Abstract
Increased transforming growth factor-β (TGF-β) expression and epidermal growth factor receptor (EGFR) amplification accompany the emergence of highly aggressive human carcinomas. Cooperative signaling between these two growth factor/receptor systems promotes cell migration and synthesis of stromal remodeling factors (i.e., proteases, protease inhibitors) that, in turn, regulate tumor invasion, neo-angiogenesis and inflammation. ranscript profiling of transformed human cells revealed that genes encoding wound healing, matrix remodeling and cell cycle proteins (i.e., the “tissue repair” transcriptome) are significantly up-regulated early after growth factor stimulation. The major inhibitor of plasmin generation, plasminogen activator inhibitor-1 (PAI-1), is among the most highly induced transcripts during the phenotypic transition initiated by TGF-β maximal expression requires EGFR signaling. PAI-1 induction occurs early in the progression of incipient epidermal squamous cell carcinoma (SCC) and is a significant indicator of poor prognosis in epithelial malignancies. Mouse modeling and molecular genetic analysis of complex systems indicates that PAI-1 regulates the temporal/spatial control of pericellular proteolysis, promotes epithelial plasticity, inhibits capillary regression and facilitates stromal invasion. Defining TGF-β1-initiated signaling events that cooperate with an activated EGFR to impact the protease-protease inhibitor balance in the tumor microenvironment is critical to the development of novel therapies for the clinical management of human cancers.
1. Introduction
Transition of a normal epithelial cell to an early malignant phenotype often involves mutation of the p53 and p21ras genes and progressive increases in autocrine TGF-β1 expression [1–10]. Elevated TGF-β1 production, in fact, typifies advanced pathologies in both mouse and human SCC [8, 10, 11]. Despite relatively high concentrations of TGF-β in the immediate tumor microenvironment, some malignant epithelial cells become refractory to TGF-β1-initiated proliferative arrest likely due to reductions in either TGF-βRII and/or SMAD4 levels as well as the now recognized p21ras-dependent antagonism of TGF-β1-mediated growth inhibition/apoptosis [10–13]. In certain epithelial malignancies, moreover, resistance to TGF-β1-mediated growth suppression is often coupled with EGFR amplification or dysregulated EGFR signaling, particularly during the later stages of tumor development [14–19]. The associated reprogramming of gene expression initiates and perpetuates TGF-β1-induced cellular “plasticity” (usually referred to as epithelial-to-mesenchymal transition or EMT) which facilites tumor invasion and metastasis [8, 20–25].
Microarray of the EMT transcriptome in several clinically relevant model systems has provided insights into the specific repertoire of “plasticity” genes. Plasminogen activator inhibitor type-1 (PAI-1; SERPINE1), the major physiologic regulator of the pericellular plasmin-generating cascade, is a prominent member of the subset of TGF-β1-induced, EMT-associated genes in human malignant keratinocytes [21, 26, 27]. In epithelial cells undergoing a mesenchymal-like conversion in response to the E-cadherin transcriptional repressors Snail, Slug or E47, PAI-1 upregulation appears to be an essential characteristic of the plastic phenotype [28]. The association between PAI-1 expression and tumor “progression” has significant clinical implications. Current data suggest that this serine protease inhibitor maintains an angiogenic “scaffold,” stabilizes nascent capillary vessel structure, and facilitates tumor cell invasion through precise control of the peritumor proteolytic microenvironment [29–31]. Increased PAI-1 expression is, in fact, an early event in the progression of epidermal SCC, often localizing to tumor cells and myofibroblasts at the invasive front [24, 32–36] and, most importantly, is a biomarker with significant prognostic value [37]. Indeed, two of the best-validated prognostic indicators (level of evidence [LOE] = 1) in breast carcinoma are the serine protease urokinase plasminogen activator (uPA) and its endogenous inhibitor PAI-1 [38]. Certain PAI-1 tumor thresholds predict both poor prognosis and reduced disease-free survival in patients with breast, lung, ovarian, and oral SCC [29, 38] with the expression amplitude frequently associated with the 4G polymorphism at the PE1 E box motif in the PAI-1 promoter [37]. Identification of PAI-1 in tumor-proximal stromal myofibroblasts, furthermore, implies a more global involvement in modulating cellular invasive potential [34–36], perhaps as a matricellular effector of epithelial motility [39], invasion and the associated angiogenic response [24, 30, 31, 40, 41].
Recent findings clearly implicate EGFR/MEK/rho-ROCK signaling as required for PAI-1 expression in TGF-β1-stimulated cells. E box motifs (CACGTG) in the PAI-1 PE1/PE2 promoter regions, moreover, are platforms for a MAP kinase-directed upstream stimulatory factor (USF) subtype switch (USF-1 → USF-2) in response to growth factor addition [42–44] suggesting that the EGFR/MEK/rho-ROCK axis impacts PAI-1 expression through USF-dependent transcriptional controls. The continued definition of TGF-β1-activated pathways that influence expression of this important target gene may lead to therapeutically useful approaches to manage human cancer. This paper, therefore, reviews data regarding the rapid transactivation of the EGFR in TGF-β1-stimulated cells suggesting cooperativity between TGF-β1 and EGFR → MAP kinase pathways in PAI-1 gene expression.
2. EGFR Signaling Is Required for TGF-β1-Induced PAI-1 Expression
TGF-β1 mobilizes both SMAD-dependent and -independent signaling [45] although the individual roles of specific cross-pathway events on PAI-1 expression are not well understood. Several recent studies demonstrated that TGF-β1-induced rapid EGFR transactivation highlighting cooperativity between TGF-β1 and EGFR signaling events in vascular, epithelial, and endothelial cells. Indeed, PAI-1 induction in response to TGF-β1 is significantly attenuated by an EGFR pharmacologic inhibitor (AG1478), by molecular targeting of EGFR activity (i.e., by adenoviral delivery of EGFRY721A kinase-dead constructs) and, more importantly, by genetic ablation of the EGFR in mouse fibroblasts [43, 46, 47] with PAI-1 “rescue” evident in EGFR−/− cells engineered to express an EGFR construct. TGF-β1 treatment, moreover, specifically increased EGFR phosphorylation at the Y845 src-target residue; either mutation of this residue (EGFRY845F) or transfection of a DN pp60c-src construct completely blocked TGF-β1-dependent PAI-1 induction. Similarly, TGF-β1 failed to stimulate PAI-1 expression in cultured mouse embryonic fibroblasts (MEFs) genetically deficient in three src family kinases (i.e., c-src, c-yes-, c-fyn- null fibroblasts; SYF−/−/−) compared to identically stimulated wild-type SYF+/+/+ cells. PAI-1 synthesis was restored in SYF−/−/− MEFs engineered to re-express a wild-type pp60c-src [47] providing proof-of-principle for involvement of this particular src kinase in the inductive response. The highly specific src family kinase inhibitor SU6656, morevover, effectively blocked TGF-β1-initiated increases in both pp60c-src and EGFR phosphorylation as well as pp60c-src and EGFR activation (at the Y416 and Y845 residues, resp.). pEGFRY845 phosphorylation in response to TGF-β1 was evident, furthermore, in wild type but not SYF−/−/− fibroblasts. The TGF-β1-dependent formation of EGFR/pp60c-src complexes [46] and EGFRY845 phosphorylation and the inhibition of TGF-β1- (but not PDGF-) induced PAI-1 expression by the EGFRY845F mutant as well as a DN-Src construct [47] collectively implicate EGFR/pp60c-src interactions and, in particular, the EGFRY845 pp60c-src site in the kinase domain activation loop in signal propagation [48]. The time course of TGF-β1-initiated SMAD2/3 activation, in contrast, was similar in both wild type and SYF−/−/− MEFs confirming that, in the context of either EGFR or src family kinase deficiency, SMAD2/3 activation occurs but is not sufficient for PAI-1 induction. TGF-β1 stimulated ERK1/2 phosphorylation in EGFR+/+ but not in EGFR−/− cells consistent with prior observations that TGF-β1-dependent ERK1/2 activation is downstream of EGFR signaling [43, 46]. EGFR−/− MEFs, however, are fully capable of responding to exogenous TGF-β1 as SMAD2 was effectively activated (i.e., phosphorylated) in both wild type and EGFR−/− fibroblasts [47].
3. The PAI-1 Gene Is a Model of TGF-β1-Initiated Cooperative EGFR Signaling
While TGF-β1 receptors phosphorylate SMADs downstream of growth factor engagement, it appears that the Rho/ROCK pathway modulates the duration of SMAD2/3 phosphorylation [47]. How Rho/ROCK impact TGF-β1-initiated SMAD2/3 activation and subcellular localization [49, 50] is not known but this pathway may function to provide efficient SMAD2/3 activation for extended periods. Alternatively, Rho/ROCK signaling may be required to inhibit negative regulation of SMAD2/3 function by inactivation of SMAD phosphatases sustaining, thereby, SMAD2/3 transcriptional actions (e.g., [51, 52]). TGF-β1-induced SMAD2 phosphorylation is not altered by EGFR blockade either pharmacologically (with AG1478), molecularly (by expression of EGFRY721A or EGFRY845F), or by the genetic absence of EGFR [47]. Clearly, while SMAD2/3 activation may be necessary it is not sufficient for TGF-β1-stimulated PAI-1 expression in the absence of EGFR signaling.
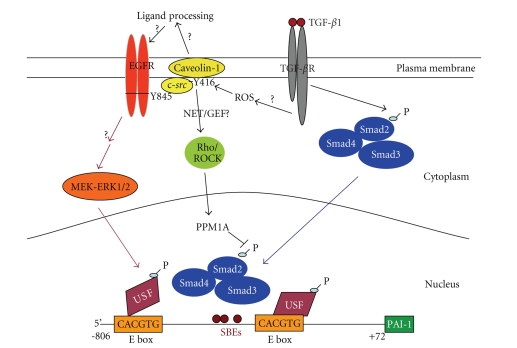
It is apparent, therefore, that TGF-β1 stimulates PAI-1 expression through two distinct but cooperating pathways that involve EGFR/pp60c-src → MEK/ERK signaling and EGFR-independent, but Rho/ROCK-modulated, TGF-βR-directed SMAD and ERK activation [47]. Interference with any of the specific individual elements in this dual cascade (EGFR/pp60c-src/MEK or Rho/p160ROCK) markedly reduced, and in some cases, completely inhibited PAI-1 expression. One model consistent with the available data [24, 40, 43, 44, 47, 53] suggests that SMADs and specific MAP kinase-targeted bHLH-LZ factors (such as USF) occupy their separate binding motifs at the critical TGF-β1-responsive PE2 region E box in the PAI-1 promoter (Figure 1). Dominant-negative interference with USF DNA-binding ability significantly reduced TGF-β1-mediated PAI-1 transcription [43, 44, 53]. Since MAP kinases regulate the DNA-binding and transcriptional activites of USF [40, 43], TGF-βR signaling through SMAD2/3 may actually cooperate with EGFR/MEK-ERK-activated USF to attain high level PAI-1 expression [40, 47]. SMADs are known to interact with E box-binding HLH-LZ factors such as TFE3 at the PE2 site in the PAI-1 geneat least in one cell type [54]. There is evidence, in fact, to suggest that such interacting complexes impact PAI-1 gene control since USF occupancy of the PAI-1 PE2 region E box site, which is juxtaposed to three SMAD-recognition elements, modulates transcription in response to TGF-β1 or serum [40, 43, 44, 53]. Current data indicate that recruitment of this multicomponent complex likely requires participation of the TGF-β1-stimulated EGFR → MEK/ERK and Rho/ROCK pathways for the optimal response of the PAI-1 gene to TGF-β1.
Figure 1.
Model for TGF-β1-induced PAI-1 expression. TGF-β1 activates two distinct signaling pathways to initiate PAI-1 transcription. Rho/ROCK are required to maintain SMAD phosphorylation and ERK activation (through to be defined mechanisms) while the pp60c-src-activated EGFR (at the Y845 site) signals to MEK-ERK initiating ERK/USF interactions resulting in USF phosphorylation and a subtype (USF-1 → USF-2) switch (e.g., [44]) at the PAI-1 PE1/PE2 E box sites. Collectively, these promoter-level events stimulate high level PAI-1 expression in response to TGF-βR occupancy. The actual mechanism underlying EGFR activation in response to TGF-β1 may involve direct recruitment of src kinases to the EGFR or the processing/release of a membrane-anchored EGFR ligand (e.g., HB-EGF). Events associated with TGF-β1 stimulation of the RhoA/ROCK pathway are similarly unclear. Rho/ROCK may regulate the activity and/or function of the SMAD phosphatase PPM1A impacting, thereby, the duration of SMAD-dependent transcription of target genes such as PAI-1. (modified from [47]).
The mechanism of MAP kinase activation in TGF-β1-stimulated cells is just becoming clear. Upon ligand binding, the TGF-βRII undergoes autophosphorylation on three tyrosines (Y259, Y336, Y424), while Y284 is a target site for src kinases [55]. TGF-βRI is also subject to tyrosine phosphorylation postreceptor accupancy [56]. Such phosphorylated tyrosine residues provide docking sites for recruitment of Grb2/Shc/SOS complexes with subsequent mobilization of the ras-raf-MEK-ERK cascade [46, 47, 55]. Although ERKs are prominently activated in response to TGF-β1 [40, 43], perhaps the JNK and p38 MAP kinase pathways are better characterized targets of TGF-β1-initiated signaling. TGF-β1 rapidly activates JNK through MKK4 and p38 via MKK3/6 perhaps even in a cell type-specific fashion contributing to the mechanistic complexity of pathway cross-talk. Each of these kinase systems, moreover, has been implicated in a cell type-dependency of PAI-1 gene control [40, 43, 55]. Should such pathways prove uniquely or, at least, preferentially utilized in specific cellular lineages, they may provide tumor type-specific targets for intervention therapy.
4. EGFR as a Potential Therapeutic Target for Regulating PAI-1 Expression
Modulation of EGFR/HER1 signaling by specific receptor function (kinase domain) inhibitors or neutralizing antibodies against specific EGFR1 ligands (e.g., HB-EGF antibodies) can be an attractive therapeutic modality (particularly in the context of neoplastic diseases associated with elevated TGF-β1 levels). This strategy would likely impact not only PAI-1 suppression but has the potential to regulate other proinvasive target genes. There is, in fact, increasing evidence that TGF-β1-induced connective tissue growth factor and fibronectin expression similarly involve EGFR/HER1 cooperative pathways (Samarakoon and Higgins, unpublished data). Moreover, PAI-1 repression by EGFR signaling blockade may also suppress tumor angiogenesis consistent with the well-established role of PAI-1 as an inhibitor of endothelial apoptosis and neovessel regression [40]. Combinatorial targeting of PAI-1 function using established small molecule PAI-1 inhibitors and genetic-based PAI-1 expression attenuation [40] coupled with disruption of EGFR signaling (e.g., with cetuximab or erlotinib) may impact, therefore, both cancer invasion and the associated angiogenic response, particularly in the context of a TGF-β1-rich tumor microenvironment.
Acknowledgment
This research is supported by NIH Grant GM57242 to PJH.
References
- 1.Breitkreutz D, Boukamp P, Ryle CM, Stark H-J, Roop DR, Fusenig NE. Epidermal morphogenesis and keratin expression in c-Ha-ras-transfected tumorigenic clones of the human HaCaT cell line. Cancer Research. 1991;51(16):4402–4409. [PubMed] [Google Scholar]
- 2.Dlugosz A, Merlino G, Yuspa SH. Progress in cutaneous cancer research. Journal of Investigative Dermatology Symposium Proceedings. 2002;7(1):17–26. doi: 10.1046/j.1523-1747.2002.19631.x. [DOI] [PubMed] [Google Scholar]
- 3.Boukamp P. UV-induced skin cancer: similarities—variations. Journal der Deutschen Dermatologischen Gesellschaft. 2005;3(7):493–503. doi: 10.1111/j.1610-0387.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- 4.Boukamp P, Peter W, Pascheberg U, et al. Step-wise progression in human skin carcinogenesis in vitro involves mutational inactivation of p53, rasH oncogene activation and additional chromosome loss. Oncogene. 1995;11(5):961–969. [PubMed] [Google Scholar]
- 5.Smoller BR. Squamous cell carcinoma: from precursor lesions to high-risk variants. Modern Pathology. 2006;19(supplement 2):S88–S92. doi: 10.1038/modpathol.3800509. [DOI] [PubMed] [Google Scholar]
- 6.Tsai KY, Tsao H. The genetics of skin cancer. American Journal of Medical Genetics Part C. 2004;131(1):82–92. doi: 10.1002/ajmg.c.30037. [DOI] [PubMed] [Google Scholar]
- 7.Akhurst RJ, Balmain A. Genetic events and the role of TGFβ in epithelial tumour progression. The Journal of Pathology. 1999;187(1):82–90. doi: 10.1002/(SICI)1096-9896(199901)187:1<82::AID-PATH248>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Cui W, Fowlis DJ, Bryson S, et al. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86(4):531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 9.Portella G, Cumming SA, Liddell J, et al. Transforming growth factor β is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth & Differentiation. 1998;9(5):393–404. [PubMed] [Google Scholar]
- 10.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nature Genetics. 2001;29(2):117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 11.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequental elevation of H-ras and Smad2 levels. Nature Cell Biology. 2002;4(7):487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 12.Han G, Lu S-L, Li AG, et al. Distinct mechanisms of TGF-β1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. The Journal of Clinical Investigation. 2005;115(7):1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFfβ/Smad signaling by oncogenic Ras. Genes & Development. 1999;13(7):804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rho O, Beltrán LM, Gimenez-Conti IB, DiGiovanni J. Altered expression of the epidermal growth factor receptor and transforming growth factor-α during multistage skin carcinogenesis in SENCAR mice. Molecular Carcinogenesis. 1994;11(1):19–28. doi: 10.1002/mc.2940110105. [DOI] [PubMed] [Google Scholar]
- 15.Yuspa SH. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. Journal of Dermatological Science. 1998;17(1):1–7. doi: 10.1016/s0923-1811(97)00071-6. [DOI] [PubMed] [Google Scholar]
- 16.O-Charoenrat P, Rhys-Evans P, Modjtahedi H, Court W, Box G, Eccles S. Overexpression of epidermal growth factor receptor in human head and neck squamous carcinoma cell lines correlates with matrix metalloproteinase-9 expression and in vitro invasion. International Journal of Cancer. 2000;86(3):307–317. doi: 10.1002/(sici)1097-0215(20000501)86:3<307::aid-ijc2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.O-Charoenrat P, Rhys-Evans PH, Archer DJ, Eccles SA. C-erbB receptors in squamous cell carcinomas of the head and neck: clinical significance and correlation with matrix metalloproteinases and vascular endothelial growth factors. Oral Oncology. 2002;38(1):73–80. doi: 10.1016/s1368-8375(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 18.Moghal N, Sternberg PW. Multiple positive and negative regulators of signaling by the EGF-receptor. Current Opinion in Cell Biology. 1999;11(2):190–196. doi: 10.1016/s0955-0674(99)80025-8. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu T, Izumi H, Oga A, et al. Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous-cell carcinoma of the skin. Dermatology. 2001;202(3):203–206. doi: 10.1159/000051637. [DOI] [PubMed] [Google Scholar]
- 20.Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 21.Zavadil J, Bitzer M, Liang D, et al. Genetic programs of epithelial cell plasticity directed by transforming growth factor-β . Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Reviews Molecular Cell Biology. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 23.Peinado H, Quintanilla M, Cano A. Transforming growth factor β-1 induces snail transcription factor in epithelial cell lines. Mechanisms for epithelial mesenchymal transitions. The Journal of Biological Chemistry. 2003;278(23):21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 24.Wilkins-Port CE, Higgins CE, Freytag J, Higgins SP, Carlson JA, Higgins PJ. PAI-1 is a critical upstream regulator of the TGF-β1/EGF-induced invasive phenotype in mutant p53 human cutaneous squamous cell carcinoma. Journal of Biomedicine and Biotechnology. 2007;2007:8 pages. doi: 10.1155/2007/85208. Article ID 85208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins-Port CE, Higgins PJ. Regulation of extracellular matrix remodeling following transforming growth factor-β1/epidermal growth factor-stimulated epithelial-mesenchymal transition in human premalignant keratinocytes. Cells Tissues Organs. 2007;185(1–3):116–122. doi: 10.1159/000101312. [DOI] [PubMed] [Google Scholar]
- 26.Qi L, Higgins SP, Lu Q, et al. SERPINE1 (PAI-1) is a prominent member of the early G0 → G1 transition “wound repair” transcriptome in p53 mutant human keratinocytes. Journal of Investigative Dermatology. 2008;128(3):749–753. doi: 10.1038/sj.jid.5701068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyoshi S, Ishii M, Nemoto N, Kawabata M, Aburatani H, Miyazono K. Targets of transcriptional regulation by transforming growth factor-β: expression profile analysis using oligonucleotide arrays. Japanese Journal of Cancer Research. 2001;92(3):257–268. doi: 10.1111/j.1349-7006.2001.tb01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-Bueno G, Cubillo E, Sarrió D, et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for snail, slug, and E47 Factors in epithelial- mesenchymal transition. Cancer Research. 2006;66(19):9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- 29.Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. International Journal of Cancer. 1997;72(1):1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Bajou K, Masson V, Gerard RD, et al. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interaction with proteases, not vitronectin: implications for antiangiogenic strategies. Journal of Cell Biology. 2001;152(4):777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajou K, Noël A, Gerard RD, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nature Medicine. 1998;4(8):923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y-J, Lin S-C, Kao T, et al. Genome-wide profiling of oral squamous cell carcinoma. The Journal of Pathology. 2004;204(3):326–332. doi: 10.1002/path.1640. [DOI] [PubMed] [Google Scholar]
- 33.Lindberg P, Larsson Å, Nielsen BS. Expression of plasminogen activator inhibitor-1, urokinase receptor and laminin γ-2 chain is an early coordinated event in incipient oral squamous cell carcinoma. International Journal of Cancer. 2006;118(12):2948–2956. doi: 10.1002/ijc.21568. [DOI] [PubMed] [Google Scholar]
- 34.Illemann M, Hansen U, Nielsen HJ, et al. Leading-edge myofibroblasts in human colon cancer express plasminogen activator inhibitor-1. American Journal of Clinical Pathology. 2004;122(2):256–265. doi: 10.1309/F32X-WQ20-T568-H8VP. [DOI] [PubMed] [Google Scholar]
- 35.Offersen BV, Nielsen BS, Høyer-Hansen G, et al. The myofibroblast is the predominant plasminogen activator inhibitor-1-expressing cell type in human breast carcinomas. American Journal of Pathology. 2003;163(5):1887–1899. doi: 10.1016/S0002-9440(10)63547-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert C, Bolon I, Gazzeri S, Veyrenc S, Brambilla C, Brambilla E. Expression of plasminogen activator inhibitors 1 and 2 in lung cancer and their role in tumor progression. Clinical Cancer Research. 1999;5(8):2094–2102. [PubMed] [Google Scholar]
- 37.Vairaktaris E, Yapijakis C, Serefoglou Z, et al. Plasminogen activator inhibitor-1 polymorphism is associated with increased risk for oral cancer. Oral Oncology. 2006;42(9):888–892. doi: 10.1016/j.oraloncology.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Hundsdorfer B, Zeilhofer H-F, Bock KP, et al. Tumour-associated urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1 in normal and neoplastic tissues of patients with squamous cell cancer of the oral cavity-clinical relevance and prognostic value. Journal of Cranio-Maxillofacial Surgery. 2005;33(3):191–196. doi: 10.1016/j.jcms.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Maquerlot F, Galiacy S, Malo M, et al. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. American Journal of Pathology. 2006;169(5):1624–1632. doi: 10.2353/ajpath.2006.051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins PJ. TGF-β1-stimulated p21ras-ERK signaling regulates expression of the angiogenic SERPIN PAI-1. Recent Research Developments in Biochemistry. 2006;7:31–45. [Google Scholar]
- 41.Maillard C, Jost M, Rømer MU, et al. Host plasminogen activator inhibitor-1 promotes human skin carcinoma progression in a stage-dependent manner. Neoplasia. 2005;7(1):57–66. doi: 10.1593/neo.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galibert M-D, Carreira S, Goding CR. The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. The EMBO Journal. 2001;20(17):5022–5031. doi: 10.1093/emboj/20.17.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kutz SM, Higgins CE, Samarakoon R, et al. TGF-β1-induced PAI-1 expression is e box/USF-dependent and requires EGFR signaling. Experimental Cell Research. 2006;312(7):1093–1105. doi: 10.1016/j.yexcr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Qi L, Allen RR, Lu Q, Higgins CE, Garone R, Staiano-Coico L, Higgins PJ. PAI-1 transcriptional regulation during the G0 → G1 transition in human epidermal keratinocytes. Journal of Cellular Biochemistry. 2006;99(2):495–507. doi: 10.1002/jcb.20885. [DOI] [PubMed] [Google Scholar]
- 45.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425(6958):577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 46.Samarakoon R, Higgins CE, Higgins SP, Kutz SM, Higgins PJ. Plasminogen activator inhibitor type-1 gene expression and induced migration in TGF-β1-stimulated smooth muscle cells is pp60c-src/MEK-dependent. Journal of Cellular Physiology. 2005;204(1):236–246. doi: 10.1002/jcp.20279. [DOI] [PubMed] [Google Scholar]
- 47.Samarakoon R, Higgins SP, Higgins CE, Higgins PJ. TGF-β1-induced plasminogen activator inhibitor-1 expression in vascular smooth muscle cells requires pp60c-src/EGFRY845 and Rho/ROCK signaling. Journal of Molecular and Cellular Cardiology. 2008;44(3):527–538. doi: 10.1016/j.yjmcc.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishizawar R, Parsons SJ. C-Src and cooperating partners in human cancer. Cancer Cell. 2004;6(3):209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Crawford M, Day RM, et al. RhoA modulates Smad signaling during transforming growth factor-β-induced smooth muscle differentiation. The Journal of Biological Chemistry. 2006;281(3):1765–1770. doi: 10.1074/jbc.M507771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kita T, Hata Y, Kano K, et al. Transforming growth factor-β2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes. 2007;56(1):231–238. doi: 10.2337/db06-0581. [DOI] [PubMed] [Google Scholar]
- 51.Itoh S, ten Dijke P. Negative regulation of TGF-β receptor/Smad signal transduction. Current Opinion in Cell Biology. 2007;19(2):176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 52.Lin X, Duan X, Liang Y-Y, et al. PPM1A functions as a Smad phosphatase to terminate TGFβ signaling. Cell. 2006;125(5):915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen RR, Qi L, Higgins PJ. Upstream stimulatory factor regulates E box-dependent PAI-1 transcription in human epidermal keratinocytes. Journal of Cellular Physiology. 2005;203(1):156–165. doi: 10.1002/jcp.20211. [DOI] [PubMed] [Google Scholar]
- 54.Hua X, Miller ZA, Wu G, Shi Y, Lodish HF. Specificity in transforming growth factor β-induced transcription of the plasminogen activator inhibitor-1 gene: interactions of promoter DNA, transcription factor μE3, and Smad proteins. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13130–13135. doi: 10.1073/pnas.96.23.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Research. 2009;19(1):128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee MK, Pardoux C, Hall MC, et al. TGF-β activates Erk MAP kinase signalling through direct phsophorylation of ShcA. The EMBO Journal. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]