Abstract
Translesion synthesis (TLS) is a DNA damage tolerance mechanism that allows continued DNA synthesis, even in the presence of damaged DNA templates. Mammals have multiple DNA polymerases specialized for TLS, including Polη, Polι, and Polκ. These enzymes show preferential bypass for different lesions. Proliferating cell nuclear antigen (PCNA), which functions as a sliding clamp for the replicative polymerase Polδ, also interacts with the three TLS polymerases. Although many PCNA-binding proteins have a highly conserved sequence termed the PCNA-interacting protein box (PIP-box), Polη, Polι, and Polκ have a noncanonical PIP-box sequence. In response to DNA damage, Lys-164 of PCNA undergoes ubiquitination by the RAD6-RAD18 complex, and the ubiquitination is considered to facilitate TLS. Consistent with this, these three TLS polymerases have one or two ubiquitin binding domains and are recruited to replication forks via interactions with ubiquitinated PCNA involving the noncanonical PIP-box and ubiquitin binding domain. However, it is unclear how these TLS polymerases interact with PCNA. To address the structural basis for interactions between different TLS polymerases and PCNA, we determined crystal structures of PCNA bound to peptides containing the noncanonical PIP-box of these polymerases. We show that the three PIP-box peptides interact with PCNA in different ways, both from one another and from canonical PIP-box peptides. Especially, the PIP-box of Polι adopts a novel structure. Furthermore, these structures enable us to speculate how these TLS polymerases interact with Lys-164-monoubiquitinated PCNA. Our results will provide clues to understanding the mechanism of preferential recruitment of TLS polymerases to the stalled forks.
Genomic DNA carrying genetic information is constantly damaged by various internal and external agents. Most types of DNA damage are removed by multiple DNA repair mechanisms, but some of them, especially those generating relatively small distortion of the DNA double helix structure, may escape DNA repair and persist in S-phase. When a replicative DNA polymerase encounters such a persisting lesion, it often stalls there. One way to continue replication past the lesion site is to replace the stalled replicative polymerase with a DNA polymerase specialized for translesion synthesis (TLS)4 that is able to incorporate nucleotides opposite DNA lesions. Because TLS polymerases have low fidelity and processivity, they are subsequently replaced by replicative polymerases after the lesion bypass is completed. To date, several TLS polymerases have been found in mammals, including Polη, Polι, Polκ, and REV1, which are all classified as Y-family DNA polymerases on the basis of similarity in their primary sequences (1).
Human Polη, Polι, and Polκ have been purified and extensively studied by in vitro experiments with DNA containing one of various types of lesion on the template strand (reviewed by Vaisman et al. (2) and Ohmori et al. (3)). Polη can efficiently incorporate two adenines opposite a thymine-thymine (T-T) cyclobutane pyrimidine dimer (4), one of the major photoproducts induced by UV irradiation. This function is consistent with the fact that defects in Polη result in a sunlight-sensitive and cancer-prone syndrome, xeroderma pigmentosum variant (5, 6). Polι efficiently incorporates an adenine opposite the 3′-T of the T-T (6-4)-photoproduct (7, 8). Although Polκ is unable to bypass a T-T cyclobutane pyrimidine dimer or (6-4)-photoproduct, it bypasses benzo[a]pyrene diol epoxide-adducted guanines by inserting the correct cytosine opposite the bulky lesion (9–11). Mouse cells lacking Polκ show high sensitivity to treatment with benzo[a]pyrene (12, 13). Thus, TLS polymerases have different lesion specificity.
Proliferating cell nuclear antigen (PCNA) is a ring-shaped homotrimeric protein that encircles DNA and tethers DNA polymerases to the primer terminus, thereby functioning as the sliding processivity factor of DNA replication. Each PCNA monomer is composed of two similar domains (N- and C-terminal domains) linked by a long loop termed the interdomain connecting loop (IDCL). PCNA interacts with a large number of proteins involved in replication, repair, cell cycle, chromatin assembly, and sister-chromatid cohesion (14). Most of these proteins have a conserved sequence, the so-called “PCNA-interacting protein box” (PIP-box) (15). The canonical PIP-box is composed of eight amino acid residues, QXXhXXaa, where the residue at position 1 (p1) is a conserved Gln, “h” at p4 is a hydrophobic residue, such as Met, Leu, or Ile, “a” at p7 and p8 is an aromatic residue, such as Phe or Tyr, and X is any residue. So far, the structures of human PCNA bound to a peptide derived from some proteins, such as the human p21 protein (16), or bound to the full-length protein of human FEN1 (flap endonuclease I) (17) have been determined, both of which include a canonical PIP-box. Those structures revealed that the interactions of canonical PIP-boxes with PCNA are very similar. In fact, the side chain of the conserved Gln at p1 in the canonical PIP-box intrudes into a pocket in PCNA (termed the “Q-pocket” in this work). Residues from p4 to p8 in the canonical PIP-box form a 310-helix, in which the hydrophobic residue at p4 and the two aromatic residues at p7 and p8 form a three-forked hydrophobic plug and fit into a hydrophobic pocket in PCNA (termed the “socket” in this work). In addition to these interactions of the PIP-box with PCNA, the C-terminal residues following the PIP-box in p21 (16) and FEN1 (17) form an extensive antiparallel β-sheet with the IDCL, whereas those of the p66 subunit of Polδ (18) and PL peptide (19), which also possess a canonical PIP-box, are not long enough to interact extensively with the IDCL.
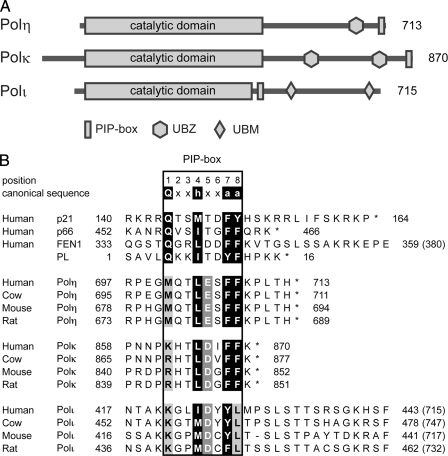
Previous studies have assigned a PCNA-binding sequence in human Polη (20), Polι (21, 22), and Polκ (23, 24), none of which has a canonical PIP-box sequence; for instance, the conserved Gln at p1 is replaced with Met in Polη and Lys in Polι and Polκ. Unlike these three TLS polymerases, REV1 does not seem to have any sequence similar to the PIP-box; rather, the BRCT domain located near the N terminus was reported to interact with nonmodified PCNA (25). The fact that Y-family DNA polymerases do not have a canonical PIP-box may be consistent with the notion that TLS polymerases, which are intrinsically error-prone, have lower affinity for PCNA than replicative polymerases, and hence TLS polymerases are recruited to the replication fork only when required. Polη physically and functionally interacts with PCNA, and it has a sequence similar to the PIP-box at its extreme C terminus, 702QTLESFFKPLTH713 (His-713 is the last residue in the entire protein (Fig. 1A), in which F707A/F708A substitution has been shown to notably reduce the interaction with PCNA in vitro (20). In vitro the DNA synthetic activity of Polκ is also stimulated by the addition of PCNA, RFC (replication factor C) and RPA (replication protein A), and, from its similarity to the Polη C-terminal sequence, the Polκ sequence 862KHTLDIFFK870 (Lys-870 is the last residue in the entire protein) located at the extreme C terminus has been postulated to be a PCNA-binding site (26). Although enhanced green fluorescent protein-tagged Polκ was found to form nuclear foci that mostly co-localized with PCNA in cells after UV irradiation or hydroxyurea treatment, the same construct with an alteration in the above putative PIP-box sequence (either deletion of the nine amino acid residues spanning positions 862–870 or the F868A/F869A substitution) did not form such foci (24). Thus, PCNA binding is considered to be one of the requirements for the formation of nuclear foci of Polκ and Polη in DNA-damaged cells (24, 27). Unlike Polη and Polκ, which contain a PIP-box at the extreme C terminus, the PCNA-binding site of Polι (the total length is 715 amino acids) is located in a central region of the protein; two research groups assigned the PIP-box to residues 420KKGLIDYY427 by showing that the Y426A/Y427A substitution greatly reduced Polι interaction with PCNA (22, 28). Furthermore, green fluorescent protein-tagged Polι formed nuclear foci in cells after UV irradiation, but a derivative with Y426A/Y427A substitution did not (22).
FIGURE 1.
A, schematic features of the primary structures of human Polη, Polκ, and Polι. Polη has a UBD termed the UBZ domain, which is a CCHH-type zinc finger domain. Polκ has two UBZ domains, which are CCHC-type zinc finger domains. Polι has two UBDs termed the UBM domains. B, sequence alignment of PCNA-interacting regions that include canonical and noncanonical PIP-boxes. The box delineates the eight residues of canonical and noncanonical PIP-boxes, which are numbered as shown above the canonical sequence. The canonical PIP-box elements at positions 1, 4, 7, and 8 are shown on a black background. Acidic residues conserved at p5 of the three TLS polymerases are shown on a gray background. The conserved methionines of Polη, the basic residues of Polκ and Polι at p1, and the leucine of Polι at p8 are shown on a light gray background. Asterisks indicate the C termini of the proteins, and numbers in parentheses are the total number of residues in the full-length proteins.
Recently, PCNA was shown to undergo ubiquitination at Lys-164 by the RAD6-RAD18 complex, which is E2-E3 ubiquitin ligase, in DNA-damaged cells (29); this ubiquitination is now considered to be an important signal for DNA synthesis by TLS polymerase at a site of DNA damage in both yeast and human cells (30–32). Consistent with this, all four human Y-family TLS polymerases contain one or two ubiquitin-binding domains (UBDs) (33, 34). Here we present the first crystal structures of PCNA bound to the noncanonical PIP-box peptides of Polη, Polι, and Polκ and reveal that those noncanonical PIP-boxes interact with PCNA in different ways, both from one another and from canonical PIP-box peptides. Our results demonstrate that the PIP-box of Polκ has much lower affinity for PCNA than those of Polη and Polι. Furthermore, mutational and structural analyses reveal that the PIP-box sequence of Polι differs from that previously assigned by one residue and that it has a novel structure with multiple intramolecular interactions. We discuss how the different interactions between PCNA and the noncanonical PIP-boxes of the three TLS polymerases correlate with interactions between monoubiquitinated PCNA and the UBDs of these polymerases.
EXPERIMENTAL PROCEDURES
PCNA and TLS Polymerase Peptides
Recombinant human PCNA was expressed in Escherichia coli BL21(DE3) harboring a pT7-PCNA expression vector (35). The protein was purified by HiTrap Q HP (GE Healthcare), HiTrap Phenyl HP (GE Healthcare), and HiLoad 26/60 Superdex 200 (GE Healthcare), as described for the PCNA G178S mutant (36). All peptides used in this work were commercially synthesized (GenScript Co.).
Surface Plasmon Resonance (SPR) Assay
The SPR assay was performed with BIACORE 2000. Synthetic peptides were immobilized on the carboxylated dextran matrix of CM5 sensor chips through the sulfhydryl group of the N-terminal cysteine by thiol activation chemistry, according to the manufacturer's protocol. Varying concentrations of PCNA protein in running buffer (10 mm HEPES-NaOH, pH 7.5, 150 mm NaCl, 3 mm EDTA, 0.005% surfactant P20) were injected over the sensor chip surface at a flow rate of 20 μl/min at 25 °C. The contact and dissociation times were 60 and 120 s, respectively. Binding constants were determined from the titration curves using BIAevaluation 3.0 software. The peptide-protein interaction signal minus the control cell signal was analyzed. The Kd value was calculated according to equilibrium value analysis by averages of measurement signals at respective concentrations. Peptides used in the SPR experiments are shown in Table 1.
TABLE 1.
Affinity (Kd) of Polη, Polκ, and Polι peptides interacting with PCNA Boldface and italic characters indicate PIP-box residues and notable alterations, respectively. Asterisks indicate the C termini of the corresponding proteins. ND, not detected.
| Peptide (region) | Property | Sequence | Kd |
|---|---|---|---|
| μm | |||
| Polη-(694–713) | Wild type | CKRPRPEGMQTLESFFKPLTH* | 0.40 |
| M701Q | CKRPRPEGQQTLESFFKPLTH* | 0.10 | |
| F707A/F708A | CKRPRPEGMQTLESAAKPLTH* | ND | |
| ΔPLTH | CKRPRPEGMQTLESFFK–– | ND | |
| Polκ-(851–870) | Wild type | CSTSKKIKPNNPKHTLDIFFK* | ND |
| Polκ-(856–870) | Wild type | CIKPNNPKHTLDIFFK* | ND |
| K862Q | CIKPNNPQHTLDIFFK* | ND | |
| +PLTH | CIKPNNPKHTLDIFFKPLTH | 4.9 | |
| Polι-(419–434) | Wild type | CAKKGLIDYYLMPSLST | 0.39 |
| Y426F/Y427F | CAKKGLIDFFLMPSLST | ND | |
| Y426A/Y427A | CAKKGLIDAALMPSLST | ND | |
| Y426F | CAKKGLIDFYLMPSLST | 1.5 | |
| Y427F | CAKKGLIDYFLMPSLST | ND | |
| L428A | CAKKGLIDYYAMPSLST | 0.67 |
Crystallizations of PCNA in Complex with TLS Polymerase Peptides
PCNA Bound to Polη Peptide—A 13-fold molar excess of the Polη-(694–713) peptide with an N-terminal cysteine used for the SPR assay was incubated with PCNA (5.4 mg/ml). Co-crystals of PCNA bound to the Polη-(694–713) peptide were grown by vapor diffusion in hanging drops at 20 °C. Typical hanging drops were prepared by mixing 0.72 μl of the PCNA-peptide solution, 0.16 μl of 100 mm praseodymium(III) acetate, 0.16 μl of 1.0 m glycine, and 0.72 μl of a reservoir solution containing 5% polyethylene glycol 10,000, 100 mm sodium citrate buffer, pH 3.6, 200 mm ammonium sulfate, and 5% glycerol. Crystals were transferred to a cryobuffer (20% polyethylene glycol 10,000, 100 mm citric acid, pH 3.6, 200 mm ammonium sulfate, and 20% glycerol) for cryoprotection.
PCNA Bound to Polκ Peptide—A 10-fold molar excess of the Polκ-(861–870) peptide with Pro-Leu-Thr-His fused to the C terminus termed Polκ-(861–870)PLTH was incubated with PCNA (6.3 mg/ml). Co-crystals of PCNA bound to the Polκ-(861–870)PLTH peptide were grown by vapor diffusion in hanging drops at 20 °C. Typical hanging drops were prepared by mixing 2.25 μl of the PCNA-peptide solution, 0.5 μl of 1.0 m glycine, and 2.25 μl of a reservoir solution containing 9% (w/w) polyethylene glycol 10,000, 100 mm trisodium citrate, pH 5.2, and 200 mm zinc sulfate. Crystals were transferred to a cryobuffer (9% polyethylene glycol 10,000, 100 mm trisodium citrate, pH 5.2, 200 mm zinc sulfate, and 20% glycerol) for cryoprotection.
PCNA Bound to Polι Peptide—PCNA bound to the Polι-(415–437) peptide was co-crystallized, and the crystals were cryoprotected, as described (37).
Data Collection and Structure Analysis
The crystals used for data collection were flash-frozen under an N2 gas stream at 100 K. X-ray diffraction data for PCNA bound to the Polκ-(861–870)PLTH peptide were collected by using an Jupiter210 CCD detector on beamline BL38B1 at SPring-8, and those for PCNA bound to the Polη-(694–713) and Polι-(415–437) peptides were collected by using an ADSC Quantum 210 CCD detector on beamline NW12A at the Photon Factory Advanced Ring. All diffraction data were processed by using the program HKL2000 (38). Data collection statistics are given in Table 2. The structures were solved by molecular replacement with the program MOLREP (39) using human PCNA (Protein Data Bank code 1VYM (19)) as a search model. The structures were manually improved with the program COOT (40) and refined with the programs CNS (41) and REFMAC (42). Geometries of the final structures were validated with the program PROCHECK (43). Refinement statistics are summarized in Table 2. Coordinates and structure factors have been deposited in the Protein Data Bank. Figs. 2 and 3 were prepared by the program PyMOL (available on the World Wide Web), and all figures were modified by the programs PHOTOSHOP and ILLUSTRATOR (Adobe Systems).
TABLE 2.
Data collection and refinement statistics Values in parentheses are those for the highest resolution shell (2.80-2.70, 2.59-2.50, or 2.38-2.30 Å for PCNA bound to the Polη, Polκ, or Polι peptide, respectively).
| PCNA-Polη | PCNA-Polκ | PCNA-Polι | |
|---|---|---|---|
| Data collection | |||
| Wavelength (Å) | 1.0000 | 1.0000 | 1.0000 |
| Space group | P43212 | P1 | C2 |
| a (Å) | 82.0 | 74.8 | 167.6 |
| b (Å) | 82.0 | 74.7 | 68.8 |
| c (Å) | 310.4 | 109.0 | 90.2 |
| α (degrees) | 90 | 87.3 | 90 |
| β (degrees) | 90 | 77.5 | 95.0 |
| γ (degrees) | 90 | 80.3 | 90 |
| Resolution (Å) | 50.0-2.70 | 50.0-2.50 | 50.0-2.30 |
| Observed reflections | 72,590 | 191,578 | 181,530 |
| Unique reflections | 27,933 | 71,006 | 44,815 |
| R-mergea (%) | 9.3 (32.8) | 8.2 (24.1) | 6.7 (33.8) |
| Completeness (%) | 92.1 (81.3) | 90.6 (84.3) | 98.0 (86.4) |
| 〈I〉/σ〈I〉 | 8.1 (2.5) | 2.7 (2.3) | 13.5 (3.2) |
| Refinement | |||
| Resolution (Å) | 20.0-2.70 | 20.0-2.50 | 20.0-2.30 |
| Refined reflections | 26,440 | 67,254 | 42,715 |
| Free reflections | 1,398 | 3,575 | 2,249 |
| R-factorb (%) | 21.8 | 22.8 | 19.6 |
| R-freeb (%) | 28.5 | 28.9 | 25.1 |
| Root mean square deviation bond lengths (Å) | 0.019 | 0.020 | 0.020 |
| Root mean square deviation bond angles (degrees) | 1.902 | 1.872 | 1.790 |
| Ramachandran plot | |||
| Most favored (%) | 83.9 | 89.3 | 91.6 |
| Additional allowed (%) | 14.4 | 8.9 | 7.9 |
| Generously allowed (%) | 1.7 | 1.7 | 0.4 |
| Disallowed (%) | 0.0 | 0.0 | 0.0 |
| Protein Data Bank ID | 2ZVK | 2ZVL | 2ZVM |
R-merge = ΣhklΣi|Ii(hkl)i – 〈I(hkl)〉|/ΣhklΣiIi(hkl)
R-factor and R-free = Σhkl||Fo| – |Fc||/Σhkl|Fo|, where R-free is calculated by using the free reflection set (5% of the total reflection), which was held aside throughout refinement
FIGURE 2.
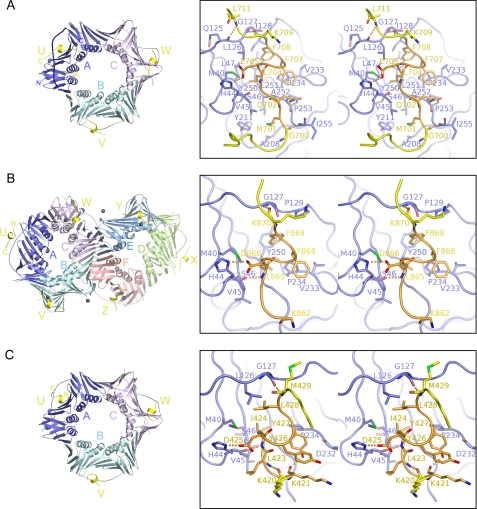
Structure of PCNA bound to the Polη (A), Polκ (B), or Polι (C) peptide. Overall structures in the asymmetric units are shown by ribbon representations (left). TLS polymerase peptides are shown in yellow. In A, the IDCL in the A-molecule is shown by a thick coil. The black spheres in B are zinc ions from the crystallization buffer. The interactions of the U-molecules of Polη, Polκ, and Polι with the A-molecule of PCNA (blue) are shown as representative structures by stereo pairs (right). For clarity, the noncanonical PIP-boxes are colored orange. The regions preceding and following the PIP-boxes are shown in yellow. Electrostatic interactions are indicated by orange dots.
FIGURE 3.
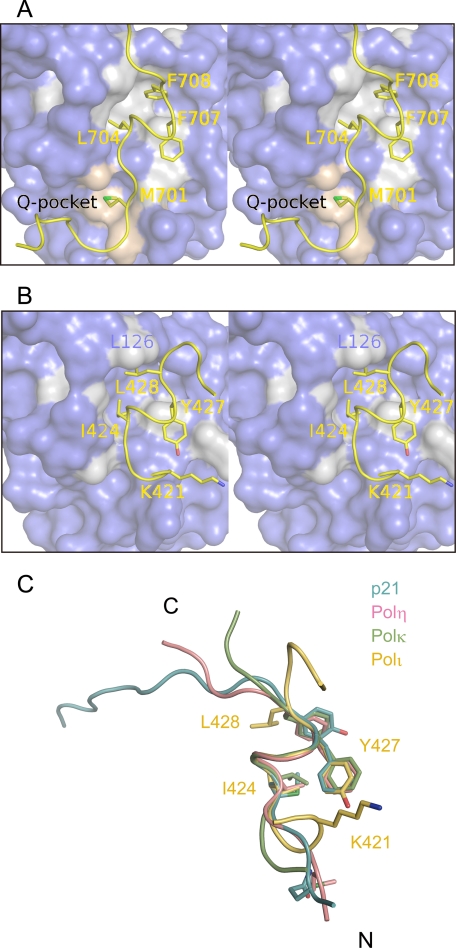
Hydrophobic plug-socket interaction of the Polη (A) or Polι (B) peptide with PCNA and superimposition of PIP-box structures bound to PCNA (C). A and B, PCNA is shown by a surface model, and residues of PCNA that interact with the three-forked hydrophobic plug are colored gray. PIP-box residues are shown by stick models. Residues of PCNA that interact with the Met-701 (p1) residue of Polη in the Q-pocket are colored light pink. C, structures of p21, Polη, Polκ, and Polι bound to PCNA are shown in light blue, pink, green, and yellow, respectively. PIP-box residues are shown by stick models, and only some PIP-box residues of Polι are denoted.
Pull-down Assays
DNA fragments encoding full-length human Polη and Polι were subcloned into pGEX6P1 and modified pCold-GST (44), resulting in pGEX6P1-Polη and pCold-GST-Polι, respectively. E. coli strains JM109(DE3) harboring a plasmid encoding tRNAs corresponding to rare codons, and BL21-Codon-Plus(DE3)-RIPL (Stratagene) were transformed by pGEX6P1-Polη and pCold-GST-Polι, respectively. Both GST-Polη and GST-Polι were expressed and purified by the following procedure. Transformants were grown at 37 °C, protein expression was induced by 0.5 mm IPTG at OD600 = 0.50, and the cells were allowed to grow for 22 h at 18 °C. All subsequent procedures were performed at 4 °C. Bacterial cells were harvested by centrifugation for 10 min at 5,000 × g, and the pellets were suspended in buffer I (40 mm HEPES-NaOH, pH 7.4, 10% glycerol, 10 mm β-mercaptoethanol, 50 μm Zn(OAc)2 and 1 mm phenylmethylsulfonyl fluoride) containing 500 mm NaCl. Cells were lysed by sonication, and cell lysates were clarified by centrifugation at 40,800 × g for 45 min. The supernatants were loaded onto glutathione-Sepharose 4B beads (GE Healthcare) equilibrated with buffer I containing 500 mm NaCl. The beads were washed with buffer I containing 2 m NaCl and equilibrated in buffer I containing 500 mm NaCl. GST-fused Polη and Polι were eluted with buffer I containing 500 mm NaCl, 10 mm MgCl2, 0.1 mm EDTA, and 50 mm reduced glutathione. The samples were concentrated with Amicon Ultra 50,000 molecular weight cut-off (Millipore) and dialyzed by buffer II (40 mm HEPES-NaOH, pH 7.4, 150 mm NaCl, 10% glycerol, 10 mm β-mercaptoethanol, 50 μm Zn(OAc)2, 0.1 mm EDTA) containing 0.05% Tween 20. To constitute complexes, 200 μg of GST-Polη or GST-Polι was mixed with 200 μg of wild type (WT) PCNA or the H44A mutant PCNA in 400 μl of buffer II containing 0.05% Tween 20. The solutions were incubated for 10 h at 4 °C and then immobilized on 40 μl of glutathione-Sepharose 4B beads. Next, the beads were washed three times with 1 ml of buffer II containing 0.1% Tween 20, and the bound proteins were eluted with 35 μl of buffer II containing 40 mm reduced glutathione, 10 mm MgCl2, and 0.05% Tween 20. The eluted proteins were separated by 12.5% SDS-PAGE and subjected to Western blot with anti-GST (GE Healthcare) or anti-PCNA antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Densitometry analyses were performed using ImageJ software.
RESULTS
Characterization of PIP-boxes of Human Polη, Polκ, and Polι—To investigate direct interactions of a peptide carrying the PIP-box of Polη, Polκ, or Polι with PCNA in vitro, we employed an SPR assay (Table 1). We examined a 21-mer peptide designated Polη-(694–713), which carries the 694–713 region of Polη plus an N-terminal cysteine added for coupling to the sensor chip, for binding to PCNA. As a control, we used a 23-mer peptide carrying the PIP-box of the human p21 protein that is known to have a very strong affinity for PCNA. The SPR measurement for the p21 peptide allowed us to estimate the dissociation constant (Kd) as 65 nm, which is in good agreement with the value of 87.7 nm obtained previously by isothermal titration calorimetry (45). The Polη-(694–713) peptide showed a weaker affinity for PCNA with an estimated Kd of 0.40 μm. Another Polη peptide with F707A/F708A substitution showed no SPR signal for PCNA binding, indicating that the interaction between PCNA and the Polη-(694–713) peptide was sequence-specific.
In contrast to the Polη peptide, we could not detect any signal for PCNA binding with either a 16-mer Polκ-(856–870) or a 21-mer Polκ-(851–870) peptide carrying the C-terminal PIP-box of Polκ. Even a 16-mer peptide in which Lys-862 was changed to Gln, the residue conserved at p1 in PIP-box sequences, did not show an SPR signal for PCNA binding. However, we found that the addition of the four residues PLTH, corresponding to the C-terminal four residues of Polη, to the C terminus of Polκ-(856–870) peptide markedly increased the affinity for PCNA, up to the estimated Kd value of 4.9 μm. Conversely, deletion of the C-terminal four residues PLTH from the Polη-(694–713) peptide strongly decreased binding to PCNA. These results indicate that the presence of several residues following FF is crucial for the noncanonical PIP-boxes to interact with PCNA and that the original PIP-box sequence of Polκ has a much lower affinity for PCNA as compared with that of Polη.
In our SPR assays, a 17-mer Polι-(419–434) peptide containing the PCNA-binding sequence of Polι (420KKGLIDYY427) showed affinity for PCNA with an estimated Kd value of 0.39 μm, which was very similar to that of the Polη-(694–713) peptide. Unexpectedly, however, not only Y426A/Y427A substitution but also Y426F/Y427F substitution in the Polι-(419–434) peptide resulted in greatly reduced binding to PCNA. Furthermore, Y427F substitution also abolished binding to PCNA, whereas Y426F substitution did not cause a big reduction in PCNA binding. These results indicate that the two Tyr residues in the noncanonical PIP-box of Polι are not equivalent but that Tyr-427 has a stronger role than Tyr-426. In fact, the YY sequence is replaced with CY or CF in the corresponding sequence of the mouse or rat homologues of Polι (Fig. 1B). When we tested a peptide carrying the PIP-box sequence of mouse or rat Polι (418AKKGPMDCYLTSLST432 or 438AKKGPMDCFLTSSLST453 with N-terminal cysteine) for binding to PCNA by SPR, we detected a strong PCNA-binding signal with the mouse Polι peptide, similar to that of the human Polι peptide, but observed little signal with the rat Polι peptide (supplemental Fig. 1). As described below, our results from structural analysis reveal that the noncanonical PIP-box of Polι is 421KGLIDYYL428 and not 420KKGLIDYY427 as previously considered. As shown in Fig. 1B, this revised alignment therefore indicates that an acidic residue is present in common at p5 in all of the three TLS polymerases.
Overall Structures of PCNA in Complex with TLS Polymerase Peptides—One PCNA trimer bound to three Polη-(694–713) peptides is present in the asymmetric unit (Fig. 2A). One of the three Polη peptides (designated U-, V-, and W-molecules) is bound to each subunit of the PCNA trimer (A-, B-, and C-molecules, respectively). All residues of the U-molecule are assigned unambiguously in the electron density map except for the C-terminal end residue His-713. The N-terminal cysteine preceding Lys-694 in the U-molecule, which was added for SPR experiments, is linked to Cys-27 of the symmetry-related PCNA trimer, but the structures of the PIP-boxes of the U-, V-, and W-molecules are essentially the same. Similar to PCNA binding to the Polη-(694–713) peptide, one PCNA trimer is bound to three Polι-(415–437) peptides in the asymmetric unit (Fig. 2C), and one of the three Polι peptides (U-, V-, and W-molecules) is bound to each subunit of the PCNA trimer (A-, B-, and C-molecules, respectively). Structures of U-, V-, and W-molecules of Polι peptides are also essentially the same. As described above, the original Polκ peptides showed no detectable binding to PCNA, but the addition of four residues (PLTH) to the C terminus of the Polκ peptide resulted in a remarkable increase in the affinity for PCNA. Thus, we successfully co-crystallized PCNA bound to a Polκ-(861–870) peptide with PLTH fused to the C terminus, designated Polκ-(861–870)PLTH. The asymmetric unit contains two PCNA trimers (Fig. 2B); one comprises A-, B-, and C-molecules, the other comprises D-, E-, and F-molecules, and both are bound to three Polκ-(861–870)PLTH peptides. The peptides binding to two subunits of each trimer (V-, W-, Y-, and Z-molecules) contain a zinc ion from the crystallization buffer, resulting in a conformational change in the N-terminal regions of V-, W-, Y-, and Z-molecules of the Polκ-(861–870)PLTH peptide (data not shown). Below, therefore, we discuss the structures of the U-molecules of the Polη-(694–713), Polι-(415–437), and Polκ-(861–870)PLTH peptides as representative structures to investigate the interaction with PCNA. These PIP-box peptides in the complexes are simply abbreviated as the Polη, Polι, and Polκ peptides, respectively, unless otherwise noted.
Interactions of the Polη and Polκ Peptides with PCNA—The structure of the noncanonical PIP-box of the Polη peptide bound to PCNA is apparently similar to that of the canonical PIP-box (Figs. 2A and 3C), but the PCNA interaction with the residue at p1 differs between the noncanonical and canonical PIP-boxes. In fact, the side chain of Met-701 (p1) in the noncanonical PIP-box of Polη intrudes into the Q-pocket and interacts with the Val-45, Ala-208, Tyr-211, and Leu-251 residues of PCNA in a hydrophobic manner (Figs. 2A and 3A), whereas the conserved Gln at p1 in the canonical PIP-box interacts with the Q-pocket in a hydrophilic manner (16–19). Interestingly, a Polη peptide with an M701Q substitution shows higher affinity for PCNA than the wild type Polη peptide (Table 1 and supplemental Fig. 2, A and B). On the other hand, the side chain of Lys-862 (p1) in the noncanonical PIP-box of the Polκ peptide is highly disordered (Fig. 2B), thereby indicating that Lys-862 (p1) is unlikely to interact with the Q-pocket. This observation is consistent with the expectation that the side chain of Lys is too large to fit into the Q-pocket. In the Polκ homologues of cows, mice, and rats, this Lys residue is replaced by Arg, which has a more bulky side chain and is probably also unable to intrude into the Q-pocket as well (Fig. 1B).
By contrast, interactions by the p4–p8 residues in the PIP-boxes of Polη and Polκ are very similar to each other and to those of the corresponding residues in the canonical PIP-box (Fig. 3C). These five residues form helical conformations, and a hydrophobic plug formed by three forks corresponding to the side chains of Leu-704 (p4), Phe-707 (p7), and Phe-708 (p8) in Polη or Leu-865 (p4), Phe-868 (p7), and Phe-869 (p8) in Polκ fits into a hydrophobic socket comprising the Met-40, Val-45, Ser-46, Leu-47, Leu-126, Ile-128, Pro-234, Tyr-250, and Pro-252 residues of PCNA (Figs. 2 (A and B) and 3 (A and C)). F707A/F708A substitution in the Polη peptide abolishes binding to PCNA (Table 1), indicating the significance of the hydrophobic plug-socket interactions between the PIP-boxes and PCNA.
The structures of the regions following the PIP-boxes of Polη and Polκ peptides are distinct from those of the corresponding regions of p21 and FEN1. The regions of p21 and FEN1 form an extensive antiparallel β-sheet with the IDCL of PCNA. However, the corresponding region of the Polη or Polκ peptide is much shorter than those of p21 and FEN1 and forms only one or a few hydrogen bonds with the IDCL, as in the cases of the p66 and PL peptides that have short regions following the PIP-box. Lys-709 and Leu-711 of Polη in the U-molecule form hydrogen bonds via main-chain atoms with Gly-127 and Gln-125 in the IDCL, respectively (Fig. 2A). In contrast, Leu-711 of Polη is disordered in both the V- and W-molecules. Deletion of PLTH in the Polη peptide results in a significantly reduced interaction with PCNA (Table 1), implying that these C-terminal residues contribute to stabilizing the interaction between Lys-709 of Polη and Gly-127 of PCNA. This implication is consistent with the interaction of Polκ-(861–870)PLTH with PCNA. Lys-870, the end C-terminal residue in the original Polκ sequence, only interacts with IDCL and the PLTH extension does not interact with PCNA (Fig. 2B), suggesting that the extended residues function to stabilize the interaction between Lys-870 of Polκ and Gly-127 of PCNA by possibly preventing hydration of the terminal oxygen of Lys-870, as in Polη.
Interactions of the Polι Peptide with PCNA—Previously, the noncanonical PIP-box of Polι was assigned to residues 420KKGLIDYY427 (22, 28). Our structure shows, however, that Ile-424, Tyr-427, and Leu-428 of Polι form a hydrophobic plug, instead of Leu-423, Tyr-426, and Tyr-427, as expected from the previous PIP-box assignment (Figs. 2C and 3B), thus indicating that the noncanonical PIP-box of Polι should be 421KGLIDYYL428. In addition, our results show that the structure of the noncanonical PIP-box of Polι differs markedly from those of the PIP-boxes of Polη and Polκ in the following features.
First, Lys-421 (p1) of Polι does not interact with the Q-pocket, as might be expected by comparison with Lys-862 (p1) of Polκ. Unexpectedly, however, the carbonyl oxygen of the Lys-421 (p1) residue forms an intramolecular hydrogen bond with Oη of Tyr-427 (p7), and the aliphatic moiety of the side chain of Lys-421 (p1) is in close contact with the aromatic ring of Tyr-426 (p6). Furthermore, the carbonyl oxygen of the preceding Lys-420 residue also forms an intramolecular hydrogen bond with the amide nitrogen of Leu-423 (p3), thereby inducing a β-bend-like structure. Importantly, our SPR experiment shows that Y427F substitution abolishes affinity for PCNA (Table 1), indicating that the intramolecule hydrogen bond between the carbonyl oxygen of Lys-421 (p1) and Oη of Tyr-427 (p7) is crucial for the interaction with PCNA.
Second, the hydrophobic plug-socket interaction of Polι with PCNA differs locally but significantly from the interactions of Polη, Polκ, and the canonical PIP-boxes (Fig. 3). The side chain of Leu-428 (p8) in the three-forked hydrophobic plug of Polι does not intrude into the inner side of the hydrophobic socket, because the side chain of Leu is smaller than that of Phe or Tyr. Thus, the side chain of Leu-428 (p8) moves out of the socket, and instead, Leu-428 (p8) appears to form van der Waals contact with Leu-126 in the IDCL of PCNA; indeed, a Polι peptide with L428A substitution has a larger Kd for PCNA as compared with the WT peptide. This structural feature of Polι crucially influences the structure of the region following the PIP-box, as described below. The structure of the Polι peptide also differs in the region following the PIP-box from those of the Polη and Polκ peptides, as well as the p21, p66, PL peptides, and FEN1. As described above, the C-terminal region of the Polη or Polκ peptide forms only one or a few hydrogen bonds with the IDCL (Fig. 2, A and B), similar to the p66 and PL peptides. On the other hand, because the PIP-box of Polι lies in an internal region, as opposed to near the C terminus, as in Polη and Polκ, we expected that the region following the PIP-box of Polι might form an extensive antiparallel β-sheet with IDCL, as observed in p21 and FEN1. Surprisingly, however, the corresponding residues do not form an antiparallel β-sheet with the IDCL and are thereby exposed to solvent (Figs. 2C and 3C). In Polη, Polκ, and canonical PIP-boxes, the carbonyl oxygen of the residue just following p8 interacts with the amide nitrogen of Gly-127 in the IDCL (Lys-709 and Lys-870 in Fig. 2, A and B, respectively). However, the structural feature of p8 described above prevents the carbonyl oxygen of the corresponding residue of Polι (Met-429) from forming a hydrogen bond with the amide nitrogen of Gly-127 in the IDCL. Instead, an alternative hydrogen bond is formed between the amide nitrogen of the Met-429 residue and the carbonyl oxygen of Gly-127 in the IDCL (Fig. 2C). As a consequence, the region following the PIP-box of the Polι peptide cannot form an antiparallel β-sheet with the IDCL, resulting in exposure to solvent. Notable interactions of Polι with PCNA appear to be much fewer as compared with Polη (Fig. 2, A and C). Nevertheless, the Polι peptide achieves an affinity for PCNA comparable with that of the Polη peptide (Table 1), which is discussed in more detail under “Discussion.”
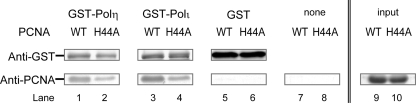
The revised alignment of the noncanonical PIP-boxes of Polη, Polκ, and Polι has revealed that they share an acidic residue at p5 (Fig. 1B). Remarkably, each of these acidic residues, Glu-705 of Polη, Asp-866 of Polκ, and Asp-425 of Polι, forms an ion pair with His-44 of PCNA (Fig. 2). Such an ion pair is also observed in the human FEN1-PCNA complex, where the Asp-341 (p5) residue interacts with His-44 (17, 18). Our SPR assay indicated that a PCNA mutant protein with an H44A substitution shows much weaker binding to the Polη peptide as compared with the WT PCNA protein (supplemental Fig. 2A). Similarly, a Polη peptide with an E705A substitution shows little binding to either WT or the H44A mutant PCNA protein (supplemental Fig. 2C). Furthermore, to examine whether the interaction at p5 contributes to the interaction between PCNA and full-length Polη or Polι, we carried out a pull-down assay using WT and H44A mutant PCNA proteins. As shown in Fig. 4, compared with WT, the efficiency of binding of H44A mutant PCNA with Polη or Polι was markedly reduced to about 25%. These results indicate that this interaction at p5 directly contributes to stable complex formation between PCNA and these TLS polymerases. As shown in Fig. 1B, the acidic residue is highly conserved, indicating that such an interaction is a common feature in the noncanonical PIP-box of these TLS polymerases in higher eukaryotes.
FIGURE 4.
Physical interactions of Polη and Polι with PCNA. GST-fused TLS polymerase (GST-Polη or GST-Polι) was mixed with WT or H44A mutant PCNA (lanes 1–4). As controls, GST or buffer was mixed with PCNA (WT or H44A mutant) (lanes 5–8). Samples were bound to glutathione-Sepharose beads, which were then washed and eluted by glutathione-containing buffer. Eluents were analyzed by Western blotting. Inputs of WT and H44A are also shown in lanes 9 and 10, respectively.
DISCUSSION
We have successfully determined the crystal structures of PCNA bound to three peptides, each containing the noncanonical PIP-box of Polη, Polι, or Polκ. The structures reveal that the interactions of these PIP-boxes with PCNA are distinct from one another and also from those of peptides containing a canonical PIP-box (Figs. 2 and 3C). To our knowledge, our structural data are the first to describe the interaction of a noncanonical PIP-box with PCNA, and they reveal many novel features of PCNA interactions with PIP-boxes. The structural features of each TLS polymerase must be widely conserved in higher eukaryotes, because the noncanonical PIP-box of each TLS polymerase shows considerable conservation (Fig. 1B).
Met-701 (p1) of Polη interacts with the Q-pocket of PCNA in a hydrophobic manner, which is unprecedented in PIP-box-PCNA interactions. We found that M701Q substitution results in a clear increase in binding to PCNA (Table 1), indicating that Met-701 (p1) has special importance in modulating the binding affinity of Polη for PCNA such that the error-prone DNA polymerase avoids competition with replicative polymerases in the normal replication fork. This also seems to be the case for the yeast Polη, which has Ser-621 at p1. Because serine is too short to intrude into the Q-pocket, the affinity of yeast Polη for PCNA is thought to be weaker than that of yeast Pol32, which is the PCNA-interacting subunit of Polδ and has the canonical PIP-box. Thus, regulation of affinity for PCNA by the residue at p1 could be generalized in the case of Polη homologues.
Furthermore, we have clearly demonstrated that the PIP-box sequence of Polι is 421KGLIDYYL428. The side chains of Lys-421 (p1) of Polι and Lys-862 (p1) of Polκ do not interact with the Q-pocket unlike that of Met-701 (p1). It therefore seems that no residue preceding p4 in the Polι and Polκ peptides forms a hydrogen bond with PCNA (Fig. 2, B and C). Therefore, given that the number of hydrogen bonds between the Polι peptide and PCNA is much fewer than that between the Polη peptide and PCNA, how does the Polι peptide interact with PCNA with an affinity comparable with that of the Polη peptide? Our structure reveals that Polι forms multiple intramolecular hydrogen bonds and stable conformation, which is crucial in its interaction with PCNA. In addition, the high affinity of Polι might be also explained by shape complementarity of the protein-protein interfaces (SC), which provides an idea of the goodness of fit between two protein surfaces (46). PCNA-Polη and PCNA-Polι have SC values of 0.75 and 0.78, respectively, whereas PCNA-Polκ has a value of 0.66; thus, these SC values are consistent with affinities for PCNA. Although the number of marked interactions of the Polι peptide with PCNA is less than that of the Polη peptide, the formation of a Polι shape compatible with PCNA is stabilized by multiple intramolecular interactions such that the Polι peptide achieves a high affinity for PCNA comparable with that of the Polη peptide. The results shown in this study also demonstrate that the PIP-box of Polκ has much weaker affinity for PCNA as compared with Polη or Polι.
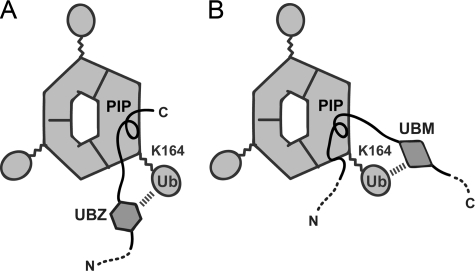
PCNA is monoubiquitinated at Lys-164 by the RAD6-RAD18 complex upon DNA damage in Saccharomyces cerevisiae and human cells (29–32). This monoubiquitination is believed to be essential for recruitment of TLS polymerase at a site of DNA damage; however, precisely how ubiquitination at Lys-164 of PCNA influences its interaction with a TLS polymerase containing a UBD remains elusive. The present study provides significant clues to the interaction of the UBDs of the three TLS polymerases with Lys-164-monoubiquitinated PCNA. Polη and Polκ have one and two UBDs (called the UBZ domain in both Polη and Polκ), respectively, at the N-terminal side of their PIP-box (Fig. 1A). As shown in Fig. 5A, the single UBZ domain in Polη and one of the two UBZ domains in Polκ (the UBZ closer to the PIP-box) are more likely to interact with the ubiquitin moiety linked to Lys-164 with the subunit of trimeric PCNA bound to the PIP-box than to interact with the ubiquitin moiety linked to Lys-164 with one of the adjacent subunits, because the distances between the UBZ and PIP-box are not so long (∼40 residues in Polη and ∼60 in Polκ). By contrast, Polι has two UBDs (called UBM domains in Polι and REV1) at the C-terminal side of the PIP-box (Fig. 1A). As mentioned above, the residues following the PIP-box of Polι are not engaged in an extensive β-sheet formation with the IDCL of PCNA. This would enable the proximal UBM domain to be positioned properly to interact with the ubiquitin moiety linked to Lys-164 with the subunit bound to the PIP-box, as shown in Fig. 5B. Although the relationship between the positions of the UBD and the PIP-box differs between these TLS polymerases, the respective PIP-box and UBD of each TLS polymerase interact within the same subunit of the monoubiquitinated PCNA.
FIGURE 5.
Proposed models of the interaction of Lys-164-monoubiquitinated PCNA with Polη or Polκ (A) and Polι (B). The ubiquitin moieties linked to Lys-164 are shown by ellipsoids. N- and C-terminal sides of TLS polymerase fragments are indicated by N and C, respectively.
More recently, the solution structure of UBZ domain of Polη was determined by NMR (47). The Kd in the interaction between the UBZ domain and free ubiquitin was estimated to be around 73 (by NMR) to ∼81 μm (by isothermal titration calorimetry), which is similar to the Kd values of many other UBDs (48) but much larger than that of the Polη PIP-box peptide to nonmodified PCNA (0.40 μm). This implies that the PIP-box makes a more significant contribution to the interaction between Polη and ubiquitinated PCNA than does the UBZ domain, thereby limiting the binding of Polη to ubiquitinated PCNA rather than other ubiquitinated proteins that are abundantly generated in DNA-damaged cells.
In summary, this study has revealed that the noncanonical PIP-boxes of Polη, Polι, and Polκ interact with PCNA differently from one another, explaining why Polη, Polι, and Polκ each has a lower affinity for PCNA than replicative polymerase with a canonical PIP-box. Our results also provided a structural basis to explain why Polη is recruited to sites of DNA damage, in preference to Polκ. This study revealed a very novel structure of the Polι PIP-box, probably correlated with its position relative to the UBDs that coordinately strengthen binding to ubiquitinated PCNA for TLS.
Supplementary Material
Acknowledgments
We thank Drs. N. Shimizu, K. Hasegawa, H. Sakai, M. Kawamoto, and M. Yamamoto for data collection at SPring-8 and Drs. Y. Yamada, N. Matsugaki, N. Igarashi, and Prof. S. Wakatsuki for data collection at Photon Factory. We also thank Drs. T. Tsurimoto and C. Vaziri for valuable advice to improve the manuscript.
This work was supported by Grants-in-aid for Young Scientists (B) from the Japan Society of the Promotion of Science 16770080 and 18770091 (to H. H.), Grants-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) 16048226, 17048023, and 19036025 (to H. H.), 20052023 (to T. S.), 18054026 and 20051020 (to M. S.), and 16370077 and 17013041 (to H. O.), and the national projects on protein structural and functional analyses (Protein 3000 Project) and the Target Protein Research Programs from MEXT (to M. S., T. S., and H. H.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: TLS, translesion synthesis; PCNA, proliferating cell nuclear antigen; IDCL, interdomain connecting loop; PIP-box, PCNA-interacting protein box; E2, ubiquitin carrier protein; E3, ubiquitin-protein isopeptide ligase; UBD, ubiquitin-binding domain; SPR, surface plasmon resonance; WT, wild type; GST, glutathione S-transferase.
References
- 1.Ohmori, H., Friedberg, E. C., Fuchs, R. P., Goodman, M. F., Hanaoka, F., Hinkle, D., Kunkel, T. A., Lawrence, C. W., Livneh, Z., Nohmi, T., Prakash, L., Prakash, S., Todo, T., Walker, G. C., Wang, Z., and Woodgate, R. (2001) Mol. Cell 8 7-8 [DOI] [PubMed] [Google Scholar]
- 2.Vaisman, A., Lehmann, A. R., and Woodgate, R. (2004) Adv. Protein Chem. 69 205-228 [DOI] [PubMed] [Google Scholar]
- 3.Ohmori, H., Ohashi, E., and Ogi, T. (2004) Adv. Protein Chem. 69 265-278 [DOI] [PubMed] [Google Scholar]
- 4.Masutani, C., Kusumoto, R., Iwai, S., and Hanaoka, F. (2000) EMBO J. 19 3100-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masutani, C., Kusumoto, R., Yamada, A., Dohmae, N., Yokoi, M., Yuasa, M., Araki, M., Iwai, S., Takio, K., and Hanaoka, F. (1999) Nature 399 700-704 [DOI] [PubMed] [Google Scholar]
- 6.Johnson, R. E., Kondratick, C. M., Prakash, S., and Prakash, L. (1999) Science 285 263-265 [DOI] [PubMed] [Google Scholar]
- 7.Tissier, A., Frank, E. G., McDonald, J. P., Iwai, S., Hanaoka, F., and Woodgate, R. (2000) EMBO J. 19 5259-5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaisman, A., Frank, E. G., Iwai, S., Ohashi, E., Ohmori, H., Hanaoka, F., and Woodgate, R. (2003) DNA Repair 2 991-1006 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki, N., Ohashi, E., Kolbanovskiy, A., Geacintov, N. E., Grollman, A. P., Ohmori, H., and Shibutani, S. (2002) Biochemistry 41 6100-6106 [DOI] [PubMed] [Google Scholar]
- 10.Zhang, Y., Yuan, F., Wu, X., Wang, M., Rechkoblit, O., Taylor, J. S., Geacintov, N. E., and Wang, Z. (2000) Nucleic Acids Res. 28 4138-4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, Y., Wu, X., Guo, D., Rechkoblit, O., and Wang, Z. (2002) DNA Repair 1 559-569 [DOI] [PubMed] [Google Scholar]
- 12.Ogi, T., Shinkai, Y., Tanaka, K., and Ohmori, H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 15548-15553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi, X., Slater, D. M., Ohmori, H., and Vaziri, C. (2005) J. Biol. Chem. 280 22343-22355 [DOI] [PubMed] [Google Scholar]
- 14.Moldovan, G. L., Pfander, B., and Jentsch, S. (2007) Cell 129 665-679 [DOI] [PubMed] [Google Scholar]
- 15.Warbrick, E. (1998) BioEssays 20 195-199 [DOI] [PubMed] [Google Scholar]
- 16.Gulbis, J. M., Kelman, Z., Hurwitz, J., O'Donnell, M., and Kuriyan, J. (1996) Cell 87 297-306 [DOI] [PubMed] [Google Scholar]
- 17.Sakurai, S., Kitano, K., Yamaguchi, H., Hamada, K., Okada, K., Fukuda, K., Uchida, M., Ohtsuka, E., Morioka, H., and Hakoshima, T. (2005) EMBO J. 24 683-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruning, J. B., and Shamoo, Y. (2004) Structure 12 2209-2219 [DOI] [PubMed] [Google Scholar]
- 19.Kontopidis, G., Wu, S.-Y., Zheleva, D. I., Taylor, P., McInnes, C., Lane, D. P., Fischer, P. M., and Walkinshaw, M. D. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 1871-1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haracska, L., Johnson, R. E., Unk, I., Phillips, B., Hurwitz, J., Prakash, L., and Prakash, S. (2001) Mol. Cell. Biol. 21 7199-7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haracska, L., Kondratick, C. M., Unk, I., Prakash, S., and Prakash, L. (2001) Mol. Cell 8 407-415 [DOI] [PubMed] [Google Scholar]
- 22.Vidal, A. E., Kannouche, P., Podust, V. N., Yang, W., Lehmann, A. R., and Woodgate, R. (2004) J. Biol. Chem. 279 48360-48368 [DOI] [PubMed] [Google Scholar]
- 23.Haracska, L., Johnson, R. E., Unk, I., Phillips, B. B., Hurwitz, J., Prakash, L., and Prakash, S. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 14256-14261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogi, T., Kannouche, P., and Lehmann, A. R. (2005) J. Cell Sci. 118 129-136 [DOI] [PubMed] [Google Scholar]
- 25.Guo, C., Sonoda, E., Tang, T. S., Parker, J. L., Bielen, A. B., Takeda, S., Ulrich, H. D., and Friedberg, E. C. (2006) Mol. Cell 23 265-271 [DOI] [PubMed] [Google Scholar]
- 26.Haracska, L., Unk, I., Johnson, R. E., Phillips, B. B., Hurwitz, J., Prakash, L., and Prakash, S. (2002) Mol. Cell. Biol. 22 784-791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannouche, P., Fernandez de Henestrosa, A. R., Coull, B., Vidal, A. E., Gray, C., Zicha, D., Woodgate, R., and Lehmann, A. R. (2002) EMBO J. 21 6246-6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haracska, L., Acharya, N., Unk, I., Johnson, R. E., Hurwitz, J., Prakash, L., and Prakash, S. (2005) Mol. Cell. Biol. 25 1183-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoege, C., Pfander, B., Moldovan, G. L., Pyrowolakis, G., and Jentsch, S. (2002) Nature 419 135-141 [DOI] [PubMed] [Google Scholar]
- 30.Stelter, P., and Ulrich, H. D. (2003) Nature 425 188-191 [DOI] [PubMed] [Google Scholar]
- 31.Kannouche, P. L., Wing, J., and Lehmann, A. R. (2004) Mol. Cell 14 491-500 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe, K., Tateishi, S., Kawasuji, M., Tsurimoto, T., Inoue, H., and Yamaizumi, M. (2004) EMBO J. 23 3886-3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bienko, M., Green, C. M., Crosetto, N., Rudolf, F., Zapart, G., Coull, B., Kannouche, P., Wider, G., Peter, M., Lehmann, A. R., Hofmann, K., and Dikic, I. (2005) Science 310 1821-1824 [DOI] [PubMed] [Google Scholar]
- 34.Plosky, B. S., Vidal, A. E., de Henestrosa, A. R., McLenigan, M. P., McDonald, J. P., Mead, S., and Woodgate, R. (2006) EMBO J. 25 2847-2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda, K., Morioka, H., Imajou, S., Ikeda, S., Ohtsuka, E., and Tsurimoto, T. (1995) J. Biol. Chem. 270 22527-22534 [DOI] [PubMed] [Google Scholar]
- 36.Hishiki, A., Shimizu, T., Serizawa, A., Ohmori, H., Sato, M., and Hashimoto, H. (2008) Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 64 819-821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hishiki, A., Shimizu, T., Hanafusa, T., Ohmori, H., Sato, M., and Hashimoto, H. (2008) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 64 954-956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otwinowski, Z., Minor, W. (1997) Methods Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 39.Vagin, A., Teplyakov, A. (1997) J. Appl. Crystallogr. 30 1022-1025 [Google Scholar]
- 40.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 41.Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T., and Warren, G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905-921 [DOI] [PubMed] [Google Scholar]
- 42.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. Sect. D Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 43.Laskowski, R. A., MacArthur, M. W., Moss, D. S., Thornton, J. M. (1993) J. Appl. Crystallogr. 26 283-291 [Google Scholar]
- 44.Hayashi, K., and Kojima, C. (2008) Protein Expression Purif. 62 120-127 [DOI] [PubMed] [Google Scholar]
- 45.Zheleva, D. I., Zhelev, N. Z., Fischer, P. M., Duff, S. V., Warbrick, E., Blake, D. G., and Lane, D. P. (2000) Biochemistry 39 7388-7397 [DOI] [PubMed] [Google Scholar]
- 46.Lawrence, M. C., and Colman, P. M. (1993) J. Mol. Biol. 234 946-950 [DOI] [PubMed] [Google Scholar]
- 47.Bomar, M. G., Pai, M. T., Tzeng, S. R., Li, S. S., and Zhou, P. (2007) EMBO Rep. 8 247-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hicke, L., Schubert, H. L., and Hill, C. P. (2005) Nat. Rev. 6 610-621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.