Abstract
The identification of two biologically active fatty acid amides, N-arachidonoylethanolamine (anandamide) and oleamide, has generated a great deal of excitement and stimulated considerable research. However, anandamide and oleamide are merely the best-known and best-understood members of a much larger family of biologically-occurring fatty acid amides. In this review, we will outline which fatty acid amides have been isolated from mammalian sources, detail what is known about how these molecules are made and degraded in vivo, and highlight their potential for the development of novel therapeutics.
Keywords: N-acylamino acid, N-acyldopamine, N-acylethanolamine, primary fatty acid amide, N-acylamide
The fatty acid amide bond has long been recognized in nature, being important in the structure of the ceramides [1] and the sphingolipids [2]. The first non-sphingosine based fatty acid amide isolated from a natural source was N-palmitoylethanolamine from egg yolk in 1957 [3]. Interest in the N-acylethanolamines (NAEs) dramatically increased upon the identification of N-arachidonoylethanolamine (anandamide) as the endogenous ligand for the cannabinoid receptors in the mammalian brain [4]. It is now known that a family of NAEs is found in the brain and in other tissues [5,6].
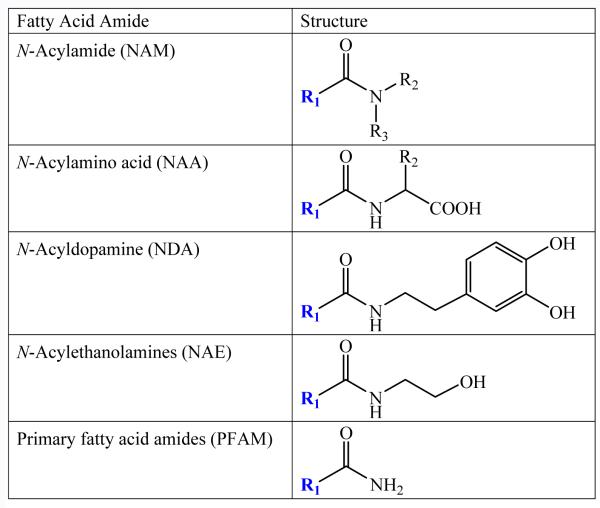
In addition to the NAEs, other classes of fatty acid amides have been characterized, namely the N-acylamino acids (NAAs) [7], the N-acyldopamines (NADAs) [8] and the primary fatty acid amides (PFAMs) [9,10] (Figure 1). Relative to NAEs, much less is currently known about the NAAs, the NADAs and the PFAMs, except that they are found in biological systems. The goal of this review is to summarize the current state of knowledge regarding the different classes of endogenous fatty acid amides and highlight their potential for drug discovery (see refs. [11-13] for earlier reviews)
Figure 1.
The Structures of the Fatty Acid Amides aR2 represents the functional groups that define the different amino acids The arrow points to carbon-2 in the fatty acid chain. R1 is an acyl group, making these structures fatty acids. R2 and R3 are also acyl groups.
N-Acylethanolamines
A series of long-chain NAEs have been identified in the mammalian brain, the most abundant being N-palmitoyl-, N-stearoyl- and N-oleoylethanolamine [5,11], each compromising ≥25% of total brain NAEs. Other less abundant NAEs found in the brain are anandamide, N-linoleoyl-, N-linolenoyl-, N-dihomo-γ-linolenoyl- and N-docosatetraenoylethanolamine [11]. In addition to the brain, the NAEs are widespread in the peripheral tissues [5].
The function of anandamide in mammals is mediated largely by its binding to the CB1 receptors (Kd = ∼80 nM) [14]. Anandamide is also known to bind to CB2 receptors (Kd = ∼500 nM) [14], peroxisome proliferators-activated receptors (PPARα, Kd = 20μM and PPARγ, Kd = 10 μM) [15], to the transient receptor potential (TRP) vanilloid type 1 (TRPV1) channels (Kd ∼ 2 μM) [13], and the transient receptor potential channels of melastatin type 8 (TRPM8) (Kd ∼ 1 μM) [16]. It is currently unclear how much the binding of anandamide to the non-CB1 receptors contributes to its total activity in vivo.Anandamide is involved in the regulation of body temperature, locomotion, feeding and the perception of pain, anxiety and fear [17-21]. The functions of the other known mammalian NAEs are not as well-established as anandamide, which is ironic given that ananadamide represents only 1-10% of brain NAEs [5,11]. With the exception of N-dihomo-γ-linolenoyl-, and N-docosatetraenoylethanolamine, the other NAEs do not bind to the CB1 and CB2 receptors [13,22,23]. N-Oleoylethanolamine binds to PPARα and PPARβ, functioning to inhibit feeding behavior [15,23], as well as the TRPV1 receptor [6], and the G-protein-coupled receptor, GPR119 [24]. Stearoylethanolamine binds to specific, non-CB1 and CB2 receptors and yet exhibits activities similar to anandamide [25]. NPalmitoylethanolamine is neuroprotective and also modulates pain and inflammation [26]. The anti-inflammatory effect of N-palmitoylethanolamine is mediated by its binding to PPARα [26]. Ryberg et al. [27] recently found that N-palmitoylethanolamine is a ligand for the orphan GPR55 receptor. It has been suggested that at least some of the activities of N-palmitoylethanolamine, N-oleoylethanolamine and N-stearoylethanolamine result from the “entourage effect”: cellular levels of anandamide are stabilized or increased because the other NAEs compete with anandamide for enzymatic degradation [22].
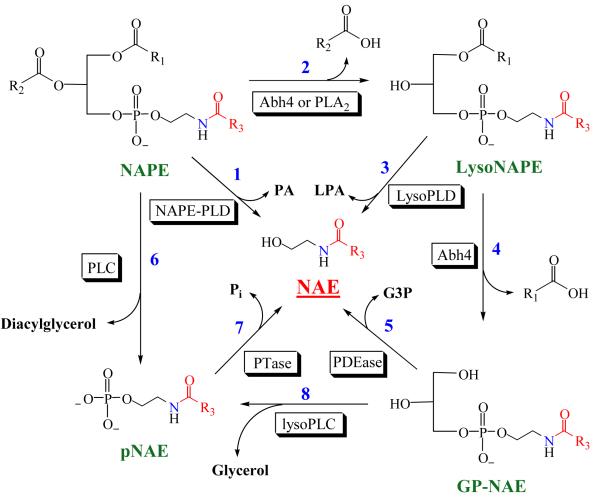
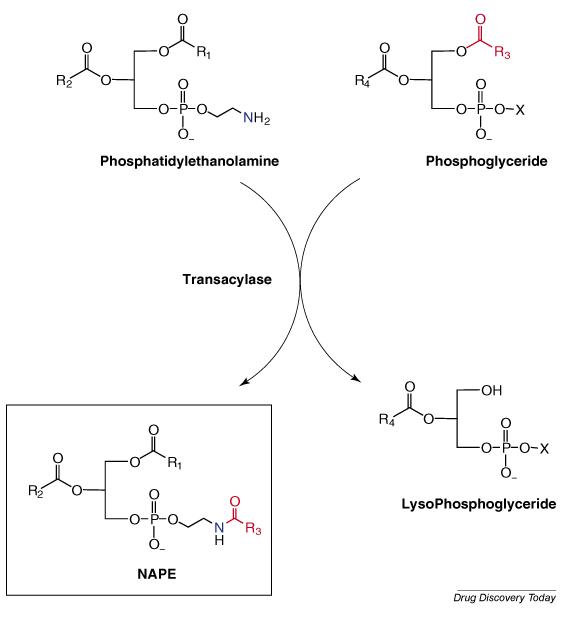
The most widely-accepted biosynthetic pathway for NAEs involves the NAPE-specific phospholipase D (NAPE-PLD)-mediated cleavage of N-acylphosphatidylethanolamine (NAPE) to the corresponding NAE and phosphatidic acid (PA) (reaction 1 in Figure 2) [28,29]. NAPE is produced by the N-acylation of phosphatidylethanolamine in a reaction catalyzed by a calcium activated transacylase (Figure 3) [28]. Recent evidence suggests that there are other PLD-independent pathways for NAE biosynthesis [30,31]. One alternative pathway involves the phospholipase C-mediated cleavage of NAPE to yield a phospho-NAE (pNAE) which is then cleaved by a phosphatase to yield the NAE and inorganic phosphate (reactions 6 and 7 in Fig. 2). Another alternative pathway involves sequential hydrolysis of the O-acyl chains of NAPE to produce free fatty acids and glycerophospho-NAE (GP-NAE) (reactions 2 and 4 in Fig. 2). Simon and Cravatt [30] have found that a serine hydrolase, α/β-hydrolase 4 (Abh4), can catalyze both O-deacylation steps required to convert NAPE to GP-NAE. Phosphodiesterase cleavage of GP-NAE will yield the NAE and glycerol 3-phosphate (reaction 5 in Fig. 2). Other possible routes to the NAEs are direct hydrolysis of lysoNAPE (reaction 3 in Fig. 2) or the 2-step conversion of GP-NAE to the NAE via phospho-NAE (reactions 8 and 7 in Fig. 2). The PLD-independent pathways for NAE biosynthesis are exciting discoveries, suggesting that the body has redundant “back-up” methods to produce these important bioactive lipid amides that are made “on demand” [12,31]. Future work will determine how these three pathways function to supply the required NAE levels.
Figure 2.
Biosynthetic Pathways for N-Acylethanolamines (NAEs) The enzymes catalyzing the individual reactions are in the shaded boxes and the numbers that refer to reactions in the text are in bold blue. The reader is referred to Simon and Cravatt [23] and Liu et al. [31] for greater details on NAE biosynthesis. Abdh4, α,β-hydrolase 4, G3P, glyercol-3-phosphate, LPA, lysophosphatic acid, LysoPLD, lysophospholipase D, NAPE-PLD, NAPE-specific phospholipase D, PA, phosphatidic acid, PDEase, phosphodiesterase, PLA2, phopholipase A2, PLC, phopholipase C, PTase, phosphatase (most likely tyrosine phosphatase, PTPN22, or inositol-5′-phosphatase, SHIP1, in vivo)
Figure 3.
Biosynthesis of the N-Acylphosphatidylethanolamine (NAPE)
Any review of NAE biosynthesis would be incomplete if one last synthetic strategy is not discussed. There is data going back more than 40 years, showing that the NAEs can be produced in vitro from ethanolamine and free fatty acids, in a reaction that did not require ATP or CoA-SH [32]. The in vivo significance of this chemistry is unclear.
NAE degradation is by hydrolysis to fatty acid and ethanolamine: R-CO-NH-CH2-OH + H2O → R-COOH + H2N-CH2-OH. Three enzymes are known to catalyze this reaction: two fatty acid amide hydrolases (FAAH-1 and FAAH-2) [33] and N-acylethanolamine-hydrolyzing acid amidase (NAAA) [34]. FAAH-1 and FAAH-2 both hydrolyze NAEs, but have different acyl group specificities. Note that FAAH inhibitors are currently being developed as potential analgesics [35-37].
N-Acyldopamines
A relatively small number of long-chain N-fatty acyldopamines have been isolated and characterized from mammalian systems, including N-palmitoyl-, N-stearoyl-, N-oleoyl- and N-arachidonoyldopamine. All of these NADAs are found in the mammalian brain, with the highest concentrations in the striatum, hippocampus and cerebellum [38].
N-Arachidonoyldopamine and N-oleoyldopamine were first identified as capsaicin-like endovanilloids that bound tightly to the TRPV1 receptor [13,38,39]. As a consequence of their binding to the TRPV1 receptors, both of these N-fatty acyldopamines stimulated calcium influx in HEK293 cells over-expressing either rat or human TRPV1 and produced hyperalgesia in rats [38,39]. N-Arachidonoyldopamine also binds tightly to the CB1 receptor (Kd = 250-500 nM) [39,40] and a non-CB1/CB2 GPR in the aorta [41].[42] Other endogenous N-fatty acyldopamines include N-palmitoyldopamine and N-stearoyldopamine, both of which bind with to the TRPV1 or CB1 receptors with relatively low affinity (Kd values >5 μM) [39]. The biological role(s) fulfilled by N-palmitoyldopamine and N-stearoyldopamine are unclear, but there is evidence that both enhance the activity of N-arachidonoyldopamine via the entourage effect [42].
In addition to the long-chain N-fatty acyldopamines, N-acetyldopamine is a known metabolite in mammals. The function of N-acetyldopamine is unclear, but it has been shown to inhibit mammalian sepiapterin reductase (an enzyme in the tetrahydrobiopterin biosynthetic pathway) with a Ki = 400 nM [43].
There has been little work on the pathways for the biosynthesis and degradation of the N-acyldopamines. N-Acetyldopamine is produced by the acetyl-CoA-dependent N-acetylation of dopamine [44] and has been found in the urine, kidney and liver [44,45]. It has been proposed that the long-chain N-acyldopamines are made in vivo in a similar fashion, with the acyl donors being the corresponding acyl-CoA thioesters [38]. Alternatively, the N-acyldopamines could be produced by the tyrosine hydroxylase-mediated oxidation of N-acyltyrosines (currently unknown metabolites in mammals). Huang et al. [38] provide data in support of both biosynthetic pathways.
Degradation of the N-acyldopamines is thought to occur by FAAH-catalyzed hydrolysis to the fatty acid and dopamine [38] or O-methylation by catechol-O-methyltransferase [38]. N-Acetyldopamine can serve as a substrate for tyrosinase; thus, the long-chain N-acyldopamines could also be oxidized to a quinone by this enzyme [46]. N-Acetylnoradrenaline is a known human metabolite [47] suggesting that N-acetyldopamine and the longer-chain N-acyldopamines could serve as substrates for dopamine β-monooxygenase.
N-Acylamino acids
Mammalian N-acylamino acids have a long history, tracing their discovery to the conjugation of glycine to benzoate to form N-benzoylglycine (hippurate) in the 1840s (see Caldwell et al. [7] and reference cited therein). Nα-Acetyl conjugates for all 20 of the common amino acids have been identified in mammals. In addition, the Nα-acetyl conjugates of other amino acids, including β-alanine, allo-isoleucine, α-aminobutyric acid, GABA, 2-aminooctanoic acid, citrulline and Nε-acetyllysine have also been characterized from mammalian sources [48-61]. With the exception of N-acetylglutamate, which serves as an allosteric activator of carbamoyl phosphate synthetase I [62], the N-acetylamino acid conjugates are trace metabolites that function in the excretion/detoxification of abnormally high levels of a particular amino acid. [63]Similarly, a set of N-isovaleroylamino acids have been identified from patents suffering from isovaleric acidemia, with N-isovaleroylglycine being the most abundant metabolite [55,60,63-65] The function of these N-isovaleroylamino acids is also in excretion; one patient suffering from isovaleric academia was excreting 1.7 grams of N-isovaleroylglycine per day [66].
[63][65] N-Conjugation of fatty acids to amino acids forming the long-chain N-fatty acylglycines is known, but is relatively uncommon in mammals. [64][55][66]The most common mammalian N-fatty acylamino acids are conjugates of glycine, glutamine and taurine (Table 1). Like the shorter chain N-acetyl and N-isovaleroyl amino acids, the major function of these longer chain amino acid conjugates would appear to be in the detoxification and excretion of xenobiotic carboxylates [7]. Glycine conjugation is particularly important in detoxification and elimination, as a careful analysis of the metabolism of most xenobiotic carboxylates reveals at least a trace of the corresponding N-acylglycine conjugate [67]. In fact, the list of N-acylglycines shown in Table 1 is incomplete as glycine conjugates of many other carboxylates also have been reported [67,68].
Table 1.
Mammalian N-Fatty Acylamino Acids
| Amino Acidb | N-Acyl Group | References |
|---|---|---|
| Alanine | Arachidonoyl | [76] |
| γ-Aminobutyric acid | Arachidonoyl | [76] |
| Glutamic Acid | β-Citryl and phenylacetyl | [54,55,114] |
| Glutamine | Phenylacetyl, other arylacetyls, and 4- phenylbutyryl |
[55,114] |
| Glycinec | Arachidonoyl, benzoyl, butyryl, bile acids, decanoyl, hexanoyl, isobutyryl, 2-methylbutyryl, 3-methylcrotonyl, octanoyl, phenylacetyl and other arylacetyls, propionyl, suberyl, and tiglyl |
[7,55,57,65,76,84,115] |
| Isoleucine | Lactyl | [116] |
| Leucine | Lactyl | [116] |
| Phenylalanine | Succinoyl | [117] |
| Pyroglutamic acid | Phenylacetyl | [57] |
| Serine | Arachidonoyl | [71] |
| Taurine | Bile acids, phenylacetyl and other arylacetyls, long chain, saturaturated acyl groups from C16:0-C26:0d, long-chain, monounsaturated acyl groups from C18:1-C24:1d |
[7,84,118] |
| Valine | Lactyl | [116] |
N-Acetyl and N-isovaleroylamino acids were not included in this table.
Amino acids not commonly found in proteins are italicized.
Included here most of the more common N-acylglycine conjugates known. Many others have been identified as metabolites in various organic acid acidemias or in the detoxification of a xenobiotic carboxylate.
Included in the family of long-chain fatty acyl groups found N-conjugated to taurine were odd-numbered acyl chains including C21:0, C21:1, C23:0, C23:1, C25:0, and C25:1. NTricosanoyltaurine was found to be one of the more abundant N-acyltaurines in mouse brain [49].
Amino acid N-fatty conjugation may function primarily in excretion/detoxification; however, this chemistry does serve other roles in mammals. [69][70]Bile acid conjugation to glycine or taurine increases bile acid solubility, renders the bile acids impermeable to cell membranes and is essential to proper liver function [69]. In addition, β-citrylglutamate may have a role in spermatogenesis [54] and in the differentiation of lens epithelial cells into fiber cells [70].
Most intriguing are the emerging roles of the long-chain N-fatty acylamino acids. Milman et al. [71] recently isolated and characterized N-arachidonoyl-L-serine from bovine brain and showed that this novel N-fatty acylserine had vasodilatory properties. We have proposed that the N-fatty acylglycines are biosynthetic precursors to the PFAMs, being oxidatively cleaved to the corresponding PFAM and glyoxylate in a reaction catalyzed by peptidylglycine α-amidating monooxygenase (PAM) [72]. Recent evidence suggests that the N-fatty acylglycines may serve as more than simple PFAM pathway intermediates and may have independent functions: N-oleoylglycine regulates body temperature and locomotion [73], N-arachidonoyltaurine activates TRPV1 and TRPV4 calcium channels of the kidney [74], N-arachidonoylglycine is an endogenous ligand for the orphan GPR18 receptor, [75], N-arachidonoyl-γ-aminobutyric acid is analgesic [76], and N-arachidonoylglycine is analgesic, and inhibits FAAH [77] and the GLYT2a glycine transporter [78]. The function(s) served by N-arachidonoylalanine is currently not understood. Another set of N-acyl amino acid conjugates that warrant some discussion are related to the conjugation of fatty acids to either the α-amino group of an N-terminal glycine residue or to the ε-amino group of internal lysine residue. The most common N-terminal acyl group found in eukaryotes is myristic acid, but other fatty acids, including lauric acid, (cis-Δ5)-tetradecaenoic acid (physeteric acid), (cis,cis-Δ5,Δ8)-tetradecadienoyl, and palmitic acid, have been identified as N-terminal fatty acids [79-81]. Mammalian proteins decorated via an amide linkage between the ε-amino group of an internal lysine and myristic acid [82] or palmitic acid [83] have been identified. Proteolytic degradation of N-terminal or ε-acyllysyl lipidated proteins could release the corresponding N-acylglycine or Nε-acyllysine, but we could not find any reports showing that such metabolites have been detected in mammals.
One biosynthetic route to the N-acylamino acids utilizes the acyl-CoA thioester as the acyl group donor: acyl-CoA + amino acid → N-acyl-amino acid + CoA-SH. Enzymes known to catalyze this reaction include N-acetylglutamate synthase [62] bile acid coenzyme A:amino acid N-acyltransferase (BAAT) for the formation of the bile acid glycine and taurine conjugates [84], acyl-CoA:glycine N-acyltransferase (ACGNAT) for the formation of the short-chain and branched chain N-acylglycines [85], a peroxisomal acyl-CoA:amino acid N-acyltransferase (ACNAT1) for the formation of the N-acyltaurines [86], and acyl-CoA: L-glutamine N-acyltransferase for the formation of the N-acylglutamines [87]. N-Terminal acylation is catalyzed by N-myristoyl transferase (NMT), an enzyme which strongly prefers myristoyl-CoA as a substrate, and only transfers the acyl group to the α-amino moiety of an N-terminal glycine. Glycine and the α-amino moiety of other N-terminal amino acids are not NMT substrates [79]. Evidence suggests that myristoyl-CoA or palmitoyl-CoA are also the acyl donors for the acylation of ε-amino group of internal lysine groups [81].
The data regarding the biosynthesis of the long-chain N-fatty acylglycines is not clear. N-conjugation of fatty acids to glycine via a fatty acyl-CoA thioester is an attractive possibility. The availabl evidence strongly suggests that ACGNAT does not catalyze this reaction in vivo: long-chain acyl-CoA thioesters are not ACGNAT substrates [85], and [87]ACGNAT is found primarily in the liver and kidney [85] while the PFAMs have been isolated from the brain [10].For that matter, ACGNAT is not likely involved in the biosynthesis of other N-fatty acylamino acids as amino acids other than glycine are very poor ACGNAT substrates [88]. Other possible candidates that might catalyze this reaction in vivo include bile BAAT, which will produce N-fatty acylglycines at a low rate relative to the bile acid conjugates [89], [89]or cytchrome c [90,91]. The recent report that cytochrome c can catalyze the formation of N-oleoylglycine and N-arachidonoylglycine from the corresponding CoA thioester in a reaction stimulated by H2O2 is very intriguing [90,91] and could provide the in vivo route to the to the N-fatty acylglycines. One last fascinating possible route to the N-fatty acylglycines might be the NAD+-dependent oxidation of the NAEs to the N-fatty acylglycines by the sequential actions of a fatty alcohol and a fatty aldehyde dehydrogenase [92].
The catabolic fates of the N-acylamino acids are not well-defined. FAAH will hydrolyze the N-acyltaurines and N-arachidonoylglycine to the corresponding fatty acid and amino acid [33,76], but the other N-acylamino acids are not degraded by FAAH [77]. We have shown that N-acylglycines are biosynthetic precursors to the PFAMs using purified PAM [72] and in PAM-expressing neuroblastoma cells [93][73][92][E1]. Marnett and co-workers have found that the N-arachidonoylamino acids are substrates for lipoxygenase and cyclooxygenase in vitro [94,95], pointing either to a mechanism for the inactivation of the N-arachidonoylamino acids or for the formation of other bioactive, oxidized amino acid conjugates. Clearly, there is much work remaining to better define the pathways of biosynthesis and degradation for the N-acylamino acids.
Primary Fatty Acid Amides
Arafat et al. [9] first isolated and characterized five PFAMs (palmitamide, palmitoleamide, oleamide, elaidamide and linoleamide) from luteal phase plasma in 1989. Because the function of the PFAMs was initially unknown, interest in these molecules was modest until Cravatt et al. [10]isolated oleamide and erucamide from the cerebrospinal fluid (CSF) of cat, rat and human and further demonstrated that the intraperitoneal injection of nanomole quantities of oleamide induced physiological sleep in rats. Research concerning oleamide has progressed rapidly since this first report and, in addition to its role in regulating the sleep/wake cycle, this PFAM has been shown to block gap junction communication in glial cells, to regulate memory processes, to decrease body temperature and locomotor activity, to stimulate Ca2+ release, to modulate depressant drug receptors in the CNS, and to allosterically activate the GABAA receptors and specific serotonin receptor subtypes [see refs.[96,97] for reviews]. Like oleamide, other members of the PFAM are bioactive: linoleamide increases Ca2+ flux [98] and inhibits the erg current in pituitary cells [99], erucamide stimulates the growth of blood vessels [100] and regulates fluid imbalance [101] and elaidamide might function as an endogenous inhibitor of epoxide hydrolase [102].
The PFAMs are degraded by fatty acid amide hydrolase, being hydrolyzed to the fatty acid and ammonia [6,77]. One of the key unanswered questions regarding the PFAMs is how these novel brain lipid amides are produced in the body. A number of reactions have been proposed to account for PFAM production. Sugiura et al. [103] found that FAAH catalyzed the in vitro production of oleamide from oleic acid and NH3. This reaction is unlikely to occur in vivo because the KM for ammonia was high (65 mM), and the pH optimum for oleamide synthesis was > 9. Mouse neuroblastoma N18TG2 cells secrete [1-14C]-oleamide when cultured in the presence of [1-14C]-oleic acid [93,104]; thus, these cells must contain the enzymatic machinery required for oleamide biosynthesis. Oleamide production in the N18TG2 cells increases upon the inhibition of FAAH, providing further evidence against a role for this enzyme in PFAM production in vivo. Bisogno et al. [104] proposed that PFAMs were produced by phospholipid aminolysis. However, incubation of [14C]-oleic acid-containing phospholipids with NH4OH in the presence of N18TG2 cell homogenates did not result in the formation of [14C]-oleamide.
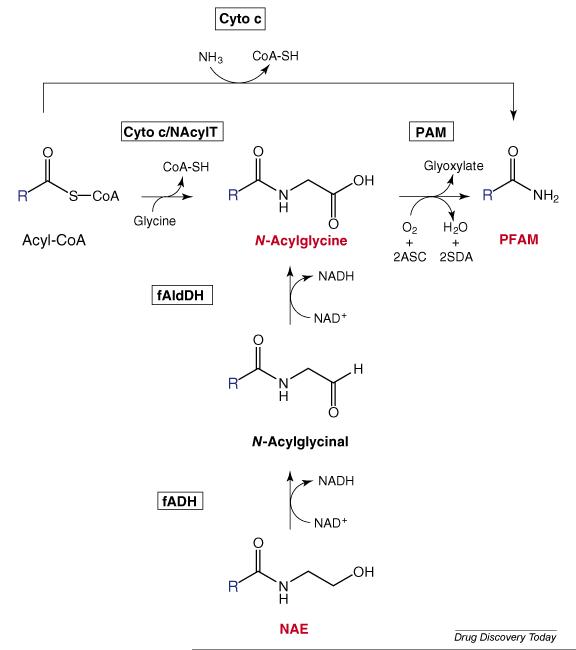
Currently, there are two proposed pathways for the biosynthesis of the PFAMs that have some experimental support. One is the direct amidation of fatty acyl-CoA thioesters by ammonia as catalyzed by cytochrome c [105]. The PFAM-synthesizing activity of cytochrome c yields a number of PFAMs, exhibits Michealis-Menton kinetics with a KM value for oleoyl-CoA of 21μM and a pH optimum of 7.5, and is stimulated by H2O2. A second proposed pathway for PFAM biosynthesis involves the PAM-mediated cleavage of N-fatty acylglycines [73,94], as mentioned above. We have shown that PAM is expressed in the oleamide-synthesizing N18TG2 cells and further demonstrated that inhibition of PAM in N18TG2 cells results in the accumulation of N-oleoylglycine [93,106]. A melding of the two proposed pathways could also lead to PFAMs: first the cytochrome c-mediated production of the N-fatty acylglycine followed by PAM oxidation to the corresponding PFAM. As discussed by Mueller and Driscoll [90], there may be more than one pathway for the in vivo production of the PFAMs, consistent with the fact that there are at a number of pathways known for the in vivo production of the NAEs (Figure 2). Outlined in Figure 4 are potential pathways for the biosynthesis of the PFAMs that metabolically link together the PFAMs to the N-fatty acylglycines and the NAEs. The potential conversion of one class of fatty acid amide to another only adds another fascinating dimension to this family of bioactive compounds.
Figure 4.
Proposed Biosynthetic Pathways for the Primary Fatty Acid Amides (PFAMs) The enzymes catalyzing the individual reactions are in the shaded boxes and the fatty acyl group are represent by the bold blue “R”. The fatty acid amides discussed in this review are highlighted in red. The reader is referred to Mueller and Driscoll [73] and Merkler et al.[74] for greater details on PFAM biosynthesis. ASC, ascorbic acid, Cyto c, cytochrome c, fADH, fatty alcohol dehydrogenase, fAldDH, fatty aldehyde dehydrogenase, NAcylT, a novel acyl-CoA:N-amino acid transferase, PAM, peptidyglycine α-amidating monooxygenase, SDA, semidehydroascorbic acid
N-Acylamides
N-Acylamides, R1-CO-NR2R3 for which R1 ≠ H, represents a broad class of molecules found in mammals (and other organisms) and is beyond the scope of this review. A few examples of mammalian N-acylamides are the acetylated polyamines, the ceramides, and sphingomyleins. The identification of N-stearoylisopropylamine [107] and the phophocholine-NAE conjugates [108] from mouse brain suggests that many other mammalian fatty acid amides await discovery.
Pharmacological Importance of the Fatty Acid Amides
Because of the broad functions exhibited by the various members of the fatty acid amide family, a wide range of indications could benefit from a fatty acid amide-targeted drug, including cancer, cardiovascular disease, inflammation, pain, drug addition, eating disorders, anxiety, and depression (see refs. [12,13,109,110] for recent reviews). Potential drug targets include the enzymes involved in fatty acid amide biosynthesis and degradation [111,112], transporters responsible for moving the fatty acid amides across the cell membranes [110], and analogs of the fatty acid amides themselves as agonists or antagonists for their respective receptors (Table 2) [13,113]. As detailed by Felder et al. [110], the potential existence of specific transporters for anandamide and the other fatty acid amides is controversial, but accumulating evidence suggests that the simple passive diffusion of the these hydrophobic compounds across the membrane driven by FAAH-hydrolysis is insufficient to account for published anandamide uptake data. The fatty acid amides represent an exciting opportunity for the development of new drugs for the treatment of human disease. Much work remains to be done, but the potential for a fatty acid amide-targeted therapeutic is high.
Table 2.
| A. N-Acylethanolamines | ||
|---|---|---|
| NAE | Receptors(s) | Reference |
| Anandamide | CB1, CB2, PPARα, PPARγ, TRPV1, and TRPM8 |
[6,13-16] |
| N-Dihomo-γlinolenoylethanolamine | CB1 and CB2 | [119] |
| 5Z,8Z,11Z- Eicosatrienoylethanolamine |
CB1 and CB2 | [119] |
| N-Oleoylethanolamine | PPARα, PPARγ, TRPV1, and GPR119 | [6,15,24] |
| N-Palmitoylethanolamine | PPARα, GPR55 | [15,27] |
| N-Linolenoylethanolamine | TRPV1 | [6] |
| N-Linoleoylethanolamine | TRPV1 | [6] |
| B. N-Acyldopamines | ||
| NDA | Receptors(s) | Reference |
| N-Arachidonoyldopamine | CB1, TRPV1, and non-CB1/CB2 GPCR (in the aorta) |
[38,39,41] |
| N-Oleoyldopamine | PPARα, PPARγ, and TRPV1 | [38,39] |
| C. N-Acylamino acidsb,c | ||
| NAA Receptors(s) | Reference | N-Arachidonoyltaurine |
| N-Arachidonoyltaurine | TRPV1 and TRPV4 | [74] |
| N-Arachidonoylglycinec | GPR18 | [75] |
| D. Primary Fatty Acid Amides | ||
| PFAM | Receptors(s) | Reference |
| Oleamide | GABAA receptor, 5-HT2A, 5-HT2C, and 5-HT7 | [120-122] |
In some cases, the indicated fatty acid amide has not been demonstrated to bind to the listed target by direct binding, but instead has been shown to be an agonist or antagonist to the target using a reporter assay. For exact details, the reader is pointed to the cited references.
While N-acetylglutamate is not formally a fatty acid amide, this N-acylamino acid binds a protein target as it is an allosteric activator of carbamoylphosphate synthetase I.
Fatty acid conjugation to amino acids serves largely in the detoxification and execretion of xenobiotic carboxylates. Thus, many of the N-acylamino acids are likely to bind to a membrane-bound transporter. For example, Wiles et al. [78] have recently shown that N-arachidonoylglycine inhibits the GLYT2a glycine transporter.
Conclusion
Fatty acid amides are a large family of structurally-diverse molecules found in humans and other organisms. Because many of these molecules have been shown to be bioactive, particularly in cell signaling, analogs of the fatty acid amides could prove useful as agonists or antagonists for their respective receptors. The enzymes involved in the biosynthesis and degradation, many of which are still poorly defined, also provide an exciting opportunity for the development of new drugs to treat sleep disorders, anxiety, depression, cardiovascular disease, and cancer.
Acknowledgements
This work was supported, in part, by grants from the National Institutes of Health - General Medical Sciences (R15-GM059050 and R15-GM073659), the Shirley W. & William L. Griffin Foundation, the Gustavus and Louise Pfeiffer Research Foundation, the Wendy Will Wendy Will Case Cancer Fund, the Eppley Foundation for Research, the Milheim Foundation for Cancer Research, the Eppley Foundation for Research, and the Alpha Research Foundation, Inc. to D.J.M. and a Graduate Multidisciplinary Scholars (GMS) award through the USF Thrust Life Sciences Program administered by the Florida Center of Excellence for Biomolecular Identification and Targeted Therapeutics (FCoE-BITT) to E.K.F.
Biographies

Author Blog - D. J. Merkler
David Merkler is a Professor in the Department of Chemistry at the University of South Florida (USF). He received his B.A. in Biochemistry in 1979 at the University of Maryland, Baltimore County. He then went on to earn his Ph.D. in Biochemistry at Pennsylvania State University. After a postdoctoral stint with Dr. Vern Schramm at Albert Einstein College of Medicine, he has had independent positions as a Senior Scientist at Unigene Laboratories and as an Associate Professor of Chemistry and Biochemistry at Duquesne University before coming to USF. His current research focuses on enzyme mechanisms, α-amidated peptides, lipid amides, and proteomic profiling.

Emma K. Farrell is a 4th year Ph.D student in the chemistry department at USF. Her research is to determine how primary fatty acid amides and N-acylamino acids are made in vivo. Her work has earned her several travel awards, including one from the ASBMB, IGERT and a departmental award at USF. She s also the recipient of a Graduate Multidisciplinary Scholars (GMS) award through the USF Thrust Life Sciences Program administered by the Florida Center of Excellence for Biomolecular Identification and Targeted Therapeutics (FCoE-BITT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser SentenceFatty acid amides are a family of mammalian bioactive compounds. These molecules and the enzymes involved in their metabolism provide an opportunity to develop new drugs to treat human disease.
References
- 1.Thudichum JLW. Chemical Constitution of the Brain. Archon Books; 1962. [Google Scholar]
- 2.Levene PA. Sphingomyelin. III. J. Biol. Chem. 1916;24:69–89. [Google Scholar]
- 3.Kuehl KA, Jr., et al. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Amer. Chem Soc. 1957;79:5577–5578. [Google Scholar]
- 4.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 5.Koga D, et al. Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric determination of anandamide and its analogs in rat brain and peripheral tissues. J Chromatogr B Biomed Sci Appl. 1997;690(12):7–13. doi: 10.1016/s0378-4347(96)00391-x. [DOI] [PubMed] [Google Scholar]
- 6.Movahed P, et al. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280(46):38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell J.e.a. The amino acid conjugations. In: Gram TE, editor. Extrahepatic Metabolism of Drugs and Other Compounds. Spectrum Publications; 1980. pp. 453–492. [Google Scholar]
- 8.Walker JM, et al. Targeted lipidomics: fatty acid amides and pain modulation. Prostaglandins Other Lipid Mediat. 2005;77(14):35–45. doi: 10.1016/j.prostaglandins.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Arafat ES, et al. Identification of fatty acid amides in human plasma. Life Sci. 1989;45(18):1679–1687. doi: 10.1016/0024-3205(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 10.Cravatt BF, et al. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268(5216):1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- 11.Mechoulam R, et al. Endocannabinoids. Eur J Pharmacol. 1998;359(1):1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 12.Di Marzo V, et al. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14(7):741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Starowicz K, et al. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114(1):13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Palmer SL, et al. Cannabinergic ligands. Chem Phys Lipids. 2002;121(12):3–19. doi: 10.1016/s0009-3084(02)00143-3. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152(5):576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Petrocellis L, et al. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res. 2007;313(9):1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Walker JM, et al. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci U S A. 1999;96(21):12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams C.M.a.K. Ananadamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- 19.Cravatt BF, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98(16):9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathura S.e.a. Modulation of anxiety through blockage of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 21.Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 22.Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas : are these two fatty acid amides cannabimimetic? Curr Med Chem. 1999;6(8):757–773. [PubMed] [Google Scholar]
- 23.Fu J, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425(6953):90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 24.Overton HA, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3(3):167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Maccarrone M, et al. Cannabimimetic activity, binding, and degradation of stearoylethanolamide within the mouse central nervous system. Mol Cell Neurosci. 2002;21(1):126–140. doi: 10.1006/mcne.2002.1164. [DOI] [PubMed] [Google Scholar]
- 26.LoVerme J.e.a. The nuclear receptor peroxisome proliferators-activated receptor-α mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharm. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 27.Ryberg E, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid HH, Berdyshev EV. Cannabinoid receptor-inactive N-acylethanolamines and other fatty acid amides: metabolism and function. Prostaglandins Leukot Essent Fatty Acids. 2002;66(23):363–376. doi: 10.1054/plef.2001.0348. [DOI] [PubMed] [Google Scholar]
- 29.Sugiura T, et al. Transacylase-mediated and phosphodiesterase-mediated synthesis of N-arachidonoylethanolamine, an endogenous cannabinoid-receptor ligand, in rat brain microsomes. Comparison with synthesis from free arachidonic acid and ethanolamine. Eur J Biochem. 1996;240(1):53–62. doi: 10.1111/j.1432-1033.1996.0053h.x. [DOI] [PubMed] [Google Scholar]
- 30.Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281(36):26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachur NR, Udenfriend S. Microsomal synthesis of fatty acid amides. J Biol Chem. 1966;241(6):1308–1313. [PubMed] [Google Scholar]
- 33.Wei BQ, et al. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281(48):36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- 34.Tsuboi K, et al. The N-acylethanolamine-hydrolyzing acid amidase (NAAA) Chem Biodivers. 2007;4(8):1914–1925. doi: 10.1002/cbdv.200790159. [DOI] [PubMed] [Google Scholar]
- 35.Maccarrone M. Fatty acid amide hydrolase: a potential target for next generation therapeutics. Curr Pharm Des. 2006;12(6):759–772. doi: 10.2174/138161206775474279. [DOI] [PubMed] [Google Scholar]
- 36.Sit SY, et al. Novel inhibitors of fatty acid amide hydrolase. Bioorg Med Chem Lett. 2007;17(12):3287–3291. doi: 10.1016/j.bmcl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Wallace VC, et al. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. Br J Pharmacol. 2007;151(7):1117–1128. doi: 10.1038/sj.bjp.0707326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang SM, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99(12):8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu CJ, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278(16):13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- 40.Bisogno T, et al. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351(Pt 3):817–824. [PMC free article] [PubMed] [Google Scholar]
- 41.O’Sullivan SE, et al. Vascular effects of delta 9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur J Pharmacol. 2005;507(13):211–221. doi: 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 42.De Petrocellis L, et al. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143(2):251–256. doi: 10.1038/sj.bjp.0705924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith G.R.e.a. New inhibitors of sepiapterin reductase. J. Biol. Chem. 1992;267:5599–5607. [PubMed] [Google Scholar]
- 44.Goldstein M, Musacchio JM. The formation in vivo of N-acetyldopamine and N-acetyl-3-methoxydopamine. Biochim Biophys Acta. 1962;58:607–608. doi: 10.1016/0006-3002(62)90078-1. [DOI] [PubMed] [Google Scholar]
- 45.Tyce GM. Metabolism of 3,4-dihydroxyphenylalanine by isolated perfused rat liver. Biochem Pharmacol. 1971;20(12):3447–3462. doi: 10.1016/0006-2952(71)90450-3. [DOI] [PubMed] [Google Scholar]
- 46.Borovansky J, et al. Mechanistic studies of melanogenesis: the influence of N-substitution on dopamine quinone cyclization. Pigment Cell Res. 2006;19(2):170–178. doi: 10.1111/j.1600-0749.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 47.Herrlich P, Sekeris CE. Identification of N-acetyl-noradrenaline in the urine of a patient with neuroblastoma. Hoppe Seylers Z Physiol Chem. 1964;339(1):249–250. [PubMed] [Google Scholar]
- 48.Goldstein FB. Studies on phenylketonuria. II. The excretion of N-acetyl-L-phenylalanine in phenylketonuria. Biochim Biophys Acta. 1963;71:204–206. doi: 10.1016/0006-3002(63)91006-0. [DOI] [PubMed] [Google Scholar]
- 49.Strandholm JJ, et al. Excretion of -N-acetylcitrulline in citrullinaemia. Biochim Biophys Acta. 1971;244(1):214–216. doi: 10.1016/0304-4165(71)90140-1. [DOI] [PubMed] [Google Scholar]
- 50.Wadman SK, et al. Automatic column chromatographic analysis of urinary and serum imidazoles in patients with histidinaemia and normals. Clin Chim Acta. 1971;31(1):215–224. doi: 10.1016/0009-8981(71)90380-9. [DOI] [PubMed] [Google Scholar]
- 51.Auditore JV, Wade LH. N-acetyl-L-asparagine in human brain. Neuropharmacology. 1972;11(3):385–394. doi: 10.1016/0028-3908(72)90024-x. [DOI] [PubMed] [Google Scholar]
- 52.Seiler N, Al-Therib MJ. Putrescine catabolism in mammalian brain. Biochem J. 1974;144(1):29–35. doi: 10.1042/bj1440029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heiden C.V.D.e.a. Familial hyperlysinaemia due to L-lysine α-ketoglutarate reductase deficiency: results of attempted treatment. J. Inher. Metab. Dis. 1978;1:89–94. doi: 10.1007/BF01805679. [DOI] [PubMed] [Google Scholar]
- 54.Miyake M, et al. Correlation of the level of beta-citryl-L-glutamic acid with spermatogenesis in rat testes. Biochim Biophys Acta. 1982;719(3):495–500. doi: 10.1016/0304-4165(82)90238-0. [DOI] [PubMed] [Google Scholar]
- 55.Divry P, et al. Routine gas chromatographic/mass spectrometric analysis of urinary organic acids. Results over a three-year period. Biomed Environ Mass Spectrom. 1987;14(11):663–668. doi: 10.1002/bms.1200141117. [DOI] [PubMed] [Google Scholar]
- 56.Lehnert W, Werle E. Elevated excretion of N-acetylated branched-chain amino acids in maple syrup urine disease. Clin Chim Acta. 1988;172(1):123–126. doi: 10.1016/0009-8981(88)90128-3. [DOI] [PubMed] [Google Scholar]
- 57.Liebich HM, Forst C. Basic profiles of organic acids in urine. J Chromatogr. 1990;525(1):1–14. doi: 10.1016/s0378-4347(00)83375-7. [DOI] [PubMed] [Google Scholar]
- 58.Sugahara K, et al. Liquid chromatographic-mass spectrometric analysis of N-acetylamino acids in human urine. J Chromatogr B Biomed Appl. 1994;657(1):15–21. doi: 10.1016/0378-4347(94)80064-2. [DOI] [PubMed] [Google Scholar]
- 59.Hiramatsu M. A role for guanidine compounds in the brain. Mol. Cell. Biochem. 2003;244:57–66. [PubMed] [Google Scholar]
- 60.Loots DT, et al. Identification of 19 new metabolites induced by abnormal amino acid conjugation in isovaleric acidemia. Clin Chem. 2005;51(8):1510–1512. doi: 10.1373/clinchem.2005.048421. [DOI] [PubMed] [Google Scholar]
- 61.Gerlo E, et al. Gas chromatographic-mass spectrometric analysis of N-acetylated amino acids: the first case of aminoacylase I deficiency. Anal Chim Acta. 2006;571(2):191–199. doi: 10.1016/j.aca.2006.04.079. [DOI] [PubMed] [Google Scholar]
- 62.Caldovic L, et al. Biochemical properties of recombinant human and mouse N-acetylglutamate synthase. Mol Genet Metab. 2006;87(3):226–232. doi: 10.1016/j.ymgme.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Lehnert W. Excretion of N-isovalerylglutamic acid in isovaleric acidemia. Clin Chim Acta. 1981;116(2):249–252. doi: 10.1016/0009-8981(81)90030-9. [DOI] [PubMed] [Google Scholar]
- 64.Lehnert W. N-Isovalerylalanine and N-isovalerylsarcosine: two new minor metabolites in isovaleric acidemia. Clin Chim Acta. 1983;134(12):207–212. doi: 10.1016/0009-8981(83)90198-5. [DOI] [PubMed] [Google Scholar]
- 65.Ito T, et al. Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric analysis of glycine conjugates and urinary isovalerylglycine in isovaleric acidemia. J Chromatogr B Biomed Appl. 1995;670(2):317–322. doi: 10.1016/0378-4347(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka K, Isselbacher KJ. The isolation and identification of N-isovalerylglycine from urine of patients with isovaleric acidemia. J Biol Chem. 1967;242(12):2966–2972. [PubMed] [Google Scholar]
- 67.Knights KM, et al. Amino acid conjugation: contribution to the metabolism and toxicity of xenobiotic carboxylic acids. Expert Opin Drug Metab Toxicol. 2007;3(2):159–168. doi: 10.1517/17425255.3.2.159. [DOI] [PubMed] [Google Scholar]
- 68.Bonafe L, et al. Evaluation of urinary acylglycines by electrospray tandem mass spectrometry in mitochondrial energy metabolism defects and organic acidurias. Mol Genet Metab. 2000;69(4):302–311. doi: 10.1006/mgme.2000.2982. [DOI] [PubMed] [Google Scholar]
- 69.Trottier J, et al. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol Pharm. 2006;3(3):212–222. doi: 10.1021/mp060020t. [DOI] [PubMed] [Google Scholar]
- 70.Tsumori M, et al. Presence of beta-citryl-L-glutamic acid in the lens: its possible role in the differentiation of lens epithelial cells into fiber cells. Exp Eye Res. 1995;61(4):403–411. doi: 10.1016/s0014-4835(05)80135-6. [DOI] [PubMed] [Google Scholar]
- 71.Milman G, et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103(7):2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilcox BJ, et al. N-acylglycine amidation: implications for the biosynthesis of fatty acid primary amides. Biochemistry. 1999;38(11):3235–3245. doi: 10.1021/bi982255j. [DOI] [PubMed] [Google Scholar]
- 73.Chaturvedi S, et al. In vivo evidence that N-oleoylglycine acts independently of its conversion to oleamide. Prostaglandins Other Lipid Mediat. 2006;81(34):136–149. doi: 10.1016/j.prostaglandins.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saghatelian A, et al. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45(30):9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- 75.Kohno M, et al. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347(3):827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 76.Huang SM, et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276(46):42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 77.Grazia Cascio M, et al. A structure-activity relationship study on N-arachidonoyl-amino acids as possible endogenous inhibitors of fatty acid amide hydrolase. Biochem Biophys Res Commun. 2004;314(1):192–196. doi: 10.1016/j.bbrc.2003.12.075. [DOI] [PubMed] [Google Scholar]
- 78.Wiles AL, et al. N-Arachidonyl-glycine inhibits the glycine transporter, GLYT2a. J Neurochem. 2006;99(3):781–786. doi: 10.1111/j.1471-4159.2006.04107.x. [DOI] [PubMed] [Google Scholar]
- 79.Rajala RV, et al. N-myristoyltransferase. Mol Cell Biochem. 2000;204(12):135–155. doi: 10.1023/a:1007012622030. [DOI] [PubMed] [Google Scholar]
- 80.Neubert TA, et al. The rod transducin alpha subunit amino terminus is heterogeneously fatty acylated. J Biol Chem. 1992;267(26):18274–18277. [PubMed] [Google Scholar]
- 81.Kleuss C, Krause E. Galpha(s) is palmitoylated at the N-terminal glycine. EMBO J. 2003;22(4):826–832. doi: 10.1093/emboj/cdg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevenson FT, et al. The 31-kDa precursor of interleukin 1 alpha is myristoylated on specific lysines within the 16-kDa N-terminal propiece. Proc Natl Acad Sci U S A. 1993;90(15):7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sachon E, et al. Characterization of N-palmitoylated human growth hormone by in situ liquid-liquid extraction and MALDI tandem mass spectrometry. J Mass Spectrom. 2007;42(6):724–734. doi: 10.1002/jms.1207. [DOI] [PubMed] [Google Scholar]
- 84.Falany CN, et al. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994;269(30):19375–19379. [PubMed] [Google Scholar]
- 85.Kelley M, Vessey DA. Characterization of the acyl-CoA:amino acid N-acyltransferases from primate liver mitochondria. J Biochem Toxicol. 1994;9(3):153–158. doi: 10.1002/jbt.2570090307. [DOI] [PubMed] [Google Scholar]
- 86.Reilly SJ, et al. A peroxisomal acyltransferase in mouse identifies a novel pathway for taurine conjugation of fatty acids. FASEB J. 2007;21(1):99–107. doi: 10.1096/fj.06-6919com. [DOI] [PubMed] [Google Scholar]
- 87.Webster LT, et al. Identification of separate acyl- CoA:glycine and acyl-CoA:L-glutamine N-acyltransferase activities in mitochondrial fractions from liver of rhesus monkey and man. J Biol Chem. 1976;251(11):3352–3358. [PubMed] [Google Scholar]
- 88.van der Westhuizen F.H.e.a. The ulitization of alanine, glutamic acid, and serine as amino acid substrates for glycine N-acyltransferase. J. Biochem. Mol. Toxicol. 2000;14:102–109. doi: 10.1002/(sici)1099-0461(2000)14:2<102::aid-jbt6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 89.O’Byrne J, et al. The human bile acid-CoA:amino acid N-acyltransferase functions in the conjugation of fatty acids to glycine. J Biol Chem. 2003;278(36):34237–34244. doi: 10.1074/jbc.M300987200. [DOI] [PubMed] [Google Scholar]
- 90.Mueller GP, Driscoll WJ. In vitro synthesis of oleoylglycine by cytochrome c points to a novel pathway for the production of lipid signaling molecules. J Biol Chem. 2007;282(31):22364–22369. doi: 10.1074/jbc.M701801200. [DOI] [PubMed] [Google Scholar]
- 91.McCue JM, et al. Cytochrome c catalyzes the in vitro synthesis of arachidonoyl glycine. Biochem Biophys Res Commun. 2008;365(2):322–327. doi: 10.1016/j.bbrc.2007.10.175. [DOI] [PubMed] [Google Scholar]
- 92.Burstein SH, et al. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61(12):29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- 93.Merkler DJ, et al. Oleic acid derived metabolites in mouse neuroblastoma N18TG2 cells. Biochemistry. 2004;43(39):12667–12674. doi: 10.1021/bi049529p. [DOI] [PubMed] [Google Scholar]
- 94.Prusakiewicz JJ, et al. Selective oxygenation of N-arachidonylglycine by cyclooxygenase-2. Biochem Biophys Res Commun. 2002;296(3):612–617. doi: 10.1016/s0006-291x(02)00915-4. [DOI] [PubMed] [Google Scholar]
- 95.Prusakiewicz JJ, et al. Oxidative metabolism of lipoamino acids and vanilloids by lipoxygenases and cyclooxygenases. Arch Biochem Biophys. 2007;464(2):260–268. doi: 10.1016/j.abb.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mendelson WB, Basile AS. The hypnotic actions of the fatty acid amide, oleamide. Neuropsychopharmacology. 2001;25(5 Suppl):S36–39. doi: 10.1016/S0893-133X(01)00341-4. [DOI] [PubMed] [Google Scholar]
- 97.Hiley CR, Hoi PM. Oleamide: a fatty acid amide signaling molecule in the cardiovascular system? Cardiovasc Drug Rev. 2007;25(1):46–60. doi: 10.1111/j.1527-3466.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 98.Lo YK, et al. Effect of oleamide on Ca(2+) signaling in human bladder cancer cells. Biochem Pharmacol. 2001;62(10):1363–1369. doi: 10.1016/s0006-2952(01)00772-9. [DOI] [PubMed] [Google Scholar]
- 99.Liu YC, Wu SN. Block of erg current by linoleoylamide, a sleep-inducing agent, in pituitary GH3 cells. Eur J Pharmacol. 2003;458(12):37–47. doi: 10.1016/s0014-2999(02)02728-0. [DOI] [PubMed] [Google Scholar]
- 100.Wakamatsu K, et al. Isolation of fatty acid amide as an angiogenic principle from bovine mesentery. Biochem Biophys Res Commun. 1990;168(2):423–429. doi: 10.1016/0006-291x(90)92338-z. [DOI] [PubMed] [Google Scholar]
- 101.Hamberger A, Stenhagen G. Erucamide as a modulator of water balance: new function of a fatty acid amide. Neurochem Res. 2003;28(2):177–185. doi: 10.1023/a:1022364830421. [DOI] [PubMed] [Google Scholar]
- 102.Morisseau C, et al. Inhibition of microsomal epoxide hydrolases by ureas, amides, and amines. Chem Res Toxicol. 2001;14(4):409–415. doi: 10.1021/tx0001732. [DOI] [PubMed] [Google Scholar]
- 103.Sugiura T, et al. Enzymatic synthesis of oleamide (cis-9, 10-octadecenoamide), an endogenous sleep-inducing lipid, by rat brain microsomes. Biochem Mol Biol Int. 1996;40(5):931–938. doi: 10.1080/15216549600201553. [DOI] [PubMed] [Google Scholar]
- 104.Bisogno T, et al. The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem Biophys Res Commun. 1997;239(2):473–479. doi: 10.1006/bbrc.1997.7431. [DOI] [PubMed] [Google Scholar]
- 105.Driscoll WJ, et al. Oleamide synthesizing activity from rat kidney: identification as cytochrome c. J Biol Chem. 2007;282(31):22353–22363. doi: 10.1074/jbc.M610070200. [DOI] [PubMed] [Google Scholar]
- 106.Ritenour-Rodgers KJ, et al. Induction of peptidylglycine alpha-amidating monooxygenase in N(18)TG(2) cells: a model for studying oleamide biosynthesis. Biochem Biophys Res Commun. 2000;267(2):521–526. doi: 10.1006/bbrc.1999.1977. [DOI] [PubMed] [Google Scholar]
- 107.Dalle Carbonare M, et al. Identification of an unusual naturally occurring apolar fatty acid amide in mammalian brain and a method for its quantitative determination. Rapid Commun Mass Spectrom. 2006;20(3):353–360. doi: 10.1002/rcm.2313. [DOI] [PubMed] [Google Scholar]
- 108.Mulder AM, Cravatt BF. Endocannabinoid metabolism in the absence of fatty acid amide hydrolase (FAAH): discovery of phosphorylcholine derivatives of N-acyl ethanolamines. Biochemistry. 2006;45(38):11267–11277. doi: 10.1021/bi061122s. [DOI] [PubMed] [Google Scholar]
- 109.Karanian DA, Bahr BA. Cannabinoid drugs and enhancement of endocannabinoid responses: strategies for a wide array of disease states. Curr Mol Med. 2006;6(6):677–684. doi: 10.2174/156652406778194991. [DOI] [PubMed] [Google Scholar]
- 110.Felder CC, et al. Cannabinoids biology: the search for new therapeutic targets. Mol Interv. 2006;6(3):149–161. doi: 10.1124/mi.6.3.6. [DOI] [PubMed] [Google Scholar]
- 111.Labar G, Michaux C. Fatty acid amide hydrolase: from characterization to therapeutics. Chem Biodivers. 2007;4(8):1882–1902. doi: 10.1002/cbdv.200790157. [DOI] [PubMed] [Google Scholar]
- 112.Jhaveri MD, et al. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007;152(5):624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahadevan A, Razdan RK. Further advances in the synthesis of endocannabinoid-related ligands. AAPS J. 2005;7(2):E496–502. doi: 10.1208/aapsj070250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Comte B, et al. Identification of phenylbutyrylglutamine, a new metabolite of phenylbutyrate metabolism in humans. J Mass Spectrom. 2002;37(6):581–590. doi: 10.1002/jms.316. [DOI] [PubMed] [Google Scholar]
- 115.Gregersen N, et al. General (medium-chain) acyl-CoA dehydrogenase deficiency (non-ketotic dicarboxylic aciduria): quantitative urinary excretion pattern of 23 biologically significant organic acids in three cases. Clin Chim Acta. 1983;132(2):181–191. doi: 10.1016/0009-8981(83)90246-2. [DOI] [PubMed] [Google Scholar]
- 116.Hagenfeldt L, Naglo AS. New conjugated urinary metabolites in intermediate type maple syrup urine disease. Clin Chim Acta. 1987;169(1):77–83. doi: 10.1016/0009-8981(87)90395-0. [DOI] [PubMed] [Google Scholar]
- 117.Grupe A, Spiteller G. New polar acid metabolites in human urine. J Chromatogr. 1981;226(2):301–314. doi: 10.1016/s0378-4347(00)86064-8. [DOI] [PubMed] [Google Scholar]
- 118.Saghatelian A, et al. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry. 2004;43(45):14332–14339. doi: 10.1021/bi0480335. [DOI] [PubMed] [Google Scholar]
- 119.Felder CC, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48(3):443–450. [PubMed] [Google Scholar]
- 120.Verdon B, et al. Stereoselective modulatory actions of oleamide on GABA(A) receptors and voltage-gated Na(+) channels in vitro: a putative endogenous ligand for depressant drug sites in CNS. Br J Pharmacol. 2000;129(2):283–290. doi: 10.1038/sj.bjp.0703051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huidobro-Toro JP, Harris RA. Brain lipids that induce sleep are novel modulators of 5-hydroxytrypamine receptors. Proc Natl Acad Sci U S A. 1996;93(15):8078–8082. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas EA, et al. Unique allosteric regulation of 5-hydroxytryptamine receptor-mediated signal transduction by oleamide. Proc Natl Acad Sci U S A. 1997;94(25):14115–14119. doi: 10.1073/pnas.94.25.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]