). We would also like to thank Perrotta Enrico for excellent technical assistance, and Serena and Maria Clelia Gervasi for language revision.
References
- Andò S, Aquila S. Arguments raised by the recent discovery that insulin and leptin are expressed in and secreted by human ejaculated spermatozoa. Mol Cell Endocrinol. 2005;245:1–6. doi: 10.1016/j.mce.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Andò S. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87:3385–3390. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile M, et al. Estrogen receptor (ER)alpha and ER beta are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J Clin Endocrinol Metab. 2004;89:1443–1451. doi: 10.1210/jc.2003-031681. [DOI] [PubMed] [Google Scholar]
- Aquila S, Gentile M, Middea E, et al. Leptin secretion by human ejaculated spermatozoa. J Clin Endocrinol Metab. 2005;90:4753–4761. doi: 10.1210/jc.2004-2233. [DOI] [PubMed] [Google Scholar]
- Aquila S, Bonofiglio D, Gentile M, et al. Peroxisome proliferator-activated receptor (PPAR)gamma is expressed by human spermatozoa: its potential role on the sperm physiology. J Cell Physiol. 2006;209:977–986. doi: 10.1002/jcp.20807. [DOI] [PubMed] [Google Scholar]
- Aquila S, Middea E, Catalano S, et al. Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum Reprod. 2007;22:2594–2605. doi: 10.1093/humrep/dem243. [DOI] [PubMed] [Google Scholar]
- Avila E, Díaz L, Barrera D, et al. Regulation of Vitamin D hydroxylases gene expression by 1,25-dihydroxyvitamin D3 and cyclic AMP in cultured human syncytiotrophoblasts. J Steroid Biochem Mol Biol. 2007;103:90–96. doi: 10.1016/j.jsbmb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Barsony J, Marx SJ. Rapid accumulation of cyclic GMP near activated vitamin D receptors. Proc Natl Acad Sci U S A. 1991;15:1436–1440. doi: 10.1073/pnas.88.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrago C, Gonzalez PV, Russo DB, Boland R. Activation of RAF-1 through RAS and PKC mediates 1,25(OH)2D3 regulation of the map kinase pathway in muscle cells. J Biol Chem. 2002;278:2199–2205. doi: 10.1074/jbc.M205732200. [DOI] [PubMed] [Google Scholar]
- Chatterjee M. Vitamin D and genomic stability. Mutat Res. 2001;18:69–87. doi: 10.1016/s0027-5107(01)00080-x. [DOI] [PubMed] [Google Scholar]
- Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006;68:1345–1349. doi: 10.1016/j.urology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- De Boland AR, Norman AW. Influx of extracellular calcium mediates 1,25-dihydroxyvitamin D3-dependent transcaltachia (the rapid stimulation of duodenal Ca2+ transport) Endocrinology. 1990;127:2475–2480. doi: 10.1210/endo-127-5-2475. [DOI] [PubMed] [Google Scholar]
- El-Hefnawy T, Manna PR, Luconi M, Baldi E, Slotte PJ. Progesterone action in a murine Leydig tumor cell line (mLTC-1), Possibly through a nonclassical receptor type. Endocrinology. 2000;141:247–255. doi: 10.1210/endo.141.1.7253. [DOI] [PubMed] [Google Scholar]
- Feldman EC, Nelson RW. In: Canine and Feline Endocrinology and Reproduction. Pedersen E, editor. Philadelphia: WB Saunders; 1987. pp. 420–421. [Google Scholar]
- Friedlaender MH. Observations on the structure of human spermatozoa; an electron microscope inquiry. Proc R Soc Lond B Biol Sci. 1952;27:60–69. doi: 10.1098/rspb.1952.0044. [DOI] [PubMed] [Google Scholar]
- Grunewald S, Paasch U, Glander HJ, Anderegg U. Mature human spermatozoa do not transcribe novel RNA. Andrologia. 2005;37:69–71. doi: 10.1111/j.1439-0272.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- Habib FK, Maddy SQ, Gelly KJ. Characterisation of receptors for 1,25-dihydroxyvitamin D3 in the human testis. Steroid Biochem. 1990;35:195–199. doi: 10.1016/0022-4731(90)90274-v. [DOI] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The Vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivoand in vitro. Mol Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D3 receptor in rat reproductive tissues. Histochem Cell Biol. 1996;105:7–15. doi: 10.1007/BF01450873. [DOI] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF. Vitamin D receptor null mutant mice fed high levels of calcium are fertile. J Nutr Jun. 2001;131:1787–1791. doi: 10.1093/jn/131.6.1787. [DOI] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- Kuritzky LA, Finlay-Jones JJ, Hart PH. The controversial role of vitamin D in the skin: immunosuppression vs. photoprotection. Clin Exp Dermatol. 2008;33:167–170. doi: 10.1111/j.1365-2230.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- Levin R. Cellular function of plasma membrane estrogen receptor. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Losel RM, Falkeistein E, Feuring M, et al. Nongenomic steroid action: controversies, questions and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Lung B. Ultrastructure and chromatin disaggregation of human sperm head with thioglycolate treatment. Journal of Cell Biology. 1972;52:179–186. doi: 10.1083/jcb.52.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massheimer V, Boland R, De Boland AR. Rapid 1,25(OH)2-vitamin D3 stimulation of calcium uptake by rat intestinal cells involves a dihydropyridine-sensitive cAMP-dependent pathway. Cell Signal. 1994;3:299–304. doi: 10.1016/0898-6568(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Masuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- Nangia AK, Butcher JL, Konety BR, Vietmeie BN, Getzenberg RH. Association of vitamin D receptors with the nuclear matrix of human and rat genitourinary tissues. Steroid Biochem. 1998;66:241–246. doi: 10.1016/s0960-0760(98)00039-9. [DOI] [PubMed] [Google Scholar]
- Naz RK. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol Reprod. 1999;60:1402–1409. doi: 10.1095/biolreprod60.6.1402. [DOI] [PubMed] [Google Scholar]
- Norman AW. Minireview: Vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- Norman AW, Okamura WH, Hammond MW, et al. Comparison of 6-s-cisand 6-s-translocked analogs of 1,25(OH)2-vitamin D3 indicates that the 6-s-cisconformation is preferred for rapid nongenomic biological responses and that neither 6-s-cisnor 6-s-translocked analogs are preferred for genomic biological responses. Mol Endocrinol. 1997;11:1518–1531. doi: 10.1210/mend.11.10.9993. [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat Rev Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Gregory JA, Kopf S. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;11:1017–1026. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Rebsamen MC, Sun J, Norman AW, Liao JK. 1α,25-Dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res. 2002;91:17–24. doi: 10.1161/01.res.0000025269.60668.0f. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Sylvia VL, Larsson D, et al. 1α,25(OH)2D3 regulates chondrocyte matrix vesicle protein kinase C (PKC) directly via G-protein-dependent mechanisms and indirectly via incorporation of PKC during matrix vesicle biogenesis. J Biol Chem. 2002;277:11828–11837. doi: 10.1074/jbc.M110398200. [DOI] [PubMed] [Google Scholar]
- Shaffer PL, McDonnell DP, Gewirth DT. Characterization of transcriptional activation and DNA-binding functions in the hinge region of the vitamin receptor. Biochemestry. 2005;44:2678–2685. doi: 10.1021/bi0477182. [DOI] [PubMed] [Google Scholar]
- Shaman JA, Yamauchi Y, Ward WS. Function of the sperm nuclear matrix. J Reprod Syst. 2007;53:135–140. doi: 10.1080/01485010701329378. [DOI] [PubMed] [Google Scholar]
- Viring S, Eirnasson S. Sperm distribution within the genital tract of naturally inseminated gilts. Nord Vet Med. 1981;33:145–149. [PubMed] [Google Scholar]
- Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interactions. 4th edn. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]