Abstract
A gene cluster (pol) essential for the biosynthesis of polyoxin, a nucleoside antibiotic widely used for the control of phytopathogenic fungi, was cloned from Streptomyces cacaoi. A 46,066-bp region was sequenced, and 20 of 39 of the putative open reading frames were defined as necessary for polyoxin biosynthesis as evidenced by its production in a heterologous host, Streptomyces lividans TK24. The role of PolO and PolA in polyoxin synthesis was demonstrated by in vivo experiments, and their functions were unambiguously characterized as O-carbamoyltransferase and UMP-enolpyruvyltransferase, respectively, by in vitro experiments, which enabled the production of a modified compound differing slightly from that proposed earlier. These studies should provide a solid foundation for the elucidation of the molecular mechanisms for polyoxin biosynthesis, and set the stage for combinatorial biosynthesis using genes encoding different pathways for nucleoside antibiotics.
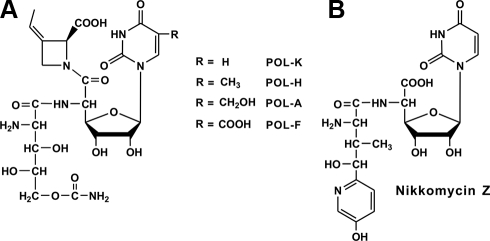
Nucleoside antibiotics are a family of important microbial secondary metabolites with a wide range of bioactive properties. They originate by a combination of several primary metabolic pathways, including those of nucleic acids, proteins, and glycans (1). Polyoxins (Fig. 1A), a group of structurally related nucleoside antibiotics produced by Streptomyces cacaoi var. asoensis (S. cacaoi hereafter) (2, 3) and Streptomyces aureochromogenes (4), exhibit powerful bioactivity against phytopathogenic fungi (1, 5). As the first discovered nucleoside antibiotic inhibiting fungal cell wall biosynthesis, polyoxin was known to act as a competitive inhibitor of the chitin synthetase (1, 5) because of its intrinsic structural mimic of UDP-N-acetylglucosamine, a substrate for chitin biosynthesis. Polyoxin has played an outstanding role as an efficient agricultural fungicide without unwanted toxicity ever since its discovery in 1965 (6).
FIGURE 1.
Chemical structures of polyoxins (A) and nikkomycin Z (B). Different polyoxin components were indicated as polyoxin-A, -F, -H, and -K, respectively.
Polyoxin was composed of three moieties (1), including a nucleoside skeleton and two modified amino acids, polyoximic acid (POIA)3 and carbamoylpolyoxamic acid (CPOAA) (2, 3, 6). Isotope feeding experiments demonstrated that the nucleoside skeleton was initiated using uridine and phosphoenolpyruvate (PEP) as substrates, and the two modified amino acids originated from l-isoleucine and l-glutamate (1) independently.
Reports on the cloning of biosynthetic gene clusters for nucleoside antibiotics were limited to complete pathways for a purine nucleoside antibiotic puromycin (7), a pyrimidine nucleoside antibiotic nikkomycin, as well as a partial pyrimidine nucleoside antibiotic blasticidin S (8). Whereas the puromycin biosynthetic pathway was completely demonstrated, the biosynthetic mechanism for blasticidin S was incompletely understood. A tentative biosynthetic pathway for nikkomycin Z (Fig. 1B), produced by Streptomyces tendae Tü901 (1) and Streptomyces ansochromogenes (9), has been elucidated (10–17) to derive from two moieties involving a nucleoside skeleton and a modified amino acid originating from l-tyrosine, but the precise mechanism for the biosynthesis of its nucleoside skeleton, which seemed similar to polyoxin, remains to be clarified (16). NikO, a key enzyme essential for biosynthesis of the nikkomycin nucleoside skeleton, was recently demonstrated to use UMP, instead of uridine, with PEP to form a novel and unexpected product 3′-enolpyruvyl-UMP (3′-EUMP) (16). This is totally different from the previously proposed pathway for the biosynthesis of the polyoxin nucleoside skeleton.
Here we describe the cloning and functional analysis of a complete polyoxin biosynthetic gene cluster. The heterologous production of polyoxin H in a nonproducer, Streptomyces lividans TK24, helped us to pinpoint an essential region consisting of 20 putative pol genes of 39 predicted open reading frames in a sequenced region as large as 46 kb, which led to a proposal of putative pathway for polyoxin biosynthesis. The availability of these genes will significantly help the elucidation of the exact biosynthetic mechanism of polyoxin, and for the more rational generation of polyoxin derivatives with novel or enhanced bioactivities via strategies involving pathway engineering or combinatorial biosynthesis.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids (Cosmids), General Methods, and Culture Conditions—Bacterial strains and plasmids (cosmids) used are described in supplemental Table S1. S. cacaoi and its derivatives were grown on MS agar (18) or in TSB liquid medium (18) at 30 °C. Liquid fermentation medium (containing the following per liter: 20 g of soy powder, 15 g of corn powder, 10 g of glucose, 10 g of yeast extract, 4 g of CaCO3, 2 g of KH2PO4, 2 g of NaCl) was used for polyoxin production. General approaches for Escherichia coli or Streptomyces manipulation were according to the standard methods of Sambrook et al. (19) or Kieser et al. (18). The final antibiotic concentrations used for selection of E. coli or Streptomyces were as follows: 100 μg/ml ampicillin, 30 μg/ml apramycin, 50 μg/ml kanamycin, and 12.5 μg/ml thiostrepton. Milli-Q water purified by Milli-Q® ultrapure water purification systems was used throughout except for medium preparation.
PCR Primers, DNA Probes, and Southern Blot—PCR primers used are listed in supplemental Table S2. For Southern blot experiments, S. cacaoi genomic DNA was digested with specific restriction enzymes, separated on 0.7% agarose gels overnight, and transferred onto Hybond-N+ nylon membrane (Amersham Biosciences). With use of [α-32P]dCTP-labeled radioactive probes by the random priming kit (Takara), Southern blot experiments were performed based on the standard protocol by Sambrook et al. (19).
Genomic Library Construction for S. cacaoi and the Strategy for Screening Positive Cosmids—For construction of the pOJ446-derived genomic library for S. cacaoi, the standard method (18, 19) was adopted, and the competent EPI300-T1R E. coli was selected as host. For screening the cosmid genomic library by PCR, the primers (PolAF and PolAR) were used, and cosmid (mix) was used as template. The positive clones were identified in 96-well plates.
Nucleotide Sequence Accession Number—The nucleotide sequence reported in this paper is available in the GenBank™ data base under accession number EU158805.
DNA Sequencing and Sequence Analysis—DNA sequencing was accomplished at Invitrogen using pIJ2925 (18) as vector, and sequencing reactions were carried out in an Applied Biosystems model 3730 automated DNA sequencer. Sequence data analysis was performed with FramePlot 3.0beta online program (20). Nucleotide and amino acid sequence homology searches were performed using BLAST (21).
Heterologous Production of Polyoxin H—For heterologous production of polyoxin in S. lividans TK24, an EcoRI-XbaI engineered fragment bearing the int gene and the attp site from pSET152 was amplified by PCR with primers 5a7mF and 5a7mR, and this engineered fragment was used to replace the corresponding region in cosmid 5A7 containing the unstable SCP2 replicon to generate the modified 5A7 (m5A7), which was introduced into S. lividans TK24 for the engineered production of polyoxin.
Construction and Identification of a polA Mutant—For construction of a polA targeted disruption vector, an ∼3.0-kb PvuII fragment harboring polA from cosmid 8B9 was cloned into the SmaI site of pIJ2925 to generate pJTU2158. With the primers Podf and Podr carrying XbaI and EcoRI site individually, the ∼3.0-kb fragment was amplified by KOD-plus DNA polymerase (Toyobo) using pJTU2158 as template; subsequently, this XbaI-EcoRI engineered fragment was cloned into pJTU1278,4 a derivative of pHZ1358 (22), to give pJTU2161, and a BamHI fragment containing aac(3)IV (apramycin resistance gene) from pHZ1070 (23) was inserted into the internal counterpart site of polA in pJTU2161 to produce the polA targeted disruption vector pJTU2165.
Both PCR and Southern blot experiments were carried out to identify the CY1 mutants. For PCR identification, the primers PiomF and PiomR were used. The additional Southern blot validation used an ∼0.7-kb polA fragment obtained from pJTU2152 as a probe.
Complementation of a Mutant CY1 with nikO and polA—With primers NikO-EXF and NikO-EXR, the complete nikO structural gene (with start codon GTG changed to ATG) was amplified with KOD-plus polymerase, treated with EcoRI, and then cloned into pIJ2925 (cleaved with EcoRI and SmaI) to generate pJTU2173. With primers polAef and polAer, the polA structural gene (with start codon GTG changed to ATG) was amplified, cut with EcoRI, and inserted into pBluescript II (SK+) (digested with EcoRI and SmaI) to produce pJTU2810. Two sequencing-confirmed EcoRI-NdeI fragments harboring the structural genes nikO from pJTU2173 and polA from pJTU2810, respectively, were cloned into the corresponding sites of pJTU695 (24) to form pJTU2179 and pJTU2198.
Construction and Identification of a polO Mutant—A 4.1-kb PvuII fragment from cosmid 5A7 containing intact polO was cloned into pBlueScriptII SK(+) to generate pJTU2900, from which a HindIII-SpeI fragment was cloned into the HindIII-XbaI site of pJTU12894 to give pJTU2940, and an aac(3)IV+oriT cassette was recombined into the pJTU2940 by PCR targeting technology (25) to produce pJTU2941 as a vector for polO disruption. Primers (polOIDF and polOIDR) were used for the identification of polO mutant.
Polyoxin Purification and Assay—Polyoxin produced by S. cacaoi var. asoensis and its derivatives was detected by bioassay, HPLC (Waters 220), and LC/MS (Agilent 1100 series LC/MSD Trap system). For the bioassay, Trichosporon cutaneum was used as indicator strain. For HPLC and LC/MS analysis, the prepared broth containing polyoxin was purified by Dowex 50W×8(H+) resin, and the targeted fraction was collected and condensed before HPLC and LC/MS analysis. The conditions for HPLC analysis were according to the method of Fiedler (26) except that the flow rate was 0.5 ml/min. The elution was monitored at 263.6 nm with a PDA (DAD) detector, and the data were analyzed with Waters Millennium Chromatography Manager (Agilent data analysis software).
Quantification of Polyoxin H/A—For quantification of polyoxin H/A, polyoxin H/A standards were independently prepared by Shimadzu LC-8A preparative liquid chromatography. The HPLC conditions were as follows: 0.15% trifluoroacetic acid was increased to 50% in 30 min, and acetonitrile was correspondingly decreased to 50%; flow rate was 5 ml/min; after that, the standard curve was drawn according HPLC peak area of series polyoxin H/A concentrations (50, 100, 200, and 500 μg/ml, respectively) (see above for HPLC under “Polyoxin Purification and Assay”) before the quantity of the polyoxin H/A in crude broth was calculated.
Conditions for MS Analysis—The ion trap mass spectrometer (Agilent 1100 series LC/MSD trap system was operated with the electrospray ionization source) analysis for polyoxin and ACV (AHV) was in positive mode and for 3′-EUMP (UMP) in negative mode. The parameters for all MS analysis are as follows: drying gas flow was 10 liters/ml, and nebulizer pressure was 30 p.s.i.; drying gas temperature was 325 °C.
Heterologous Overexpression of Recombinant His6-tagged PolA, NikO, and PolO—For heterologous expression of PolA, NikO, and PolO in E. coli BL21(DE3)/pLysE (Stratagene), both EcoRI-NdeI fragments harboring the structural genes nikO from pJTU2173, polA from pJTU2810, and polO from pJTU2896 were individually cloned into the corresponding sites of pET28a (Stratagene) to obtain pJTU2178, pJTU2197, and pET28a/polO. The plasmid DNA was transformed into E. coli BL21 (DE3)/pLysE. The transformants were grown in 600 ml of LB medium containing kanamycin and chloramphenicol at 37 °C to A600 of 0.6 before addition of isopropyl β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.1 mm, and the incubation was continued at 30 °C for 3 h. The cells were then harvested by centrifugation at 6000 rpm for 10 min, and the pellet resuspended in 60 ml of binding buffer (150 mm NaCl, 20 mm Tris-HCl, pH 7.5) was stored frozen at -70 °C until further use.
Preparation of Cell-free Extracts and Purification of His6-tagged PolA, NikO, and PolO—After thawing in water, the E. coli cells were lysed by sonication, and the cell debris was removed by centrifugation at 15,000 rpm for at least 30 min. Recombinant His-tagged protein was purified from the supernatant by gravity-flow chromatography on nickel-nitrilotriacetic acid-agarose (Qiagen). The bound recombinant protein was then eluted off the nickel column with gradient elution buffer (500 mm imidazole, 150 mm NaCl, 20 mm Tris-HCl, pH 7.5) at a flow rate 0.5 ml/min by Amersham Biosciences ÄKTA FPLC, and glycerol was added to the purified protein to a final concentration of 10% to prevent precipitation. After that, the purified recombinant protein was aliquoted and stored at -70 °C.
In Vitro Enzyme Assay for PolA and NikO—In vitro characterization of PolA and NikO was according to the method of Ginj et al. (16). The LC/MS analysis conditions used to judge the enzyme-catalyzed product were as follows: 91% of 0.15% trifluoroacetic acid, 9% of methanol, flow rate 0.3 ml/min. Compounds were analyzed by recording the absorbancy at 260 nm (for UMP and 3′-EUMP). Before injection of samples into LC/MS, protein was precipitated by adding an equal volume of 14% trichloroacetic acid to the reaction mix.
In Vitro Enzyme Assay for PolO—In vitro enzyme assay of PolO was carried out at 30 °C overnight (under the following conditions: 200 μl volume containing phosphate buffer, pH 7.2, 50 mm, AHV 1 mm, MgSO4 5 mm, ATP 2 mm, dithiothreitol 2 mm, carbamoylphosphate 1 mm, PolO 10 μm). Before LC/MS analysis, protein was precipitated using the conditions described above, and the LC/MS analysis conditions used to judge the enzyme-catalyzed product were as follows: 90% of 0.15% trifluoroacetic acid, 10% of acetonitrile, flow rate 0.3 ml/min. The procedure for TLC analysis of PolO-catalyzed product was as follows: PolO-catalyzed product was initially purified by Dowex 50W×8(H+), separated by TLC with the solvent system butanol/acetic acid/water (4:1:2), and visualized by ninhydrin solution (1.5 g of ninhydrin dissolved in 100 ml of butanol and 3 ml of acetic acid).
Synthesis of AHV—Synthesis of AHV was according to method of Garcia et al. (27). The synthesized AHV was confirmed by 1H NMR (D2O, 400 MHz) δ 3.58 (t, J = 5.6 Hz, 1H), 3.45 (t, J = 6.4 Hz, 2H), 1.70–1.77 (m, 2H), 1.38–1.51 (m, 2H).
RESULTS
Genetic Organization of the Cloned Polyoxin Gene Cluster—When an ∼0.7-kb nikO fragment from S. tendae Tü901 for the nikkomycin biosynthesis was amplified with primers NikOTF and NikOTR for use to probe the BamHI-digested genomic DNA of the two polyoxin producers (S. cacaoi var. asoensis and S. aureochromogenes (4)) at low stringency, weak hybridization signals at ∼1.6 and ∼3.5 kb, respectively (see supplemental Fig. S1), appeared, which indicates that both strains may contain nikO homologs.
To isolate a nikO homolog as a probe for screening cosmids from a genomic library of S. cacaoi, a distinct PCR product of 723 bp, deduced to encode a 240-amino acid NikO homolog, was first amplified from S. aureochromogenes using specific primers NikOTF and NikOTR, from which another pair of primers (PolAf and PolAr) was designed for the amplification of a 549-bp PCR product simultaneously from S. cacaoi and S. aureochromogenes. Both PCR products showed 99% identity within the shared 549-bp region.
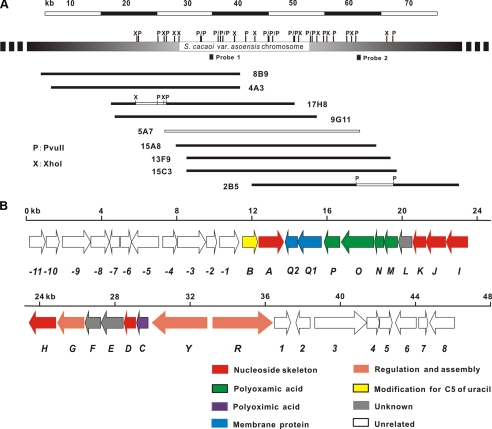
A pOJ446-derived cosmid library was screened by PCR (see “Experimental Procedures”). Of ∼2,000 cosmids, 8 gave positive signals, which spanned ∼65-kb contiguous region of S. cacaoi as revealed by mapping with PvuII (Fig. 2A).
FIGURE 2.
Restriction map (A) and genetic organization of the polyoxin biosynthetic gene cluster (B). ∼46-kb sequenced region from S. cacaoi var. asoensis covered by nine overlapping cosmids is indicated by open bars, and the region used as probes by solid squares. See Table 1 for the proposed functions of the pol genes.
A contiguous 46,066-bp sequenced region arising from sequencing one of the centrally located cosmids 5A7 (∼35 kb), and subsequently an additional XhoI (4.8 kb) and two internal PvuII (1.5 and 6.7 kb) fragments (GenBank™ accession number EU158805, Fig. 2A), had an overall G + C content of 72.58%, similar to that of a typical Streptomyces genome exemplified by Streptomyces coelicolor A3(2) (28) and Streptomyces avermitilis (29). The frame plot 3.0 beta online program revealed 39 complete open reading frames whose organization is shown in Fig. 2B, with putative functions described in Table 1.
TABLE 1.
Deduced functions of the open reading frames in the pol gene cluster
| Protein | Amino acids | Proposed function | Homolog, origin | Identity, similarity | Accession no. |
|---|---|---|---|---|---|
| % | |||||
| PolB | 257 | Thymidylate synthase | SCO5743, S. coelicolor A3(2) | 60, 72 | NP_629868 |
| PolAa | 445 | UMP-enolpyruvyltransferase | NikO, S. tendae | 62, 71 | CAC80913 |
| PolQ2 | 188 | Adenylate kinase | NikN, S. tendae | 59, 68 | CAC80912 |
| PolQ1 | 419 | Membrane protein | NikN, S. tendae | 47, 61 | CAC80912 |
| PolP | 280 | Acetylglutamate kinase | SCO1578, S. coelicolor A3(2) | 55, 68 | NP_625855 |
| PolOa | 573 | Carbamoyltransferase | NodU, Sinorhizobium sp. | 49, 64 | CAH04371 |
| PolN | 168 | Amino acid N-acetyltransferase | ArgA, Rhodoferax ferrireducens | 30, 46 | YP_523360 |
| PolM | 255 | Short chain dehydrogenase | PA4907, P. aeruginosa PAO1 | 49, 67 | NP_253594 |
| PolL | 243 | Unknown | ORF3, Agrobacterium vitis | 35, 51 | ABG82019 |
| PolK | 213 | Hydroxylase | SanC, S. ansochromogenes | 58, 69 | AAF61921 |
| PolJ | 273 | Phosphatase | SanB, S. ansochromogenes | 63, 71 | AAF61216 |
| PolI | 380 | Aminotransferase | NikK, S. tendae | 61, 69 | CAC80909 |
| PolH | 469 | Radical S-adenosylmethionine protein | NikJ, S. tendae | 70, 80 | CAC80908 |
| PolG | 430 | Amide synthetase | NikS, Streptomyces tendae | 57, 70 | CAC11141 |
| PolF | 275 | Molybdopterin oxidoreductase | Aave_0767, Acidovorx avenae subsp. citrulli | 28, 41 | YP_969139 |
| PolE | 370 | Unknown | SAML1083, Streptomyces ambofaciens | 30, 46 | CAJ90069 |
| PolD | 215 | Hydroxylase | NikI, S. tendae | 50, 65 | CAC80907 |
| PolC | 204 | Hydroxylase | SanF, S. ansochromogenes | 29, 49 | AAF74976 |
| PolY | 962 | Regulator | ORF4, Streptomyces echinatus | 39, 53 | ABL09952 |
| PolR | 1111 | Regulator | ORFR, S. tendae | 55, 66 | CAC80806 |
Functions were confirmed in vitro
Genes Essential for the Biosynthesis of the Polyoxin Nucleoside Skeleton—Six noncontiguous genes (polA, -D, -H, -I, -J, and -K) were identified as similar to an isolated gene (nikO) and five contiguous genes (nikI-nikM) involved in the biosynthesis of nikkomycin nucleoside skeleton, and were thus deduced to be responsible for the counterpart biosynthesis of the polyoxin nucleoside skeleton.
PolA shows significant homology with NikO and SanX (62 and 61% identity, respectively), both independently essential for biosynthesis of nikkomycin in S. tendae Tü901 and S. ansochromogenes (9), likely to act as enolpyruvyltransferases (13, 16). PolD exhibits 50% identity and 65% similarity to NikI, but their precise roles in the biosynthesis of the nucleoside skeleton, either for nikkomycin or polyoxin, remain ambiguous. PolH resembles NikJ, a radical S-adenosylmethionine family protein postulated to catalyze diverse reactions, including formation of cyclized intermediate. PolI is 61% identical to NikK, and they both strongly resemble the histidinol-phosphate/aromatic aminotransferase of Magnetospirillum magneticum AMB-1. The significant resemblance of PolJ (with a protein tyrosine/serine phosphatase domain identical to NikL) and PolK (identical to NikM, a hydroxylase) strongly suggests that they should play similar roles in the biosynthesis of the nucleoside skeletons for polyoxin and/or nikkomycin.
Genes for the Biosynthesis of CPOAA and POIA—Five genes (polL–P) seem to be involved in CPOAA biosynthesis, of which PolM displayed significant homology with a probable short chain dehydrogenase of Pseudomonas aeruginosa PAO1, PolN with ∼30% identity to ArgA of Rhodoferax ferrireducens, PolP with considerable homology with the corresponding protein of S. coelicolor A3(2) involved in arginine biosynthesis (30), and PolO with significant homology with NodU, a carbamoyltransferase of Sinorhizobium sp. (31). No obvious PolL homolog was found in the data base.
Three genes (polC, -E, and -F) were assumed to be responsible for the biosynthesis of POIA, a distinctive moiety present in polyoxin. PolC exhibits 29% identity with SanF, a putative hydroxylase involved in nikkomycin biosynthesis in S. ansochromogenes (32), whereas PolE shows no obvious similarity to proteins in the data base. Meanwhile, PolF shows only a marginal homology with Ave_0767 of Acidovorax aveenae as a putative molybdopterin oxidoreductase.
Genes for the Modification of C-5 of the Nucleoside Skeleton—The methylation modification is very likely to be governed by PolB as a result of its significant homology with ThyX (a thymidylate synthase) of several bacteria, including those from S. coelicolor A3(2) (60% identity) (28) and S. avermitilis (58% identity) (29). Apart from polB, no other gene(s) related to hydroxylation/carboxylation of the methyl group of the polyoxin nucleoside skeleton was/were identified in the pol gene cluster.
Genes Involved in Assembly, Regulation, and Transport—Three moieties, the nucleoside skeleton, CPOAA, and POIA, seem to be independently synthesized and assembled into polyoxin A, likely by PolG showing significant homology with NikS (SanS) (57% identity), a putative amide synthetase with confirmed ATPase activity (33), as a final step.
Two candidate regulatory genes, polY and polR, were identified. polY encodes a putative member of the AfsR family of the two-component response regulators, whereas polR seems to encode a pathway-specific regulator with considerable homology with SanG (OrfR in S. tendae), a positive regulator affecting both nikkomycin biosynthesis and colony development (9).
Two likely co-transcribed transport genes (polQ1 and polQ2) seemed to encode proteins with two conserved domains aligning well with NikN, a predicted membrane protein (N-terminal putative membrane protein domain and C-terminal putative adenylate kinase domain that functions as a sensor) involved in nikkomycin biosynthesis.
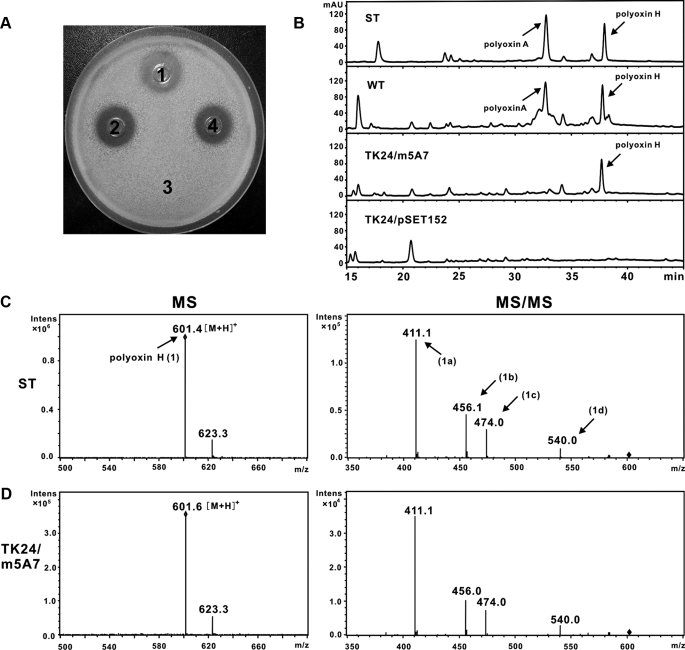
Engineered Production of Polyoxin H in S. lividans TK24—For the engineered production of polyoxin in a heterologous host, cosmid 5A7 (pOJ446-derived) carrying an insert of ∼36 kb was initially introduced into S. lividans TK24, but no inhibition of the indicator strain (T. cutaneum) was observed. A large fragment of ∼32 kb, however, was found to be deleted from this cosmid (5A7) isolated from S. lividans TK24, because of its likely instability in an autonomous state. Cosmid 5A7 was therefore modified by replacement of the SCP2 replicon with an engineered fragment containing int and attp from pSET152 (see “Experimental Procedures”) to produce a 5A7 derivative (m5A7) for introduction into S. lividans TK24. When m5A7 was found to be integrated intact into the chromosome of S. lividans TK24, as confirmed by Southern hybridization, the fermentation broth of the recombinant strains showed obvious bioactivity against the indicator strain (Fig. 3A). Samples from the TK24 recombinant strain were subjected to HPLC analysis, and a distinctive peak with a retention time at ∼37.7 min appeared at a position corresponding to where the samples isolated from the wild-type S. cacaoi and also where standard polyoxin H would run, although the sample isolated from the negative control, S. lividans/pSET152, lacked this peak (Fig. 3B). The peak from the TK24/m5A7 sample was subjected to MS analysis in positive ion mode, revealing a distinctive ion and fragmentation pattern (Fig. 3D), which corresponded exactly to the data analyzed in parallel using polyoxin H as authentic standard (Fig. 3C and supplemental Fig. S2), thus confirming the predominant component produced by the recombinant strain as polyoxin H (Fig. 3, C and D). In addition, the yield of polyoxin H produced by the S. lividans recombinant strain is ∼60.7 μg/ml as compared with ∼71.4 μg/ml of the wild-type S. cacaoi (polyoxin H in crude broth was quantified with HPLC method).
FIGURE 3.
Heterologous production of polyoxin H in S. lividans TK24. A, bioassay of the metabolites produced by the recombinant. T. cutaneum was used as indicator strain, and a volume of 35 μl of processed fermentation broths were used for each assay. Recombinants (S. lividans TK24 carrying construct m5A7) spotted individually in spots 2 and 4 were compared with wild-type S. cacaoi var. asoensis as a positive control (spot 1) and S. lividans TK24/pSET152 as a negative control (spot 3). B, HPLC analysis of the metabolites produced by the engineered recombinant. Polyoxin authentic standards (ST), and samples from wild-type (WT) of S. cacaoi var. asoensis, S. lividans TK24/m5A7 (TK24/m5A7), and S. lividans TK24/pSET152 (TK24/pSET152) were compared. C, MS/MS fragmentation of the polyoxin H (601.4) authentic standard (1) into 411.1 (1a), 456.1 (1b), 474.0 (1c), and 540.0(1d). D, identical MS/MS fragmentation pattern of the polyoxin component produced by recombinant S. lividans TK24/m5A7 as polyoxin H (C).
Boundaries of the Polyoxin Gene Cluster—Deletion of a large fragment (from orf6 to orf1) results in no influence on polyoxin production, and thus, orf1 is at the left boundary of the pol cluster (supplemental Fig. S3). To determine the right boundary, orf3 and orf4, two contiguous genes in one operon, were simultaneously disrupted, whereupon polyoxin production remained intact. The same result was obtained by disruption of orf1 (supplemental Figs. S4 and S5), adjacent to the regulatory gene polR, defining orf1 as the right boundary of the pol cluster.
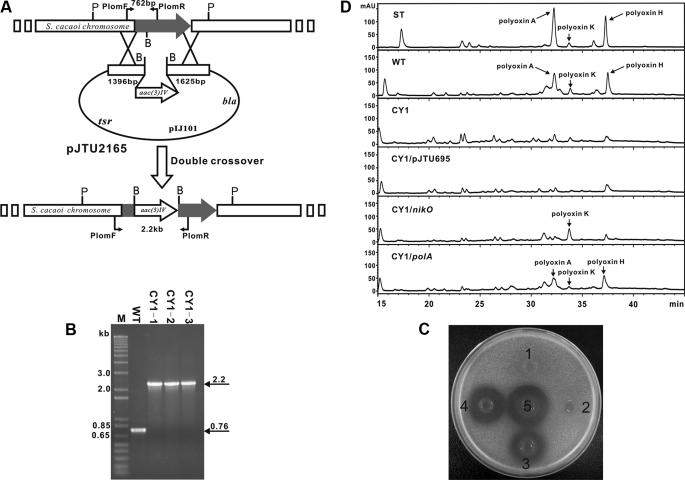
Targeted Inactivation of polA Abolishes Polyoxin A Production—To obtain direct proof that polA was involved in polyoxin biosynthesis, pJTU2165, a polA-targeted disruption vector, was constructed and transferred into S. cacaoi. Exconjugants were selected according to the standard method (18). Three random aprrthios candidates (Fig. 4A) were selected for confirmation by PCR, and they all produced a 2.2-kb PCR product, in sharp contrast with wild-type S. cacaoi which gives a 762-bp PCR product (Fig. 4B). For the final confirmation of the three preliminarily selected CY1 mutants by Southern blot analysis, genomic DNAs of CY1 mutants and wild-type S. cacaoi were digested with PvuII, and a 723-bp polA fragment from pJTU2152 was used as probe. As expected, all three preliminarily selected CY1 mutants (CY1–3) gave positive signals at 4.5 kb, whereas wild-type S. cacaoi gave a signal at 3.0 kb (supplemental Fig. S6), in agreement with the insertion of a 1.4-kb fragment carrying aac(3)IV (apramycin resistance gene).
FIGURE 4.
Insertional inactivation into polA and mutant complementation by polA. A, schematic representation for the construction of CY1 mutant. P, PvuII; B, BamHI; B, PCR confirmation of the CY1 mutants as having a 1.4-kb aac(3)IV fragment inserted into polA, as the 2.2-kb PCR product generated from mutants is ∼1.4 kb larger than that (762 bp) from the wild-type strain. C, bioassay of polA mutant CY1 (spot 1) and its complemented strain by polA(spot 3). Mutant CY1 containing empty vector pJTU695 (spot 2) was used as a negative control, and wild-type S. cacaoi (spot 5) as positive control. Mutant CY1 containing nikO (spot 4) was included to show the similar complementation effect of nikO with polA. D, HPLC analysis of the metabolites produced by the mutant CY1 and its complemented strain by polA. Polyoxins authentic standard (ST) and samples from wild-type (WT) of S. cacaoi var. asoensis, mutant CY1 (CY1), mutant CY1 complemented by polA (CY1/polA) and nikO (CY1/nikO), and mutant CY1 containing empty vector pJTU695 (CY1/pJTU695) as negative control were compared.
The CY1 mutants were found to have lost their ability to produce polyoxin by bioactivity assay against the indicator strain, T. cutaneum (Fig. 4C). HPLC results demonstrated that CY1 mutants lack characteristic peaks at retention times of ∼32.2 and 37.2 min (Fig. 4D), characteristics of the authentic polyoxin A and polyoxin H standard run in a parallel experiment, verifying unambiguously that polA is directly involved in the biosynthesis of polyoxin.
A polA Mutation in the Mutant CY1 Could Be Complemented by polA and Its Counterpart, nikO, from the Nikkomycin Pathway—The in vivo functions of polA, in comparison with its close relative nikO in the nikkomycin pathway, were further characterized by making constructs placing both genes individually under the control of the constitutively expressed ermE* promotor in pJTU695 (24). When both constructs were independently introduced into the mutant CY1, confirmed by PCR, the exconjugants were found to have resumed polyoxin production, although at a reduced yield in comparison with that of the wild type, although the mutant CY1 carrying an empty vector, pJTU695, as negative control gave no production. The restored production was confirmed by bioassay (Fig. 4C) and HPLC analysis (Fig. 4D). Compared with the wild-type polyoxin production, the complemented production by polA is ∼50.5% (polyoxin H in crude broth was quantified). As indicated by bioassay and HPLC analysis, complementation by polA differs from that by nikO in that the major polyoxin component produced by CY1/nikO is merely polyoxin K (Fig. 4D and supplemental Fig. S7), suggesting that NikO and PolA may have different substrate preferences, and thus such enzymatic steps could be tailored to obtain the in vivo component flexibilities.
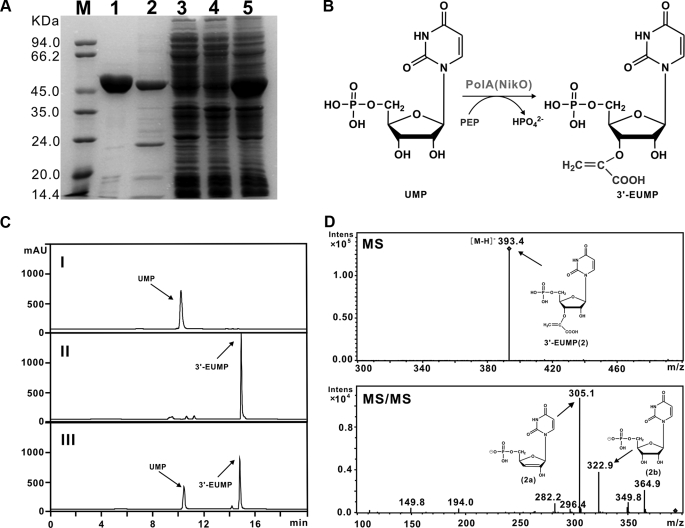
PolA Is Capable of Converting UMP and PEP to 3′-EUMP—To see if PolA could serve in vitro, as an enolpyruvyltransferase (Fig. 5B), as implied by bioinformatic analysis, it was overexpressed in E. coli BL21(DE3)/pLysE, in parallel with its homolog NikO involved in nikkomycin biosynthesis as a positive control. SDS-PAGE analysis proved PolA as an ∼47.0-kDa protein, consistent with the theoretical mass of 46.6-kDa with a purity of ∼80%, although NikO was detected as a 50.4-kDa protein of the expected size with a purity of ∼90% (Fig. 5A).
FIGURE 5.
Characterization of PolA as an enolpyruvyltransferase. A, SDS-PAGE analysis of PolA and NikO proteins. Purified His6-tagged NikO (lane 1), PolA (lane 2), soluble proteins of cell-free extract from E. coli BL21 (DE3)/pLysE/polA before (lane 4) and after (lane 3) induction with IPTG, and total proteins of cell-free extract of E. coli BL21 (DE3)/pLysE/polA after induction with IPTG (lane 5), were aligned with molecular mass markers (M). B, conversion mechanism of UMP to 3′-EUMP catalyzed by PolA. C, HPLC analysis of the products catalyzed by PolA (III) and NikO (II), respectively. UMP (I) is included as a negative control. D, MS/MS fragmentation of the products 3′-EUMP catalyzed by PolA in negative mode.
As anticipated, UMP, instead of uridine, and PEP could be catalyzed by both NikO and PolA. The NikO-catalyzed product, which was previously demonstrated to be 3′-EUMP (16), yielded a distinctive peak at ∼15.0 min (Fig. 5C) with characteristic ion [M - H]- at m/z 393.3 and principal fragmentation pattern at m/z 305.0, 323.1, 364., and 349.8 (supplemental Fig. S8). Similarly, the PolA-catalyzed product also generated a characteristic peak at the same retention time of ∼15.0 min (Fig. 5C) with [M - H]- ion at m/z 393.4 (2). In addition, MS/MS analysis indicated that the main fragment ion was generated at m/z 305.2 (2a), 322.9 (2b), 364.9, and 349.8 (Fig. 5D), whose fragmentation pattern is consistent with that generated by NikO. HPLC analysis showed that the negative control (no NikO or PolA added) could produce nothing but a peak characteristic of UMP at ∼10.0 min (Fig. 5C). These results confirmed that PolA and NikO shared an identical role for the biosynthesis of similar nucleoside skeletons of polyoxin and nikkomycin.
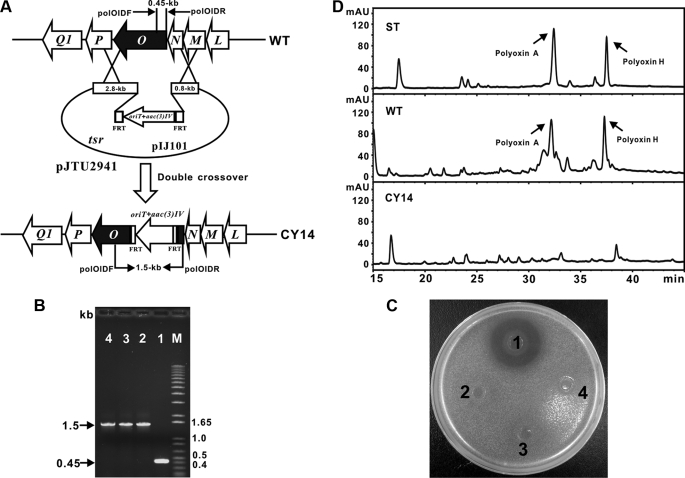
Targeted Insertional Inactivation of polO Led to the Abolished Polyoxin Production—To demonstrate that polO encoding a putative distinctive AHV O-carbamoyltransferase is involved in polyoxin biosynthesis, a vector pJTU2941, targeted for polO inactivation was constructed with PCR targeting technology (25). After its introduction into S. cacaoi, and selection of the exconjugants by the standard protocol (18) (Fig. 6A), three identical mutants (CY14) were confirmed to have generated an expected PCR fragment corresponding to 1.5 kb, whereas the fragment generated from the wild-type S. cacaoi is clearly 0.45 kb in size (Fig. 6B).
FIGURE 6.
Targeted inactivation of polO. A, schematic representation for the construction of mutant CY14. B, PCR confirmation of the CY14 mutants, as the aac(3)IV cassette was recombined into polO the mutants give an ∼1.5-kb PCR product, although the wild-type strain shows 0.45-kb PCR product. C, bioassay of polO mutant CY14 (spots 2–4) with the wild-type S. cacaoi (spot 1). D, HPLC analysis of the metabolites produced by mutant CY14 (CY14) together with the wild-type (WT) S. cacaoi and authentic polyoxin standard (ST).
None of the three CY14 mutants showed inhibition to the indicator fungi (Fig. 6C). Meanwhile, none of them produced peaks corresponding to polyoxin A (32.2 min) and polyoxin H (37.3 min), as compared with samples from standard and wild-type strain of S. cacaoi by HPLC analysis (Fig. 6D), indicating that polO is involved in polyoxin biosynthesis.
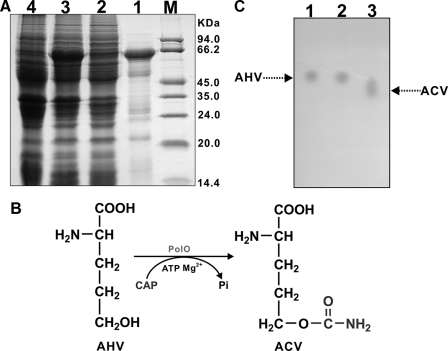
PolO Acts as AHV O-Carbamoyltransferase—To get direct evidence whether the PolO, whose likely involvement in the CPOAA biosynthesis would seem to be slightly different from that of arginine, could function as O-carbamoyltransferase in polyoxin biosynthesis, PolO was cloned into pET28a and heterologously overexpressed in E. coli BL21(DE3)/pLysE. SDS-PAGE analysis demonstrated PolO as an ∼62.0-kDa protein with a purity of ∼80% (Fig. 7A), conforming to the theoretical mass of 61.8 kDa.
FIGURE 7.
Characterization of PolO as an O-carbamoyltransferase. A, SDS-PAGE analysis of PolO proteins. Purified His6-tagged PolO (lane 1), soluble proteins of cell-free extract from E. coli BL21 (DE3)/pLysE/polO before (lane 4) and after (lane 2) induction with IPTG, and total proteins of cell-free extract of E. coli BL21 (DE3)/pLysE/polO after induction with IPTG (lane 3) were aligned with molecular weight markers (M). B, conversion mechanism of AHV to ACV catalyzed by PolO. C, TLC analysis of the products catalyzed by PolO (lane 3) AHV standard (lane 1) and reaction mix with heat-inactivated (90 °C, 10 min) PolO added was included as negative controls (lane 2).
To demonstrate whether PolO could catalyze the conversion of AHV to ACV (Fig. 7B), the PolO catalyzed product was assayed by TLC, which exhibited an Rf value clearly different from that of the negative control AHV (Fig. 7C). Further MS analysis characterized it as consistent with the molecular weight of ACV with a distinctive ion [M + H]+ at m/z 177.0 (supplemental Fig. S9), but not that of AHV whose molecular weight with an expected ion [M + H]+ would be at m/z 134.0. Moreover, the fragmentation pattern of the PolO-catalyzed product conformed well with that of the positive control sample prepared by cell-free enzyme solution (supplemental Fig. S9) (34). Thus, all of these results unambiguously characterized PolO as a distinctive AHV O-carbamoyltransferase.
DISCUSSION
Polyoxin aroused our interest at the molecular level because it has been proven as an important representative nucleoside antibiotic with a broad spectrum of antifungal activity, and is produced in increasing scale each year in China to combat a variety of plant diseases, including tobacco brown spot disease caused by Alternaria alternata, and apple Alternaria leaf spot caused by Alternaria mali. Indeed, the cloning and analysis of the gene cluster governing polyoxin biosynthesis provided a chance not only for a deeper insight into the novel aspects of the genetics of nucleoside antibiotic biosynthesis but also for strain improvement resulting in polyoxin overproduction and/or novel compounds with improved activities.
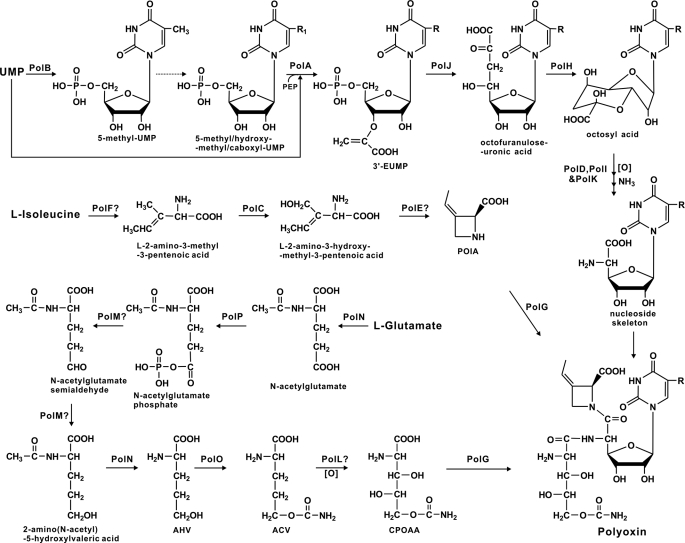
Earlier feeding experiments with isotope-labeled substrates demonstrated that three independent moieties (nucleoside skeleton, POIA, and CPOAA) of polyoxin could originate from uridine, l-isoleucine, and l-glutamate, respectively. Biosynthesis of the nucleoside skeleton is considered to be initiated by condensation of uridine with PEP to form octofuranuloseuronic acid as intermediate, followed by oxidative elimination of the two terminal carbon atoms and introduction of an amino group at the C-5′ position (35). Its biosynthesis is very likely to be catalyzed by five proteins, including PolA (enolpyruvyltransferase), PolD (hydroxylase), PolH (radical S-adenosylmethionine protein), PolI (aminotransferase), PolJ (phosphatase), and PolK (hydroxylase), respectively, because the corresponding homologs hypothetically resulting in an identical nucleoside skeleton had been characterized in the nikkomycin biosynthetic pathway. Moreover, the capacity of NikO (PolA homolog) to activate UMP for accepting the PEP moiety for the eventual formation of 3′-EUMP (16) closely resembles PolA, and both nikO and polA could complement a polA mutation. Thus, it is logical to propose that dephosphorylation by PolJ (a putative tyrosine phosphatase) and cyclization of 3′-EUMP by PolH would immediately follow the reaction of PolA to generate an intermediate, which is then converted to the aminohexuronic acid moiety with stepwise functions of PolK, PolD, and PolI, respectively. As suggested in Fig. 8, this proposal, however, would result in a slight modification of the previously proposed pathway for biosynthesis of the polyoxin nucleoside skeleton (35), whose biosynthesis was deduced to be initiated using uridine and PEP as start substrates.
FIGURE 8.
Proposed pathway for polyoxin biosynthesis. The dashed line indicates the protein(s) for the proposed catalytic reaction(s) was/were not identified. R1 = CH3,CH3OH, or COOH; R = H, CH3,CH3OH, or COOH.? indicates the highly hypothetic protein functions deduced from bioinformatic analysis only.
POIA was deduced to be stereospecifically dehydrogenated from l-isoleucine by introduction of the β, γ-double bond, followed by oxidation and cyclization (36). Three proteins: PolC (hydroxylase), PolE (unknown), and PolF (oxidoreductase), were conceived as candidate enzymes for its biosynthesis. Although the role of PolF to catalyze l-isoleucine to generate the intermediate l-2-amino-3-methyl-3-pentenoic acid could be deduced more obviously from bioinformatic analysis and putative hydroxylation by PolC occurs to generate l-2-amino-3-hydroxymethyl-3-pentenoic acid after that, how PolE might be involved in the unique cyclization reaction for POIA biosynthesis still remains obscure (Fig. 8).
The intermediates in the putative pathway for CPOAA biosynthesis were also proposed by feeding experiments with isotope-labeled substrates (34, 37). The initial reaction involves reduction of l-glutamate to l-glutamate-γ-semialdehyde and then to AHV, which is further catalyzed to ACV by transcarbamoylation, followed by the final conversion into carbamoylpolyoxamic acid after hydroxylation. Bioinformatic analysis of the pol gene cluster contradicts two aspects with the above proposal. First, the significant similarity of PolN (amino acid N-acetyltransferase) and PolP (acetylglutamate kinase) to the counterpart proteins for arginine biosynthesis in primary metabolism strongly suggested that the pathways for CPOAA and arginine biosynthesis could be very similar. Second, if the direct reductions of l-glutamate were in the order of l-glutamate-γ-semialdehyde to AHV, the proposed intermediate l-glutamate-γ-semialdehyde could self-cyclize easily for l-proline biosynthesis, which would decrease biosynthetic efficiency of CPOAA. Furthermore, the roles of PolN and PolP could not be appropriately assigned from the above proposal. Thus, we propose that CPOAA is formed by first transfer of an N-acetyl group to l-glutamate by PolN to form N-acetyl-l-glutamate, followed by phosphorylation to form N-acetyl-l-glutamate phosphate by PolP before stepwise reduction, deacetylation, transcarbamoylation, and hydroxylation (Fig. 8).
Methylation at the C-5 position of the polyoxin nucleoside skeleton was previously concluded also by isotope incorporation experiments as governed by an enzyme independent of thymidylate synthase for DNA biosynthesis (38), but neither enzyme nor catalyzed substrate was known (1).
All three of the above-described moieties were proposed to be assembled under control of a novel amide synthetase, PolG (NikS homolog) (Fig. 8), perhaps before modification by hydroxylation and carboxylation of the C-5 methyl group of the polyoxin nucleoside skeleton occurs for the biosynthesis of some of the specific polyoxin components (e.g. polyoxin K). This seemed to agree well with the production of nikkomycin nucleoside skeleton (nikkomycin Cx and nikkomycin Cz) by nikS mutant (14), which implies NikS as an amide synthetase for nikkomycin assembly. It is thus logical to propose that PolG possesses a similar function for assembly of the three moieties in polyoxin biosynthesis, although the precise mechanism for PolG catalyzing variable substrates still remained ambiguous.
It is true that the genes required for hydroxylation and carboxylation of the C-5 methyl group of the polyoxin nucleoside skeleton do not seem to exist in the pol gene cluster, whose left and right boundaries seem to have been unambiguously determined, but this does not necessarily mean that the pol gene cluster is separated into two independent loci on the S. cacaoi var. asoensis genome, as has been reported in some unprecedented rare cases (39–41). Such modification processes could be mediated by genes from other pathways, either secondary or primary. The heterologous expression of the identified pol gene cluster in S. lividans TK24 to produce polyoxin H, which is a derivative of polyoxin A without hydroxylation and carboxylation of the C-5 methyl group of the polyoxin nucleoside skeleton, strongly suggests that genes for hydroxylation/carboxylation to form polyoxin A/F are missing in TK24. Additionally, this result suggests that the genes for the specific hydroxylation and carboxylation reactions are located in an unidentified or unknown biosynthetic cluster in S. cacaoi var. asoensis, absent from TK24, rather than being involved in primary metabolism. It would be interesting to isolate the putative second gene cluster via the proposed functions involved in the specific hydroxylation and carboxylation reactions so that the mechanism(s) of a cross-talk between different biosynthetic pathways could be investigated in more detail. This work is now in progress.
Supplementary Material
Acknowledgments
We are very grateful to Prof. Sir David A. Hopwood for critical reading of the manuscript and many valuable comments. We also sincerely thank Prof. Christopher T. Walsh, Harvard University, for kindly providing us with genomic DNA of S. tendae Tü901.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) EU158805.
This work was supported by Grants 973 and 863 from the Ministry of Science and Technology, the National Science Foundation of China, the Shanghai Municipal Council of Science and Technology, and Shanghai Leading Academic Discipline Project B203.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2, Figs. S1–S9, and additional references.
Footnotes
The abbreviations used are: POIA, polyoximic acid; PEP, phosphoenolpyruvate; 3′-EUMP, 3′-enolpyruvyl-UMP; IPTG, isopropyl β-d-thiogalactopyranoside; CPOAA, carbamoylpolyoxamic acid; AHV, α-amino-δ-hydroxyvaleric acid; ACV, α-amino-δ-carbamoylhydroxyvaleric acid; LC/MS, liquid chromatography/mass spectrometry; MS, mass spectrometry; MS/MS, tandem MS; HPLC, high pressure liquid chromatography.
H. E. Yunlong, unpublished data.
References
- 1.Isono, K. (1988) J. Antibiot. (Tokyo) 41 1711-1739 [DOI] [PubMed] [Google Scholar]
- 2.Isono, K., Nagatsu, J., Kobinata, K., Sazuki, K., and Suzuki, S. (1965) Agric. Biol. Chem. 29 848-854 [Google Scholar]
- 3.Isono, K. N., Kobinata, K., Sasaki, K., and Suzuki, S. (1967) Agric. Biol. Chem. 31 190-199 [Google Scholar]
- 4.Wen, Z. (2004) Chinese J. Biol. 21 36-37 [Google Scholar]
- 5.Hori, M., Eguchi, J., Kakiki, K., and Misato, T. (1974) J. Antibiot. (Tokyo) 27 260-266 [DOI] [PubMed] [Google Scholar]
- 6.Zhang, D., and Miller, M. J. (1999) Curr. Pharm. Des. 5 73-99 [PubMed] [Google Scholar]
- 7.Lacalle, R. A., Tercero, J. A., and Jimenez, A. (1992) EMBO J. 11 785-792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cone, M. C., Yin, X., Grochowski, L. L., Parker, M. R., and Zabriskie, T. M. (2003) ChemBioChem 4 821-828 [DOI] [PubMed] [Google Scholar]
- 9.Liu, G., Tian, Y., Yang, H., and Tan, H. (2005) Mol. Microbiol. 55 1855-1866 [DOI] [PubMed] [Google Scholar]
- 10.Bruntner, C., and Bormann, C. (1998) Eur. J. Biochem. 254 347-355 [DOI] [PubMed] [Google Scholar]
- 11.Bruntner, C., Lauer, B., Schwarz, W., Mohrle, V., and Bormann, C. (1999) Mol. Gen. Genet. 262 102-114 [DOI] [PubMed] [Google Scholar]
- 12.Lauer, B., Russwurm, R., and Bormann, C. (2000) Eur. J. Biochem. 267 1698-1706 [DOI] [PubMed] [Google Scholar]
- 13.Lauer, B., Sussmuth, R., Kaiser, D., Jung, G., and Bormann, C. (2000) J. Antibiot. (Tokyo) 53 385-392 [DOI] [PubMed] [Google Scholar]
- 14.Lauer, B., Russwurm, R., Schwarz, W., Kalmanczhelyi, A., Bruntner, C., Rosemeier, A., and Bormann, C. (2001) Mol. Gen. Genet. 264 662-673 [DOI] [PubMed] [Google Scholar]
- 15.Chen, H., Hubbard, B. K., O'Connor, S. E., and Walsh, C. T. (2002) Chem. Biol. 9 103-112 [DOI] [PubMed] [Google Scholar]
- 16.Ginj, C., Ruegger, H., Amrhein, N., and Macheroux, P. (2005) ChemBioChem 6 1974-1976 [DOI] [PubMed] [Google Scholar]
- 17.Carrell, C. J., Bruckner, R. C., Venci, D., Zhao, G., Jorns, M. S., and Mathews, F. S. (2007) Structure (Lond.) 15 928-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieser, T., Bibb, M. J., Chater, K. F., Butter, M. J., and Hopwood, D. A. (2000) Practical Streptomyces Genetics, 2nd Ed., pp. 169-170, John Innes Foundation, Norwich, UK
- 19.Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 20.Ishikawa, J., and Hotta, K. (1999) FEMS Microbiol. Lett. 174 251-253 [DOI] [PubMed] [Google Scholar]
- 21.Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990) J. Mol. Biol. 215 403-410 [DOI] [PubMed] [Google Scholar]
- 22.Sun, Y., Zhou, X., Liu, J., Bao, K., Zhang, G., Tu, G., Kieser, T., and Deng, Z. (2002) Microbiology 148 361-371 [DOI] [PubMed] [Google Scholar]
- 23.Li, W., Ying, X., Guo, Y., Yu, Z., Zhou, X., Deng, Z., Kieser, H., Chater, K. F., and Tao, M. (2006) J. Bacteriol. 188 8368-8375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai, L., Li, L., Xu, H., Minagawa, K., Yu, Y., Zhang, Y., Zhou, X., Floss, H. G., Mahmud, T., and Deng, Z. (2006) Chem. Biol. 13 387-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gust, B., Challis, G. L., Fowler, K., Kieser, T., and Chater, K. F. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 1541-1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiedler, H. P. (1984) J. Chromatogr. 316 487-494 [DOI] [PubMed] [Google Scholar]
- 27.Garcia, M., Serra, A., Rubiralta, M., Diez, A., Segarra, V., Lozoya, E., Ryder, H., and Palacios, J. M. (2000) Tetrahedron Asymmetry 11 991-994 [Google Scholar]
- 28.Bentley, S. D., Chater, K. F., Cerdeno-Tarraga, A. M., Challis, G. L., Thomson, N. R., James, K. D., Harris, D. E., Quail, M. A., Kieser, H., Harper, D., Bateman, A., Brown, S., Chandra, G., Chen, C. W., Collins, M., Cronin, A., Fraser, A., Goble, A., Hidalgo, J., Hornsby, T., Howarth, S., Huang, C. H., Kieser, T., Larke, L., Murphy, L., Oliver, K., O'Neil, S., Rabbinowitsch, E., Rajandream, M. A., Rutherford, K., Rutter, S., Seeger, K., Saunders, D., Sharp, S., Squares, R., Squares, S., Taylor, K., Warren, T., Wietzorrek, A., Woodward, J., Barrell, B. G., Parkhill, J., and Hopwood, D. A. (2002) Nature 417 141-147 [DOI] [PubMed] [Google Scholar]
- 29.Omura, S., Ikeda, H., Ishikawa, J., Hanamoto, A., Takahashi, C., Shinose, M., Takahashi, Y., Horikawa, H., Nakazawa, H., Osonoe, T., Kikuchi, H., Shiba, T., Sakaki, Y., and Hattori, M. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 12215-12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hindle, Z., Callis, R., Dowden, S., Rudd, B. A., and Baumberg, S. (1994) Microbiology 140 311-320 [DOI] [PubMed] [Google Scholar]
- 31.Van Rhijn, P., Desair, J., Vlassak, K., and Vanderleyden, J. (1994) Appl. Environ. Microbiol. 60 3615-3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, W., Zeng, H., and Tan, H. (2000) Curr. Microbiol. 41 312-316 [DOI] [PubMed] [Google Scholar]
- 33.Li, Y., Zeng, H., and Tan, H. (2004) Curr. Microbiol. 49 128-132 [DOI] [PubMed] [Google Scholar]
- 34.Funayama, S., and Isono, K. (1975) Biochemistry 14 5568- [DOI] [PubMed] [Google Scholar]
- 35.Isono, K., Sato, T., Hirasawa, K., Funayama, S., and Suzuki, S. (1978) J. Am. Chem. Soc. 100 3937-3939 [Google Scholar]
- 36.Isono, K., Funayama, S., and Suhadolnik, R. J. (1975) Biochemistry 14 2992-2996 [DOI] [PubMed] [Google Scholar]
- 37.Funayama, S., and Isono, K. (1977) Biochemistry 16 3121-3127 [DOI] [PubMed] [Google Scholar]
- 38.Isono, K., and Suhadolnik, R. J. (1976) Arch. Biochem. Biophys. 173 141-153 [DOI] [PubMed] [Google Scholar]
- 39.Yu, T. W., Bai, L., Clade, D., Hoffmann, D., Toelzer, S., Trinh, K. Q., Xu, J., Moss, S. J., Leistner, E., and Floss, H. G. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 7968-7973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tahlan, K., Park, H. U., and Jensen, S. E. (2004) Can. J. Microbiol. 50 803-810 [DOI] [PubMed] [Google Scholar]
- 41.Ostash, B., Saghatelian, A., and Walker, S. (2007) Chem. Biol. 14 257-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.