Abstract
Francisella tularensis is an intracellular gram-negative bacterium and the etiological agent of pulmonary tularemia. Given the high degrees of infectivity in the host and of dissemination of bacteria following respiratory infection, immunization strategies that target mucosal surfaces are critical for the development of effective vaccines against this organism. In this study, we have characterized the efficacy of protective immunity against pneumonic tularemia following oral vaccination with F. tularensis LVS (live vaccine strain). Mice vaccinated orally with LVS displayed colocalization of LVS with intestinal M cells, with subsequent enhanced production of splenic antigen-specific gamma interferon and of systemic and mucosal antibodies, including immunoglobulin A (IgA). LVS-vaccinated BALB/c mice were highly protected against intranasal (i.n.) SCHU S4 challenge and exhibited significantly less bacterial replication in the lungs, liver, and spleen than mock-immunized animals. Depletion of CD4+ T cells significantly abrogated the protective immunity, and mice deficient in B cells or IgA displayed partial protection against SCHU S4 challenge. These results suggest that oral vaccination with LVS induces protective immunity against i.n. challenge with F. tularensis SCHU S4 by a process mediated cooperatively by CD4+ T cells and antibodies, including IgA.
Francisella tularensis is an intracellular gram-negative bacterium that can cause acute pneumonic disease in humans (18, 54). F. tularensis can be classified into several subspecies, including Francisella tularensis subsp. tularensis (type A), Francisella tularensis subsp. holarctica (type B), “Francisella tularensis subsp. novicida,” and Francisella tularensis subsp. mediasiatica (55). The ease of aerosol dissemination and the ability to cause pneumonic disease by inhalation of as few as 10 organisms of a type A strain have made this organism a potential biothreat agent (48). An attenuated strain of F. tularensis subsp. holarctica (type B), the live vaccine strain (LVS), has been evaluated for protection of humans and animals (14, 48). Parenteral administration of LVS to humans by scarification has been shown to provide protection against intradermal (i.d.) challenge with type A but afforded minimal protection from exposure to aerosols with large particles (7, 22, 47, 48).
Most vaccines delivered parenterally do not induce significant mucosal immunity in the respiratory compartment (58), which is the initial site of exposure in pulmonary infection. Although there may be compartmentalization within the mucosal immune system, there is evidence to demonstrate the efficacy of immunization at distant mucosal inductive sites, particularly with the ability of oral vaccination to prevent infection of the lungs (66). To this end, membranous or microfold cells (M cells) are located in the follicle-associated epithelium of intestinal Peyer's patches and have been shown to be specialized in the transport and uptake of luminal antigens for the robust induction of systemic and mucosal immunity (10, 28). Targeting of vaccine antigens to M cells has gained considerable attention as a means to deliver effective mucosal vaccines (29, 51). Given the success of oral vaccines for human use, including the Sabin polio vaccine and the licensed typhoid vaccine, the oral route of immunization may be important in the development of defined vaccines against pulmonary tularemia (51).
Protective immunity against F. tularensis requires the efficient induction of cellular immunity, including T cells, and gamma interferon (IFN-γ) induction (16, 17, 52, 63). Moreover, evidence for the role of antibodies (26, 41, 44, 45, 53), and particularly immunoglobulin A (IgA) (4), in mucosal immunity against Francisella infection has been accumulating. IgA is the principal immunoglobulin isotype involved in the inhibition of bacterial attachment and the neutralization of viruses at mucosal surfaces (31). Moreover, serum IgA and secretory IgA have been shown to suppress inflammatory pathology by reducing inflammatory cytokine production or the oxidative burst (21, 37, 60). Thus, a targeted vaccination regimen that induces cellular and mucosal immunity in the respiratory compartment may be highly beneficial in defense against an F. tularensis type A strain.
In this study, we examined various mechanisms that underlie protective immunity induced by oral LVS vaccination against murine pulmonary tularemia. Mice vaccinated orally with LVS were remarkably protected against subsequent intranasal (i.n.) or i.d. challenge with the F. tularensis type A strain SCHU S4. The significant protection conferred by oral LVS immunization was reflected in reductions in the degrees of bacterial replication and dissemination following pulmonary challenge. The oral vaccination regimen induced splenic antigen-specific IFN-γ responses and serum IgG2a responses. Moreover, orally vaccinated mice produced LVS-specific fecal and respiratory secretory IgA. The respiratory protection conferred by oral LVS vaccination was partially dependent on B cells and on IgA production and required the presence of CD4+ T cells.
MATERIALS AND METHODS
Bacteria.
Francisella tularensis LVS (lot 703-0303-016) was obtained from Rick Lyons at the University of New Mexico, and F. tularensis subsp. tularensis (strain SCHU S4) was obtained from the Centers for Disease Control and Prevention. The bacteria were grown at 37°C in Trypticase soy broth (TSB) or on Trypticase soy agar (TSA), each supplemented with 0.1% (wt/vol) cysteine (25). mCherry-labeled LVS (KKF314) was prepared as follows. mCherry was PCR amplified from pmCherry (Clontech, Mountain View, CA) using primers mFruit NdeI (forward) (5′-CCCGGGCATATGGTGAGCAAGGGCGAGGAG-3′) and mFruit XhoI (reverse) (5′-GGCTCGAGTTACTTGTACAGCTCGTCCATGCC-3′), where underlining indicates the restriction sites. The PCR fragment was cut and ligated into the Francisella expression plasmid pKEK894 (65), and the mCherry expression plasmid (pKEK1124) was then electroporated into LVS as described previously (33). Bacteria were grown at 37°C in TSB-cysteine plus 10 μg/ml of tetracycline (Sigma-Aldrich, St. Louis, MO). LVS expressing mCherry was visualized by fluorescent microscopy (wavelength, 587 to 610 nm) using a Axioskop 2 Plus microscope (Zeiss, Thornwood, NY) and by confocal microscopy (with a 510 Meta laser scanning confocal microscope [Zeiss]).
Mice.
Four- to 8-week-old BALB/c and C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD). BALB/c IFN-γ−/− mice (11) and C57BL/6 IFN-γ receptor-deficient (IFN-γR−/−) (23) and μMT (B-cell-deficient) mice (27) were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 × 129 IgA−/− mice were generated as described previously (37), and C57BL/6 × 129 F2 mice were used as controls. Mice were maintained at the University of Texas at San Antonio Animal Facility, and all experimental procedures were performed in compliance with the Institutional Care and Use Committee guidelines.
Oral immunization and pulmonary or i.d. challenge.
Mice were anesthetized with 3% isoflurane by use of a rodent anesthesia machine (Harvard Apparatus, Holliston, MA) (40, 41). Mice were either vaccinated orally, using a 22-gauge, 25-mm-long, 1.25-mm-tip feeding needle (Fine Science Tools Inc., Foster City, CA) (20), with 103 CFU of LVS in 200 μl of phosphate-buffered saline (PBS) or mock immunized with PBS alone. We have determined the 50% lethal dose (LD50) of LVS administered orally to be approximately 104 CFU. Vaccinated mice were rested for the indicated periods and challenged i.n. with 50, 100, or 500 LD50s of SCHU S4 (LD50, <10 CFU [50, 56]) in 25 μl PBS at 3 or 8 weeks after oral LVS vaccination. For i.d. challenge, mice were injected at the base of the tail with either 100 or 500 LD50s of F. tularensis SCHU S4 in 50 μl of PBS. Some mice received a second oral vaccination boost (103 CFU) of LVS 8 weeks after the first inoculation and then were challenged i.n. with SCHU S4 after 4 weeks. The actual vaccination and challenge doses administered in each experiment were determined by dilution plating on TSA plus cysteine. Animals were monitored daily for morbidity and mortality.
Splenocyte culture for analysis of cytokine production.
Mice were either immunized orally with 103 CFU of LVS or mock immunized with PBS alone and were euthanized 14 days after immunization. Spleens were then collected. Single-cell suspensions were prepared and cultured (1 × 106 cells/well) for 72 h in Dulbecco's modified Eagle medium supplemented with 10% (vol/vol) fetal bovine serum (FBS) with or without 104 or 105 CFU of UV-inactivated LVS. Bacteria were inactivated by exposure to a 30-W short-wavelength UV light source for 15 min at a distance of 15 cm. The inactivation was confirmed by the absence of bacterial growth on TSA-plus-cysteine plates. Some cells were also cultured with the unrelated antigen hen egg lysozyme (HEL). Culture supernatants were harvested for IFN-γ, interleukin 2 (IL-2), and IL-4 analysis by enzyme-linked immunosorbent assay (ELISA) as described previously (40, 41).
Detection of antibody and isotype levels by ELISA.
Three weeks after oral LVS vaccination, mice were bled and sera prepared. For collection of bronchoalveolar lavage (BAL) fluid, the mice were sacrificed and the tracheae were intubated using a 0.58-mm (outer diameter) polyethylene catheter (Becton Dickinson, Sparks, MD). The lungs were then lavaged twice with Hanks balanced salt solution (Invitrogen, Carlsbad, CA). The recovered BAL fluid (1 ml) was centrifuged at 9,500 × g for 7 min at 4°C, and the supernatant was stored at −70°C until use. For analysis of fecal supernatants, 0.1 g of fresh fecal pellets was collected and dissolved in 1 ml of PBS containing protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), and supernatants were collected by centrifugation. Microtiter plates were coated overnight with 106 CFU of UV-inactivated LVS in sodium bicarbonate buffer (pH 9.5), washed with PBS containing 0.3% Brij 35 (Sigma), and blocked for 2 h at room temperature with PBS containing 5% FBS and 0.1% Brij 35 as described previously (44). Serial dilutions of serum (starting at a 1:50 dilution), undiluted BAL fluid, or fecal supernatants were added to wells and incubated at room temperature for 2 h. The plates were then washed and incubated for an additional 2 h with goat anti-mouse total Ig, IgG1, IgG2a, IgA, and IgM conjugated to horseradish peroxidase (Southern Biotechnology Associates, Birmingham, AL). After incubation, the plates were washed, and a tetramethylbenzidine substrate (BD Biosciences, San Diego, CA) was added for color development. Absorbance at 630 nm was measured using an ELISA microplate reader (Bio-Tek Instruments). The reciprocal serum dilutions corresponding to 50% maximal binding were used to obtain titers. However, because of the large dilution involved in the procedures for collection of BAL fluid and fecal supernatants, these samples were tested undiluted, and results were reported as absorbance units. No binding of immune serum was detected in plates coated with the unrelated antigen HEL.
M-cell and LVS costaining.
Groups of BALB/c mice were immunized orally with 106 CFU of mCherry-labeled LVS. After 90 min, mice were euthanized, and sections of small intestine were removed, embedded in freezing medium (Triangle Biomedical Sciences, Durham, NC), and stored at −80°C. Cryosections (thickness, 5 μm) were prepared, fixed with 4% paraformaldehyde at 4°C for 1 h, and blocked for an additional 1 h in PBS containing 10% FBS. Sections were stained at room temperature for 1 h with the fluorescein isothiocyanate-conjugated lectin Ulex europaeus agglutinin 1 (UEA-1) (20 μg/ml; Sigma) to visualize M cells (34) and with Hoechst nuclear stain (Sigma). Images were acquired using a 510 Meta laser scanning confocal microscope (Zeiss) and were analyzed using Imaris software (Bitplane, Saint Paul, MN).
CD4+ T-cell depletion.
The hybridoma cell line GK1.5 (36) was purchased from the ATCC and grown in HyQ serum-free medium (HyClone) supplemented with decreasing amounts (20% to 1.25%) of FBS to produce an anti-CD4 neutralizing antibody. Ammonium sulfate precipitation was performed on cell culture supernatants to produce a purified antibody, and a Bradford assay was performed to determine the protein concentration by using known concentrations of bovine serum albumin (Fisher Scientific) as standards and an ELISA plate reader (Bio-Tek Instruments, Winooski, VT). A rat Ig (Sigma-Aldrich) was used as an isotype control. BALB/c mice were either immunized orally with 103 CFU of LVS or mock immunized (PBS). Three weeks later, mice were injected intraperitoneally (i.p.) with either 0.25 mg of a monoclonal anti-CD4 antibody or an isotype control on day −2, day −1, the day of challenge, and every third day thereafter until day 15 after challenge. The level of CD4+ T-cell depletion was measured by flow cytometry using an anti-CD4 monoclonal antibody conjugated with allophycocyanin-Cy7 (BD Biosciences).
Statistical analyses.
SigmaStat (Systat Software Inc., San Jose, CA) was used to perform all the tests of significance. Statistical analysis for survival experiments was performed using the Kaplan-Meier test, and the Student t test was used to determine differences in cytokine and antibody production. All data are reported as the mean ± standard error from each experimental animal group and are representative of at least two independent experiments.
RESULTS
Oral LVS vaccination induces systemic and mucosal cellular and humoral immune responses.
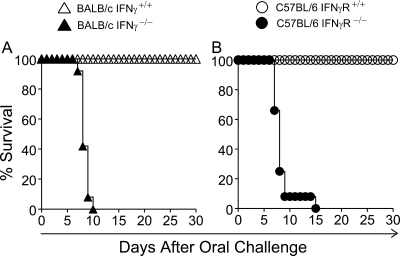
BALB/c mice were orally challenged with escalating doses (102 to 108 CFU) of LVS and were monitored daily for morbidity and mortality. These analyses revealed that the LD50 of LVS administered orally was approximately 104 CFU (data not shown). A challenge dose of 103 CFU of LVS delivered orally did not induce apparent morbidity in either BALB/c or C57BL/6 mice but induced 100% mortality in BALB/c IFN-γ−/− and C57BL/6 IFN-γR−/− mice by days 10 and 15, respectively (Fig. 1), consistent with previous studies demonstrating the importance of IFN-γ production in the initial control of parenteral LVS infection (1, 15, 32).
FIG. 1.
IFN-γ is required for survival following oral challenge with LVS. Groups (n = 12) of wild-type and IFN-γ−/− BALB/c mice or wild-type and IFN-γR−/− C57BL/6 mice were challenged orally with 103 CFU of LVS. Mice were monitored daily for morbidity and mortality. (A) Survival profile for wild-type and IFN-γ−/− BALB/c mice. Differences in survival between wild-type and IFN-γ−/− mice were significant at a P value of <0.001 (statistical power, 0.94 with an alpha of 0.50). (B) Survival profile for wild-type and IFN-γR−/− C57BL/6 mice. Differences in survival between wild-type and IFN-γR−/− mice were significant at a P value of <0.001 (statistical power, 1 with an alpha of 0.50). Results are representative of two separate experiments.
In addition, antigen-specific cell-mediated and humoral responses were characterized in BALB/c mice following oral LVS vaccination. Splenocytes were removed at day 14 after oral immunization, and single cells were stimulated with 104 or 105 CFU of UV-inactivated LVS. These analyses revealed that antigen-specific IFN-γ production was significantly (P < 0.001) increased in a dose-dependent manner (0.326 ± 0.15 ng/ml and 0.762 ± 0.77 ng/ml, respectively) in splenocytes from mice orally vaccinated with LVS (Fig. 2A). Moreover, the cervical lymph node cells from orally vaccinated mice also produced an appreciable IFN-γ response in culture, in a dose-dependent manner (data not shown). In contrast, there was negligible induction of IFN-γ production in cells from mock-vaccinated mice and in cells from both groups that were incubated with the unrelated antigen HEL or with medium alone. Moreover, antigen-specific IL-2 production was also detected upon oral LVS vaccination, but IL-4 production was not detectable in any of the cell cultures (data not shown).
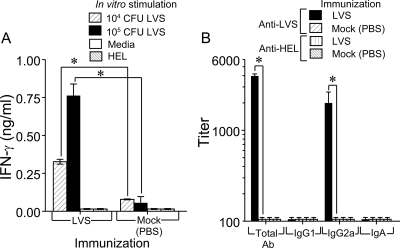
FIG. 2.
Oral LVS vaccination induces LVS-specific IFN-γ and serum antibodies. (A) Groups of BALB/c mice (n = 5) were either vaccinated orally with 103 CFU of LVS or mock immunized with PBS. Spleens were removed 14 days later, and single cells were prepared and incubated for 72 h in the presence of UV-inactivated LVS at two doses (104 and 105), medium alone, or the unrelated antigen HEL. Supernatants were analyzed for IFN-γ production. *, differences in IFN-γ production between LVS- and mock-immunized (PBS) mice were significant at a P value of <0.001 (statistical power, 1 with an alpha of 0.50). (B) Groups of BALB/c mice (n = 8) were immunized orally with 103 CFU of LVS in PBS and were rested for 21 days. Blood was collected, and prepared sera were analyzed by isotype-specific ELISAs using microtiter plates coated with UV-inactivated LVS. The results are reported as 50% end point titers. *, differences in antibody titers between immune and nonimmune sera were significant at a P value of <0.001 for total antibody (Ab) (statistical power, 1 with an alpha of 0.50) and at a P value of 0.015 for IgG2a (statistical power, 0.767 with an alpha of 0.50). Results are representative of two separate experiments.
Mice were bled 21 days following oral vaccination, and sera were analyzed for anti-LVS antibody responses. As shown in Fig. 2B, there was significant induction of total (3,948 ± 224; P < 0.001) and IgG2a (1,993 ± 641; P < 0.015) serum antibodies. In contrast, there was minimal induction of LVS-specific IgG1 and IgA in the sera. Anti-LVS antibody responses in mock-vaccinated mice were negligible. No binding of immune serum was detected in plates coated with HEL. To further determine the effects of oral LVS vaccination at inductive and distal mucosal sites (13), we examined antibody responses in fecal supernatants and BAL fluids collected from immunized animals. Oral LVS immunization induced significant (P < 0.001) IgA production but minimal IgM production in the intestines (Fig. 3A). No LVS-specific IgG was detected in fecal supernatants (data not shown). In the respiratory compartments of orally vaccinated animals, LVS induced significant levels of antibodies, including total antibodies, IgG1, IgG2a, and IgM (P < 0.001), and IgA (P < 0.015) (Fig. 3B). As expected, there was negligible induction of antibodies in the fecal supernatants and BAL fluids of mock-vaccinated mice. Collectively, these results demonstrate the efficacy of the oral route of vaccination in inducing antigen-specific systemic and mucosal cellular and humoral immune responses.
FIG. 3.
Oral LVS vaccination induces LVS-specific fecal and respiratory antibodies. Groups of BALB/c mice (n = 6) were vaccinated orally with 103 CFU of LVS and were rested for 21 days. Fecal (A) and BAL (B) samples were collected, and the processed supernatants were analyzed by isotype-specific ELISAs using microtiter plates coated with UV-inactivated LVS. The results are reported as optical densities (OD) at 630 nm. Differences in OD between immune and nonimmune fecal supernatants were significant at a P value of <0.001 for IgA (statistical power, 1 with an alpha of 0.50). Differences in OD between immune and nonimmune BAL fluids were significant at a P value of <0.001 for total antibody (Ab), IgG1, IgG2a, and IgM (statistical power, 1 with an alpha of 0.50), and a P value of 0.015 for IgA (statistical power, 0.537 with an alpha of 0.50). Results are representative of two separate experiments.
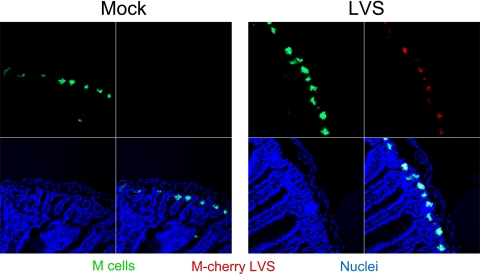
M cells located in the follicle-associated epithelium of intestinal tissue play an important role in the sampling and transport of antigens for processing and the initiation of immune responses (10, 28). To determine whether orally delivered LVS localizes to M cells following vaccination, we administered mCherry-labeled LVS and examined the gastrointestinal tract for the presence of these bacteria by confocal laser scanning microscopy. As shown in Fig. 4, M cells (green) were identified by staining with UEA-1 (34) and were apparent in the crypts of the small intestine. Interestingly, orally administered LVS cells expressing mCherry (red) were visible within the small intestine after 90 min and colocalized (yellow) to M cells. These results suggest that orally administered LVS may be trafficking to M cells for the initiation of mucosal immune responses.
FIG. 4.
LVS administered orally is trafficked through M cells. Groups (n = 4) of BALB/c mice were either vaccinated orally with 106 CFU of mCherry-labeled LVS (red) or mock immunized with PBS. Mice were sacrificed 90 min later, and intestinal sections were removed and snap-frozen. Tissue sections were incubated with fluorescein isothiocyanate-conjugated UEA-1 (green) in order to visualize M cells and with the Hoechst nuclear stain (blue). Stained intestinal sections were examined by confocal laser scanning microscopy.
Oral LVS vaccination induces robust protection against pulmonary and i.d. challenges with F. tularensis SCHU S4.
To determine the efficacy of oral LVS immunization in conferring protective immunity, BALB/c mice were vaccinated with LVS (103 CFU) and were challenged i.n. or i.d. with F. tularensis SCHU S4 3 weeks later. As shown in Fig. 5A, mice orally vaccinated with LVS exhibited significant protection (100% survival with 126 CFU and 80% survival with 580 CFU) against i.n. pulmonary challenge during the monitoring period of 1 month. Additionally, oral LVS vaccination induced 100% protection against both infectious doses following SCHU S4 challenge administered i.d. (Fig. 5C), another common route of infection. All vaccinated mice exhibited minimal loss of body weight following type A bacterial challenge (Fig. 5B and D), and as expected, mock-vaccinated mice showed a rapid decline in body weight and succumbed to the infection by day 6 after challenge.
FIG. 5.
Oral LVS vaccination protects against pulmonary and systemic SCHU S4 challenge. Groups of BALB/c mice (n = 6) were either vaccinated orally with 103 CFU of LVS in PBS or mock immunized with PBS. Three weeks later, mice were challenged i.n. or i.d. with 126 CFU or 580 CFU of F. tularensis SCHU S4. Mice were monitored daily for survival and weight loss. (A and B) Survival profile (A) and weight loss (B) after i.n. challenge. Differences in survival between LVS- and mock-immunized mice were significant at a P value of <0.001 (statistical power, 0.94 with an alpha of 0.50). (C and D) Survival profile (C) and weight loss (D) after i.d. challenge. Differences in survival between LVS- and mock-immunized mice were significant at a P value of <0.001 (statistical power, 0.94 with an alpha of 0.50). Results are representative of two separate experiments.
Since the duration of protective immunity against virulent type A strains induced by LVS immunization has been shown to be short (9, 30, 59, 61), we examined the extent of protection conferred by the oral vaccination regimen in this study. Given that oral LVS vaccination induced protective immunity against pulmonary SCHU S4 challenge after 3 weeks (Fig. 5), we evaluated the protective efficacy at 8 weeks postvaccination. Mice vaccinated orally with LVS (103 CFU) were rested for 8 weeks and subsequently challenged i.n. with 86 or 375 CFU of SCHU S4. As shown in Fig. 6A, immunized mice exhibited 33% and 50% survival rates (at 86 and 375 CFU, respectively) at this extended time following oral LVS vaccination, indicating a waning of protective immunity over time, with no significant difference in the survival rate between mice that received either dose. Therefore, some immunized mice were boosted with LVS (103 CFU) orally after 8 weeks, rested for an additional 4 weeks, and challenged i.n. with SCHU S4. As shown in Fig. 6B, mice receiving a secondary boost of LVS orally were highly protected (80% survival with 86 CFU and 60% survival with 375 CFU) against i.n. challenge with SCHU S4. These analyses reveal that maintenance of long-term protective immunity by oral LVS vaccination may require additional booster immunizations.
FIG. 6.
Duration of pulmonary protection conferred by oral LVS vaccination. Groups of BALB/c mice (n = 6) were either immunized orally with 103 CFU of LVS or mock immunized. (A) Survival profiles of mice challenged i.n. with 86 CFU or 375 CFU of F. tularensis SCHU S4 8 weeks later. Differences in survival between LVS-immunized and mock-immunized (PBS) mice were significant at a P value of <0.001 (statistical power, 0.94 with an alpha of 0.50). (B) Survival profiles of mice given an oral boost of 103 CFU of LVS in PBS or a mock boost (PBS only) 8 weeks later. Four weeks after the boost, mice were challenged i.n. with 86 CFU or 375 CFU of F. tularensis SCHU S4. Differences in survival between LVS- and mock-immunized mice were significant at a P value of <0.001 (statistical power, 0.94 with an alpha of 0.50). Results are representative of two separate experiments.
To further evaluate the efficacy of the oral vaccination regimen, mice vaccinated with LVS (103 CFU) or PBS were euthanized following pulmonary SCHU S4 challenge, and bacterial replication in the lungs, liver, and spleen was examined. As shown in Fig. 7A, small, comparable numbers of bacteria were recovered from the target organs of both LVS- and mock-vaccinated animals 1 day after challenge. However, by days 3 and 4, mock-vaccinated animals exhibited extensive bacterial replication within the lungs (∼106 CFU), liver (105 to 107 CFU), and spleen (105 to 108 CFU). Moreover, as shown in Fig. 5A and B, the mock-vaccinated mice exhibited a significant loss of body weight and rapidly succumbed to the infection. In contrast, animals orally vaccinated with LVS exhibited significantly (P < 0.05) lower levels of recoverable viable bacteria in the target organs than mock-immunized animals during this initial period and up to day 14 (Fig. 7A). In parallel, histological analyses of the lungs were performed on both sets of animals at day 3 post-SCHU S4 pulmonary challenge. These experiments revealed that mock-vaccinated, SCHU S4-challenged mice exhibited minimal signs of cellular infiltration, with otherwise normal lung architecture (Fig. 7BI), like that of naïve animals (Fig. 7BIII). The lung sections of mice orally vaccinated with LVS and challenged with SCHU S4 were generally comparable to those of mock-vaccinated animals, with the exception of foci of peribronchiolar mononuclear lymphocytic infiltration (Fig. 7BII). Collectively, these results demonstrate the efficacy of the oral vaccination route with LVS at inducing effective control of SCHU S4 replication and dissemination, presumably via the initiation of an early cellular influx into the primary site of infection.
FIG. 7.
Oral LVS vaccination leads to a reduction in bacterial replication and an increase in inflammatory response following pulmonary SCHU S4 challenge. Groups of BALB/c mice (n = 5) were either vaccinated orally with 103 CFU of LVS or mock immunized with PBS. Three weeks later, mice were challenged i.n. with 130 CFU of F. tularensis SCHU S4. (A) Mice were sacrificed at different time points (1, 3, 4, 8, and 14 days) after challenge, and various organs were removed. Bacterial numbers were enumerated by homogenization of whole individual organs and serial dilution plating. *, differences in bacterial numbers between LVS- and mock-immunized mice were significant at a P value of <0.05 (statistical power, 0.999 with an alpha of 0.50). (B) Mice were sacrificed 3 days after challenge, and lungs were collected for hematoxylin-and-eosin analyses. (I) Mock-vaccinated, SCHU S4-challenged mice. The arrow indicates the absence of peribronchiolar cellular infiltration. (II) LVS-vaccinated, SCHU S4-challenged mice. The arrow indicates the presence of peribronchiolar mononuclear lymphocytic infiltration. (III) Mock-vaccinated mice, no challenge. The arrow indicates normal bronchiolar architecture. Images are shown at a magnification of ×50. Results are representative of two separate experiments.
Pulmonary immunity against SCHU S4 challenge is mediated by CD4+ T cells and antibodies, including IgA.
Since the protective immunity against SCHU S4 challenge after oral LVS vaccination correlated with the early infiltration of mononuclear lymphocytes to the sites of infection, and given the demonstrated role of CD4+ T cells and IFN-γ in the control of Francisella infections (16, 63), we also examined the role of CD4+ T cells in orally vaccinated mice by treatment with an anti-CD4 neutralizing antibody (36). i.p. injection of the neutralizing anti-CD4 antibody markedly depleted splenic CD4+ T cells (0.5% of total splenocytes after treatment) in contrast to injection of a control rat Ig (17.6% of total splenocytes after treatment) (data not shown). As shown in Fig. 8, depletion of antigen-specific CD4+ T cells following pulmonary challenge with SCHU S4 (80 CFU) had a pronounced effect on the survival (25%) of the vaccinated animals in comparison to that of vaccinated animals not receiving the CD4+ T-cell depletion treatment (87%) or vaccinated mice injected with a control rat Ig (87%). As expected, all mock-immunized animals succumbed to the infection by day 6. These results suggest the significant contribution of CD4+ T cells to the protective immunity induced by oral LVS vaccination.
FIG. 8.
Contribution of LVS-specific CD4+ T cells to protective immunity against SCHU S4 challenge. Groups of BALB/c mice (n = 8) were either vaccinated orally with 103 CFU of LVS or mock immunized with PBS. Mice were rested for 3 weeks and received i.p. injections of either an anti-CD4 neutralizing antibody, a control rat Ig, or PBS at day −2, day −1, the day of i.n. challenge with 80 CFU of SCHU S4 (day 0), and every subsequent third day. Mice were monitored daily for morbidity and mortality. Differences in survival between vaccinated mice receiving rat Ig and those receiving anti-CD4 antibody treatment were significant at a P value of 0.0185 (statistical power, 0.996 with an alpha of 0.50). Results are representative of two separate experiments.
Apart from cellular immunity, antibodies have been shown to play a role in immunity against Francisella (26, 41, 44, 45, 53). Elevated levels of serum and mucosal antibodies were detected in mice after oral LVS vaccination (Fig. 3 and 4). IgG2a is the major murine isotype involved in the opsonization and phagocytosis of bacteria (2), while IgA has been shown to be the principal immunoglobulin isotype involved in the inhibition of bacterial attachment and the neutralization of viruses at mucosal surfaces (31). Moreover, there is evidence to suggest that IgA is required for the effective priming of T cells and the development of Th1 type immunity (3). To elucidate the role of humoral immunity in protection, μMT (B-cell-deficient) and wild-type C57BL/6 mice were vaccinated orally with LVS and challenged i.n. 3 weeks later with 50 and 102 CFU of SCHU S4. As shown in Fig. 9A, at an inoculum of 50 CFU, vaccinated B-cell-deficient mice exhibited 50% survival, while similarly challenged wild-type animals were completely (100%) protected, suggesting that protective immunity was partially dependent on antibodies. When the SCHU S4 challenge inoculum was doubled to 102 CFU (Fig. 9B), vaccinated B-cell-deficient mice still exhibited 56% survival, comparable to the 50% survival at the 50-CFU challenge dose, further suggesting that B cells contributed only partially to protective immunity and that the deficiency of B cells did not affect the immunity afforded by non-B-cell-responses. Moreover, vaccinated wild-type mice challenged at 102 CFU and monitored for 1 month exhibited only 66% survival (compared to 100% survival at the 50-CFU challenge dose), a level comparable to the survival rate of B-cell-deficient mice (56%) challenged at the same dose, indicating that increasing doses of challenge inocula may overwhelm the protective effects mediated by antibodies.
FIG. 9.
Contribution of antibodies, including IgA, to protective immunity against SCHU S4 challenge. (A and B) Groups (n = 6) of wild-type and B-cell-deficient (μMT) C57BL/6 mice were either vaccinated orally with 103 CFU of LVS or mock immunized with PBS. Three weeks later, mice were challenged i.n. with either 50 CFU (A) or 102 CFU (B) of F. tularensis SCHU S4; then they were monitored daily for survival. Differences in survival between immunized wild-type and B-cell-deficient mice at a challenge dose of 50 CFU were significant at a P value of <0.01 (statistical power, 0.996 with an alpha of 0.50). Differences in survival between LVS- and mock-immunized mice were significant at a P value of <0.001 (statistical power, 0.996 with an alpha of 0.50). (C) Groups (n = 6) of wild-type and IgA−/− C57BL/6 × 129 mice were either immunized orally with 103 CFU of LVS in PBS or mock immunized; 3 weeks later, they were challenged i.n. with 98 CFU of F. tularensis SCHU S4. Differences in survival between LVS-immunized wild-type and IgA−/− mice were significant at a P value of 0.0179 (statistical power, 0.996 with an alpha of 0.50). Results are representative of two separate experiments.
Oral LVS vaccination was shown to induce fecal and respiratory IgA responses (Fig. 3) that may contribute to protective immunity against mucosal pathogens. To evaluate the contribution of IgA to protective immunity, we vaccinated IgA−/− and wild-type C57BL/6 × 129 mice orally with LVS and challenged these animals i.n. with 98 CFU of SCHU S4. IgA−/− mice exhibited 50% survival, while 87% of the corresponding wild-type animals were protected against bacterial challenge (Fig. 9C). As expected, all mock-immunized animals in both experiments succumbed to the infection by day 8 after challenge (Fig. 9). These results further suggest that IgA may contribute significantly to the protective immunity mediated by antibodies in general. Collectively, these results suggest that antibodies, including IgA, and CD4+ T cells cooperatively play an important role in the induction of effective pulmonary immunity to SCHU S4 challenge following oral LVS vaccination.
DISCUSSION
Vaccination strategies that effectively induce mucosal immunity and control F. tularensis growth and pneumonic and systemic disease are important considerations for the development of effective vaccines against tularemia. We have now reported, further validating the findings of Chen and colleagues (30), that the oral route of vaccination using LVS effectively induces mucosal and systemic immunity and confers significant protection against both respiratory and i.d. challenges with the type A strain SCHU S4. Moreover, the protective efficacy of the oral vaccination regimen may involve the antigen-sampling mechanisms of M cells within the intestinal tract and appears to be mediated by CD4+ T cells and antibodies, including IgA.
Recent studies have suggested that the oral route of vaccination with LVS may be preferential for inducing protective pulmonary immunity against human-virulent F. tularensis (30). Specifically, Chen and colleagues have shown that BALB/c mice vaccinated orally with 108 CFU of LVS exhibited lower bacterial burdens than sham-immunized animals (30). Although protection in their study was seen with challenges up to 50 CFU i.n. and 20 CFU by aerosol exposure, mice challenged i.n. with higher inocula were not protected by oral LVS vaccination. The primary differences between the previously reported study and this study include the oral vaccination doses (108 CFU versus 103 CFU, respectively) of LVS and the type A strains (FSC33/snMF [strain FSC033] versus SCHU S4, respectively) used for challenges. Various studies (12, 49, 61) have shown significant differences in the virulence of LVS that may arise from culture with different media or different growth conditions, as well as from differences in the source of the strain. The strain of LVS used by Chen et al. was acquired from a source different from that used for this study, which may account for the differences in the oral LD50 between the studies. Nevertheless, evidence from both independent studies clearly indicates the feasibility of the oral route of vaccination in inducing significant respiratory immunity against virulent F. tularensis type A strains. Given the successful history of oral vaccines for humans, this immunization route may be highly viable for inducing both systemic and mucosal immune protection against F. tularensis. For example, oral vaccination with a Salmonella enterica delivery system expressing Yersinia pestis F1 and V antigens has been reported to be protective against bubonic and pneumonic plague (62). The efficacy of the oral vaccination regimen in protection against pulmonary tularemia may be a result of the effective delivery of vaccine antigens to M cells, which are located in the follicle-associated epithelium of Peyer's patches (10, 28, 29). M cells have been shown to play an important role in the sampling and uptake of luminal antigens (46) and to play a role in the release of costimulatory signals for effective induction of T- and B-cell proliferation (42). In this regard, Kiyono and colleagues (38) have recently shown the feasibility of targeting vaccine antigens to the M-cell-specific carbohydrate moiety as a highly effective strategy for inducing mucosal immunity. To this end, the uptake of microorganisms and microparticles from the small intestine may occur both through the M cells of Peyer's patches (35) and through intestinal villous M cells, described recently (24), as well as by an alternative mechanism of villous transepithelial passage, originally termed persorption (57). Early studies have shown that oral administration of suspensions of a large variety of different solid particles, the size of microorganisms and larger, to animals and human volunteers resulted in passage in less than an hour from the small intestine through the lymphatic and portal systems to the peripheral blood and a variety of body organs (57). Given that larger inocula of bacteria may result in greater systemic spread and induce some degree of morbidity themselves, the size of the immunizing oral LVS inoculum may be an important consideration and may affect the protective efficacy of the vaccination.
Effective mucosal defenses have been shown to be mediated by both cell-mediated and humoral mechanisms that operate in concert at major portals of entry for microorganisms (6). In the respiratory system, distinct mechanisms may be involved in the clearance of bacteria from the upper airways and deeper alveolar spaces. To this end, phagocytic cells such as macrophages and neutrophils may be involved in the removal of microorganisms that reach the deeper alveolar spaces by cognate interaction with antibodies through Fc-receptor-mediated processes (43). Whereas infection with SCHU S4 provokes a minimal inflammatory response in the lungs early after pneumonic challenge, as seen in this study and others (5, 8), the lungs of orally vaccinated and challenged mice exhibited an increase in the number of inflammatory cells, which were primarily lymphocytic. This influx of lymphocytes, which was evident only in vaccinated mice, may have contributed to the effective local control of bacterial replication. Moreover, depletion of antigen-specific CD4+ T cells at the time of infection remarkably abrogated the protective effects of oral LVS vaccination, indicating the importance of this cell type and of the production of cytokines such as IFN-γ for optimal bacterial clearance and protection against i.n. Francisella challenge. Oral LVS vaccination induced significant levels of antibodies in the respiratory compartment. LVS-mediated protection against pulmonary SCHU S4 challenge was also partially abrogated in the absence of B-cell and IgA expression. Antibodies have been shown by us (41, 44) and others (26, 53) to play an important role in the control of pulmonary Francisella infection. The mechanisms by which antibodies may facilitate the control of bacterial replication may include the neutralization of infectious organisms and Fc-receptor-mediated killing (44, 45). Both of these mechanisms may act in concert during an infection and limit the early dissemination of the organism, given that Francisella bacteremia occurs both in intra- and extracellular phases (19, 64).
The protection conferred by oral LVS vaccination began to wane by 2 months. Both the magnitude of the antibody responses and that of the antigen-specific cell-mediated IFN-γ response in vaccinated mice were reduced by factors of 2 and 4, respectively, by 2 to 3 months postimmunization (H. J. Ray and B. P. Arulanandam, unpublished data). A similar waning of LVS-mediated immunity against pulmonary tularemia has been reported previously (9, 30, 59, 61), following immunization by different routes. Given that the correlates of protective immunity against SCHU S4 have yet to be defined, the question of the long-term efficacy of LVS vaccination in the mouse model remains to be resolved. However, we have now shown that an additional boost of LVS given orally can be used to maintain protective immunity for an extended period.
In summary, with the significant interest in the development of a licensed vaccine for use against F. tularensis, consideration also has to be given to routes of delivery that induce optimal immunity at sites of infection. The advantages of an oral vaccine include (i) the ease of delivery, (ii) the possibility of fewer adverse effects than those with parenteral injection of dead whole or subunit vaccines, and (iii) the effective induction of both systemic and mucosal immunity, particularly in the upper respiratory system. While LVS continues to be used only to treat certain at-risk individuals, it may be unlikely to be licensed for use in the general population with the current level of understanding of the exact conditions under which it was generated, the mutations responsible for its attenuation, and the residual (dose-dependent) morbidity and mortality (39). However, LVS is a useful organism to be used in animal models for the evaluation of immune mechanisms that confer protective immunity, particularly against the virulent type A Francisella strains. Further studies to determine the efficacy of the oral vaccination route with defined attenuated Francisella vaccine strains and in other animal models of pulmonary tularemia are warranted and are currently under development.
Acknowledgments
This project has been funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN266200500040C and grant PO1 AI057986.
We thank the UTSA Imaging Center (and Colleen Witt) for access to confocal facilities.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Anthony, L. S., E. Ghadirian, F. P. Nestel, and P. A. Kongshavn. 1989. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb. Pathog. 7421-428. [DOI] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 696718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., R. H. Raeder, J. G. Nedrud, D. J. Bucher, J. Le, and D. W. Metzger. 2001. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J. Immunol. 166226-231. [DOI] [PubMed] [Google Scholar]
- 4.Baron, S. D., R. Singh, and D. W. Metzger. 2007. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect. Immun. 752152-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosio, C. M., H. Bielefeldt-Ohmann, and J. T. Belisle. 2007. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 1784538-4547. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg, P., E. S. Baekkevold, I. N. Farstad, F. L. Jahnsen, F. E. Johansen, E. M. Nilsen, and T. Yamanaka. 1999. Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol. Today 20141-151. [DOI] [PubMed] [Google Scholar]
- 7.Burke, D. S. 1977. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J. Infect. Dis. 13555-60. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W., R. KuoLee, H. Shen, M. Busa, and J. W. Conlan. 2004. Toll-like receptor 4 (TLR4) does not confer a resistance advantage on mice against low-dose aerosol infection with virulent type A Francisella tularensis. Microb. Pathog. 37185-191. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W., H. Shen, A. Webb, R. KuoLee, and J. W. Conlan. 2003. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 213690-3700. [DOI] [PubMed] [Google Scholar]
- 10.Corr, S. C., C. C. Gahan, and C. Hill. 2008. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 522-12. [DOI] [PubMed] [Google Scholar]
- 11.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 2591739-1742. [DOI] [PubMed] [Google Scholar]
- 12.Duckett, N. S., S. Olmos, D. M. Durrant, and D. W. Metzger. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 732306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwinell, M. B., and M. F. Kagnoff. 1999. Mucosal immunity. Curr. Opin. Gastroenterol. 1533-38. [DOI] [PubMed] [Google Scholar]
- 14.Eigelsbach, H. T., and C. M. Downs. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J. Immunol. 87415-425. [PubMed] [Google Scholar]
- 15.Elkins, K. L., T. Rhinehart-Jones, C. A. Nacy, R. K. Winegar, and A. H. Fortier. 1993. T-cell-independent resistance to infection and generation of immunity to Francisella tularensis. Infect. Immun. 61823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkins, K. L., T. R. Rhinehart-Jones, S. J. Culkin, D. Yee, and R. K. Winegar. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 643288-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkins, K. L., R. K. Winegar, C. A. Nacy, and A. H. Fortier. 1992. Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb. Pathog. 13417-421. [DOI] [PubMed] [Google Scholar]
- 18.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forestal, C. A., M. Malik, S. V. Catlett, A. G. Savitt, J. L. Benach, T. J. Sellati, and M. B. Furie. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J. Infect. Dis. 196134-137. [DOI] [PubMed] [Google Scholar]
- 20.Hamrick, T. S., J. R. Horton, P. A. Spears, E. A. Havell, I. W. Smoak, and P. E. Orndorff. 2003. Influence of pregnancy on the pathogenesis of listeriosis in mice inoculated intragastrically. Infect. Immun. 715202-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honorio-França, A. C., P. Launay, M. M. Carneiro-Sampaio, and R. C. Monteiro. 2001. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J. Leukoc. Biol. 69289-296. [PubMed] [Google Scholar]
- 22.Hornick, R. B., and H. T. Eigelsbach. 1966. Aerogenic immunization of man with live tularemia vaccine. Bacteriol. Rev. 30532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 2591742-1745. [DOI] [PubMed] [Google Scholar]
- 24.Jang, M. H., M. N. Kweon, K. Iwatani, M. Yamamoto, K. Terahara, C. Sasakawa, T. Suzuki, T. Nochi, Y. Yokota, P. D. Rennert, T. Hiroi, H. Tamagawa, H. Iijima, J. Kunisawa, Y. Yuki, and H. Kiyono. 2004. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA 1016110-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketavarapu, J. M., A. R. Rodriguez, J. J. Yu, Y. Cong, A. K. Murthy, T. G. Forsthuber, M. N. Guentzel, K. E. Klose, M. T. Berton, and B. P. Arulanandam. 2008. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc. Natl. Acad. Sci. USA 1059313-9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirimanjeswara, G. S., J. M. Golden, C. S. Bakshi, and D. W. Metzger. 2007. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J. Immunol. 179532-539. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350423-426. [DOI] [PubMed] [Google Scholar]
- 28.Kiyono, H., and S. Fukuyama. 2004. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KuoLee, R., and W. Chen. 2008. M cell-targeted delivery of vaccines and therapeutics. Expert Opin. Drug Deliv. 5693-702. [DOI] [PubMed] [Google Scholar]
- 30.KuoLee, R., G. Harris, J. W. Conlan, and W. Chen. 2007. Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis. Vaccine 253781-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51311-340. [DOI] [PubMed] [Google Scholar]
- 32.Leiby, D. A., A. H. Fortier, R. M. Crawford, R. D. Schreiber, and C. A. Nacy. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 6084-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maier, T. M., A. Havig, M. Casey, F. E. Nano, D. W. Frank, and T. C. Zahrt. 2004. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 707511-7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantis, N. J., M. C. Cheung, K. R. Chintalacharuvu, J. Rey, B. Corthesy, and M. R. Neutra. 2002. Selective adherence of IgA to murine Peyer's patch M cells: evidence for a novel IgA receptor. J. Immunol. 1691844-1851. [DOI] [PubMed] [Google Scholar]
- 35.Miller, H., J. Zhang, R. KuoLee, G. B. Patel, and W. Chen. 2007. Intestinal M cells: the fallible sentinels? World J. Gastroenterol. 131477-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphey, C., A. K. Murthy, P. A. Meier, G. M. Neal, G. Zhong, and B. P. Arulanandam. 2006. The protective efficacy of chlamydial protease-like activity factor vaccination is dependent upon CD4+ T cells. Cell. Immunol. 242110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murthy, A. K., J. Sharma, J. J. Coalson, G. Zhong, and B. P. Arulanandam. 2004. Chlamydia trachomatis pulmonary infection induces greater inflammatory pathology in immunoglobulin A deficient mice. Cell. Immunol. 23056-64. [DOI] [PubMed] [Google Scholar]
- 38.Nochi, T., Y. Yuki, A. Matsumura, M. Mejima, K. Terahara, D. Y. Kim, S. Fukuyama, K. Iwatsuki-Horimoto, Y. Kawaoka, T. Kohda, S. Kozaki, O. Igarashi, and H. Kiyono. 2007. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 2042789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oyston, P. C., and J. E. Quarry. 2005. Tularemia vaccine: past, present and future. Antonie van Leeuwenhoek 87277-281. [DOI] [PubMed] [Google Scholar]
- 40.Pammit, M. A., V. N. Budhavarapu, E. K. Raulie, K. E. Klose, J. M. Teale, and B. P. Arulanandam. 2004. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob. Agents Chemother. 484513-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pammit, M. A., E. K. Raulie, C. M. Lauriano, K. E. Klose, and B. P. Arulanandam. 2006. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect. Immun. 742063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pappo, J., and R. T. Mahlman. 1993. Follicle epithelial M cells are a source of interleukin-1 in Peyer's patches. Immunology 78505-507. [PMC free article] [PubMed] [Google Scholar]
- 43.Pilette, C., Y. Ouadrhiri, V. Godding, J. P. Vaerman, and Y. Sibille. 2001. Lung mucosal immunity: immunoglobulin-A revisited. Eur. Respir. J. 18571-588. [DOI] [PubMed] [Google Scholar]
- 44.Powell, H. J., Y. Cong, J. J. Yu, M. N. Guentzel, M. T. Berton, K. E. Klose, A. K. Murthy, and B. P. Arulanandam. 2008. CD4+ T cells are required during priming but not the effector phase of antibody-mediated IFN-γ-dependent protective immunity against pulmonary Francisella novicida infection. Immunol. Cell Biol. 86515-522. [DOI] [PubMed] [Google Scholar]
- 45.Rawool, D. B., C. Bitsaktsis, Y. Li, D. R. Gosselin, Y. Lin, N. V. Kurkure, D. W. Metzger, and E. J. Gosselin. 2008. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J. Immunol. 1805548-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansonetti, P. J., and A. Phalipon. 1999. M cells as ports of entry for enteroinvasive pathogens: mechanisms of interaction, consequences for the disease process. Semin. Immunol. 11193-203. [DOI] [PubMed] [Google Scholar]
- 47.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 107689-701. [DOI] [PubMed] [Google Scholar]
- 48.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107702-714. [DOI] [PubMed] [Google Scholar]
- 49.Sebastian, S., S. T. Dillon, J. G. Lynch, L. T. Blalock, E. Balon, K. T. Lee, L. E. Comstock, J. W. Conlan, E. J. Rubin, A. O. Tzianabos, and D. L. Kasper. 2007. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect. Immun. 752591-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen, H., W. Chen, and J. W. Conlan. 2004. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine 222116-2121. [DOI] [PubMed] [Google Scholar]
- 51.Silin, D. S., O. V. Lyubomska, V. Jirathitikal, and A. S. Bourinbaiar. 2007. Oral vaccination: where we are? Expert Opin. Drug Deliv. 4323-340. [DOI] [PubMed] [Google Scholar]
- 52.Sjöstedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 1421369-1374. [DOI] [PubMed] [Google Scholar]
- 53.Stenmark, S., H. Lindgren, A. Tärnvik, and A. Sjöstedt. 2003. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb. Pathog. 3573-80. [DOI] [PubMed] [Google Scholar]
- 54.Tärnvik, A. 1989. Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11440-451. [PubMed] [Google Scholar]
- 55.Titball, R. W., A. Johansson, and M. Forsman. 2003. Will the enigma of Francisella tularensis virulence soon be solved? Trends Microbiol. 11118-123. [DOI] [PubMed] [Google Scholar]
- 56.Twine, S. M., H. Shen, J. F. Kelly, W. Chen, A. Sjostedt, and J. W. Conlan. 2006. Virulence comparison in mice of distinct isolates of type A Francisella tularensis. Microb. Pathog. 40133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkheimer, G. 1974. Passage of particles through the wall of the gastrointestinal tract. Environ. Health Perspect. 9215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker, R. I. 1994. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine 12387-400. [DOI] [PubMed] [Google Scholar]
- 59.Wayne Conlan, J., H. Shen, R. KuoLee, X. Zhao, and W. Chen. 2005. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an αβ T cell- and interferon gamma-dependent mechanism. Vaccine 232477-2485. [DOI] [PubMed] [Google Scholar]
- 60.Wolf, H. M., M. B. Fischer, H. Puhringer, A. Samstag, E. Vogel, and M. M. Eibl. 1994. Human serum IgA down regulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 831278-1288. [PubMed] [Google Scholar]
- 61.Wu, T. H., J. A. Hutt, K. A. Garrison, L. S. Berliba, Y. Zhou, and C. R. Lyons. 2005. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect. Immun. 732644-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, X., B. J. Hinnebusch, T. Trunkle, C. M. Bosio, Z. Suo, M. Tighe, A. Harmsen, T. Becker, K. Crist, N. Walters, R. Avci, and D. W. Pascual. 2007. Oral vaccination with Salmonella simultaneously expressing Yersinia pestis F1 and V antigens protects against bubonic and pneumonic plague. J. Immunol. 1781059-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yee, D., T. R. Rhinehart-Jones, and K. L. Elkins. 1996. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 1575042-5048. [PubMed] [Google Scholar]
- 64.Yu, J. J., E. K. Raulie, A. K. Murthy, M. N. Guentzel, K. E. Klose, and B. P. Arulanandam. 2008. The presence of infectious extracellular Francisella tularensis subsp. novicida in murine plasma after pulmonary challenge. Eur. J. Clin. Microbiol. Infect. Dis. 27323-325. [DOI] [PubMed] [Google Scholar]
- 65.Zogaj, X., S. Chakraborty, J. Liu, D. G. Thanassi, and K. E. Klose. 2008. Characterization of the Francisella tularensis subsp. novicida type IV pilus. Microbiology 1542139-2150. [DOI] [PubMed] [Google Scholar]
- 66.Zuercher, A. W. 2003. Upper respiratory tract immunity. Viral Immunol. 16279-289. [DOI] [PubMed] [Google Scholar]