Abstract
Although fasciae have long interested clinicians in a multitude of different clinical and paramedical disciplines, there have been few attempts to unite the ensuing diverse literature into a single review. The current article gives an anatomical perspective that extends from the gross to the molecular level. For expediency, it deals only with fascia in the limbs and back. Particular focus is directed towards deep fascia and thus consideration is given to structures such as the fascia lata, thoracolumbar fascia, plantar and palmar fascia, along with regional specializations of deep fascia such as retinacula and fibrous pulleys. However, equal emphasis is placed on general aspects of fascial structure and function, including its innervation and cellular composition. Among the many functions of fascia considered in detail are its ectoskeletal role (as a soft tissue skeleton for muscle attachments), its importance for creating osteofascial compartments for muscles, encouraging venous return in the lower limb, dissipating stress concentration at entheses and acting as a protective sheet for underlying structures. Emphasis is placed on recognizing the continuity of fascia between regions and appreciating its key role in coordinating muscular activity and acting as a body-wide proprioceptive organ. Such considerations far outweigh the significance of viewing fascia in a regional context alone.
Keywords: deep fascia, ectoskeleton, iliotibial tract, osteofascial compartments, palmar fascia, plantar fascia, superficial fascia, thoracolumbar fascia
Introduction
‘Fascia’ is a vague term that is derived from the Latin for a band or bandage. It has long been used by gross anatomists to embrace a spectrum of undifferentiated mesenchymal tissues that wrap around what are sometimes regarded as being the more ‘specialized’ organs and tissues of the body, or form a packing material between them. The inherent implication of this traditional view is that fasciae are inconsequential residues that are less important than the tissues with which they are associated. Increasingly, the errors of this assumption are being exposed and undoubtedly fascia is of considerable importance to many surgeons, physiotherapists, orthotists, osteopaths, massage therapists and other professionals working in health-related disciplines. They are involved in the spread or containment of infections and/or pus, oedematous effusions and a multitude of other conditions, including compartment syndromes, fibromyalgia, Dupuytren's contracture and plantar fasciitis or fasciosis.
Encouragingly, there has been a strong resurgence of interest into both basic and applied research in fasciae in recent times – as evidenced by the first international fascia research congress in Boston, USA (Findley & Schleip, 2007a) and the publication of a book by Lindsay (2008) that is aimed at health-care professionals and students. This has been an important motivation for tackling the current review, together with the observation that there has been no recent broad anatomical overview of fascia in any journal, which could help to stimulate further research. Relatively few scientists are interested primarily in fascia – many who publish on it have a different principal focus and fascia is often just part of a grander portfolio. However, the clinical significance of fascia could tempt others to enter the field if contemporary issues are addressed in a multidisciplinary fashion with the tools that modern science affords. Fasciae probably hold many of the keys for understanding muscle action and musculoskeletal pain, and maybe of pivotal importance in understanding the basis of acupuncture and a wide range of alternative therapies (Langevin et al. 2001, 2002, 2006a; Langevin & Yandow, 2002; Iatridis et al. 2003). Intriguingly, Langevin et al. (2007) have shown that subtle differences in the way that acupuncture needles are manipulated can change how the cells in fascia respond. The continuum of connective tissue throughout the body, the mechanical role of fascia and the ability of fibroblasts to communicate with each other via gap junctions, mean that fascia is likely to serve as a body-wide mechanosensitive signaling system with an integrating function analogous to that of the nervous system (Langevin et al. 2004; Langevin, 2006). It is indeed a key component of a tensegrity system that operates at various levels throughout the body and which has been considered in detail by Lindsay (2008) in the context of fascia.
Anatomists have long distinguished between superficial and deep fascia (Fig. 1), although to many surgeons, ‘fascia’ is simply ‘deep fascia’. The superficial fascia is traditionally regarded as a layer of areolar connective or adipose tissue immediately beneath the skin, whereas deep fascia is a tougher, dense connective tissue continuous with it. Deep fascia is commonly arranged as sheets and typically forms a stocking around the muscles and tendons beneath it. However, regions of dense connective can also be found within the superficial fascia – e.g. the hollow canals of fibrous tissue enclosing the saphenous veins (Shah & Srivastava, 1966; Papadopoulos et al. 1981). According to Caggiati (1999), these saphenous canals reduce the risk of varicosities. They point out that varicosities more commonly affect the tributaries of the (great) saphenous veins, which lie outside the saphenous canal (Caggiati, 2000). There are also fascial canals in the fingers that are most familiar to hand surgeons and which are formed from Grayson's and Cleland's ligaments (Doyle, 2003). They form a protective tunnel for the digital vessels and nerves, preventing them from bowstringing when the finger is flexed.
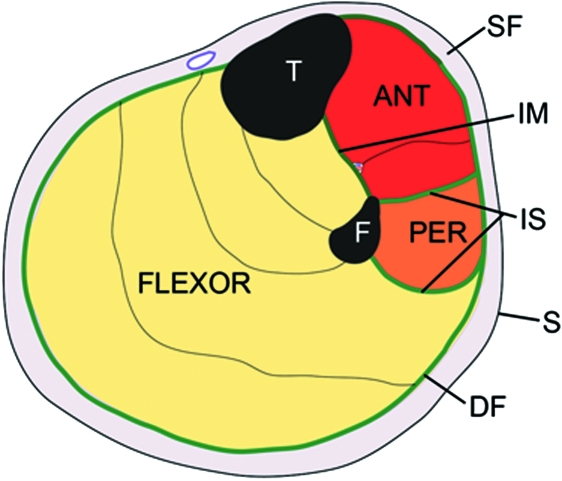
Fig. 1.
A diagrammatic representation of a transverse section through the upper part of the leg showing the relative positions of the superficial (SF) and deep fascia (DF) in relation to the skin (S) and muscles. Note how the deep fascia, in association with the bones [tibia (T) and fibula (F)] and intermuscular septa (IS) forms a series of osteofascial compartments housing the extensor, peroneal (PER) and flexor muscles. If pressure builds up within a compartment because of an acute or overuse injury, then the vascular supply to the muscles within it can be compromised and ischaemia results. ANT, anterior compartment; IM, interosseous membrane.
All fasciae are forms of soft connective tissue, i.e. the ‘connective tissue proper’ of standard histology texts (Bloom & Fawcett, 1975). Fasciae are continuous with each other throughout the limbs – a principal long recognized by anatomists. Gerrish (1899), for example, implores the students of his day to note the continuity of fibrous membranes, i.e. how tendons, ligaments and fasciae blend with periosteum, how both tendons and fasciae can function as ligaments and how tendons can become fascial expansions. More recently, Gerlach & Lierse (1990) have emphasized the unifying role of connective tissue in the limbs and refer to an integrating ‘bone–fascia–tendon system’. Thus, in one sense it is fitting that modern authors advocate a very broad definition of fascia that embraces all forms of soft connective tissue (Findley & Schleip, 2007b). On the other hand, it is confusing to regard more specialized structures such as tendons and ligaments as ‘fascia’ and thus similar to, for example, the areolar connective tissue beneath the skin. Furthermore, it is impractical in an article such as the current one to consider ‘fascia’ synonymous with ‘connective tissue’. Thus, as tendons have been the subject of a recent review by the author (Benjamin et al. 2008), limited coverage will be given to this area in particular, or to the related topic of ligaments. In a further effort to keep the review within manageable bounds and because of the particular interests and expertise of the author, only fasciae in the limbs and back are considered and the primary focus is on deep fascia. It is recognized, however, that fasciae are also prominent and important elsewhere – particularly in the head, neck and pelvis. An attempt has been made to balance general principles of fascial biology with matters of specific regional interest that pertain largely to certain named fasciae alone.
General principles of fascial biology
The anatomical and histological forms of fascia
The presence of a significant layer of fat in the superficial fascia is a distinctive human trait (the panniculus adiposus), compensating for the paucity of body hair. It thus plays an important role in heat insulation. In hairy mammals, the same fascia is typically an areolar tissue that allows the skin to be readily stripped from the underlying tissues (Le Gros Clark, 1945). Where fat is prominent in the superficial fascia (as in man), it may be organized into distinctive layers, or laminae (Johnston & Whillis, 1950), although Gardner et al. (1960) caution that these may sometimes be a characteristic of embalmed cadavers and not evident in the living person. Furthermore, Le Gros Clark (1945) also argues that fascial planes can be artefactually created by dissection. Conversely, however, some layers of deep fascia are more easily defined in fresh than in fixed cadavers (Lytle, 1979).
The superficial fascia conveys blood vessels and nerves to and from the skin and often promotes movement between the integument and underlying structures. This is particularly evident over highly mobile joints and on the dorsum of the hand, where the skin has considerable freedom of movement so that it can slide easily over the extensor tendons during finger movements. Skin mobility protects both the integument and the structures deep to it from physical damage. Mobility is promoted by multiple sheets of collagen fibres coupled with the presence of elastin (Kawamata et al. 2003). The relative independence of the collagen sheets from each other promotes skin sliding and further stretching is afforded by a re-alignment of collagen fibres within the lamellae. The skin is brought back to its original shape and position by elastic recoil when the deforming forces are removed. As Kawamata et al. (2003) point out, one of the consequences of the movement-promoting characteristics of the superficial fascia is that the blood vessels and nerves within it must run a tortuous route so that they can adapt to an altered position of the skin, relative to the deeper structures.
There are some sites where the skin is tightly bound to the underlying tissues to prevent or restrict movement – as in the palmar or plantar aspects of the hands and feet (Fig. 2). If movement were allowed to occur here within fascial planes, it would conflict with the requirements of facilitating a firm grip. Hence, loose connective tissue is sparse beneath the skin in the palms and soles. Indeed, it is completely absent at the finger creases on the palmar sides of the interphalangeal joints, so that the skin immediately covers fascial tendon sheaths. This explains why puncture wounds at the creases carry a risk of infection to these structures (Fifield, 1939). Although deep fascia elsewhere in the limbs is often not so tightly bound to the skin, nevertheless cutaneous ligaments extending from deep fascia to anchor the integument are much more widespread than generally recognized and serve to resist a wide variety of forces, including gravitational influences (Nash et al. 2004).
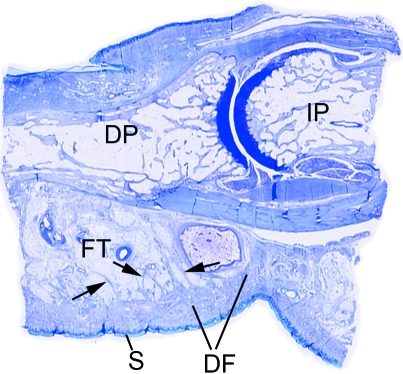
Fig. 2.
A low power view of a sagittal section through a finger in the region of the distal interphalangeal joint. Note how the skin (S) on the palmar side is intimately associated with a thick region of dense fascia (DF) that anchors it in position and stops it sliding in the interests of a firm grip. At a deeper level, the bundles of fascial fibres (arrows) are mixed with fat, to form a pressure-tolerant, fibro-adipose tissue (FT). DP, distal phalanx; IP, intermediate phalanx.
Fascia in the form of loose, areolar connective tissue surrounds skeletal muscle fibres (forming the endo- and epimysium) and creates thin films of tissue between adjacent muscles. Again, such fasciae are important in promoting movement – by allowing one muscle or fibre to move independently of its neighbour. By contrast, the deep fasciae in the limbs and back are typically dense connective tissue sheets that have large numbers of closely packed collagen fibres. The dominant cell is a fibroblast, although the accumulation of actin stress fibres within these cells in response to mechanical loading has led some authors to consider many of these cells to be myofibroblasts (Schleip et al. 2007) – see below. However, where dense fascia is subject to significant levels of compression [e.g. in certain retinacula (Benjamin & Ralphs, 1998, Kumai & Benjamin, 2002) or in parts of the plantar fascia], the cells have a chondrocytic phenotype and the tissue can be regarded as fibrocartilage. Yet other forms of fascia can be considered mixtures of fibrous and adipose tissues –‘fibro-adipose tissue’. This is particularly characteristic of the palms, the soles and the palmar/plantar pads of the fingers and toes (Figs 2 and 3). At all these locations, fibroadipose tissue functions as an important pressure-tolerant device (see below).
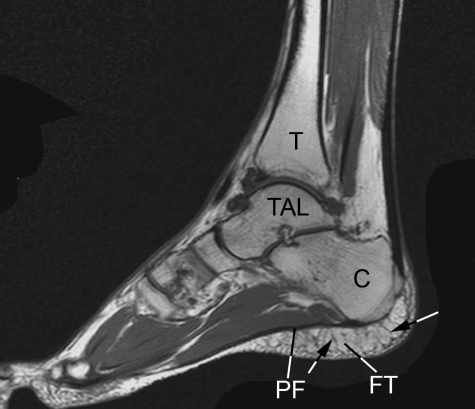
Fig. 3.
A T2-weighted sagittal-plane MRI of the foot showing the extensive areas of fibro-adipose tissue (FT) that are characteristic of the heel, deep to the plantar fascia (PF). C, calcaneus; TAL, talus; T, tibia. The fibrous septa within the fibroadipose tissue are arrowed. Image kindly provided by D. McGonagle.
Of the different histological forms of fascia, it is areolar tissue that is characterized by the greatest diversity of cell types. Its resident fibroblasts are integral to mechanotransduction. They communicate with each other via gap junctions and respond to tissue stretch by shape changes mediated via the cytoskeleton (Langevin et al. 2005; Langevin, 2006). In stretched tissue, the cells are sheet-like and have large cell bodies, whereas in unstretched tissue, they are smaller and dendritic in shape, and have numerous, attenuated cell processes (Langevin et al. 2005). According to Bouffard et al. (2008), brief stretching decreases TGF-β1-mediated fibrillogenesis, which may be pertinent to the deployment of manual therapy techniques for reducing the risk of scarring/fibrosis after an injury. As Langevin et al. (2005) point out, such striking cell responses to mechanical load suggest changes in cell signaling, gene expression and cell-matrix adhesion. The cell shape changes may also influence tension with the connective tissue itself. The many important contributions of Langevin and her colleagues in recent years have significant implications for understanding the basis of the therapeutic responses to the physical manipulation of fascia that is central to numerous ‘alternative therapies’ (e.g. massage, yoga, Rolfing, etc.).
Mast cells are also found in areolar connective tissue (Fig. 4) together with a variable population of ‘immigrant’ cells that have entered the fascia by migrating across the walls of thin blood vessels coursing through them. These essentially represent white blood cells or their differentiation products and thus comprise lymphocytes, granulocytes and macrophages. All forms of fascia contain collagen and/or elastic fibres, although clearly deep fascia is more densely packed with fibres than areolar connective tissue (Fig. 5).
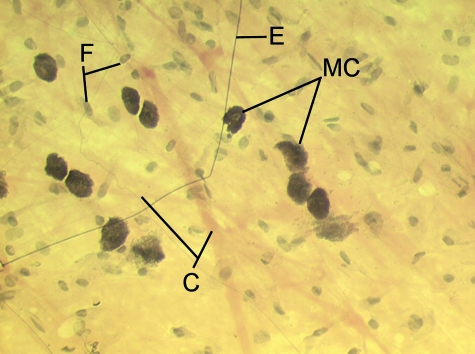
Fig. 4.
A spread of rat superficial fascia showing the presence of pale bundles of collagen fibres (C) of different thicknesses and dark, uniformly-thin, elastic (E) fibres. There are also a number of large, heavily granulated mast cells (MC). The majority of the other cells are only recognizable by their nuclei and are likely to be fibroblasts (F).
Fig. 5.
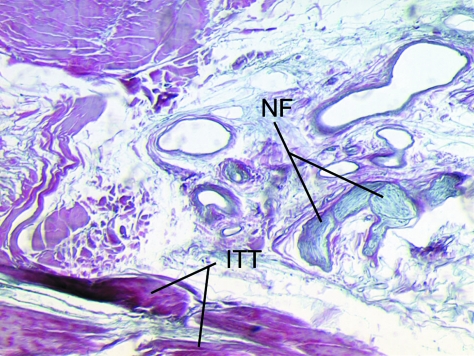
The iliotibial tract (ITT) is a fascial structure that is composed of dense connective tissue. In the region of the lateral femoral epicondyle, it is juxtaposed to an area of adipose tissue that lies immediately deep to it, which contains prominent nerve fibres (NF).
Standard histology texts give a broad treatment of connective tissue cell types and the subject has also been briefly addressed by Lindsay (2008) in his recent textbook on fascia. Although the fibroblast is obviously a key cell within fascia, extensive attention has already been paid to this cell by connective tissue biologists and thus interest is focused in the current article on the suggestion that many fascial ‘fibroblasts’ are really better viewed as myofibroblasts.
Myofibroblasts
The term ‘myofibroblast’ arose from wound-healing studies in the 1950s that were being conducted at the time the cytoskeletal concept was emerging (Gabbiani, 2003, 2007). Myofibroblasts were regarded as fibroblasts that acquire smooth muscle cell features when granulation tissue develops in a wound. The accumulation of actin stress fibres and the development of adherens and gap junction between cells, enable the cells to become contractile and help to close the edges of a wound (Gabbiani, 2007). After wound closure, myofibroblasts normally diminish in number by apoptosis (Desmouliere et al. 1997). However, in certain pathological circumstances, they can remain and their long-term presence is thought to be associated with a whole spectrum of fibrotic conditions (Gabbiani, 2003). Many affect fascia. A good example is Dupuytren's contracture – considered in further detail below. It is associated with the development of nodules of proliferative cells in the palmar aponeurosis which progress to form contractile, collagenous cords (Satish et al. 2008). Both the nodules and particularly the cords contain myofibroblasts. Typically, the myofibroblasts are surrounded by a region of extracellular matrix (ECM) that has certain similarities to the basal lamina around true muscle cells (Berndt et al. 1994). Myofibroblasts also appear in other fibrotic conditions. Fidzianska & Jablonska (2000) have drawn attention to a rare scleroderma-like condition known as congenital fascial dystrophy in which patients have difficulty in walking properly and adopt a tip-toe gait pattern, have limb contractures, limited joint mobility and very hard skin. The principle abnormalities visible in the superficial fascia and fascia lata of the lower limb are the formation of large-diameter collagen fibrils and the appearance of myofibroblasts.
It is worth noting that although there are changes to the actin cytoskeleton in loose connective tissue fibroblasts subjected to stretch, the cells do not form true stress fibres (or zonula occludens) and are thus not myofibroblasts (Langevin et al. 2006b). In contrast, Schleip et al. (2007) have reported myofibroblasts in the rat lumbar fascia (a dense connective tissue). The cells can contract in vitro and Schleip et al. (2007) speculate that similar contractions in vivo may be strong enough to influence lower back mechanics. Although this is an intriguing suggestion that is worthy of further exploration, it should be noted that tendon cells immunolabel just as strongly for actin stress fibres as do fascial cells and this may be associated with tendon recovery from passive stretch (Ralphs et al. 2002). Finally, the reader should also note that true muscle fibres (both smooth and skeletal) can sometimes be found in fascia. Smooth muscle fibres form the dartos muscle in the superficial fascia of the scrotum and skeletal muscle fibres form the muscles of fascial expression in the superficial fascia of the head and neck.
Fascial expansions of entheses
The region where a tendon, ligament or joint capsule attaches to a bone (its ‘enthesis’) is an area of great stress concentration, for it represents the meeting point between hard and soft tissues. Consequently, entheses are designed to reduce this stress concentration, and the anatomical adaptations for so doing are evident at the gross, histological and molecular levels. Thus many tendons and ligaments flare out at their attachment site to gain a wide grip on the bone and commonly have fascial expansions linking them with neighbouring structures. Perhaps the best known of these is the bicipital aponeurosis that extends from the tendon of the short head of biceps brachii to encircle the forearm flexor muscles and blend with the antebrachial deep fascia (Fig. 6). Eames et al. (2007) have suggested that this aponeurosis may stabilize the tendon of biceps brachii distally. In doing so, it reduces movement near the enthesis and thus stress concentration at that site.
Fig. 6.
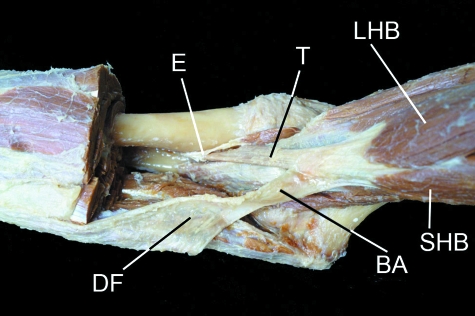
The bicipital aponeurosis (BA) is a classic example of a fascial expansion which arises from a tendon (T) and dissipates some of the load away from its enthesis (E). It originates from that part of the tendon associated with the short head of biceps brachii (SHB) and blends with the deep fascia (DF) covering the muscles of the forearm. The presence of such an expansion at one end of the muscle only, means that the force transmitted through the proximal and distal tendons cannot be equal. LHB, long head of biceps brachii. Photograph courtesy of S. Milz and E. Kaiser.
A further example of a fascial expansion arising from a tendon is that which emanates from the quadriceps tendon as it attaches to the upper pole of the patella. Here, there is a fascial sheet that passes anterior to the patella to contribute superficial fibres to the patellar tendon (Toumi et al. 2006). In a similar manner, the Achilles tendon not only attaches to the posterior aspect of the calcaneus, but also has fascial continuity both with the plantar aponeurosis over the back of the heel (Wood Jones, 1944; Snow et al. 1995; Milz et al. 2002) and with the fibrous septa of the heel fat pad (M. Benjamin, unpublished observations). As teachers of gross anatomy well know, there are numerous, largely unrecognized fascial sheets interconnecting tendons and ligaments in the foot. Among the better known are the tendinous expansions of tibialis posterior that attach to every tarsal bone in the foot except the talus.
Retinacula
Retinacula are strap-like thickenings of dense connective tissue that bind down structures near joints (Fig. 7) and are sometimes used as autografts in the repair of torn ligaments (Saragaglia et al. 1997). Where the levels of compression between tendon and retinacula are significant, both may be fibrocartilaginous (Benjamin et al. 1995). Retinacula not only prevent tendons from bowstringing, but also provide a smooth contact surface for them to slide longitudinally when their associated muscle contracts. As they attach to bone to create tunnels for their associated tendons, a pathological mismatch can develop between the size of the tunnel and that of the tendons that is commonly referred to as stenosing tenosynovitis (Palmborg, 1952; Crimmins & Jones, 1995; Taki et al. 2007).
Fig. 7.
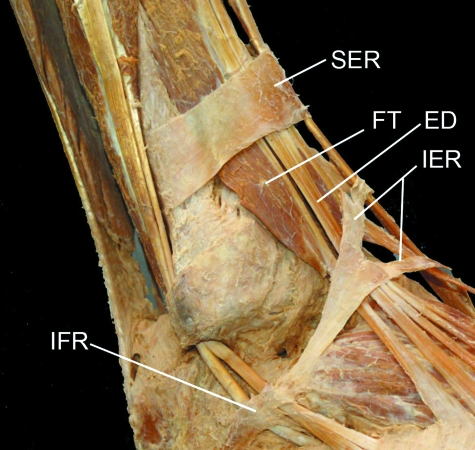
The retinacula of the ankle region dissected artificially away from the rest of the deep fascia in traditional manner. Note how muscle fibres of fibularis (peroneus) tertius (FT) pass beneath the superior extensor retinaculum (SER), but how extensor digitorum (ED) is entirely tendinous at that level. IER, inferior extensor retinaculum; IFR, inferior fibularis (peroneal) retinaculum. Photograph courtesy of S. Milz and E. Kaiser.
The various retinacula at the ankle have a criss-cross arrangement of collagen fibres that enhances their stability. Abu-Hijleh & Harris (2007) compare this arrangement to the straps that cross in a sandal or the laces in a shoe. The retinacula around the ankle average 1 mm in thickness, but variations are considerable (Numkarunarunrote et al. 2007). Retinacula can occasionally be congenitally absent and are commonly subject to traumatic ruptures (Numkarunarunrote et al. 2007). Either circumstance can result in a marked subluxation of the tendons beneath them.
Even where a retinaculum is dense fibrous connective tissue, its entheses may be fibrocartilaginous (Fig. 8). This is of particular significance in relation to the superior peroneal retinaculum that binds down the peroneal tendons behind the lateral malleolus. If the retinaculum is torn (e.g. as a skiing injury), the stiffening associated with its fibrocartilaginous entheses creates a sharp edge to the tissue that can ‘razor’ through the subluxed peroneal tendons (Kumai & Benjamin, 2003).
Fig. 8.
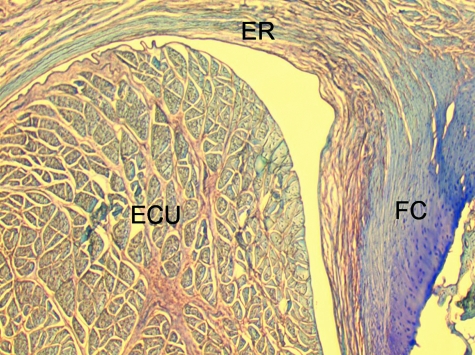
A transverse section through the extensor retinaculum (ER) of the forearm in the region of the tendon of extensor carpi ulnaris (ECU). Note how the retinaculum itself is largely fibrous, but that its ulnar enthesis is fibrocartilaginous (FC).
Retinacula are typically regional specializations of the deep fascia, though as Abu-Hijleh & Harris's (2007) excellent illustrations show, they are nowhere near as distinct as most elementary texts depict. There are also other regions of deep fascia that have a retinacular function, but are not formally referred to as ‘retinacula’ (Abu-Hijleh & Harris, 2007). Thus, there are thickenings of deep fascia along the medial and lateral borders of the foot that the authors compare to the stitches on the side of a shoe, helping to anchor the deep fascia on the dorsum of the foot and augment its stabilizing action on the extensor tendons. The deep fascia also acts as a retinaculum over the extensor tendons in the region of the metatarsophalangeal joints (Abu-Hijleh & Harris, 2007) and over the Achilles tendon in the lower part of the leg (Benjamin et al. 2007).
Typically, it is tendons rather than muscle bellies that pass beneath retinacula, because the compressive forces acting here are not conducive to the survival of highly vascularized tissues such as skeletal muscle. However, notable exceptions are flexor hallucis longus and the inconstant fibularis tertius, where muscle fibres also can lie deep to the superior extensor retinaculum (Haumont et al. 2007; Fig. 7). Significantly though, muscle fibres are retracted in a dorsiflexed foot, when pressure between the retinaculum and the structures deep to it is likely to be greatest. Nevertheless, the anatomical relationship between the muscle–tendon unit and the retinaculum is important clinically for it means that flexor hallucis longus in particular can be vulnerable to the effects of compression syndrome as it passes under the retinaculum. This may necessitate surgery on both the crural fascia and the retinaculum itself (Haumont et al. 2007).
Fascial innervation
Several reports suggest that fascia is richly innervated, and abundant free and encapsulated nerve endings (including Ruffini and Pacinian corpuscles) have been described at a number of sites, including the thoracolumbar fascia, the bicipital aponeurosis and various retinacula (Stilwell, 1957; Tanaka & Ito, 1977; Palmieri et al. 1986; Yahia et al. 1992; Sanchis-Alfonso & Rosello-Sastre, 2000; Stecco et al. 2007a). However, it is sometimes difficult to decide from the literature whether a particular piece of deep fascia is itself innervated or whether the nerve fibres lie on its surface or in areolar or adipose tissue associated with it. Changes in innervation can occur pathologically in fascia, and Sanchis-Alfonso & Rosello-Sastre (2000) report the ingrowth of nociceptive fibres, immunoreactive to substance P, into the lateral knee retinaculum of patients with patello-femoral malignment problems.
Stecco et al. (2008) argue that the innervation of deep fascia should be considered in relation to its association with muscle. They point out, as others have as well (see below in ‘Functions of fascia’) that many muscles transfer their pull to fascial expansions as well as to tendons. By such means, parts of a particular fascia may be tensioned selectively so that a specific pattern of proprioceptors is activated.
Despite the contribution of the above studies, our understanding of fascial innervation is still very incomplete and it is likely that there are regional differences of functional significance, as with ligaments. It is worth noting therefore that Hagert et al. (2007) distinguish between ligaments at the wrist that are mechanically important yet poorly innervated, and ligaments with a key role in sensory perception that are richly innervated. There is a corresponding histological difference, with the sensory ligaments having more conspicuous loose connective tissue in their outer regions (in which the nerves are located). Comparable studies are not available for deep fascia, although Stecco et al. (2007a) report that the bicipital aponeurosis and the tendinous expansion of pectoralis major are both less heavily innervated than the fascia with which they fuse. Where nerves are abundant in ligaments, blood vessels are also prominent (Hagert et al. 2005). One would anticipate similar findings in deep fascia.
From comparisons with tendons and ligaments, where nerve and blood vessels are generally a feature of the associated loose connective tissue sheaths (Hagert et al. 2007), it might be anticipated that fascial nerves would also be commonly surrounded by areolar connective tissue. The levels of mechanical loading to which dense connective tissue is adapted are not likely to be conducive to having nerves and densely packed collagen fibres too close together. Abnormal levels of mechanical loading have been suggested to cause nerve damage in knee retinacula (Sanchis-Alfonso & Rosello-Sastre, 2000). Nevertheless, Stecco et al. (2007a) do show evidence of fascial nerves that are closely related to densely packed collagen fibres and thus lie within the fascia itself. In the distal part of the iliotibial, however, it is the tissue adjacent to deep fascia that is more conspicuously innervated than the fascia itself (Fairclough et al. 2006; Fig. 5).
Some of the nerve fibres associated with fascia are adrenergic and likely to be involved in controlling local blood flow, but others may have a proprioceptive role. Curiously, however, Bednar et al. (1995) failed to find any nerve fibres in thoracolumbar fascia taken at surgery from patients with low back pain.
The functions of fascia
Some of the numerous functions that have been associated with fascia have inevitably been mentioned above in relation to its basic organization. Further important functions are dealt with specifically below.
The role of fascia in creating distinctive compartments for muscles and in acting as an ectoskeleton for their attachment
The unyielding character of the deep fascia enables it to serve as a means of containing and separating groups of muscles into relatively well-defined spaces called ‘compartments’. The deep fascia integrates these compartments and transmits load between them. As the compartmentalizing role of deep fascia is a function it performs in conjunction with the associated bones and intermuscular septa (Fig. 1), the compartments are sometimes called osteofascial compartments. Each segment of the limbs (e.g. arm, forearm, thigh, leg or foot) has its own characteristic compartments separating functional groups of muscles with distinctive embryological origins, blood and nerve supplies. The compartments are generally named according to their position (anterior, posterior, medial, lateral, etc.) or the actions of their contained muscles (flexors, extensors, evertors, adductors, etc.).
The fascial intermuscular septa are often attached to periosteum, and as these two structures share the same developmental origin, it means that fascia rarely makes contact with bone without also attaching to it (Grant, 1948). It is not surprising therefore that fascial entheses have been implicated in overuse injuries – e.g. medial tibial stress syndrome (Bouche & Johnson, 2007). These authors have suggested that the tenting effect of tendons associated with eccentric muscle contraction in the posterior compartment of the leg increases the tensile load on the deep fascia. This is ultimately relayed to the fascial attachment site on the tibial crest. Bouche & Johnson (2007) reported a linear relationship between tension of any one (or all) of the tendons involved and strain levels in the tibial fascia. Simple inspection of cadaveric specimens in which muscle contraction has been simulated by pulling on the tendons with a pneumatic actuator, shows clear evidence of fascial tenting. The authors point out that (1) eccentric contraction of the implicated muscles is increased when exercising on hard surfaces to promote shock dissipation and that individuals with marked foot pronation also have increased eccentric contractions; (2) both running on hard ground and over-pronation are known risk factors for medial tibial stress syndrome (‘shin splints’). Their fascial theory is supported by the common finding of inflammatory changes in the crural fascia of patients with shin splints.
Where deep fascia and intermuscular septa partition muscles, they may also serve for their attachment and this makes such fascia particularly thick (Grant, 1948). One of the most influential anatomists of the 20th century, Professor Frederic Wood Jones, coined the term ‘ectoskeleton’ to capture the idea that fascia could serve as a significant site of muscle attachment – a ‘soft tissue skeleton’ complementing that created by the bones themselves (Wood Jones, 1944). It is clearly related to the modern-day concept of ‘myofascia’ that is popular with manual therapists and to the idea of myofascial force transmission within skeletal muscle, i.e. the view that force generated by skeletal muscle fibres is transmitted not only directly to the tendon, but also to connective tissue elements inside and outside the skeletal muscle itself (Huijing et al. 1998; Huijing, 1999). Several authors have commented on the extent to which muscles attach to fascia as well as bone and on how this has been overlooked in the past. Thus Kalin & Hirsch (1987) found that only eight of the 69 interossei muscles they studied in the feet of dissecting room cadavers had attachments that were limited to bone. In the vast majority of cases the muscles had extensive attachments to ligaments and fascia that effectively link the muscles together to promote their contraction as a co-ordinated unit. A similar point is made by Chang & Blair (1985) in relation to the attachments of adductor pollicis. These authors described prominent attachments of its transverse head to fascia covering the palmar interossei that had not been recorded previously. As the palmar interossei and adductor pollicis are supplied by the same nerve and are both adductors of the thumb (Mardel & Underwood, 1991), it is likely that fascial interconnections promote their coordinated activity.
An interesting implication of recognizing the existence of fascial routes of force transmission is that in muscles with tendons at both ends, the forces within these tendons may be unequal (Huijing & Baan, 2001a). Furthermore, as Huijing et al. (2003) point out, what are generally taken to be morphologically discrete muscles from an anatomical perspective, cannot be considered isolated units controlling forces and moments. One can even extend this idea to embrace the concept that agonists and antagonists are mechanically coupled via fascia (Huijing, 2007). Thus Huijing (2007) argues that forces generated within a prime mover may be exerted at the tendon of an antagonistic muscle and indeed that myofascial force transmission can occur between all muscles of a particular limb segment.
It is intriguing to note that Huijing et al. (2003) follow Wood Jones's (1944) lead and liken compartment-forming fascia to a skeleton. Indeed, they suggest that one of the functions of myofascial force transmission is to stiffen this skeleton and hence augment its function. Whether surgery to this ‘skeleton’ (notably fasciotomies undertaken to reduce intracompartmental pressure – see below) changes the force-generating capacity of limb muscles is a valid question that does not seem to have been addressed, although Huijing & Baan (2001b) report significant changes in the forces generated by muscles in the anterior compartment of the rat leg following fasciotomy. Furthermore, as neighbouring muscles can be strongly attached to each other by extramuscular connective tissue, it may well be that the tenotomies favoured by some surgeons in the treatment of tennis elbow affect the force transmission of neighbouring muscles in way that has not been thoroughly explored.
Wood Jones (1944) was particularly intrigued by the ectoskeletal function of fascia in the lower limb. He related this to man's upright stance and thus to the importance of certain muscles gaining a generalized attachment to the lower limb when it is viewed as a whole weight-supporting column, rather than a series of levers promoting movement. He singled out gluteus maximus and tensor fascia latae as examples of muscles that attach predominantly to deep fascia rather than bone (Wood Jones, 1944). Viewing the deep fascia as an ectoskeleton emphasizes the importance of considering the responses of this tissue to distraction procedures designed to lengthen a limb segment. According to Wang et al. (2007), a lengthening rate of 1 mm day−1 in a rabbit model of distraction in which the tibia is ultimately increased in length by 20%, results in a corresponding re-modelling of the deep fascia. This ensures that the tensile forces operating in the fascia match the increasing length of the limb so that the fascia does not impede distraction.
Although gastrocnemius is strikingly lacking in any significant attachment to its overlying deep fascia (see above), it nevertheless gains extensive anchorage to a thick fascial sheet on its deep surface. As with any such well-developed aponeurosis on the surface of a muscle, this inevitably restricts the range of movement. Indeed, a surgical approach that has been advocated to improve the range of motion in cerebral palsy patients with an equinus deformity is to make a series of transverse incisions through this fascia (Saraph et al. 2000).
This ectoskeletal role of fascia is particularly obvious in relation to the intrinsic muscles of the foot and tibialis anterior in the upper part of the leg. The firm fascial attachments of tibialis anterior account for the longitudinal orientation of the fascial fibres at this site (i.e. parallel to the long axis of the muscle) and for the difficulty in making a clean dissection. In marked contrast, the deep fascia on the back of the leg covering the muscle bellies of gastrocnemius, does not serve for muscle attachment at all (Grant, 1948). This indicates the importance of this particular muscle belly to be able to move independently of its fascia during the powerful contractions that it performs in its weight-bearing capacity. Advantage is taken of the absence of muscle–fascia attachment in this location in the design of surgical interventions to augment calf size, either for purely aesthetic reasons or to correct a deformity resulting from illness (Niechajev, 2005). Silicone implants can be placed between the investing deep fascia covering gastrocnemius and the muscle itself (Niechajev, 2005).
The general thinness of the deep fascia covering the large, flat pectoral muscles in the chest is in line with the need for the thorax to expand and contract during breathing (Grant, 1948). In contrast, the deep fascia is particularly thick in the leg in line with its compartmentalizing and ectoskeletal roles. Strong intermuscular septa pass inwards from the deep fascia to fuse with the periosteum of the tibia and fibula and create separate compartments for the dorsiflexor, peroneal and plantarflexor muscles (Fig. 1). The anterior compartment houses the dorsiflexor muscles (which include tibialis anterior, discussed above) and is of particular clinical significance in relation to compartment syndrome. This is a painful and potentially limb-threatening condition that occurs when pressure builds up within the space that is limited by the tough and unyielding deep fascia impairing blood flow. It can occur as a result of sudden trauma (e.g. a haematoma) or as a consequence of overuse. The resulting muscle ischaemia may require an emergency fasciotomy to reduce the pressure. If surgery is unduly delayed, serious systemic complications can arise, including renal failure (Mubarak & Owen, 1975). Anterior compartment syndrome is perhaps the best known, but compartment syndrome can occur elsewhere as well. From an anatomical perspective, it is worth remembering that the fascial compartments characterizing the hands and feet are small (matching the size of the muscles; Grant, 1948) and thus pressure can quickly build up from a haematoma and trigger a collapse of the local circulation.
The attachment of seemingly diverse muscles to a common fascia means that fascia is in a strategic position to co-ordinate muscle activity. This is not surprising bearing in mind the integrating role of the connective tissue sheaths within a given muscle, harnessing the activity of its muscle fibres into an integrated whole. In an influential paper, Vleeming et al. (1995) have highlighted the importance of the thoracolumbar fascia in integrating the activity of muscles traditionally regarded as belonging to the lower limb, upper limb, spine or pelvis and whose action is thus often considered in that territory alone. They have argued that a common attachment to the thoracolumbar fascia means that the latter has an important role in integrating load transfer between different regions. In particular, Vleeming et al. (1995) have proposed that gluteus maximus and latissimus dorsi (two of the largest muscles of the body) contribute to co-ordinating the contralateral pendulum like motions of the upper and lower limbs that characterize running or swimming. They suggest that the muscles do so because of a shared attachment to the posterior layer of the thoracolumbar fascia. Others, too, have been attracted by the concept of muscle-integrating properties of fascia. Thus Barker et al. (2007) have argued for a mechanical link between transversus abdominis and movement in the segmental neutral zone of the back, via the thoracolumbar fascia. They feel that the existence of such fascial links gives an anatomical/biomechanical foundation to the practice in manual therapy of recommending exercises that provoke a submaximal contraction of transversus abdominis in the treatment of certain forms of low back pain. Stecco et al. (2007a,b, 2008) have also given us further examples of how fascia in the upper limb links numerous different muscles together. They suggest that a basal level of tension that is loaded onto the fascia by flexor and extensor muscles alike, contributes to myofascial continuity and possibly activates specific patterns of proprioceptors associated with the fascia.
One of the most striking examples of how fascia links muscles together concerns the extensor muscles of the forearm in the region of the lateral epicondyle. Although standard anatomy texts often simplify the position greatly by saying that the extensor muscles attach to the common extensor origin, the reality is rather more complex. Clearly, the area of bone provided by the lateral epicondyle is insufficient to attach the numerous muscles on the back of the forearm that arise from the common extensor origin. What happens instead is that the muscles attach to each other in this region via fascia and by this means they can be crowded onto a limited surface of bone. This is well illustrated by the work of Briggs & Elliott (1985) who dissected 139 limbs, and found that extensor carpi radialis brevis (the muscle most commonly associated with tennis elbow) actually attached directly to the epicondylar region in only 29 cases. Much more frequently it was linked by fascia to extensor carpi radialis longus, extensor digitorum communis, supinator and the radial collateral ligament.
The circulatory-support function of deep fascia – the muscle pump phenomenon
An important function of deep fascia in the limbs is to act as a restraining envelope for muscles lying deep to them. When these muscles contract against a tough, thick and resistant fascia, the thin-walled veins and lymphatics within the muscles are squeezed and their unidirectional valves ensure that blood and lymph are directed towards the heart. Wood Jones (1944) contests that the importance of muscle pumping for venous and lymphatic return is one of the reasons why the deep fascia in the lower limb is generally more prominent than in the upper – because of the distance of the leg and foot below the heart. However, there is also a thick, inelastic fascia covering the scapular muscles above the level of the heart. This has a bearing on the high intramuscular pressure in supraspinatus that is implicated in shoulder pain and muscle isthemia (Jarvholm et al. 1988).
That the deep fascia is under tension from the contraction of its contained muscles is evident when it is punctured, for the muscle bulges through it (Le Gros Clark, 1945). The protrusions are known as fascial herniations and may merit a fasciotomy if muscle ischaemia is a risk (de Fijter et al. 2006). The anti-gravitational nature of the ‘muscle pump’ associated with the crural fascia is commonly mentioned in anatomy texts and has traditionally been reinforced to generations of medical students by the observation that long periods of standing still (e.g. as with soldiers on ceremonial duty) can cause blood to pool in the legs and feet, resulting in inadequate venous return and fainting. More recently, the emphasis has turned to deep vein thrombosis as a way of illustrating the importance of the muscle pump. The stagnancy of peripheral blood flow that comes from long periods of static posture (again linked to inadequate muscle activity) can lead to the formation of blood clots – which can be fatal. Understanding the role of fascia in the muscle-pumping actions of the lower limb depends on a sound understanding of the venous system. This is adequately covered in standard anatomy teaching texts and has also been addressed in a recent review article by Meissner et al. (2007). Briefly, a distinction is made between superficial and deep veins according to their relative position with respect to the deep fascia. The two sets of veins are linked by ‘perforating veins’ that penetrate the deep fascia, linking veins either side. All three sets of vessels have valves that prevent backflow and help to divide the hydrostatic column of blood into segments (Meissner et al. 2007). Valvular incompetence in the leg thus diminishes muscle pump function. Curiously, however, the perforating veins in the foot lack valves and thus do allow bidirectional flow. According to Meissner et al. (2007), the calf muscle pump is the most significant and has the largest capacitance, but is primed by muscle pumps in the foot. They view the influence of similar pumps in the thigh as being minimal.
On occasions, the deep fascia may have too severe a restraining influence on its contained muscles, so that they are in danger of inadequate perfusion because their vessels are occluded for prolonged periods. The outcome is known as a ‘compartment syndrome’ and can be acute or chronic. Acute compartment syndromes may be associated with trauma where there is bleeding within the compartment or may be elicited by a plaster-cast applied too tightly to a limb. Chronic compartment syndromes stem from an exercise-induced increase in intra-compartmental pressure that compromises normal neuromuscular function (Bourne & Rorabeck, 1989). The muscle ischaemia stemming from acute compartment syndrome can be limb- (and sometimes life-)threatening and represents a surgical emergency. The confining deep fascia must be cut to reduce the pressure. The urgency with which a fasciotomy needs to be performed in severe acute cases is indicated by the observation that significant necrosis can occur within 3 h (Vaillancourt et al. 2004). In mild cases of exercise-induced compartment syndrome, the pressure on the muscles may be reduced by applying ice.
In view of the importance of gravitational influences in accounting for the prominence of deep fascia in the leg, it might be surmised that fasciotomies would seriously impair the venous calf pumps. This is the conclusion of Bermudez et al. (1998) who caution that patients who have had fasciotomies are at risk of developing chronic venous insufficiency in the long term. This, however, contrasts with the earlier findings of Ris et al. (1993).
The protective role of fascia
In certain regions of the body, fascia has a protective function. Thus, the bicipital aponeurosis (lacertus fibrosus), a fascial expansion arising from the tendon of the short head of biceps brachii (Athwal et al. 2007), protects the underlying vessels. It also has mechanical influences on force transmission and stabilizes the tendon itself distally (Eames et al. 2007). There are also very tough sheets of connective tissue in the palms and soles. The palmar aponeurosis in the former, and the plantar aponeurosis in the latter (Fig. 9), protect the vessels and nerves that run deep to them between the proximal and distal parts of the hand and foot. They also tie the skin to the skeleton, controlling its displacement during locomotion (Bojsen-Moller & Flagstad, 1976). However, fascia in the form of dense connective tissue is not best suited to protect against the compressive forces that act during walking or the creation of a power grip. As fat is fluid and thus incompressible, it is far better for this task. This is well illustrated by the presence and regional distribution of tubular sleeves of adipose tissue around the digital nerves in the foot. The fat is strikingly present as the nerves pass through the weight-bearing ball of the foot en route to the toes (see Fig. 2 in Bojsen-Moller & Flagstad, 1976). Fat also protects the metatarsals themselves in the region – a point recognized by Bojsen-Moller & Flagstad, who referred to the fat in this region as creating sub-metatarsal cushions. Indeed, these cushions also protect the flexor tendons and their sheaths as they pass through the area.
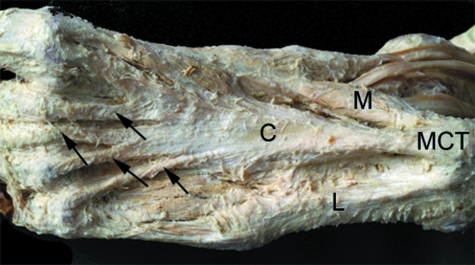
Fig. 9.
The central (C), medial (M) and lateral (L) parts of the plantar aponeurosis in the sole of the foot. Note the extensive attachment of the aponeurosis to the medial calcaneal tubercle (MCT) and the distal expansions of the aponeurosis passing to the lesser toes (arrows). Photograph kindly provided by S. Milz and E. Kaiser.
Regional considerations of fascia
Even a cursory inspection of any large anatomy tome will inform the reader of a bewildering diversity of named fascia, reflecting the requirements of regional anatomy. In the limbs and back, these include the fascia lata and iliotibial tract, the clavipectoral, axillary, brachial, antebrachial, thoracolumbar, plantar, palmar, crural and gluteal fascia, and many retinacula and pulleys that are increasingly prominent towards the distal parts of the limbs. However, as Wood Jones (1944) points out, there is also an argument for considering such a daunting list of terms to be redundant and a hindrance to understanding general principles of fascial biology. It is with his cautionary note in mind that only a limited number of fascia are considered here individually by name. It is more important to see fascia as a connective tissue continuum throughout the body, uniting and integrating its different regions. Yes, the names are convenient labels – but little more than that. Just as Myers (1987) has argued persuasively that the traditional approach of anatomical dissection viewing muscles as independent units has obscured the ‘trains of muscle continuity’ that he eloquently describes, so too is the naming and study of a plethora of fasciae in isolation, a barrier to understanding the bigger picture of fascial function. It is thus critical to appreciate that loose connective tissue forms one of the great highways of the body – a favoured route by which blood vessels, nerves and lymphatics journey between regions (Wood Jones, 1944; Le Gros Clark, 1945). Evidence is also accumulating to suggest that fascia have an important integrating function as a proprioceptive organ and that they can coordinate the action of different muscles by acting as a common ectoskeleton that provides a large area of grip for muscle attachment (Wood Jones, 1944).
The palmar and plantar fasciae
Both the palm of the hand and the sole of the foot contain a tough sheet of dense connective tissue that protects the underlying vessels and nerves from the pressure associated with grip or body weight. Both are firmly attached to the thick skin overlying them, so as to limit movement between the integument and neighbouring structures and both send prolongations extending into the digits. The former sheet of connective tissue is called the palmar fascia and the latter the plantar fascia. Both are closely related to the tendon of a vestigial muscle, but Caughell et al. (1988) argue that the palmar fascia and the tendon of palmaris longus are best viewed as separate anatomical structures that develop independently. Nevertheless, palmaris longus does serve as a tensor of both the palmar and thenar fasciae (Stecco et al. 2007b). As Botte (2003) points out, this is an aid to grip, as by providing a firm anchorage for the palmar aponeurosis, the muscle helps to resist shearing forces in this region of the hand.
Palmar fascia
The palmar fascia (synonym palmar aponeurosis) is triangular-shaped and located in the hollow of the palm. Its apex lies proximally near the wrist and is continuous with the flexor retinaculum and the tendon of palmaris longus, and its base lies distally near the fingers and sends four prolongations (pretendinous bands) into the fingers to blend with the fibrous flexor sheaths surrounding the flexor tendons (Fifield, 1939; de-Ary-Pires et al. 2007). Branches of the bands give rise to cutaneous ligaments, eponymously referred to as Cleland's and Grayson's ligaments. These are thought to stabilize the neurovascular bundles within the digits during finger movements and to anchor the skin in a way that limits its displacement during finger flexion (de-Ary-Pires et al. 2007). Between the four digital processes are three fat-filled intervals containing neurovascular bundles and the lumbrical muscles. Their existence is of great importance to hand surgeons in connection with the spread of any infection from the fingers into the subaponeurotic spaces of the palm (Fifield, 1939). Similarly, there is a sheet of interosseous fascia jointly covering the interossei and the metacarpals that contributes to the formation of a potential deep fascial space of the palm between it and the deep flexor tendons and their lumbricals (Fifield, 1939). Again, this is significant in relation to the spread of pus. There is a vertical septum attached to the 3rd metacarpal bone that divides the deep fascial space into medial and lateral compartments and confines any pus to one side alone (Fifield, 1939). The existence of two other potential spaces also hinges on the presence of fascial sheets, i.e. the thenar and mid-palmar spaces (Fifield, 1939). Again, their importance relates to the tracking and confinement of pus.
The palmar fascia and its pretendinous bands are of particular interest in relation to Dupuytren's contracture, one of the most common conditions managed by hand surgeons, but also one frequently seen by many other doctors as well (Hart & Hooper, 2005). Patients present with various degrees of digital flexure, especially on the medial side of the hand, an observation that has occasionally led to the erroneous assumption that the ulnar nerve is implicated (Clay, 1944). The condition particularly involves the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints and is regarded as irreversible. Non-surgical attempts at correction are usually unsuccessful, though local injections of collagenase in the region of the cords are reported to be beneficial (Badalamente et al. 2002; de-Ary-Pires et al. 2007). Nevertheless, surgery remains the preferred option for patients who have more than 30o of MCP joint contracture (Townley et al. 2006). A palmar fasciotomy, fascietomy or even finger amputation may be advocated to alleviate the condition. The disease classically commences with thickening and pitting of the skin, followed by the development of fibrous nodules. These are firmly linked to the skin and eventually form cords (thickenings of the pretendinous fascia) which contract to flex the MCP and PIP joints and produce the characteristic deformity (Townley et al. 2006). It is a proliferative fascial affliction that has features in common with wound healing, the formation of keloid scars and fibrosarcomas (Jemec et al. 1999; Fitzgerald et al. 1999; Townley et al. 2006). Murrell et al. (1987) have argued that an increased free radical production could lead to a degree of tissue damage that subsequently triggers the reparative fibrosis that is characteristic of the contracture.
Dupuytren's disease has an interesting and colourful history (Elliot, 1988; Flatt, 2001). It is thought to have originated with the Vikings, has sometimes been compared to the ‘hand of Benediction’ or referred to as the ‘curse of the MacCrimmon clan’. The last name stems from its reputation for having interfered with their bagpipe-playing skills! It is commonly bilateral and characteristically affects men over the age of 50 years, impinging significantly on daily work and home life (Townley et al. 2006; de-Ary-Pires et al. 2007). Sufferers may complain about difficulties in performing manual tasks, wearing gloves, shaking hands with another person, washing or dressing (Townley et al. 2006). As it often affects older individuals, it may be a threat to independent living. In view of the predominance of Dupuytren's disease in males, it is interesting that androgen receptors are more highly expressed in the palmar fascia of patients with the condition than in normal hands (Pagnotta et al. 2002).
The considerable clinical significance of Dupuytren's contracture has led to a comprehensive evaluation of the gene-expression profiles of cells in the palmar fascia and the composition of the fascial ECM. Predictably, many of the molecules characteristic of tendons and ligaments are present in fascia as well. As in tendons and ligaments, the predominant collagen is type I (Brickley-Parsons et al. 1981). Type III collagen is virtually absent in normal palmar fascia, but accumulates in patients with Dupuytren's disease (Brickley-Parsons et al. 1981; Melling et al. 1999). Murrell et al. (1991) suggest that this may be related to the high density of fibroblasts in Dupuytren fascia, for they mimicked the altered type III/I collagen balance both in cultured Dupuytren's fibroblasts and in control cells simply by increasing cell density. Similarly, there is increased expression of type IV collagen (together with fibronectin, laminin and tenascin) in the myofibroblast-rich areas of the palmar fascia from Dupuytren's patients (Berndt et al. 1994). Decorin (a small dermatan sulphate-rich proteoglycan, DSPG) is the principal small proteoglycan (PG), and biglycan and large chondroitin sulphate/DSPG are present as minor components (Kozma et al. 2005). Decorin and biglycan have also been reported in the fascia lata and large chondroitin sulphate/DSPG appears in scarred fascia (Kozma et al. 2000).
ECM composition changes substantially with pathology, not only in the palmar fascia in Dupuytren's contracture (reviewed by Cordova et al. 2005) but also in the fascia lata subject to scarring (Kozma et al. 2000). In both cases, there is an increase in the expression of biglycan relative to that of decorin (Kozma et al. 2000, 2005). This is indicative of overall changes in dermatan sulphate expression that may influence collagen–glycosaminoglycan interaction and thus fibrillogenesis (Kozma et al. 2007). An increase in gene expression for a wide variety of ECM components has been reported in microarray studies of the palmar fascia of patients with Dupuytren's disease (Qian et al. 2004). Of particular interest are numerous changes in mRNA levels reported by these authors that can be interpreted as attempts to deal with the fibrosis that is characteristic of the disease-defining tissue nodules. Thus, Qian et al. (2004) report a marked upregulation of a variety of genes involved in apoptosis, inflammation and proteolysis including the Alzheimer protein, amyloid A4 protein precursor (consistent with inflammatory reactions in general), pro-apoptotic Rho proteins (involved not only in apoptosis, but also in cell contractility and myofibroblast differentiation), several matrix metalloproteinases (MMPs – enzymes important for matrix turnover) and contactin (a gene involved in actin cytoskeleton remodelling related to myofibroblast activity). More recently still, Satish et al. (2008) have highlighted a downregulation of genes coding for proteoglycan 4 (PRG4), fibulin-1 (FBLN-1) transcript variant D, and collagen type XV alpha 1 chain, and Forsman et al. (2008) have reported a dramatic downregulation of myoglobin and an upregulation of tyrosine kinase-like orphan receptor 2 (ROR2). Augoff et al. (2006) have found an increased activity of MMP2 activity, and Johnston et al. (2007) have highlighted a marked increase in the expression of MMPs 1, 13, and 14 and of ADAMTS14 in Dupuytren nodules. They also found that TIMP1 expression is much higher in nodules compared with control tissue. Lee et al. (2006) have encouraged us to note the significant upregulation of MafB in view of the role of Maf proteins in tissue development and regulation. In immunohistochemical preparations, MafB co-localizes with actin stress fibres in Dupuytren myofibroblasts. As a generality, Rehman et al. (2008) point out that many of the genes that are dysregulated in tissue taken from Dupuytren cords showed an increase in fold change compared with tissue taken from nodules. Interestingly, they draw attention to a particular involvement of genes associated with cytoskeletal formation – clearly a pertinent issue in view of the myofibroblastic phenotype of many cells from Dupuytren-associated fascia. Hopefully, further gene-profiling studies will help to guide the development of agents that could be used for therapeutic or diagnostic purposes. However, at the moment, the gap between our scientific knowledge of the changes that characterize Dupuytren tissue and the clinical application of this knowledge remains considerable (Cordova et al. 2005). It is pertinent to note the observation that 29 of the dysregulated genes identified by Satish et al. (2008) in fascial fibroblasts from Dupuytren's tissue were of unknown function, making it likely that novel pathways are operating in the disease about which we are currently ignorant.
Dupuytren's disease shares with fibrosarcomas an elevated expression of the c-myc oncogene – a gene known to be associated with the control of cell proliferation, apoptosis and differentiation (Evan et al. 1994). The parallels with wound healing are particularly intriguing, especially the prominence of myofibroblasts and the possible role of these cells in tissue contraction (Bisson et al. 2003, 2004). According to Bisson et al. (2003), myofibroblasts are most common in Dupuytren nodules (where approximately 10% of the cells express alpha smooth muscle actin), less common in the cords (roughly 3%) and least common in normal flexor retinaculum sampled as a control (just over 1%). Intriguingly, Bisson et al. (2003) reported a marked increase in the number of myofibroblasts from both Dupuytren cords and nodules in response to TGF-β1 treatment compared with control fascia. The percentage of myofibroblasts increased to approximately 25% in both cords and nodules, whereas the cells of control fascia showed no response. The authors suggest that this may relate to the high recurrence rate of the disease or its progression following injury. It is also of interest that high levels of nerve growth factor have been associated with the proliferative stage of the disease, when myofibroblast number increases markedly (Lubahn et al. 2007). The expression of platelet-derived growth factor is also upregulated in Dupuytren's disease (Terek et al. 1995) and Augoff et al. (2005) has reported changes in the expression of epidermal growth factor.
Plantar fascia
The plantar fascia (synonym plantar aponeurosis) is a superficially placed sheet of dense connective tissue that covers and protects the intrinsic muscles of the foot (Fig. 9). It helps to maintain the medial longitudinal arch and transmit forces from the hind to the forefoot (Erdemir et al. 2004). It can act both as a beam and a truss (Hicks, 1954; Salathe et al. 1986) – a beam during propulsion when the metatarsals are subject to significant bending forces and a truss when the foot absorbs impact forces during landing and during the stance phase of gait. Its function as a tie-beam for the foot is particularly well explained by Sarrafian (1987). He likens the foot to a twisted plate in which the hindfoot lies in the sagittal plane and the forefoot horizontally. It is the twist that occurs between hind and forefoot that creates the characteristic arches. When the foot is loaded by body weight acting through the ankle joint, the dorsum of the foot experiences compressive loading and the plantar side is subject to tensile loading. The plantar fascia thus acts as a tie-beam in the sole to relieve the tensile loading to which this area is subject.
The plantar fascia stretches from the calcaneus to the distal aspect of the metatarsophalangeal (MTP) joints, where it divides into five slips – one for each toe. The slips pass distal to the MTP joints, blending with the volar plates, via which they are attached to the skeleton (Bojsen-Moller & Flagstad, 1976). However, they are also attached to the skin of the forefoot in this region. As the fascia inserts distal to the MTP joints, it is ‘cranked’ around the heads of the metatarsals as the body is raised onto the toes (Fig. 10). The metatarsals act as pulleys around which the fascia is tightened so that the medial longitudinal arch of the foot can be raised without muscular effort. The mechanism is commonly referred to as a ‘windlass’ (Hicks, 1954). In everyday usage, this is a cylindrical piece of equipment used to move heavy weights. Thus a windlass is effectively a barrel, rotated by turning a crank. It is commonly used by mariners to help raise heavy anchors. The tightening of the plantar fascia that occurs by this windlass mechanism at toe-off, increases the height of the medial longitudinal arch and helps to convert the foot into a rigid lever during weight bearing. As the metatarsals are firmly planted on the ground to support body weight at toe-off, the moveable end of the foot now becomes the heel. Consequently, because of the predominantly medial attachment of the plantar fascia to the calcaneus, it automatically inverts and supinates the foot, as it is tightened (Barthold, 2001).
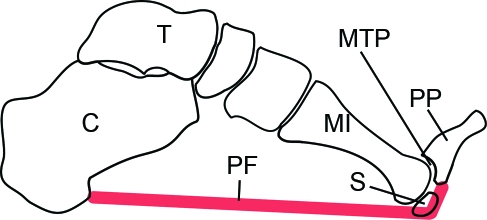
Fig. 10.
A diagrammatic representation (modified from Fig. 4 of Hicks, 1954) to show the windlass mechanism by which the plantar fascia (PF) heightens the medial longitudinal arch of the foot. The fascia extends from the calcaneus (C) to beyond the level of the metatarsophalangeal joint (MTP), thus attaching to the proximal phalanx (PP) instead. Consequently as the foot is dorsiflexed, the fascia is tightened around the plantar surface of the MTP joint and the arch of the foot is heightened. MI, 1st metatarsal bone; S, sesamoid.
From a clinical perspective, the principal interest in this fascia lies in relation to plantar fasciitis (or fasciosis) – a common cause of heel pain that is well documented as an overuse injury and is particularly common in runners (Warren, 1990). Patients complain of tenderness and pain on the infero-medial aspect of the heel, i.e. at the site where the plantar fascia attaches to the medial tubercle of the calcaneus (Neufeld & Cerrato, 2008). It is commonly worse when arising from bed in the morning, or after periods of physical inactivity. It is frequently associated with biomechanical abnormalities of the foot, including overpronation and a high medial longitudinal arch (Bolgla & Malone, 2004). Bony spurs may be present as well, though there is no clear cause–effect relationship between the presence of these spurs and heel pain. Furthermore, the spurs are rarely within the fascia itself, but lie instead on its deep surface (Kumai & Benjamin, 2002; Abreu et al. 2003). For this reason, Kumai & Benjamin (2002) have challenged the assumption that they are traction spurs and have suggested that they are more comparable to the peripheral osteophytes at the edge of articular cartilage in an osteoarthritic synovial joint.
Plantar fasciitis is normally treated conservatively, but surgery (‘fasciotomy’) is sometimes advocated for intransient cases. Among the possible consequences that are recognized by an invasive approach are a reduction in the stability of the medial longitudinal arch and an increased strain that can be placed on other foot ligaments in compensation (Cheung et al. 2004). Plantar fascial thickness is commonly determined sonographically and is known to be greater in patients with plantar fasciitis (Kane et al. 2001; Pascual Huerta & Alarcon Garcia, 2007) and in diabetics (Duffin et al. 2002). However, Pascual Huerta & Alarcon Garcia (2007) caution that the thickness of the plantar fascia varies regionally within a given individual and that the site where measurements are taken is not always clearly defined. Such methodological shortcomings mean that it is difficult to decide how thick a plantar fascia should be before it is viewed as pathologically thickened (Pascual Huerta & Alarcon Garcia, 2007). In diabetics, the thickening is probably a general sign of the hyperglycaemia that occurs in this condition, promoting glycosylation in tissues dense in collagen – hence in fascia (Barbagallo et al. 1993). Significantly, from a clinical perspective, the extent of plantar fascial thickening is thought to be a good predictor of complications known to arise from type I diabetes (Craig et al. 2008). In diabetics, thickening of the plantar fascia (and Achilles tendon) may increase the likelihood of foot ulcers because of the altered foot biomechanics that accompany such changes – particularly the formation of a cavus foot, i.e. a foot characterized by a high arch (Giacomozzi et al. 2005).
Thoracolumbar fascia
The thoracolumbar fascia (lumbar fascia or thoracodorsal fascia) is the deep fascia of the back. It lies in both the thoracic and lumbar regions of the trunk and covers the erector spinae complex. Its basic structure is dealt with in most general anatomy books (e.g. Standring, 2004) and more detailed accounts are available in the more specialized text of Vleeming et al. (2007). In the thoracic region, it forms a thin covering for the extensor muscles of the vertebral column. Medially, it is attached to the spines of the thoracic vertebrae, and laterally it is attached to the ribs, near their angles. In the lumbar region, it is also attached to the vertebral spines, but in addition it forms a strong aponeurosis that is connected laterally to the flat muscles of the abdominal wall. Medially, it splits into anterior, middle and posterior layers. The first two layers surround quadratus lumborum and the last two form a sheath for the erector spinae and multifidus muscles. Below, it is attached to the iliolumbar ligament, the iliac crest and the sacroiliac joint. Via its extensive attachment to vertebral spines, the thoracolumbar fascia is attached to the supraspinous and interspinous ligaments and to the capsule of the facet joints. Willard (2007) argues that this ‘interspinous–supraspinous–thoracolumbar ligament complex’ provides central support to the lumbar spine. It also transfers stress from a multitude of muscles to numerous facet joint capsules.
Particular interest is directed towards the posterior layer of the fascia, for this is important in transferring forces between spine, pelvis and lower limbs (Vleeming & Stoeckart, 2007). It is a superficially placed membrane that is a strong, glistening sheet of connective tissue, linking two of the largest muscles of the body – latissimus dorsi and gluteus maximus. According to Vleeming et al. (1995), it connects these muscles functionally in a way that promotes the co-ordinated activities of the upper and lower limb, notably the pendulum-like actions of the contralateral arms and legs during walking and running. As Vleeming & Stoeckart (2007) comment, the coupling between gluteus maximus and latissimus dorsi means that we need to be more cautious about categorizing a particular muscle as being an ‘upper limb’ or ‘lower limb’ muscle.
Although it has long been recognized that transversus abdominis and the internal oblique muscles are attached via the middle layer of the thoracolumbar fascia to the tips of the vertebral transverse processes, Barker et al. (2007) argue that the strength and significance of this attachment has been underestimated. Their dissections lead them to conclude that the middle layer of the thoracolumbar fascia has a sufficiently thick and strong attachment to the vertebral transverse processes that these processes could be avulsed by strong transversus abdominis contractions. Furthermore, they also suggest that the strength of the connections between the thoracolumbar fascia and the transversus abdominis muscles is such that the latter could be important in lumbar segmental control (Barker et al. 2004, 2007).
The fascia lata and the iliotibial tract
The fascia lata is the deep fascia of the thigh – a veritable stocking forming an ectoskeleton for its muscles. It is thicker where it is more exposed laterally than it is medially, and the thickening is called the iliotibial (IT) tract or band. The IT tract also serves as a tendon for tensor fascia latae and gluteus maximus. It has extensive attachments to the lateral intermuscular septum in the thigh (another fascia), is attached to the lower part of the femur and is finally anchored to Gerdy's tubercle at the upper end of the tibia (Fairclough et al. 2006). The IT tract is a common site of an overuse injury, well documented in runners and cyclists, located where the tract passes over the lateral femoral epicondyle (Fredericson & Wolf, 2005). Both the fascia lata in general and the IT tract in particular are used as sources of dense connective tissue for a wide variety of surgical procedures including sealing lung tissue (Molnar et al. 2003), correcting eyelid deformities (Flanagan & Campbell, 1981) and providing a suitable substitute for a torn anterior cruciate ligament (Joseph, 1988). The attraction of using the fascia as an autograft stems from the sparsity of its cell populations and thus its low nutritional requirements (Flanagan & Campbell, 1981).
IT tract syndrome is commonly attributed to excessive friction between the tract and the lateral femoral epicondyle just above the knee (Fredericson & Wolf, 2005), although Fairclough et al. (2006) argue that compressive forces between bone and fascia at this site are more significant. They point out that as the IT tract is part of a whole fascial stocking completely encircling the thigh, is extensively connected to the lateral intermuscular septum and constantly anchored to the lower part of the femur, it cannot move forward and backwards, as commonly surmised, during flexion and extension of the knee. They propose instead that the tract gives the illusion of a forward-backward translation, because of a shifting pattern of tension within its anterior and posterior fibres as the knee moves from full extension to 30º of flexion (at which angle, the symptoms are generally most acute). This is at odds with the views of Gerlach & Lierse (1990) that the IT tract is not part of the fascia lata and is a structure that glides in its own fascial bag. However, in a recent article, Vieira et al. (2007) also emphasize the numerous sites of bony connections of the IT tract, spanning from the hip to the tibia. The main thrust of Vieira et al.'s (2007) argument is that the IT tract has a structure in line with its importance as a lateral stabilizer or brace for the knee.
Concluding remarks
Fascia has long been of interest to surgeons and viewed with considerable importance by paramedical practitioners including manual therapists, osteopaths, chiropractors and physical therapists. ‘Fascial release techniques’ are routine working practices in such professions. However, despite the wealth and diversity of studies that have been reviewed in the current work, there is a paradoxical feeling that fascial research is still in its infancy, largely because it is not ‘mainstream’. Yet it is likely to become so in the foreseeable future if it does indeed hold the key to understanding aspects of musculoskeletal problems such as low back pain and fibromyalgia. As a substantial number of visits by patients to primary care centres relate to musculoskeletal disorders, the importance of attracting further interest in fascia from the research community is obvious.
References
- Abreu MR, Chung CB, Mendes L, Mohana-Borges A, Trudell D, Resnick D. Plantar calcaneal enthesophytes: new observations regarding sites of origin based on radiographic, MR imaging, anatomic, and paleopathologic analysis. Skeletal Radiol. 2003;32:13–21. doi: 10.1007/s00256-002-0585-x. [DOI] [PubMed] [Google Scholar]
- Abu-Hijleh MF, Harris PF. Deep fascia on the dorsum of the ankle and foot: extensor retinacula revisited. Clin Anat. 2007;20:186–195. doi: 10.1002/ca.20298. [DOI] [PubMed] [Google Scholar]
- Athwal GS, Steinmann SP, Rispoli DM. The distal biceps tendon: footprint and relevant clinical anatomy. J Hand Surg [Am] 2007;32:1225–1229. doi: 10.1016/j.jhsa.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Augoff K, Kula J, Gosk J, Rutowski R. Epidermal growth factor in Dupuytren's disease. Plast Reconstr Surg. 2005;115:128–133. [PubMed] [Google Scholar]
- Augoff K, Ratajczak K, Gosk J, Tabola R, Rutowski R. Gelatinase A activity in Dupuytren's disease. J Hand Surg [Am] 2006;31:1635–1639. doi: 10.1016/j.jhsa.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: nonoperative treatment of Dupuytren's disease. J Hand Surg [Am] 2002;27:788–798. doi: 10.1053/jhsu.2002.35299. [DOI] [PubMed] [Google Scholar]
- Barbagallo M, Novo S, Licata G, Resnick LM. Diabetes, hypertension and atherosclerosis: pathophysiological role of intracellular ions. Int Angiol. 1993;12:365–370. [PubMed] [Google Scholar]
- Barker PJ, Briggs CA, Bogeski G. Tensile transmission across the lumbar fasciae in unembalmed cadavers: effects of tension to various muscular attachments. Spine. 2004;29:129–138. doi: 10.1097/01.BRS.0000107005.62513.32. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Urquhart DM, Story IH, Fahrer M, Briggs CA. The middle layer of lumbar fascia and attachments to lumbar transverse processes: implications for segmental control and fracture. Eur Spine J. 2007;16:2232–2237. doi: 10.1007/s00586-007-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SJ. Biomechanical problems of the lower limb – the key to overuse injury? In: Maffulli N, Chan KM, MacDonald R, Malina RM, Parker AW, editors. Sports Medicine for Specific Ages and Abilities. Edinburgh: Churchill Livingstone; 2001. pp. 425–436. [Google Scholar]
- Bednar DA, Orr FW, Simon GT. Observations on the pathomorphology of the thoracolumbar fascia in chronic mechanical back pain. A microscopic study. Spine. 1995;20:1161–1164. doi: 10.1097/00007632-199505150-00010. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Qin S, Ralphs JR. Fibrocartilage associated with human tendons and their pulleys. J Anat. 1995;187:625–633. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Theobald P, Suzuki D, Toumi H. The anatomy of the Achilles tendon. In. In: Maffulli N, Almekinders L, editors. The Achilles tendon. London: Springer; 2007. pp. 5–16. [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez K, Knudson MM, Morabito D, Kessel O. Fasciotomy, chronic venous insufficiency, and the calf muscle pump. Arch Surg. 1998;133:1356–1361. doi: 10.1001/archsurg.133.12.1356. [DOI] [PubMed] [Google Scholar]
- Berndt A, Kosmehl H, Katenkamp D, Tauchmann V. Appearance of the myofibroblastic phenotype in Dupuytren's disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix. Pathobiology. 1994;62:55–58. doi: 10.1159/000163879. [DOI] [PubMed] [Google Scholar]
- Bisson MA, McGrouther DA, Mudera V, Grobbelaar AO. The different characteristics of Dupuytren's disease fibroblasts derived from either nodule or cord: expression of alpha-smooth muscle actin and the response to stimulation by TGF-beta1. J Hand Surg [Br] 2003;28:351–356. doi: 10.1016/s0266-7681(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Bisson MA, Mudera V, McGrouther DA, Grobbelaar AO. The contractile properties and responses to tensional loading of Dupuytren's disease-derived fibroblasts are altered: a cause of the contracture? Plast Reconstr Surg. 2004;113:611–621. doi: 10.1097/01.PRS.0000101527.76293.F1. [DOI] [PubMed] [Google Scholar]
- Bloom W, Fawcett DW. A Textbook of Histology. Philadelphia: W.B. Saunders; 1975. [Google Scholar]
- Bojsen-Moller F, Flagstad KE. Plantar aponeurosis and internal architecture of the ball of the foot. J Anat. 1976;121:599–611. [PMC free article] [PubMed] [Google Scholar]
- Bolgla LA, Malone TR. Plantar fasciitis and the windlass mechanism: a biomechanical link to clinical practice. J Athl Train. 2004;39:77–82. [PMC free article] [PubMed] [Google Scholar]
- Botte MJ. Muscle anatomy. In. In: Doyle JR, Botte MJ, editors. Surgical Anatomy of the Hand and Upper Extremity. Philadelphia: Lippincott, Williams & Wilkins; 2003. pp. 92–184. [Google Scholar]
- Bouche RT, Johnson CH. Medial tibial stress syndrome (tibial fasciitis): a proposed pathomechanical model involving fascial traction. J Am Podiatr Med Assoc. 2007;97:31–36. doi: 10.7547/0970031. [DOI] [PubMed] [Google Scholar]
- Bouffard NA, Cutroneo KR, Badger GJ, White SL, Buttolph TR, Ehrlich HP, Stevens-Tuttle D, Langevin HM. Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models. J Cell Physiol. 2008;214:389–395. doi: 10.1002/jcp.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne RB, Rorabeck CH. Compartment syndromes of the lower leg. Clin Orthop Relat Res. 1989;240:97–104. [PubMed] [Google Scholar]
- Brickley-Parsons D, Glimcher MJ, Smith RJ, Albin R, Adams JP. Biochemical changes in the collagen of the palmar fascia in patients with Dupuytren's disease. J Bone Joint Surg [Am] 1981;63:787–797. [PubMed] [Google Scholar]
- Briggs CA, Elliott BG. Lateral epicondylitis. A review of structures associated with tennis elbow. Anat Clin. 1985;7:149–153. doi: 10.1007/BF01654635. [DOI] [PubMed] [Google Scholar]
- Caggiati A. Fascial relationships of the long saphenous vein. Circulation. 1999;100:2547–2549. doi: 10.1161/01.cir.100.25.2547. [DOI] [PubMed] [Google Scholar]
- Caggiati A. Fascial relations and structure of the tributaries of the saphenous veins. Surg Radiol Anat. 2000;22:191–196. doi: 10.1007/s00276-000-0191-3. [DOI] [PubMed] [Google Scholar]
- Caughell KA, McFarlane RM, McGrouther DA, Martin AH. Developmental anatomy of the palmar aponeurosis and its relationship to the palmaris longus tendon. J Hand Surg [Am] 1988;13:485–493. doi: 10.1016/s0363-5023(88)80083-2. [DOI] [PubMed] [Google Scholar]
- Chang L, Blair WF. The origin and innervation of the adductor pollicis muscle. J Anat. 1985;140:381–388. [PMC free article] [PubMed] [Google Scholar]
- Cheung JT, Zhang M, An KN. Effects of plantar fascia stiffness on the biomechanical responses of the ankle-foot complex. Clin Biomech. 2004;19:839–846. doi: 10.1016/j.clinbiomech.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Clay RC. Dupuytren's contracture: fibroma of the palmar fascia. Ann Surg. 1944;120:224–231. doi: 10.1097/00000658-194408000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova A, Tripoli M, Corradino B, Napoli P, Moschella F. Dupuytren's contracture: an update of biomolecular aspects and therapeutic perspectives. J Hand Surg [Br] 2005;30:557–562. doi: 10.1016/j.jhsb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Craig ME, Duffin AC, Gallego PH, et al. Plantar fascia thickness, a measure of tissue glycation, predicts the development of complications in adolescents with type 1 diabetes. Diabetes Care. 2008;31:1201–1206. doi: 10.2337/dc07-2168. [DOI] [PubMed] [Google Scholar]
- Crimmins CA, Jones NF. Stenosing tenosynovitis of the extensor carpi ulnaris. Ann Plast Surg. 1995;35:105–107. doi: 10.1097/00000637-199507000-00020. [DOI] [PubMed] [Google Scholar]
- de Fijter WM, Scheltinga MR, Luiting MG. Minimally invasive fasciotomy in chronic exertional compartment syndrome and fascial hernias of the anterior lower leg: short- and long-term results. Mil Med. 2006;171:399–403. doi: 10.7205/milmed.171.5.399. [DOI] [PubMed] [Google Scholar]
- de-Ary-Pires B, Valdez CF, Shecaira AP, de Ary-Pires R, Ary Pires-Neto M. Cleland's and Grayson's ligaments of the hand: a morphometrical investigation. Clin Anat. 2007;20:68–76. doi: 10.1002/ca.20241. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Badid C, Bochaton-Piallat ML, Gabbiani G. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol. 1997;29:19–30. doi: 10.1016/s1357-2725(96)00117-3. [DOI] [PubMed] [Google Scholar]
- Doyle JR. Hand. In. In: Doyle JR, Botte MJ, editors. Surgical Anatomy of the Hand and Upper Extremity. Philadelphia: Lippincott, Williams & Wilkins; 2003. pp. 532–641. [Google Scholar]
- Duffin AC, Lam A, Kidd R, Chan AK, Donaghue KC. Ultrasonography of plantar soft tissues thickness in young people with diabetes. Diabet Med. 2002;19:1009–1013. doi: 10.1046/j.1464-5491.2002.00850.x. [DOI] [PubMed] [Google Scholar]
- Eames MH, Bain GI, Fogg QA, van Riet RP. Distal biceps tendon anatomy: a cadaveric study. J Bone Joint Surg [Am] 2007;89:1044–1049. doi: 10.2106/JBJS.D.02992. [DOI] [PubMed] [Google Scholar]
- Elliot D. The early history of contracture of the palmar fascia. Part 1: the origin of the disease: the curse of the MacCrimmons: the hand of benediction: Cline's contracture. J Hand Surg [Br] 1988;13:246–253. doi: 10.1016/0266-7681_88_90078-2. [DOI] [PubMed] [Google Scholar]
- Erdemir A, Hamel AJ, Fauth AR, Piazza SJ, Sharkey NA. Dynamic loading of the plantar aponeurosis in walking. J Bone Joint Surg [Am. 2004;86:546–552. doi: 10.2106/00004623-200403000-00013. [DOI] [PubMed] [Google Scholar]
- Evan G, Harrington E, Fanidi A, Land H, Amati B, Bennett M. Integrated control of cell proliferation and cell death by the c-myc oncogene. Philos Trans R Soc Lond B Biol Sci. 1994;345:269–275. doi: 10.1098/rstb.1994.0105. [DOI] [PubMed] [Google Scholar]
- Fairclough J, Hayashi K, Toumi H, et al. The functional anatomy of the iliotibial band during flexion and extension of the knee: implications for understanding iliotibial band syndrome. J Anat. 2006;208:309–316. doi: 10.1111/j.1469-7580.2006.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidzianska A, Jablonska S. Congenital fascial dystrophy: abnormal composition of the fascia. J Am Acad Dermatol. 2000;43:797–802. doi: 10.1067/mjd.2000.107961. [DOI] [PubMed] [Google Scholar]
- Fifield LR. Infections of the Hand. London: H.K. Lewis; 1939. [Google Scholar]
- Findley T, Schleip R. Introduction. In. In: Findley T, Schleip R, editors. Fascia Research. Basic Science and Implications for Conventional and Complementary Health Care. Munich: Elsevier; 2007b. pp. 2–9. [Google Scholar]
- Findley TW, Schleip R. Fascia Research. Munich: Elsevier; 2007a. [Google Scholar]
- Fitzgerald AM, Kirkpatrick JJ, Naylor IL. Dupuytren's disease. The way forward? J Hand Surg [Br] 1999;24:395–399. doi: 10.1054/jhsb.1999.0207. [DOI] [PubMed] [Google Scholar]
- Flanagan JC, Campbell CB. The use of autogenous fascia lata to correct lid and orbital deformities. Trans Am Ophthalmol Soc. 1981;79:227–242. [PMC free article] [PubMed] [Google Scholar]
- Flatt AE. The Vikings and Baron Dupuytren's disease. Proc (Bayl Univ Med Cent) 2001;14:378–384. doi: 10.1080/08998280.2001.11927791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman M, Paakkonen V, Tjaderhane L, et al. The expression of myoglobin and ROR2 protein in Dupuytren's disease. J Surg Res. 2008;146:271–275. doi: 10.1016/j.jss.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Fredericson M, Wolf C. Iliotibial band syndrome in runners: innovations in treatment. Sports Med. 2005;35:451–459. doi: 10.2165/00007256-200535050-00006. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. Evolution and clinical implications of the myofibroblast concept. In. In: Findley TW, Schleip R, editors. Fascia Research. Basic Science and Implications for Conventional and Complementary Health Care. Munich: Urban and Fischer; 2007. pp. 56–60. [Google Scholar]
- Gardner E, Gray DJ, O’Rahilly R. Anatomy. A Regional Study of Human Structure. London: Saunders; 1960. [Google Scholar]
- Gerlach UJ, Lierse W. Functional construction of the superficial and deep fascia system of the lower limb in man. Acta Anat (Basel) 1990;139:11–25. doi: 10.1159/000146973. [DOI] [PubMed] [Google Scholar]
- Gerrish FH. A Textbook of Anatomy. London: Henry Kimpton; 1899. [Google Scholar]
- Giacomozzi C, D’Ambrogi E, Uccioli L, Macellari V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin Biomech (Bristol, Avon) 2005;20:532–539. doi: 10.1016/j.clinbiomech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Grant JCB. A Method of Anatomy. London: Baillière, Tindall and Cox; 1948. [Google Scholar]
- Hagert E, Forsgren S, Ljung BO. Differences in the presence of mechanoreceptors and nerve structures between wrist ligaments may imply differential roles in wrist stabilization. J Orthop Res. 2005;23:757–763. doi: 10.1016/j.orthres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Hagert E, Garcia-Elias M, Forsgren S, Ljung BO. Immunohistochemical analysis of wrist ligament innervation in relation to their structural composition. J Hand Surg [Am] 2007;32:30–36. doi: 10.1016/j.jhsa.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hart MG, Hooper G. Clinical associations of Dupuytren's disease. Postgrad Med J. 2005;81:425–428. doi: 10.1136/pgmj.2004.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haumont T, Gauchard GC, Zabee L, Arnoux JM, Journeau P, Lascombes P. Extensor retinaculum syndrome after distal tibial fractures: anatomical basis. Surg Radiol Anat. 2007;29:303–311. doi: 10.1007/s00276-007-0215-3. [DOI] [PubMed] [Google Scholar]
- Hicks JH. The mechanics of the foot. II. The plantar aponeurosis and the arch. J Anat. 1954;88:25–30. [PMC free article] [PubMed] [Google Scholar]
- Huijing P. Muscular force transmission: a unified, dual or multiple system? A review and some explorative experimental results. Arch Physiol Biochem. 1999;107:292–311. doi: 10.1076/13813455199908107041qft292. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Epimuscular myofascial force transmission between antagonistic and synergistic muscles can explain movement limitation in spastic paresis. J Electromyogr Kinesiol. 2007;17:708–724. doi: 10.1016/j.jelekin.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Baan GC. Extramuscular myofascial force transmission within the rat anterior tibial compartment: proximo-distal differences in muscle force. Acta Physiol Scand. 2001a;173:297–311. doi: 10.1046/j.1365-201X.2001.00911.x. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Baan GC. Myofascial force transmission causes interaction between adjacent muscles and connective tissue: effects of blunt dissection and compartmental fasciotomy on length force characteristics of rat extensor digitorum longus muscle. Arch Physiol Biochem. 2001b;109:97–109. doi: 10.1076/apab.109.2.97.4269. [DOI] [PubMed] [Google Scholar]
- Huijing PA, Baan GC, Rebel GT. Non-myotendinous force transmission in rat extensor digitorum longus muscle. J Exp Biol. 1998;201:683–691. [PubMed] [Google Scholar]
- Huijing PA, Maas H, Baan GC. Compartmental fasciotomy and isolating a muscle from neighboring muscles interfere with myofascial force transmission within the rat anterior crural compartment. J Morphol. 2003;256:306–321. doi: 10.1002/jmor.10097. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Wu J, Yandow JA, Langevin HM. Subcutaneous tissue mechanical behavior is linear and viscoelastic under uniaxial tension. Connect Tissue Res. 2003;44:208–217. [PubMed] [Google Scholar]
- Jarvholm U, Palmerud G, Styf J, Herberts P, Kadefors R. Intramuscular pressure in the supraspinatus muscle. J Orthop Res. 1988;6:230–238. doi: 10.1002/jor.1100060210. [DOI] [PubMed] [Google Scholar]
- Jemec B, Grobbelaar AO, Wilson GD, Smith PJ, Sanders R, McGrouther DA. Is Dupuytren's disease caused by an imbalance between proliferation and cell death? J Hand Surg [Br] 1999;24:511–514. doi: 10.1054/jhsb.1999.0251. [DOI] [PubMed] [Google Scholar]
- Johnston P, Chojnowski AJ, Davidson RK, Riley GP, Donell ST, Clark IM. A complete expression profile of matrix-degrading metalloproteinases in Dupuytren's disease. J Hand Surg [Am] 2007;32:343–351. doi: 10.1016/j.jhsa.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Johnston TB, Whillis J. Gray's Anatomy – Descriptive and Applied. London: Longmans, Green and Co; 1950. [Google Scholar]
- Joseph DM. Reconstruction of the anterior cruciate ligament using the bone-block iliotibial-tract transfer. J Bone Joint Surg Am. 1988;70:790–791. [PubMed] [Google Scholar]
- Kalin PJ, Hirsch BE. The origins and function of the interosseous muscles of the foot. J Anat. 1987;152:83–91. [PMC free article] [PubMed] [Google Scholar]
- Kane D, Greaney T, Shanahan M, et al. The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology. 2001;40:1002–1028. doi: 10.1093/rheumatology/40.9.1002. [DOI] [PubMed] [Google Scholar]
- Kawamata S, Ozawa J, Hashimoto M, Kurose T, Shinohara H. Structure of the rat subcutaneous connective tissue in relation to its sliding mechanism. Arch Histol Cytol. 2003;66:273–279. doi: 10.1679/aohc.66.273. [DOI] [PubMed] [Google Scholar]
- Kozma EM, Olczyk K, Glowacki A, Bobinski R. An accumulation of proteoglycans in scarred fascia. Mol Cell Biochem. 2000;203:103–112. doi: 10.1023/a:1007012321333. [DOI] [PubMed] [Google Scholar]
- Kozma EM, Olczyk K, Wisowski G, Glowacki A, Bobinski R. Alterations in the extracellular matrix proteoglycan profile in Dupuytren's contracture affect the palmar fascia. J Biochem. 2005;137:463–476. doi: 10.1093/jb/mvi054. [DOI] [PubMed] [Google Scholar]
- Kozma EM, Glowacki A, Olczyk K, Ciecierska M. Dermatan sulfate remodeling associated with advanced Dupuytren's contracture. Acta Biochim Pol. 2007;54:821–830. [PubMed] [Google Scholar]
- Kumai T, Benjamin M. Heel spur formation and the subcalcaneal enthesis of the plantar fascia. J Rheumatol. 2002;29:1957–1964. [PubMed] [Google Scholar]
- Kumai T, Benjamin M. The histological structure of the malleolar groove of the fibula in man: its direct bearing on the displacement of peroneal tendons and their surgical repair. J Anat. 2003;203:257–262. doi: 10.1046/j.1469-7580.2003.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin HM. Connective tissue: a body-wide signaling network? Med Hypotheses. 2006;66:1074–1077. doi: 10.1016/j.mehy.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec. 2002;269:257–265. doi: 10.1002/ar.10185. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Churchill DL, Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. Faseb J. 2001;15:2275–2282. doi: 10.1096/fj.01-0015hyp. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Churchill DL, Wu J, et al. Evidence of connective tissue involvement in acupuncture. FASEB J. 2002;16:872–874. doi: 10.1096/fj.01-0925fje. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Cornbrooks CJ, Taatjes DJ. Fibroblasts form a body-wide cellular network. Histochem Cell Biol. 2004;122:7–15. doi: 10.1007/s00418-004-0667-z. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol. 2005;288:C747–756. doi: 10.1152/ajpcell.00420.2004. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Bouffard NA, Badger GJ, Churchill DL, Howe AK. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006a;207:767–774. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Storch KN, Cipolla MJ, White SL, Buttolph TR, Taatjes DJ. Fibroblast spreading induced by connective tissue stretch involves intracellular redistribution of alpha- and beta-actin. Histochem Cell Biol. 2006b;125:487–495. doi: 10.1007/s00418-005-0138-1. [DOI] [PubMed] [Google Scholar]
- Langevin HM, Bouffard NA, Churchill DL, Badger GJ. Connective tissue fibroblast response to acupuncture: dose-dependent effect of bidirectional needle rotation. J Altern Complement Med. 2007;13:355–360. doi: 10.1089/acm.2007.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LC, Zhang AY, Chong AK, Pham H, Longaker MT, Chang J. Expression of a novel gene, MafB, in Dupuytren's disease. J Hand Surg [Am] 2006;31:211–218. doi: 10.1016/j.jhsa.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. The Tissues of the Body. An Introduction to the Study of Anatomy. Oxford: Clarendon Press; 1945. [Google Scholar]
- Lindsay M. Fascia. Clinical Applications for Health and Human Performance. New York: Delmar Cengage Learning; 2008. [Google Scholar]
- Lubahn JD, Pollard M, Cooney T. Immunohistochemical evidence of nerve growth factor in Dupuytren's diseased palmar fascia. J Hand Surg [Am] 2007;32:337–342. doi: 10.1016/j.jhsa.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Lytle WJ. Inguinal anatomy. J Anat. 1979;128:581–594. [PMC free article] [PubMed] [Google Scholar]
- Mardel S, Underwood M. Adductor pollicis. The missing interosseous. Surg Radiol Anat. 1991;13:49–52. doi: 10.1007/BF01623142. [DOI] [PubMed] [Google Scholar]
- Meissner MH, Moneta G, Burnand K, et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(Suppl. S):4S–24S. doi: 10.1016/j.jvs.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Melling M, Reihsner R, Pfeiler W, et al. Comparison of palmar aponeuroses from individuals with diabetes mellitus and Dupuytren's contracture. Anat Rec. 1999;255:401–406. doi: 10.1002/(SICI)1097-0185(19990801)255:4<401::AID-AR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Milz S, Rufai A, Buettner A, Putz R, Ralphs JR, Benjamin M. Three-dimensional reconstructions of the Achilles tendon insertion in man. J Anat. 2002;200:145–152. doi: 10.1046/j.0021-8782.2001.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar TF, Rendeki S, Lukacs L, Horvath OP. Improvement of air tightness of stapled lung parenchyma using fascia lata. Interact Cardiovasc Thorac Surg. 2003;2:503–505. doi: 10.1016/S1569-9293(03)00126-9. [DOI] [PubMed] [Google Scholar]
- Mubarak S, Owen CA. Compartmental syndrome and its relation to the crush syndrome: a spectrum of disease. A review of 11 cases of prolonged limb compression. Clin Orthop Relat Res. 1975;113:81–89. doi: 10.1097/00003086-197511000-00012. [DOI] [PubMed] [Google Scholar]
- Murrell GA, Francis MJ, Bromley L. Free radicals and Dupuytren's contracture. Br Med J (Clin Res Ed) 1987;295:1373–1375. doi: 10.1136/bmj.295.6610.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell GA, Francis MJ, Bromley L. The collagen changes of Dupuytren's contracture. J Hand Surg [Br] 1991;16:263–266. doi: 10.1016/0266-7681(91)90050-x. [DOI] [PubMed] [Google Scholar]
- Myers TW. Anatomy Trains: Myofascial Meridians for Manual and Movement Therapists. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Nash LG, Phillips MN, Nicholson H, Barnett R, Zhang M. Skin ligaments: regional distribution and variation in morphology. Clin Anat. 2004;17:287–293. doi: 10.1002/ca.10203. [DOI] [PubMed] [Google Scholar]
- Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008;16:338–346. doi: 10.5435/00124635-200806000-00006. [DOI] [PubMed] [Google Scholar]
- Niechajev I. Calf augmentation and restoration. Plast Reconstr Surg. 2005;116:295–305. doi: 10.1097/01.prs.0000170050.86464.8e. [DOI] [PubMed] [Google Scholar]
- Numkarunarunrote N, Malik A, Aguiar RO, Trudell DJ, Resnick D. Retinacula of the foot and ankle: MRI with anatomic correlation in cadavers. Am J Roentgenol. 2007;188:W348–W354. doi: 10.2214/AJR.05.1066. [DOI] [PubMed] [Google Scholar]
- Pagnotta A, Specchia N, Greco F. Androgen receptors in Dupuytren's contracture. J Orthop Res. 2002;20:163–168. doi: 10.1016/S0736-0266(01)00072-9. [DOI] [PubMed] [Google Scholar]
- Palmborg G. Stenosing tenosynovitis. Ann Rheum Dis. 1952;11:193–195. doi: 10.1136/ard.11.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri G, Panu R, Asole A, Farina V, Sanna L, Gabbi C. Macroscopic organization and sensitive innervation of the tendinous intersection and the lacertus fibrosus of the biceps brachii muscle in the ass and horse. Arch Anat Histol Embryol. 1986;69:73–82. [PubMed] [Google Scholar]
- Papadopoulos NJ, Sherif MF, Albert EN. A fascial canal for the great saphenous vein: gross and microanatomical observations. J Anat. 1981;132:321–329. [PMC free article] [PubMed] [Google Scholar]
- Pascual Huerta J, Alarcon Garcia JM. Effect of gender, age and anthropometric variables on plantar fascia thickness at different locations in asymptomatic subjects. Eur J Radiol. 2007;62:449–453. doi: 10.1016/j.ejrad.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Qian A, Meals RA, Rajfer J, Gonzalez-Cadavid NF. Comparison of gene expression profiles between Peyronie's disease and Dupuytren's contracture. Urology. 2004;64:399–404. doi: 10.1016/j.urology.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21:67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Rehman S, Salway F, Stanley JK, Ollier WE, Day P, Bayat A. Molecular phenotypic descriptors of Dupuytren's disease defined using informatics analysis of the transcriptome. J Hand Surg [Am] 2008;33:359–372. doi: 10.1016/j.jhsa.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ris HB, Furrer M, Stronsky S, Walpoth B, Nachbur B. Four-compartment fasciotomy and venous calf-pump function: long-term results. Surgery. 1993;113:55–58. [PubMed] [Google Scholar]
- Salathe EP, Jr, Arangio GA, Salathe EP. A biomechanical model of the foot. J Biomech. 1986;19:989–1001. doi: 10.1016/0021-9290(86)90116-8. [DOI] [PubMed] [Google Scholar]
- Sanchis-Alfonso V, Rosello-Sastre E. Immunohistochemical analysis for neural markers of the lateral retinaculum in patients with isolated symptomatic patellofemoral malalignment. A neuroanatomic basis for anterior knee pain in the active young patient. Am J Sports Med. 2000;28:725–731. doi: 10.1177/03635465000280051801. [DOI] [PubMed] [Google Scholar]
- Saragaglia D, Fontanel F, Montbarbon E, Tourne Y, Picard F, Charbel A. Reconstruction of the lateral ankle ligaments using an inferior extensor retinaculum flap. Foot Ankle Int. 1997;18:723–728. doi: 10.1177/107110079701801108. [DOI] [PubMed] [Google Scholar]
- Saraph V, Zwick EB, Uitz C, Linhart W, Steinwender G. The Baumann procedure for fixed contracture of the gastrosoleus in cerebral palsy. Evaluation of function of the ankle after multilevel surgery. J Bone Joint Surg [Br] 2000;82:535–540. doi: 10.1302/0301-620x.82b4.9850. [DOI] [PubMed] [Google Scholar]
- Sarrafian SK. Functional characteristics of the foot and plantar aponeurosis under tibiotalar loading. Foot Ankle. 1987;8:4–18. doi: 10.1177/107110078700800103. [DOI] [PubMed] [Google Scholar]
- Satish L, Laframboise WA, O’Gorman DB, et al. Identification of differentially expressed genes in fibroblasts derived from patients with Dupuytren's Contracture. BMC Med Genomics. 2008;1:1–10. doi: 10.1186/1755-8794-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Schleip R, Kingler W, Lehmann-Horn F. Fascia is able to contract in a smooth muscle-like manner and thereby influence musculoskeletal mechanics. In. In: Findley TW, Schleip R, editors. Fascia Research. Basic Science and Implications for Conventional and Complementary Health Care. Munich: Urban and Fischer; 2007. pp. 76–77. [Google Scholar]
- Shah AC, Srivastava HC. Fascial canal for the small saphenous vein. J Anat. 1966;100:411–413. [PMC free article] [PubMed] [Google Scholar]
- Snow SW, Bohne WH, DiCarlo E, Chang VK. Anatomy of the Achilles tendon and plantar fascia in relation to the calcaneus in various age groups. Foot Ankle Int. 1995;16:418–421. doi: 10.1177/107110079501600707. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray's Anatomy: the Anatomical Basis of Clinical Practice. Edinburgh: Churchill Livingstone; 2004. [Google Scholar]
- Stecco C, Gagey O, Belloni A, et al. Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie. 2007a;91:38–43. doi: 10.1016/j.morpho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Stecco C, Gagey O, Macchi V, et al. Tendinous muscular insertions onto the deep fascia of the upper limb. First part: anatomical study. Morphologie. 2007b;91:29–37. doi: 10.1016/j.morpho.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Stecco C, Porzionato A, Macchi V, et al. The expansions of the pectoral girdle muscles onto the brachial fascia: morphological aspects and spatial disposition. Cells Tissues Organs. 2008. EPub ahead of Print. [DOI] [PubMed]
- Stilwell DL., Jr Regional variations in the innervation of deep fasciae and aponeuroses. Anat Rec. 1957;127:635–653. doi: 10.1002/ar.1091270402. [DOI] [PubMed] [Google Scholar]
- Taki K, Yamazaki S, Majima T, Ohura H, Minami A. Bilateral stenosing tenosynovitis of the peroneus longus tendon associated with hypertrophied peroneal tubercle in a junior soccer player: a case report. Foot Ankle Int. 2007;28:129–132. doi: 10.3113/FAI.2007.0022. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Ito T. Histochemical demonstration of adrenergic fibers in the fascia periosteum and retinaculum. Clin Orthop Relat Res. 1977;126:276–281. [PubMed] [Google Scholar]
- Terek RM, Jiranek WA, Goldberg MJ, Wolfe HJ, Alman BA. The expression of platelet-derived growth-factor gene in Dupuytren contracture. J Bone Joint Surg [Am] 1995;77:1–9. doi: 10.2106/00004623-199501000-00001. [DOI] [PubMed] [Google Scholar]
- Toumi H, Higashiyama I, Suzuki D, et al. Regional variations in human patellar trabecular architecture and the structure of the proximal patellar tendon enthesis. J Anat. 2006;208:47–57. doi: 10.1111/j.1469-7580.2006.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren's contracture unfolded. Br Med J. 2006;332:397–400. doi: 10.1136/bmj.332.7538.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt C, Shrier I, Vandal A, et al. Acute compartment syndrome: How long before muscle necrosis occurs? Can J Emergency Med Care. 2004;6:147–154. doi: 10.1017/s1481803500006837. [DOI] [PubMed] [Google Scholar]
- Vieira EL, Vieira EA, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23:269–274. doi: 10.1016/j.arthro.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Stoeckart R. The role of the pelvic girdle in coupling the spine and the legs: a clinical-anatomical persepctive on pelvic stability. In. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, Stability and Lumbopelvic Pain. Edinburgh: Churchill Livingstone; 2007. pp. 113–137. [Google Scholar]
- Vleeming A, Mooney V, Stoeckart R. Movement, Stability & Lumbopelvic Pain. Integration of Research and Therapy. Edinburgh: Churchill Livingstone Elsevier; 2007. [Google Scholar]
- Vleeming A, Pool-Goudzwaard AL, Stoeckart R, van Wingerden JP, Snijders CJ. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine. 1995;20:753–758. [PubMed] [Google Scholar]
- Wang HQ, Li MQ, Wu ZX, Zhao L. The deep fascia in response to leg lengthening with particular reference to the tension-stress principle. J Pediatr Orthop. 2007;27:41–45. doi: 10.1097/01.bpo.0000242439.88811.15. [DOI] [PubMed] [Google Scholar]
- Warren BL. Plantar fasciitis in runners. Treatment and prevention. Sports Med. 1990;10:338–345. doi: 10.2165/00007256-199010050-00004. [DOI] [PubMed] [Google Scholar]
- Willard FH. The muscular, ligamentous, and neural structure of the lumbosacrum and its relationship to low back pain. In: Vleeming A, Mooney V, Stoeckart R, editors. Movement, stability & lumbopelvic pain. Integration of Research and Therapy. Edinburgh: Churchill Livingstone; 2007. pp. 5–45. [Google Scholar]
- Wood Jones F. Structure and Function as Seen in the Foot. London: Baillière, Tindall and Cox; 1944. [Google Scholar]
- Yahia L, Rhalmi S, Newman N, Isler M. Sensory innervation of human thoracolumbar fascia. An immunohistochemical study. Acta Orthop Scand. 1992;63:195–197. doi: 10.3109/17453679209154822. [DOI] [PubMed] [Google Scholar]