Abstract
Efficient internalization of proteins from the cell surface is essential for regulating cell growth and differentiation. In a screen for yeast mutants defective in ligand-stimulated internalization of the α-factor receptor, we identified a mutant allele of TOR2, tor2G2128R. Tor proteins are known to function in translation initiation and nutrient sensing and are required for cell cycle progression through G1. Yeast Tor2 has an additional role in regulating the integrity of the cell wall by activating the Rho1 guanine nucleotide exchange factor Rom2. The endocytic defect in tor2G2128R cells is due to disruption of this Tor2 unique function. Other proteins important for cell integrity, Rom2 and the cell integrity sensor Wsc1, are also required for efficient endocytosis. A rho1 mutant specifically defective in activation of the glucan synthase Fks1/2 does not internalize α-factor efficiently, and fks1Δ cells exhibit a similar phenotype. Removal of the cell wall does not inhibit internalization, suggesting that the function of Rho1 and Fks1 in endocytosis is not through cell wall synthesis or structural integrity. These findings reveal a novel function for the Tor2-Rho1 pathway in controlling endocytosis in yeast, a function that is mediated in part through the plasma membrane protein Fks1.
INTRODUCTION
Tor proteins are phosphatidylinositol (PI) kinase-related proteins that regulate cell growth in response to nutrients and are inhibited by the immunosuppressant drug rapamycin (reviewed in Schmelzle and Hall, 2000; Gingras et al., 2001). Both yeast (Tor1 and Tor2) and mammalian (mTor) Tor proteins function as serine/threonine kinases in vivo (Cardenas and Heitman, 1995; Alarcon et al., 1999; Schmelzle and Hall, 2000; Rohde et al., 2001). Tor2 can also function as a PI-4 kinase in vitro (Cardenas and Heitman, 1995). Tor2 has a unique, essential function that is not sensitive to rapamycin and cannot be performed by Tor1. This unique function requires the Tor2 kinase domain and is involved in maintaining a normal actin cytoskeleton via the cell integrity pathway (Zheng et al., 1995; Schmidt et al., 1996; Helliwell et al., 1998b). This pathway responds to growth and stress signals to direct cell wall synthesis and is controlled by the GTPase Rho1 (Schmidt et al., 1997; reviewed in Schmelzle and Hall, 2000).
GTPases function as molecular switches, cycling between the active, GTP-bound and the inactive, GDP-bound state. Rho1 is regulated by the Rom2 guanine nucleotide exchange factor (GEF), which is activated by at least three stimuli: cell wall damage (Bickle et al., 1998), phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] (Audhya and Emr, 2002), and Tor2 (Schmidt et al., 1997). Cell wall damage is transmitted to Rom2 through cell wall integrity sensors, the integral plasma membrane proteins Wsc1 and Mid2 (Gray et al., 1997; Verna et al., 1997; Ketela et al., 1999; Rajavel et al., 1999; Philip and Levin, 2001). Production of PI(4,5)P2 at the plasma membrane is required for proper localization of Rom2, which binds to this lipid via its pleckstrin homology domain (Audhya and Emr, 2002). Rom2 activation by Tor2 is not well-defined, but this event seems to require members of the large Tor2-associated TORC complex (Loewith et al., 2002; Wedaman et al., 2003).
Rho GTPases mediate their functions by binding to and activating specific effector molecules in their GTP-bound state. Rho1 has five known effectors in yeast that together help coordinate the complex actin cytoskeletal arrangements, polarized secretion, and cell wall deposition that occur during polarized growth or when cell wall integrity is compromised. Rho1 participates directly in the production of new cell wall by functioning as the regulatory subunit of the β-1,3-glucan synthase that synthesizes glucan, the main component of the yeast cell wall. The catalytic subunit, Fks1, is the Rho1 effector for this function (Drgonova et al., 1996; Qadota et al., 1996).
Through activation of another effector, protein kinase C (Pkc1), Rho1 plays an indirect role in the formation of new cell wall by activation of a mitogen-activated protein (MAP) kinase pathway that regulates genes involved in cell wall maintenance (Levin et al., 1994; Nonaka et al., 1995; Kamada et al., 1996). Rho1 activation of Pkc1 is also required for actin cytoskeletal organization (Mazzoni et al., 1993; Delley and Hall, 1999), and Rho1 further contributes to the organization of actin filaments by interacting with the yeast formin Bni1 (Kohno et al., 1996). Other effectors of Rho1 include the transcription factor Skn7 (Alberts et al., 1998; Ketela et al., 1999) and a component of the exocyst complex, Sec3 (Guo et al., 2001).
Endocytosis, the process by which plasma membrane proteins are internalized into the cell, is choreographed by a large number of proteins and lipids that coordinate the timing and location of internalization. Sterols, sphingolipids, kinases, and components of the actin cytoskeleton have been identified as regulators of the internalization step of endocytosis in yeast (reviewed in Munn, 2001). We have now identified a role for the Tor2 signaling pathway, including Wsc1, Rom2, and Rho1 in regulating this initial step of endocytosis. We find that Rho1 controls endocytosis through its plasma membrane Fks1 effector, a novel function for the Rho1-Fks1 complex. Furthermore, our results indicate that Rho GTPases play a general role in internalization and illustrate one way by which Rho GTPases regulate endocytosis (reviewed in Qualmann and Mellor, 2003; Symons and Rusk, 2003).
MATERIALS AND METHODS
Strains and reagents
Table 1 lists the yeast strains used in this study. fks1Δ and fks2Δ mutant strains were provided by Howard Bussey (McGill University, Montreal, Canada; Dijkgraaf et al., 2002). All other strains containing single genomic deletions were obtained from EUROSCARF (Johann Wolfgang Goethe, University Frankfurt, Frankfurt, Germany) in the background strain BY4741, a derivative of the S288c background used in our laboratory. All EUROSCARF deletions were confirmed by polymerase chain reaction, and those deletions showing internalization defects were analyzed for genetic segregation of the defect with kanamycin resistance, which marks the deletion. Temperature-sensitive rho1 and fks1 fks2 strains were provided by Yoshikazu Ohya (University of Tokyo, Chiba, Japan; Saka et al., 2001; Dijkgraaf et al., 2002), and the temperature-sensitive bni1 bnr1 strain was provided by David Pellman (Harvard University, Boston, MA; Sagot et al., 2002).
Table 1.
Yeast strains
| Strain | Genotypea |
|---|---|
| LHY2632 | ura3Δ his3Δ leu2Δ met15Δ bar1Δ::URA3 |
| LHY2817 | pkc1::LEU2 leu2 ura3 his4 trp1 lys2 bar1 ppkc1-2ts [URA3] |
| LHY3421 | bni1Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY3602 | ade2 his3 leu2 lys2 trp1 ura3 rho1Δ::HIS3 ade3::rho1-2::LEU2 bar1Δ::URA3 |
| LHY3603 | ade2 his3 leu2 lys2 trp1 ura3 rho1Δ::HIS3 ade3::rho1-11::LEU2 bar1Δ::URA3 |
| LHY3604 | ade2 his3 leu2 lys2 trp1 ura3 rho1Δ::HIS3 ade3::RHO1::LEU2 bar1Δ::URA3 |
| LHY3741 | rom1Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY3742 | rom2Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY3745 | sec3ts leu2 ura3 bar1 bar1Δ::URA3 |
| LHY3746 | skn7Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY3749 | tus1Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY3863 | tor2G2128R ura3 trp1 leu2 his3 lys2 bar1 |
| LHY3865 | ura3 leu2 bar1 |
| LHY3877 | trp1 his3 ura3 leu2 bar1 |
| LHY3878 | tor2G2128R trp1 ura3 leu2 met15 bar |
| LHY3879 | sac7Δ::KanMX4 ura3 leu2 bar1 |
| LHY3880 | sac7Δ::KanMX4 tor2G2128R ura3 leu2 bar1 |
| LHY3946 | wsc1Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY4143 | mid2Δ::KanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 bar1Δ::URA3 |
| LHY4171 | bni1::bni1-FH2#1::HIS3 bnr1::KanMX4 his3Δ leu2Δ ura3Δ lys2Δ bar1Δ::URA3 |
| LHY4172 | his3Δ leu2Δ met15Δ ura3Δ bar1Δ::URA3 |
| LHY4306 | tor2G2128R ura3 trp1 leu2 his3 lys2 bar1 pTOR2 [URA3] |
| LHY4387 | ypk1Δ::KanMX4 trp1 ura3 his3 leu2 bar1Δ::URA3 |
| LHY4388 | met15Δ ura3 his3 leu2 bar1Δ::URA3 |
| LHY4389 | sac7Δ::KanMX4 met15Δ ura3 his3 leu2 bar1Δ::URA3 |
| LHY4390 | ypk1Δ::KanMX4 sac7Δ::KanMX4 met15Δ ura3 his3 leu2 bar1Δ::URA3 |
| LHY4477 | fks1Δ::HIS3 ura3::KanMX4 his3 leu2 can1 bar1Δ::URA3 |
| LHY4498 | MATa/MATα TOR2/tor2Δ::KanMX4 his3Δ/his3Δ leu2Δ/leu2Δ lys2Δ/LYS2 MET15/met15Δ ura3Δ/ura3Δ |
| LHY4514 | fks2Δ::KanMX4 ura3::KanMX4 his3 leu2 can1 bar1Δ::URA3 |
| LHY4515 | ura3::KanMX4 his3 leu2 can1 bar1Δ::URA3 |
All strains are MATa unless indicated otherwise
The mating type of yeast strains was switched by transformation with a plasmid expressing the HO gene, followed by removal of the HO plasmid on 5-fluoroorotic acid. Deletion strains containing a mutation in the Bar1 protease were made by introducing a bar1Δ::URA3 mutation by homologous recombination, or by meiotic recombination with a bar1-1 strain. All strains were grown in synthetic minimal (YNB), YNBYE (YNB supplemented with 0.2% yeast extract), or YPUAD (2% bacto-peptone, 1% yeast extract, 2% glucose supplemented with 20 mg/l adenine, uracil, and tryptophan) media as indicated in the figure legends (Sherman, 1991).
Cloning of TOR2
TOR2 was cloned from a centromere-based genomic DNA library made in the YCpKan101 vector (generously provided by Jon Binkley and David Botstein, Stanford University, Stanford, CA) by complementation of the growth defect of udi11-1 cells on YPUAD + 6 mM caffeine at 37°C. The isolated clone (LHP1557) contained a region of chromosome XI from base coordinates 52489-65453. The TOR2 gene on a CEN plasmid (pML48; Lorenz and Heitman, 1995) obtained from Joseph Heitman (Duke University, Durham, NC) was able to complement the growth and internalization defects of the udi11-1 mutant.
The mutation in udi11-1 was determined by gapped plasmid repair (Rothstein, 1991; deHart et al., 2002). A plasmid carrying TOR2 was digested with BglII, removing 5270 base pairs and leaving ∼1440 base pairs and 710 base pairs at the 5′ and 3′ ends of TOR2, respectively. The gapped plasmid was purified and transformed into tor2 and TOR2 cells. Two plasmids recovered from tor2 cells were each found to have a single point mutation in codon 2128 that was not present in two plasmids recovered from TOR2 cells. This mutation was duplicated in the TOR2 plasmid using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) with mutagenic primers. The resulting plasmid (LHP1934) was transformed into TOR2/tor2Δ::KanMX4 diploid cells (LHY4498) from EUROSCARF. Haploid progeny from diploid transformants were isolated and tor2Δ strains carrying the tor2G2128R plasmid were identified and assayed for growth on caffeine medium and for α-factor internalization.
Internalization Assays
All α-factor internalization assays were performed essentially as described previously (Dulic et al., 1991) by the continuous presence protocol. Conditions for growth and the assay temperature are indicated in the figure legends. Cells were harvested in early log phase and shifted to the assay temperature for 15 min before the addition of 35S-α-factor. Each data point represents the average of at least three experiments, and the error bars represent the SD. SDS treatment was assayed by adding SDS to a concentration of 0.005% to cells 15 min before the addition of 35S-α-factor. Rapamycin to 200 ng/ml or solvent alone (90% ethanol, 10% Tween) was added to the cells 15 min before the addition of 35S-α-factor.
For internalization assays using spheroplasts, cells were grown to early log phase in YPUAD and treated at 5 × 108 cells/ml with 10 mM dithiothreitol in 100 mM Tris, pH 9.4 for 10 min. After removing the dithiothreitol buffer, cells were incubated with 0.6-1 mg/ml yeast lytic enzyme (ICN Pharmaceuticals Biochemicals Division, Aurora, OH) in lytic buffer (0.7 M sorbitol, 0.75% yeast extract, 1.5% peptone, 0.5% glucose, 10 mM Tris) at 30°C until the OD600 had dropped to ∼10% of the nontreated cells. The spheroplasts were washed and incubated at 5 × 107 cells/ml in recovery buffer (0.7 M sorbitol, 0.75% yeast extract, 1.5% peptone, 1% glucose) for 20-30 min at 30°C. Spheroplasts were then harvested, resuspended in recovery buffer at 5 × 108 cells/ml, and incubated with 35S-α-factor. Standard pH 1.0 and pH 6.0 buffers were supplemented with 0.7 M sorbitol to provide osmotic support.
Lucifer yellow localization assays were performed as described previously (Dulic et al., 1991). Cells were grown in YPUAD at 24°C to early log phase, harvested, and resuspended in YPUAD. The cell suspension was incubated at 30°C for 15 min and Lucifer yellow (Sigma-Aldrich, St. Louis, MO) was added for 1 h at 30°C. Cells were viewed using an LSM410 confocal microscope (Carl Zeiss, Thornwood, NY) with a fluorescein isothiocyanate (FITC) filter and with differential interference contrast optics. The FITC images within a figure were taken using identical parameters.
Actin Staining
Staining of the actin cytoskeleton was performed basically as described previously (Adams and Pringle, 1984; Bénédetti et al., 1994). Cells (2 × 108) were grown in YPUAD at 24°C to early log phase, harvested by centrifugation, and resuspended in 20 ml of YPUAD. After incubation at 30°C for 3 h, cells were fixed and stored at 4°C overnight. Cells (4 × 107) were incubated with 1 μl of 0.4 mg/ml FITC-phalloidin (Sigma-Aldrich) for 2 h, washed, resuspended in 1:9 phosphate-buffered saline buffer pH 9.0:glycerol, and mounted on a slide with an equal volume of 1.6% low-melt agarose. Stained cells were viewed using an LSM410 confocal microscope with an FITC filter. For quantification of the data, poor polarization was defined as five or more actin spots in the mother cell (Delley and Hall, 1999).
RESULTS
Tor2 Is Required for Endocytosis
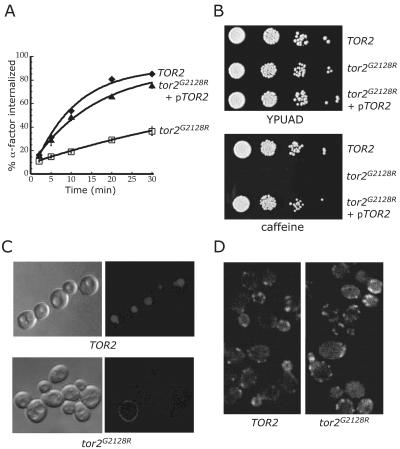
udi11-1 (ubiquitin-dependent internalization) was identified in a screen for mutants defective in the receptor-mediated internalization of α-factor pheromone (deHart et al., 2002). α-factor internalization in udi11-1 cells was significantly delayed at 24°C, 30°C, and 37°C, indicating that this phenotype was not temperature dependent (Figure 1A; our unpublished data). The growth of udi11-1 cells was not temperature-sensitive on rich or synthetic medium but was sensitive to the presence of caffeine in the medium (Figure 1B). Using this caffeine-sensitive growth phenotype, the gene defective in udi11-1 cells was cloned from a centromere-based genomic DNA library. A plasmid carrying only TOR2 was able to suppress both the caffeine sensitivity and the internalization defect of udi11-1 cells (Figure 1, A and B). Using gapped plasmid repair, we identified the mutation in udi11-1 as a single C-to-T transition, resulting in mutation of G2128 to arginine at the beginning of the Tor2 kinase domain. We introduced the G2128R mutation into wild-type TOR2 and found that expression of Tor2G2128R in tor2Δ cells resulted in cells that were defective for growth on caffeine-containing medium and for internalization (our unpublished data). These results demonstrate that UDI11 is allelic to TOR2, and hereafter we refer to udi11-1 as tor2G2128R.
Figure 1.
Tor2 is required for receptor-mediated and fluid phase internalization. (A) Internalization of 35S-α-factor was measured at 30°C after growth of cells in YPUAD at 24°C. Wild-type (LHY3865, ♦); tor2G2128R (LHY3863, □); tor2G2128R with pTOR2 (LHY4306, ▴). These same strains were used for the experiments shown in all parts of the figure. (B) Wild-type, tor2G2128R, or tor2G2128R cells carrying centromere-based pTOR2 were grown on YPUAD or YPUAD + 6 mM caffeine plates at 30°C. (C) Lucifer yellow localization was assayed in wild-type and tor2G2128R cells. Images were taken using differential interference contrast and fluorescence optics. (D) The actin cytoskeleton was stained in wild-type cells and in tor2G2128R cells. Cells were shifted to 30°C for 3 h before fixation and incubation with FITC-phalloidin. In cells with emerging buds, 29.4% of wild-type cells showed poor polarization of the cytoskeleton (see MATERIALS AND METHODS), compared with 48.4% in tor2G2128R cells.
We examined whether tor2G2128R cells are impaired in their ability to perform fluid-phase endocytosis by monitoring the internalization and localization of a hydrophilic dye, Lucifer yellow, into the lysosome-like vacuole. Whereas TOR2 cells efficiently localized the dye to the vacuole, tor2G2128R cells did not internalize the dye, and little or no cell staining was observed after 1 h incubation (Figure 1C). The actin cytoskeleton plays a critical role in endocytosis (reviewed in Munn, 2001), and the unique function of Tor2 regulates actin cytoskeletal organization (Schmidt et al., 1996). Therefore, we analyzed actin localization in tor2G2128R cells. Wild-type cells displayed a polarized actin cytoskeleton, with most of the actin localized in the bud or near the neck. In tor2G2128R cells, actin staining was more often depolarized, with actin patches seen throughout the mother cell (Figure 1D). Depolarization in tor2G2128R cells was not as dramatic as has been seen for other tor2 alleles (Schmidt et al., 1996), consistent with the weaker growth phenotypes observed with this mutant.
Rho1 Regulators Act Downstream of Tor2 in Endocytosis
tor2G2128R cells exhibited a severe endocytic defect even though wild-type Tor1 was expressed. Therefore, we reasoned that the role of Tor2 in internalization was part of its unique function. To support this idea, we assayed internalization in tor1Δ cells, and found no discernible internalization defect (our unpublished data). Furthermore, we tested whether receptor internalization was sensitive to rapamycin, a drug that affects the shared essential function of Tor1 and Tor2, but not the Tor2 unique function (Zheng et al., 1995). α-factor internalization was unaffected by the presence of rapamycin at a concentration sufficient to inhibit cell division (our unpublished data), another demonstration that the unique Tor2 function is required for endocytosis.
Because the unique function of Tor2 involves activation of the Rho1 GEF Rom2, and perhaps other Rho1 GEFs (Schmidt et al., 1997), we tested whether disruption of genes encoding these GEFs (ROM1, ROM2, and TUS1) affected α-factor internalization. rom2Δ cells were severely defective for internalization, whereas tus1Δ cells showed a more modest defect and rom1Δ cells internalized α-factor normally (Figure 2A). Deletion of each of the four known Rho1 GTPase-activating proteins (GAPs: BAG7, BEM2, LRG1, and SAC7) had little or no effect on internalization (our unpublished data), consistent with a role for GTP-bound Rho1 in endocytosis.
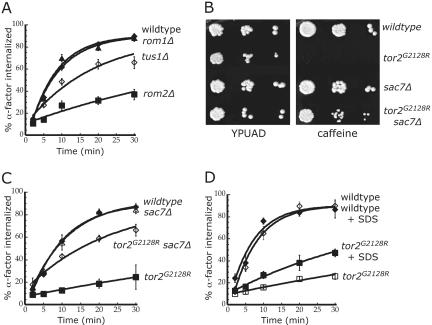
Figure 2.
The unique function of Tor2 is required for its role in receptor internalization. Internalization of 35S-α-factor was measured at 30°C after growth of cells in YPUAD at 24°C. (A) Wild-type (LHY2632, ♦), rom1Δ (LHY3741, ▴), rom2Δ (LHY3742, ▪), and tus1Δ (LHY3749, ⋄). (B) The same strains assayed in C were tested for growth on YPUAD or YPUAD + 6 mM caffeine plates at 30°C. (C) Wild-type (LHY3877, ♦), tor2G2128R (LHY3878, ▪), sac7Δ (LHY3879, Δ), and tor2G2128R sac7Δ (LHY3880, ⋄). (D) Wild-type (LHY3865; ⋄, ♦) and tor2G2128R (LHY3863; □, ▪) cells were assayed either in YPUAD (open symbol) or YPUAD + 0.005% SDS (closed symbol).
The growth defect of a temperature-sensitive tor2ts mutant is suppressed by deletion of SAC7, presumably by increasing the amount of active, GTP-bound Rho1 (Schmidt et al., 1997). Similarly, deletion of SAC7 in tor2G2128R cells suppressed the caffeine-sensitivity of these cells (Figure 2B). In addition, deletion of SAC7 substantially suppressed the internalization defect of tor2G2128R cells (Figure 2C). Suppression of tor2G2128R phenotypes was not observed with deletion of any of the other three Rho1 GAPs (our unpublished data), indicating that suppression is specifically due to loss of Sac7 function. These observations suggest that regulators of Rho1 act downstream of Tor2 to regulate receptor internalization.
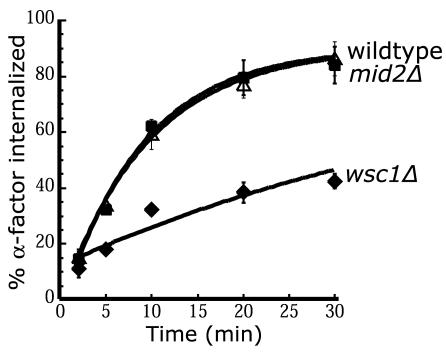
Cell wall damage activates Rom2 independently of Tor2 and suppresses the growth defect of tor2ts cells (Bickle et al., 1998). Therefore, we tested whether cell wall damage would suppress the tor2G2128R internalization defect, and we found that addition of SDS to 0.005% partially suppressed the defect (Figure 2D). Rom2 can also be activated by the cell wall sensors Wsc1 and Mid2. The WSC family of genes (WSC1-4) were identified as plasma membrane-localized, integral membrane proteins that act upstream of Pkc1 in activating the cell integrity pathway under a variety of environmental stress conditions (Gray et al., 1997; Verna et al., 1997). Mid2, though not homologous to the Wsc proteins, also functions as a sensor for cell wall damage and activates the Pkc1-controlled MAP kinase pathway (Ketela et al., 1999; Rajavel et al., 1999). Both Wsc1 and Mid2 physically interact with Rom2 and are required for efficient loading of GTP onto Rho1 (Philip and Levin, 2001). Deletion of WSC1 resulted in a significant internalization defect, whereas deletion of MID2 had no effect (Figure 3). Together, the observations described above indicate that the major function of Tor2 in regulating endocytosis is through activation of the Rho1-regulated cell integrity pathway.
Figure 3.
Cell wall integrity sensor Wsc1 is required for internalization. Wild-type (LHY2632, ▵), wsc1Δ (LHY3946, ♦), and mid2Δ (LHY4143, ▪) cells were assayed for internalization of 35S-α-factor at 30°C after growth in YPUAD at 24°C.
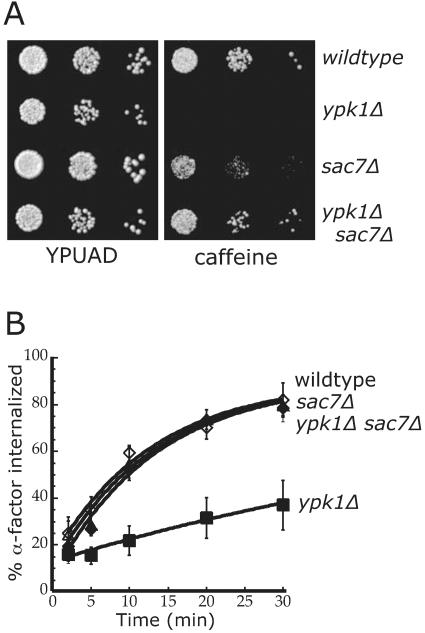
The Ypk1 kinase is required for receptor internalization (deHart et al., 2002) and influences the Rom2-Rho1 pathway (Roelants et al., 2002; Schmelzle et al., 2002). The α-factor internalization defect of ypk1Δ cells, as well as the caffeine-sensitive growth defect, was fully suppressed by deletion of SAC7 (Figure 4, A and B). These observations suggest that Ypk1 acts upstream of Rho1 in controlling receptor internalization.
Figure 4.
Ypk1 regulates receptor internalization through Rom2-Rho1. (A) Wild-type (LHY4388), ypk1Δ (LHY4387), sac7Δ (LHY4389), and ypk1Δ sac7Δ (LHY4390) cells were grown on YPUAD or YPUAD + 6 mM caffeine plates at 30°C. (B) The same strains were assayed for internalization of 35S-α-factor at 30°C after growth of cells in YPUAD at 24°C. Wild type (♦), ypk1Δ (▪), sac7Δ (Δ), and ypk1Δ sac7Δ (⋄).
Fks1 Is a Rho1 Effector Required for Internalization
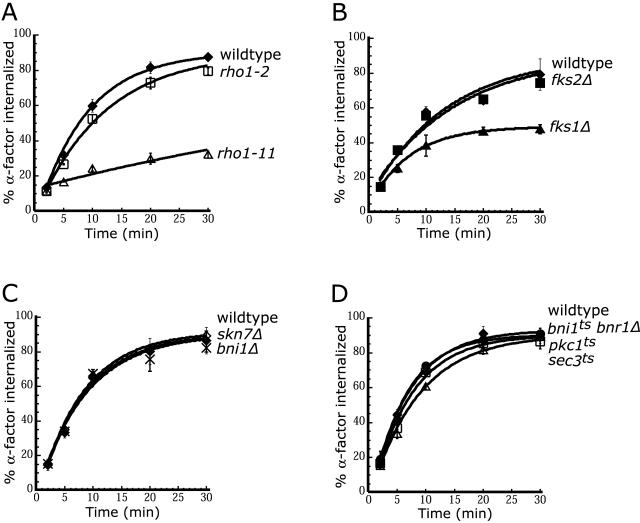
The results described above indicate that activation of Rho1 is required for efficient endocytosis. Temperature-sensitive alleles of RHO1 have been divided into at least two intragenic complementation groups: those that inhibit polarized growth and activation of Pkc1, represented by rho1-2, and those that inhibit β-1,3-glucan synthase activity, represented by rho1-11 (Drgonova et al., 1999; Saka et al., 2001). Receptor internalization in rho1-11 cells was severely compromised, whereas internalization in rho1-2 cells was unaffected (Figure 5A). Cells bearing deletions in the nonessential RHO2 or RHO4 genes internalized α-factor normally (our unpublished data). We next analyzed receptor internalization in strains carrying mutations in each of the known Rho1 effectors, bni1Δ, fks1Δ, fks2Δ, sec3ts, skn7Δ, bni1ts bnr1Δ, and pkc1ts cells. Only fks1Δ cells exhibited a defect in internalization (Figure 5, B-D; Friant et al., 2000). Furthermore, deletion of MPK1, the gene encoding the MAP kinase downstream of Pkc1, had no effect on internalization (our unpublished data). These observations indicate that Fks1 is a downstream effector of Rho1 that is required for receptor internalization.
Figure 5.
Fks1 is a Rho1 effector required for internalization. Internalization of 35S-α-factor was measured at the indicated temperature after growth of cells in YPUAD at 24°C. (A) Wild-type (LHY3604, ♦), rho1-2 (LHY3602, □), and rho1-11 (LHY3603, ▵) cells were assayed at 37°C. (B) Wild-type (LHY4515, ♦), fks1Δ (LHY4477, ▴), and fks2Δ (LHY4514, ▪) cells were assayed at 30°C. (C) Wild-type (LHY2632, ♦), skn7Δ (LHY3746, ⋄), and bni1Δ (LHY3421, X) cells were assayed at 30°C. (D) Wild-type (LHY4172, ♦), bnr1Δ bni1ts (LHY4171, ▪), pkc1ts (LHY2817, □), and sec3ts (LHY3745, ▵) cells were assayed at 37°C.
Cell Wall Damage Does Not Inhibit Internalization
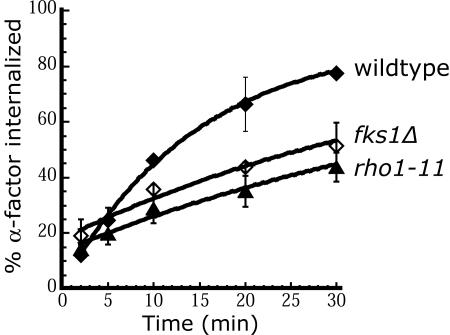
Rho1 and Fks1 together synthesize the major cell wall component, β-1,3-glucan, and it is possible that a healthy cell wall produced by normal glucan synthesis is required to internalize material from the plasma membrane. This possibility was tested by analyzing the effect of removing the cell wall on α-factor internalization. Cells were converted to spheroplasts by enzymatic removal of the cell wall, allowed to recover briefly in osmotically supported rich medium, and then assayed for internalization of α-factor. rho1-11 and fks1Δ spheroplasts exhibited a significant defect in internalization compared with wild-type spheroplasts (Figure 6). This observation indicates that Rho1 and Fks1 are required for internalization even in the absence of a cell wall. Therefore, the internalization defect in rho1 and fks1 cells is unlikely to be due to a compromised cell wall. These results suggest that Fks1 is involved in another function besides cell wall production, an idea supported by recent work that identified mutations in Fks1 that result in temperature-dependent growth, but have no effect on cell wall glucan content (Dijkgraaf et al., 2002). Several fks1ts fks2Δ strains, as well as mutants in other genes affecting the cell wall, such as ccw14Δ, cwp1Δ, and chs3Δ, were assayed for internalization, and all internalized α-factor normally (our unpublished data). These results further support our conclusion that a normal cell wall is not required for internalization.
Figure 6.
Cell wall damage does not inhibit internalization. Wild-type (LHY2632, ♦), fks1Δ (LHY3736, ⋄), and rho1-11 (LHY3603, ▴) cells were grown in YPUAD at 24°C, converted to spheroplasts, incubated for 20-30 min in recovery buffer, and then assayed for internalization of 35S-α-factor at 30°C.
DISCUSSION
In this article, we demonstrate that the unique function of Tor2 is necessary for efficient endocytosis in yeast. Tor2 acts through the GEF Rom2 to stimulate Rho1, which in turn controls receptor internalization through Fks1 (Figure 7). In addition to Tor2, the other known activators of Rom2 are also required for efficient endocytosis. The cell wall sensor, Wcs1, is required for Rom2 activation in response to cell wall damage and Wsc1 is essential for rapid receptor internalization. The PI-4 kinase Stt4 and the PI(4)P-5 kinase Mss4 synthesize the lipid Tor2 activator, PI(4,5)P2, at the plasma membrane (Audhya and Emr, 2002). A temperature-sensitive mss4 mutant is defective in receptor internalization (Desrivieres et al., 2002; our unpublished data). Although PI(4,5)P2 is also likely to be required for other roles in endocytosis (reviewed in Cullen et al., 2001; Takenawa and Itoh, 2001), these results together suggest that Rom2 requires stimulation from each of its three known inputs for receptor internalization to occur at its maximal rate.
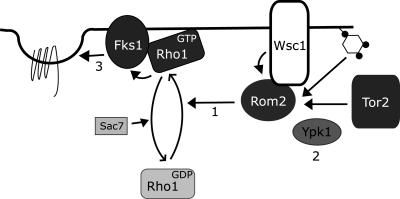
Figure 7.
Cell integrity pathway components involved in regulating receptor internalization. A schematic diagram of the proteins of the cell integrity pathway that are involved in the regulation of endocytosis. 1) Proteins required for the activation of Rho1 (Tor2, Wsc1, and Rom2) are required for efficient internalization. 2) The endocytic function of Ypk1 is mediated in part by this pathway. Our results are consistent with a role for Ypk1 upstream of Rho1. Ypk1 is likely to be activated by Tor2-dependent phosphorylation (see DISCUSSION); however, the target of Ypk1 action is not known. 3) The Rho1 effector Fks1 is required for endocytosis, perhaps by influencing the localization of components of the endocytic machinery or by affecting localization of Rho1 itself, which may be necessary for activation of an unidentified Rho1 effector and/or control of PI metabolism.
We also show that the Ypk1 kinase acts upstream of Rho1 to regulate endocytosis. Ypk1 was previously linked to the cell integrity pathway and postulated to act either upstream of Rho1 (Schmelzle et al., 2002) or through independent activation of the transcription factors, Rlm1 and Smp1 (Roelants et al., 2002). Our results support a role for the endocytic function of Ypk1 upstream of Rho1 in the cell integrity pathway. Although it has been suggested that the internalization defect of ypk1 mutants is indirect due to an effect on genes controlled by Smp1 and Rlm1 (Roelants et al., 2002), α-factor internalization is normal in smp1Δ rlm1Δ cells and in strains carrying deletions of genes regulated by these transcription factors (Jung and Levin, 1999), including hsp150Δ, exg1Δ, ccw14Δ, cwp1Δ, chs3Δ, and ktr6Δ (our unpublished data). The mechanism by which Ypk1 impinges on this pathway has not been established. One possibility is that Tor2 acts directly upstream of Ypk1 by phosphorylating the Ypk1 turn and hydrophobic motifs, an event that regulates Ypk1 homologs in other organisms (Burnett et al., 1998; Isotani et al., 1999; Oldham et al., 2000; Zhang et al., 2000; Saitoh et al., 2002; Matsuo et al., 2003). Another possibility is that Ypk1 may directly regulate a Tor2 complex, TORC2 (Loewith et al., 2002; Wedaman et al., 2003), because the Avo1-3 members of this complex each contain Ypk1 consensus phosphorylation sites (RXRXXS/TØ; Casamayor et al., 1999). These two models are not mutually exclusive.
Due to the close relationship between actin cytoskeleton function and endocytosis, we were surprised to discover that mutations that cause a defect in the protein kinase C pathway and in actin organization (rho1-2, pkcts, mpk1Δ, and bni1ts bnr1Δ) caused no internalization defect, whereas mutations affecting the glucan synthase pathway (rho1-11 and fks1Δ) caused a strong defect. Cells deleted for WSC1 are compromised in their ability to produce β-1,3-glucan, demonstrating that Wsc1 plays a role in Fks1 activation (Sekiya-Kawasaki et al., 2002). In contrast, mid2Δ cells show no decrease in β-1,3-glucan production (Sekiya-Kawasaki et al., 2002), even though these cells are defective in activation of the Pkc1-controlled MAP kinase pathway (Ketela et al., 1999; Rajavel et al., 1999). Our finding that deletion of WSC1 results in defective α-factor internalization, whereas deletion of MID2 does not, further supports an important role for Fks1 in internalization.
Although Fks1 is required for receptor internalization, an intact cell wall is not. Temperature-sensitive mutations in Fks1 that have no effect on the glucan content of the cell have been described previously (Dijkgraaf et al., 2002). This observation together with our results suggests that, in addition to β-1,3-glucan production, there is a novel Rho1-Fks1 function required for internalization. The Rho1-Fks1 complex may be involved in the proper localization or activation of a component of the endocytic machinery, or in regulating PI localization or levels, as has been suggested for mammalian Rac (Malecz et al., 2000). Several proteins that interact with Fks1 have been identified by yeast two-hybrid and large-scale complex purification and mass spectroscopy studies (Gavin et al., 2002; Poirey et al., 2002). These Fks1-interacting proteins include Fth1, a protein required for normal fluid phase endocytosis (Wiederkehr et al., 2001); Srv2, a protein involved in actin organization and internalization (Wesp et al., 1997); Ygl051w, a protein that interacts with the endocytic protein Pan1 (Wendland et al., 1996; Poirey et al., 2002); Lcb2, a protein required for receptor internalization that is in involved in the production of sphingoid bases and in activation of the Pkh kinases (Zanolari et al., 2000; Friant et al., 2001); and Stt4, a PI-4 kinase that contributes to localization of Rom2 (Audhya et al., 2000; Audhya and Emr, 2002).
Mammalian Rho family GTPases are involved in regulating endocytic traffic both positively and negatively (reviewed in Ellis and Mellor, 2000; Qualmann and Mellor, 2003; Symons and Rusk, 2003). For example, activated Rho stimulates the constitutive endocytosis of sodium pumps in Xenopus laevis oocytes, whereas activated Rho and Rac inhibit transferrin receptor and epidermal growth factor receptor internalization (Schmalzing et al., 1995; Lamaze et al., 1996; Malecz et al., 2000). The specific Rho effector(s) that regulates endocytosis has not been identified in mammalian cells, but this regulation is likely to occur through control of PI metabolism (Malecz et al., 2000). Perhaps Fks1 is required to help localize Rho1 to specific membrane domains, where Rho1 can affect local lipid synthesis or metabolism required for endocytosis.
The Tor proteins are key regulators of cell growth, which interpret cues regarding the availability of nutrients and the phase of growth of the cell to control transcription, translation, and ribosome biosynthesis (Schmelzle and Hall, 2000). Tor proteins in yeast have been shown to regulate the plasma membrane localization of amino acid permeases in a rapamycin-sensitive manner (Schmidt et al., 1998; Beck et al., 1999). Our results indicate that the unique function of Tor2, which regulates the cell integrity pathway and contributes to cellular growth by affecting actin organization, polarized growth, and cell wall deposition (Drgonova et al., 1996; Qadota et al., 1996; Helliwell et al., 1998a), also regulates the internalization step of endocytosis. Consistent with our observations, it was recently suggested that Tor proteins may participate in endocytosis (Wedaman et al., 2003) due to their plasma membrane, vacuole, and endocytic vesicle localization, and their connection to sphingolipid signaling (Cardenas and Heitman, 1995; Withers et al., 1997; Beeler et al., 1998; Sabatini et al., 1999; Kunz et al., 2000; Wedaman et al., 2003).
Why might the cell integrity pathway affect endocytosis? One possibility is that to respond to changes in pressure on the plasma membrane in the face of assaults on the cell wall or polarized growth, the cell needs to be able to modulate the rate of endocytosis to affect the cellular volume (Morris and Homann, 2001). The cell integrity signaling pathway is responsive to these conditions, and therefore is in a prime position to react to changes in the extracellular environment to modulate endocytosis. Our results support this model, reveal a role for the cell integrity pathway in regulating endocytosis and suggest a novel endocytic function for the Rho1-Fks1 complex independent of cell wall biogenesis.
Acknowledgments
We thank Jon Binkley, David Botstein, Howard Bussey, Jurgen Heinisch, Joseph Heitman, Yoshikazu Ohya, Dominick Pallota, David Pellman, and Howard Riezman for gifts of strains and plasmids. This manuscript was improved by the critical comments of Greg deHart and Adam Adler. The National Institutes of Health (DK-53257) supported the research described in this manuscript.
Abbreviations used: GAP, GTPase activating protein; GEF, guanine nucleotide exchange factor; PI, phosphatidyinositol.
References
- Adams, A.E., and Pringle, J.R. (1984). Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98, 934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon, C.M., Heitman, J., and Cardenas, M.E. (1999). Protein kinase activity and identification of a toxic effector domain of the target of rapamycin TOR proteins in yeast. Mol. Biol. Cell 10, 2531-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts, A.S., Bouquin, N., Johnston, L.H., and Treisman, R. (1998). Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 273, 8616-8622. [DOI] [PubMed] [Google Scholar]
- Audhya, A., and Emr, S.D. (2002). Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2, 593-605. [DOI] [PubMed] [Google Scholar]
- Audhya,A.,Foti,M.,andEmr,S.D.(2000).Distinctrolesfortheyeastphosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, T., Schmidt, A., and Hall, M.N. (1999). Starvation induces vacuolar targeting and degradation of the tryptophan permease in yeast. J. Cell Biol. 146, 1227-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler, T., Bacikova, D., Gable, K., Hopkins, L., Johnson, C., Slife, H., and Dunn, T. (1998). The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3-ketosphinganine reductase is identified in a screen for temperature-sensitive suppressors of the Ca2+-sensitive csg2Δ mutant. J. Biol. Chem. 273, 30688-30694. [DOI] [PubMed] [Google Scholar]
- Bénédetti, H., Raths, S., Crausaz, F., and Riezman, H. (1994). The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell 5, 1023-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle, M., Delley, P.A., Schmidt, A., and Hall, M.N. (1998). Cell wall integrity modulates RHO1 activity via the exchange factor ROM2. EMBO. J 17, 2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett, P.E., Barrow, R.K., Cohen, N.A., Snyder, S.H., and Sabatini, D.M. (1998). RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 95, 1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas, M.E., and Heitman, J. (1995). FKBP12-rapamycin target TOR2 is a vacuolar protein with an associated phosphatidylinositol-4kinase activity. EMBO J. 14, 5892-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor, A., Torrance, P.D., Kobayashi, T., Thorner, J., and Alessi, D.R. (1999). Functional counterparts of mammalian protein kinases PDK1 and SGK in budding yeast. Curr. Biol. 9, 186-197. [DOI] [PubMed] [Google Scholar]
- Cullen, P.J., Cozier, G.E., Banting, G., and Mellor, H. (2001). Modular phosphoinositide-binding domains-their role in signalling and membrane trafficking. Curr. Biol. 11, R882-R893. [DOI] [PubMed] [Google Scholar]
- deHart, A.K., Schnell, J.D., Allen, D.A., and Hicke, L. (2002). The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J. Cell Biol. 156, 241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delley, P.A., and Hall, M.N. (1999). Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivieres, S., Cooke, F.T., Morales-Johansson, H., Parker, P.J., and Hall, M.N. (2002). Calmodulin controls organization of the actin cytoskeleton via regulation of phosphatidylinositol (4,5)-bisphosphate synthesis in Saccharomyces cerevisiae. Biochem. J. 366, 945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf, G.J., Abe, M., Ohya, Y., and Bussey, H. (2002). Mutations in Fks1p affect the cell wall content of β-1,3- and β-1,6-glucan in Saccharomyces cerevisiae. Yeast 19, 671-690. [DOI] [PubMed] [Google Scholar]
- Drgonova, J., Drgon, T., Roh, D.H., and Cabib, E. (1999). The GTP-binding protein Rho1p is required for cell cycle progression and polarization of the yeast cell. J. Cell Biol. 146, 373-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drgonova, J., Drgon, T., Tanaka, K., Kollar, R., Chen, G.C., Ford, R.A., Chan, C.S., Takai, Y., and Cabib, E. (1996). Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272, 277-279. [DOI] [PubMed] [Google Scholar]
- Dulic, V., Egerton, M., Elguindi, I., Raths, S., Singer, B., and Riezman, H. (1991). Yeast endocytosis assays. Methods Enzymol. 194, 697-710. [DOI] [PubMed] [Google Scholar]
- Ellis, S., and Mellor, H. (2000). Regulation of endocytic traffic by Rho family GTPases. Trends Cell Biol. 10, 85-88. [DOI] [PubMed] [Google Scholar]
- Friant, S., Lombardi, R., Schmelzle, T., Hall, M.N., and Riezman, H. (2001). Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 20, 6783-6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friant, S., Zanolari, B., and Riezman, H. (2000). Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J. 19, 2834-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, A.C., et al. (2002). Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141-147. [DOI] [PubMed] [Google Scholar]
- Gingras, A.C., Raught, B., and Sonenberg, N. (2001). Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15, 807-826. [DOI] [PubMed] [Google Scholar]
- Gray, J.V., Ogas, J.P., Kamada, Y., Stone, M., Levin, D.E., and Herskowitz, I. (1997). A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16, 4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., Tamanoi, F., and Novick, P. (2001). Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3, 353-360. [DOI] [PubMed] [Google Scholar]
- Helliwell, S.B., Howald, I., Barbet, N., and Hall, M.N. (1998a). TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148, 99-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell, S.B., Schmidt, A., Ohya, Y., and Hall, M.N. (1998b). The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol. 8, 1211-1214. [DOI] [PubMed] [Google Scholar]
- Isotani, S., Hara, K., Tokunaga, C., Inoue, H., Avruch, J., and Yonezawa, K. (1999). Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J. Biol. Chem. 274, 34493-34498. [DOI] [PubMed] [Google Scholar]
- Jung, U.S., and Levin, D.E. (1999). Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34, 1049-1057. [DOI] [PubMed] [Google Scholar]
- Kamada, Y., Qadota, H., Python, C.P., Anraku, Y., Ohya, Y., and Levin, D.E. (1996). Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271, 9193-9196. [DOI] [PubMed] [Google Scholar]
- Ketela, T., Green, R., and Bussey, H. (1999). Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181, 3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, H., et al. (1996). Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 6060-6068. [PMC free article] [PubMed] [Google Scholar]
- Kunz, J., Schneider, U., Howald, I., Schmidt, A., and Hall, M.N. (2000). HEAT repeats mediate plasma membrane localization of Tor2p in yeast. J. Biol. Chem. 275, 37011-37020. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Chuang, T.-H., Terlecky, L.J., Bokoch, G.M., and Schmid, S.L. (1996). Regulation of receptor-mediated endocytosis by Rho and Rac. Nature 382, 177-179. [DOI] [PubMed] [Google Scholar]
- Levin, D.E., Bowers, B., Chen, C.Y., Kamada, Y., and Watanabe, M. (1994). Dissecting the protein kinase C/MAP kinase signalling pathway of Saccharomyces cerevisiae. Cell Mol. Biol. Res. 40, 229-239. [PubMed] [Google Scholar]
- Loewith, R., Jacinto, E., Wullschleger, S., Lorberg, A., Crespo, J.L., Bonenfant, D., Oppliger, W., Jenoe, P., and Hall, M.N. (2002). Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457-468. [DOI] [PubMed] [Google Scholar]
- Lorenz, M.C., and Heitman, J. (1995). TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J. Biol. Chem. 270, 27531-27537. [DOI] [PubMed] [Google Scholar]
- Malecz, N., McCabe, P.C., Spaargaren, C., Qiu, R., Chuang, Y., and Symons, M. (2000). Synaptojanin 2, a novel Rac1 effector that regulates clathrin-mediated endocytosis. Curr. Biol. 10, 1383-1386. [DOI] [PubMed] [Google Scholar]
- Matsuo, T., Kubo, Y., Watanabe, Y., and Yamamoto, M. (2003). Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 22, 3073-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni, C., Zarov, P., Rambourg, A., and Mann, C. (1993). The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123, 1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, C.E., and Homann, U. (2001). Cell surface area regulation and membrane tension. J. Membr. Biol. 179, 79-102. [DOI] [PubMed] [Google Scholar]
- Munn, A.L. (2001). Molecular requirements for the internalisation step of endocytosis: insights from yeast. Biochim. Biophys. Acta 1535, 236-257. [DOI] [PubMed] [Google Scholar]
- Nonaka, H., Tanaka, K., Hirano, H., Fujiwara, T., Kohno, H., Umikawa, M., Mino, A., and Takai, Y. (1995). A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14, 5931-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham, S., Montagne, J., Radimerski, T., Thomas, G., and Hafen, E. (2000). Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 14, 2689-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip, B., and Levin, D.E. (2001). Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell Biol. 21, 271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirey, R., Despons, L., Leh, V., Lafuente, M.J., Potier, S., Souciet, J.L., and Jauniaux, J.C. (2002). Functional analysis of the Saccharomyces cerevisiae DUP240 multigene family reveals membrane-associated proteins that are not essential for cell viability. Microbiology 148, 2111-2123. [DOI] [PubMed] [Google Scholar]
- Qadota, H., Python, C.P., Inoue, S.B., Arisawa, M., Anraku, Y., Zheng, Y., Watanabe, T., Levin, D.E., and Ohya, Y. (1996). Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-β-glucan synthase. Science 272, 279-281. [DOI] [PubMed] [Google Scholar]
- Qualmann, B., and Mellor, H. (2003). Regulation of endocytic traffic by Rho GTPases. Biochem. J. 371, 233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavel, M., Philip, B., Buehrer, B.M., Errede, B., and Levin, D.E. (1999). Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell Biol. 19, 3969-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants, F.M., Torrance, P.D., Bezman, N., and Thorner, J. (2002). Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol. Biol. Cell 13, 3005-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde, J., Heitman, J., and Cardenas, M.E. (2001). The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276, 9583-9586. [DOI] [PubMed] [Google Scholar]
- Rothstein, R. (1991). Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 194, 281-301. [DOI] [PubMed] [Google Scholar]
- Sabatini, D.M., Barrow, R.K., Blackshaw, S., Burnett, P.E., Lai, M.M., Field, M.E., Bahr, B.A., Kirsch, J., Betz, H., and Snyder, S.H. (1999). Interaction of RAFT1 with gephyrin required for rapamycin-sensitive signaling. Science 284, 1161-1164. [DOI] [PubMed] [Google Scholar]
- Sagot, I., Rodal, A.A., Moseley, J., Goode, B.L., and Pellman, D. (2002). An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4, 626-631. [DOI] [PubMed] [Google Scholar]
- Saitoh, M., Pullen, N., Brennan, P., Cantrell, D., Dennis, P.B., and Thomas, G. (2002). Regulation of an activated S6 kinase 1 variant reveals a novel mammalian target of rapamycin phosphorylation site. J. Biol. Chem. 277, 20104-20112. [DOI] [PubMed] [Google Scholar]
- Saka, A., Abe, M., Okano, H., Minemura, M., Qadota, H., Utsugi, T., Mino, A., Tanaka, K., Takai, Y., and Ohya, Y. (2001). Complementing yeast rho1 mutation groups with distinct functional defects. J. Biol. Chem. 276, 46165-46171. [DOI] [PubMed] [Google Scholar]
- Schmalzing, G., Richter, H.P., Hansen, A., Schwarz, W., Just, I., and Aktories, K. (1995). Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J. Cell Biol. 130, 1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzle, T., and Hall, M.N. (2000). TOR, a central controller of cell growth. Cell 103, 253-262. [DOI] [PubMed] [Google Scholar]
- Schmelzle, T., Helliwell, S.B., and Hall, M.N. (2002). Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast. Mol. Cell Biol. 22, 1329-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., Beck, T., Koller, A., Kunz, J., and Hall, M.N. (1998). The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17, 6924-6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, A., Bickle, M., Beck, T., and Hall, M.N. (1997). The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88, 531-542. [DOI] [PubMed] [Google Scholar]
- Schmidt, A., Kunz, J., and Hall, M.N. (1996). TOR2 is required for organization of the actin cytoskeleton in yeast. Proc. Natl. Acad. Sci. USA 93, 13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya-Kawasaki, M., Abe, M., Saka, A., Watanabe, D., Kono, K., Minemura-Asakawa, M., Ishihara, S., Watanabe, T., and Ohya, Y. (2002). Dissection of upstream regulatory components of the Rho1p effector, 1, 3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics 162, 663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3-21. [DOI] [PubMed] [Google Scholar]
- Symons, M., and Rusk, N. (2003). Control of vesicular trafficking by Rho GTPases. Curr. Biol. 13, R409-418. [DOI] [PubMed] [Google Scholar]
- Takenawa, T., and Itoh, T. (2001). Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta 1533, 190-206. [DOI] [PubMed] [Google Scholar]
- Verna, J., Lodder, A., Lee, K., Vagts, A., and Ballester, R. (1997). A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94, 13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedaman, K.P., Reinke, A., Anderson, S., Yates III, J., Mccaffrey, J.M., and Powers, T. (2003). Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 1204-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland, B., McCaffery, J.M., Xiao, Q., and Emr, S.D. (1996). A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J. Cell Biol. 135, 1485-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesp, A., Hicke, L., Palecek, J., Lombardi, R., Aust, T., Munn, A.L., and Riezman, H. (1997). End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 2291-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr, A., Meier, K.D., and Riezman, H. (2001). Identification and characterization of Saccharomyces cerevisiae mutants defective in fluid-phase endocytosis. Yeast 18, 759-773. [DOI] [PubMed] [Google Scholar]
- Withers, D.J., Ouwens, D.M., Nave, B.T., van der Zon, G.C., Alarcon, C.M., Cardenas, M.E., Heitman, J., Maassen, J.A., and Shepherd, P.R. (1997). Expression, enzyme activity, and subcellular localization of mammalian target of rapamycin in insulin-responsive cells. Biochem. Biophys. Res. Commun. 241, 704-709. [DOI] [PubMed] [Google Scholar]
- Zanolari, B., Friant, S., Funato, K., Sutterlin, C., Stevenson, B.J., and Riezman, H. (2000). Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. EMBO J. 19, 2824-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Stallock, J.P., Ng, J.C., Reinhard, C., and Neufeld, T.P. (2000). Regulation of cellular growth by the Drosophila target of rapamycin dTOR. Genes Dev. 14, 2712-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X.F., Florentino, D., Chen, J., Crabtree, G.R., and Schreiber, S.L. (1995). TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell 82, 121-130. [DOI] [PubMed] [Google Scholar]