Abstract
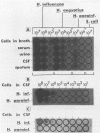
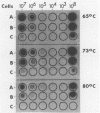
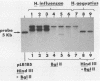
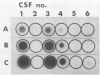
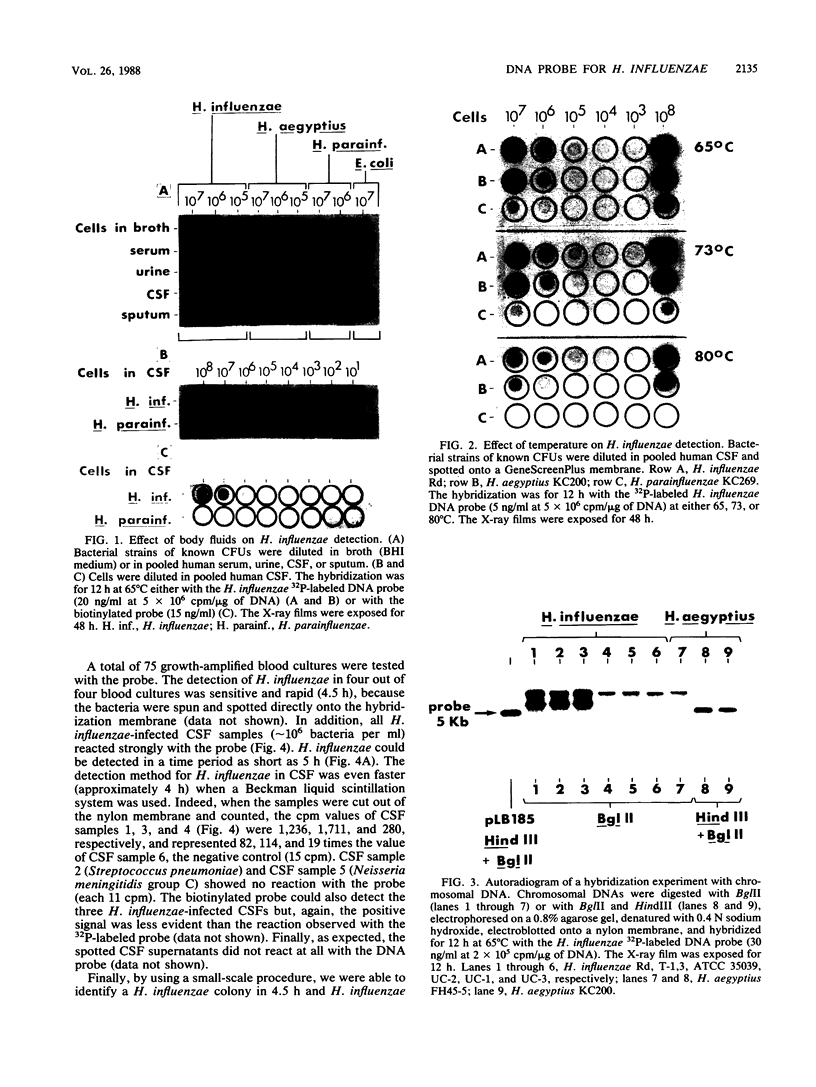
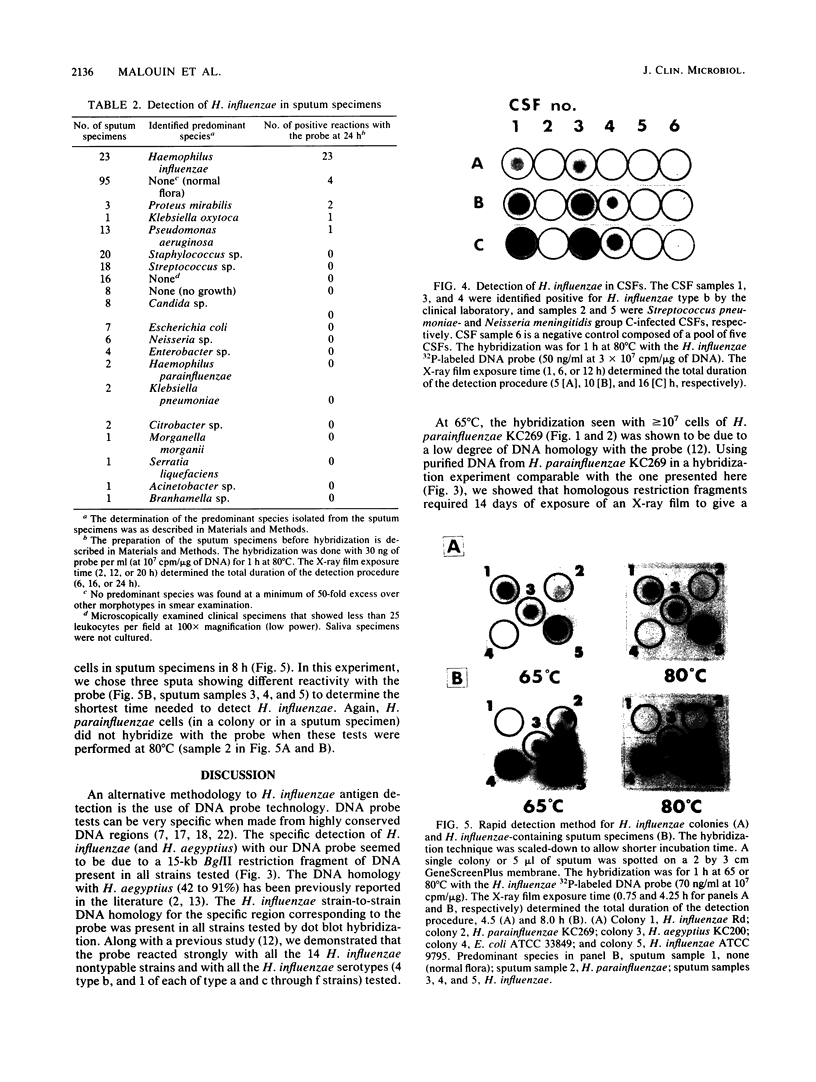
In a previous study, we reported that a 5-kilobase Haemophilus influenzae DNA fragment involved in penicillin-binding protein expression could be used as a probe for specific detection of H. influenzae strains (F. Malouin and L. E. Bryan, Mol. Cell. Probes 1:221-232, 1987). Here, we report the ability of this probe to detect H. influenzae in clinical specimens. In a bacterial dot experiment, there was strong hybridization of the 32P-labeled probe to nonencapsulated and serotype a through f H. influenzae strains. The detection of H. influenzae in body fluids was then evaluated by using pooled human serum, urine, cerebrospinal fluid, and sputum as dilution media for H. influenzae, Haemophilus aegyptius, Haemophilus parainfluenzae, and Escherichia coli cells. At 65 degrees C, the probe hybridized to H. influenzae and H. aegyptius (greater than or equal to 10(5) cells) in all fluids. There was no hybridization with the E. coli negative control, and H. parainfluenzae hybridized when greater than or equal to 10(7) cells were used. Experiments performed at 73 and 80 degrees C permitted elimination of H. parainfluenzae hybridization. The detection of H. influenzae in 232 sputa from patients with respiratory tract infections was very specific (96 to 97%) and sensitive (74 to 100%) when the total time of the procedure was sufficient (6 to 24 h) and when the experiments were performed at 80 degrees C. In addition, the probe detected three of three and four of four H. influenzae-infected cerebrospinal fluids and blood cultures, respectively, and did not react with pneumococcus- or streptococcus-infected cerebrospinal fluids. Finally, by using a small-scale procedure, the probe rapidly detected H. influenzae in cerebrospinal fluid and sputum specimens (4 and 8 h, respectively). These results imply prompt diagnosis of H. influenzae infections caused by nonencapsulated and serotype a through f strains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L. Infections due to Haemophilus species other than H. influenzae. Annu Rev Microbiol. 1982;36:199–216. doi: 10.1146/annurev.mi.36.100182.001215. [DOI] [PubMed] [Google Scholar]
- Albritton W. L., Setlow J. K., Thomas M., Sottnek F., Steigerwalt A. G. Heterospecific transformation in the genus Haemophilus. Mol Gen Genet. 1984;193(2):358–363. doi: 10.1007/BF00330693. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Finegold S. M. Bacteriology of expectorated sputum with quantitative culture and wash technique compared to transtracheal aspirates. Am Rev Respir Dis. 1978 Jun;117(6):1019–1027. doi: 10.1164/arrd.1978.117.6.1019. [DOI] [PubMed] [Google Scholar]
- Collins J. K., Kelly M. T. Comparison of Phadebact coagglutination, Bactogen latex agglutination, and counterimmunoelectrophoresis for detection of Haemophilus influenzae type b antigens in cerebrospinal fluid. J Clin Microbiol. 1983 Jun;17(6):1005–1008. doi: 10.1128/jcm.17.6.1005-1008.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drow D. L., Maki D. G., Manning D. D. Indirect sandwich enzyme-linked immunosorbent assay for rapid detection of Haemophilus influenzae type b infection. J Clin Microbiol. 1979 Oct;10(4):442–450. doi: 10.1128/jcm.10.4.442-450.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Eisenstein B. I. The impact of new cloning techniques on the diagnosis and treatment of infectious diseases. N Engl J Med. 1984 Oct 4;311(14):892–901. doi: 10.1056/NEJM198410043111406. [DOI] [PubMed] [Google Scholar]
- Jablonski E., Moomaw E. W., Tullis R. H., Ruth J. L. Preparation of oligodeoxynucleotide-alkaline phosphatase conjugates and their use as hybridization probes. Nucleic Acids Res. 1986 Aug 11;14(15):6115–6128. doi: 10.1093/nar/14.15.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen M., Käyhty H. Comparison of counter-current immunoelectrophoresis, latex agglutination, and radioimmunoassay in detection of soluble capsular polysaccharide antigens of Haemophilus influenzae type b and Neisseria meningitidis of groups A or C. J Clin Pathol. 1978 Dec;31(12):1172–1176. doi: 10.1136/jcp.31.12.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malouin F., Bryan L. E. DNA probe technology for detection of Haemophilus influenzae. Mol Cell Probes. 1987 Sep;1(3):221–232. doi: 10.1016/0890-8508(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Martel A. Y., Sottnek F. O., Thomas M. L., Albritton W. L. Susceptibility of Haemophilus aegyptius to trooleandomycin: lack of taxonomic value. Can J Microbiol. 1986 Apr;32(4):289–293. doi: 10.1139/m86-059. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987 Jan-Feb;9(1):1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- Parr T. R., Jr, Bryan L. E. Mechanism of resistance of an ampicillin-resistant, beta-lactamase-negative clinical isolate of Haemophilus influenzae type b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C. Studies of antimicrobial resistance genes using DNA probes. Antimicrob Agents Chemother. 1986 May;29(5):721–725. doi: 10.1128/aac.29.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra W. J., Schoone G. J., ter Schegget J., van Nierop J. C., Griffioen R. W. In situ hybridization for the detection of Haemophilus in sputum of patients with cystic fibrosis. Scand J Infect Dis. 1987;19(6):641–646. doi: 10.3109/00365548709117199. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Hensel D. Evaluation of Bactogen and Phadebact for detection of Haemophilus influenzae type b antigen in cerebrospinal fluid. J Clin Microbiol. 1982 Nov;16(5):905–908. doi: 10.1128/jcm.16.5.905-908.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwadyk P., Jr, Cooksey R. C. Nucleic acid probes in clinical microbiology. Crit Rev Clin Lab Sci. 1987;25(1):71–103. doi: 10.3109/10408368709105878. [DOI] [PubMed] [Google Scholar]