Viruses are intracellular parasites that require host cell functions to reproduce. They must modify the host cell environment to maximize their replication and avoid the activation of antiviral responses, while keeping the cell alive long enough to produce viral progeny. Accordingly, many of the viral oncogene products of DNA tumor viruses carry out functions which disrupt cellular processes, such as apoptosis and cell cycle checkpoint activation. By studying these genes and the proteins they encode, it is possible to gain valuable insights into the workings of fundamental cellular pathways, many of which are deregulated in cancer cells. For example, the tumor suppressor protein p53 was originally identified in complexes with the simian virus 40 large tumor antigen (87, 91). The potential significance of p53 was highlighted by the observation that a tumor antigen from another DNA virus, the adenovirus (Ad) large early region 1B 55-kDa protein (E1B-55K protein), also interacts with it (129).
Ads are a family of small DNA tumor viruses that have been extensively studied at the molecular level for over 30 years. At least 50 human serotypes (subdivided into groups A to F) have been identified to date, and much is now known about the mechanisms these viruses use to deregulate host cell functions to promote their replication. Although they are not currently thought to cause human cancers, certain Ad serotypes, such as the oncogenic type 12 virus (Ad12), can cause tumors in newborn rodents (142). In addition, Ads have been used as a model system to study the process of cell transformation, after it was shown that DNA from human Ads can transform primary rodent cells in vitro (42, 106). By convention, most Ad proteins are termed early (E1A, E1B, E2A, E2B, E3, and E4) or late (L1 to L5) (80), although this nomenclature is not strictly accurate, as L1 transcripts are detectable within 1 h postinfection before the early-to-late switch at 6 to 8 h postinfection (133). The E1A region is the first transcription unit to be activated following Ad infection (113, 133); however, although E1A alone can partially transform cells, complete and stable transformation requires complementation by the E1B region (6, 12, 45, 46, 56, 127, 145, 146).
The function of Ad E1A during viral infection is relatively straightforward, although its mode of action is complex and multifaceted. It stimulates the infected cell to enter S phase and is required to promote the transcription of other early viral genes (10, 43, 116). However, the function of the E1B-55K protein, is much more difficult to summarize simply. During viral infection, it has a number of roles, but it generally acts in combination with the Ad E4 open reading frame 6 (E4orf6) protein. The E1B-55K and E4orf6 proteins regulate the degradation of cellular proteins which may have a detrimental effect on viral replication, and they are involved in the transport of late viral mRNAs. To complicate matters, during Ad E1-mediated transformation, the E1B-55K protein functions (in the absence of the E4orf6 protein) as a cooperating oncogene with E1A, markedly increasing transformation frequency. Its mode of action in transformed cells, where it possibly acts primarily as a transcriptional repressor, is almost certainly quite different from that during viral infection.
A further significant difference between the E1B-55K protein and E1A proteins is the relatively limited number of cellular protein-binding partners identified for E1B-55K. While interest has focused on its interaction with p53, little is known about the functional significance of its other associations. In this review, we will consider in detail the properties of the E1B-55K protein, both during viral infection and in Ad E1-transformed cells. As far as we are aware, there have been no monographs dealing with the overall properties of E1B-55K, although particular aspects of its functions, either alone or in combination with those of other viral proteins, have been reviewed (10, 36, 41, 151). As we shall make clear, however, major questions about many aspects of the function of the E1B-55K protein remain unanswered.
AMINO ACID SEQUENCES IN THE E1B-55K PROTEIN
Sequence data are available for a large number of Ad E1B-55K proteins from diverse Ads. Of the human serotypes, there is at least one example from each subgroup (Fig. 1). Homology is least marked in the N-terminal 120 amino acids (aa) but is appreciable for the remainder of the protein. No evidence has so far been presented to indicate to what extent E1B-55K proteins are structured, although the Ad5 E1B-55K protein appears to be nonglobular, with an elongated structure (102). E1B-55K proteins appear to lack discrete structural domains, but they may adopt a delicate three-dimensional arrangement that is particularly prone to mutational inactivation. This statement is based on observations that small deletions/insertions scattered throughout the Ad5 E1B-55K protein interfere with p53 and E4orf6 protein interaction, indicating that binding sites probably consist of short sequences or single amino acids from disparate areas of the protein. In contrast, E1A has limited structure (62, 116). Importantly, because of its modular nature, it is possible to map precise binding sites on E1A for partner proteins, such as Rb, p300, and CtBP, with deletions of a particular site leaving other interactions relatively unaffected (43, 116). This mapping may not be possible for the E1B-55K protein.
FIG. 1.
Sequence alignment of E1B-55K proteins. A representative member of each subgroup is shown: Ad12 from group A, Ad11 from group B, Ad5 from group C, Ad9 from group D, Ad4 from group E, and Ad40 from group F. Letters with a black background are residues conserved among all subgroups; letters with a gray background are residues conserved among some subgroups. Alignments were produced and edited using the ClustalW and GeneDoc programs.
From a consideration of the amino acid sequences shown in Fig. 1, it can be seen that there is a concentration of highly conserved cysteine and histidine residues in the C-terminal half of the protein. In a number of the homologues, the spacing of the C and H residues is consistent with a zinc finger motif. For example, in all the E1B-55K proteins shown, a CX4CX16CX4C motif (aa 283 to 310 in Ad5) is present. It is also possible to distinguish C2H2 and C4 motifs unique to the Ad12 protein and to the subgroup C virus proteins (Ad1, -2, and -5) (41). Considerable time has elapsed since the unequivocal demonstration that Ad5 E1A binds zinc atoms (27), yet no definitive evidence has been presented to demonstrate zinc binding to any E1B-55K proteins. Indeed, we have been unable to show any binding of 61Zn to purified Ad5 or the Ad12 E1B-55K protein expressed in Escherichia coli (R.J.A.G., unpublished observations). In a comprehensive review of the structural features of zinc finger domains, it was noted that most zinc fingers comprise two or more β strands and a following α helix, although zinc ribbon domains tend to consist entirely of a series of β strands (84). In a structural prediction for the E1B-55K proteins (Jpred; available at http://www.compbio.dundee.ac.uk/∼www-jpred), no region of α helix was suggested to occur in close proximity to clusters of C and H residues. Zinc finger motifs have been implicated in protein binding and nucleic acid binding (24, 49, 98), both of which have been demonstrated for the Ad5 E1B-55K protein (73, 129). However, it is not clear that any interactions of the E1B-55K protein are directly mediated through zinc fingers. E1B-55K proteins with mutations in putative zinc finger motifs show defects in certain specific interactions, but they are not as stable as the wild-type (wt) protein and may also be defective in binding generally (see Table 1).
TABLE 1.
E1B-55K mutant proteins and their known phenotypesa
| E1B-55K proteinb | Protein stability | Substrate degradation
|
Substrate binding
|
E4orf6 binding | E1B-AP5 binding | Nuclear localization | Aggresome formation | Inhibition of viral genome concatemers | mRNA export and host cell shut-off | Late protein synthesis | Replication efficiency | Primary rodent cell transformation | Reference(s) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p53 | MRN complex | Ligase IV | p53 | MRN complex | ||||||||||||
| wt | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| H17in | + | ND | ND | ND | + | ND | + | + | ND | ND | ND | ± | ± | − | − | 44, 77, 126, 155 |
| L83/L87/L89A | + | ± | ± | + | + | + | ND | ND | ++ | − | ND | + | + | + | ++ | 37, 79, 132 |
| K104R | + | + | ± | ND | ND | + | ND | ND | + | + | ND | + | + | + | − | 35, 79 |
| NES/K104R | + | + | ± | ND | ND | + | ND | ND | + | + | ND | + | + | + | ND | 79 |
| A143in | + | ND | ND | ND | + | ND | − | + | ++ | ND | ND | ± | ± | ± | ++ | 44, 50, 77, 126, 155 |
| H180in | ± | ND | ND | ND | − | − | − | − | − | − | ND | − | − | − | ± | 44, 50, 77, 93, 126, 155 |
| H215in | − | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | − | − | ± | − | 50, 155 |
| H224in | + | + | + | + | + | + | ± | ± | ± | + | + | ± | ± | ± | ± | 44, 50, 77, 126, 132, 155 |
| R240A | + | − | + | + | − | + | + | ND | + | + | + | + | + | + | ND | 22, 86, 132, 134 |
| H260A | + | − | ND | ND | − | ND | − | ND | − | ND | ND | ± | + | ± | ND | 115, 134 |
| A262in | ± | − | ND | ND | − | − | − | − | − | − | ND | − | − | − | − | 44, 50, 77, 93, 119, 126, 155 |
| R309in | ± | − | ND | ND | − | − | − | − | − | − | ND | − | − | − | − | 44, 50, 77, 93, 119, 126, 155 |
| H326in | ± | − | ND | ND | − | − | − | − | − | − | ND | ± | ± | − | ± | 44, 50, 77, 93, 126, 136, 155 |
| H354in | + | + | − | + | + | − | + | ± | + | + | + | ± | ± | ± | ± | 22, 44, 50, 77, 86, 93, 126, 155 |
| C361/C366S | ± | − | − | − | ± | − | ND | ND | ± | + | − | ND | ND | ND | ND | 132 |
| H373A | + | + | ± | + | + | − | ND | ND | + | + | + | ND | ND | ND | ND | 132 |
| S380in | + | + | + | ND | ± | ND | + | − | − | + | ND | + | + | + | ± | 44, 50, 77, 93, 126, 155 |
| R443in | + | ± | − | ± | + | − | + | + | ± | ± | − | ± | ± | − | − | 35, 44, 50, 77, 126, 132, 136, 155 |
| Y444A | + | + | ± | + | + | ± | ND | ND | ± | + | + | ND | + | ± | ND | 132, 134 |
| C454/C456S | ± | − | − | − | + | − | ND | ND | ± | + | − | ND | ND | ND | − | 69, 132 |
| H474in | − | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | − | − | − | ± | 50, 155 |
| F484in | ± | ND | ND | ND | ± | ND | + | − | − | ND | ND | ± | ± | ± | + | 44, 50, 77, 126, 155 |
| S490/S491A | + | + | ± | + | + | ND | + | + | + | ND | ND | + | ± | − | − | 44, 119, 126, 132, 140 |
| S490/S491/T495A | + | − | − | − | − | − | + | ND | ± | − | − | ND | ND | ND | − | 69, 119, 132, 141 |
| S490/S491/T495D | + | ± | + | + | + | + | ND | ND | + | + | + | ND | ND | ND | ND | 132 |
List of E1B-55K mutants and their known properties in viral infection and cell transformation. Abbreviations: +, proficient; ±, partially defective; −, severely defective; ND, not determined.
The “in” suffix indicates an insertion mutant.
Even though there is no unequivocal evidence associating the cysteine residues in the E1B-55K protein with zinc binding, it is clear that they are involved in disulfide bond formation, both within the protein and between protein molecules, giving rise to dimers and multimers. It has been suggested that the E1B-55K protein is present largely as a dimer or tetramer, with the interactions being stabilized by disulfide linkages (59, 102). Implications of this for the properties of the protein are not clear at present.
It has been known for a considerable time that the Ad5 E1B-55K protein shuttles between the cytoplasm and the nucleus. It was believed that this was a function of interaction with the E4orf6 protein in infected cells (32, 33). However, it is now clear that there is a well-defined nuclear export signal (NES) of the human immunodeficiency virus type 1 Rev type in the Ad5 E1B-55K protein (Fig. 2), minimally comprising aa 83 to 93 (LYPELRRILTI) (32, 33, 83). Proteins in which the leucines in this sequence have been mutated to alanines remain in the nucleus (83). This particular sequence is highly conserved in E1B-55K proteins from group B1, B2, C, D, and E Ads (Ad1, -2, -3, -4, -5, -7, -9, -11, and -35) but not in Ad12 and Ad40 (from groups A and F, respectively). This finding may go some way toward explaining the nuclear localization of the Ad12 E1B-55K protein and the predominantly cytoplasmic/aggresomal localization of the Ad5 E1B-55K protein in Ad E1-transformed cells (16, 158). Deletional analysis has suggested the presence of a nuclear localization signal (NLS) in the C-terminal third of the Ad5 E1B-55K protein, but no specific identification of the essential amino acids has been reported (83). However, it appears that a short sequence (228GDKFKGIMFEAN239) in the Ad12 E1B-55K protein includes an NLS (61). Presumably, the lysine residues are of particular importance for nuclear localization, but this has not been confirmed.
FIG. 2.
Linear representation of the Ad5 E1B-55K protein. Arrows indicate residues known to be sumoylated (K104) or phosphorylated (S490/S491/T495). Gray boxes indicate different motifs in the E1B-55K polypeptide. RNP, RNP motif; C2H2, putative zinc finger.
E1B-55K POSTTRANSLATIONAL MODIFICATIONS
Two posttranslational modifications of the Ad5 E1B-55K protein have been identified (Fig. 2). The Ad5 E1B-55K protein is phosphorylated on serines 490 and 491, probably by casein kinase 2, and on threonine 495, which is a consensus site for casein kinase 1 (140, 141). Mutation of S490 and S491 to alanines decreases viral replication efficiency and reduces the transformation frequency of rat cells in combination with E1A (140). Although binding to p53 appears to be unaffected, the mutation of these serine residues reduces the ability of the protein to inhibit p53 transactivation (140). When all three phosphorylation sites are mutated, the protein loses both its transcriptional repression properties in in vitro assays and its ability to block E1A-induced p53-mediated apoptosis (141). This may be because although the S490/S491/T495A phosphomutant can still interact with E4orf6, it has lost the ability to bind to p53 and other cellular proteins (132). It has been concluded that inhibition of apoptosis depends on transcriptional repression by the E1B-55K protein and that this is regulated by C-terminal phosphorylation events (141); however, it is far from clear how phosphorylation affects the interaction of the Ad5 E1B-55K protein with the cellular proteins presumably required for repression. Significantly, Ad12 E1B-55K does not appear to be phosphorylated, either during viral infection or in Ad12 E1-transformed cells (R.J.A.G., unpublished). However, the residues equivalent to the Ad5 S490/S491 residues are conserved in the Ad12 protein, although T495 is not (Fig. 1).
Recently, a second modification has been demonstrated. The Ad5 E1B-55K protein is sumoylated on lysine 104, and mutation of that amino acid reduces the ability of the protein to complement E1A in transformation assays (35). Chromosome region maintenance 1 (CRM1) is the major export receptor for the Ad5 E1B-55K protein, and inactivation of its CRM1-dependent NES results in complete redistribution of the E1B-55K protein from the cytoplasm to the nucleus, where it accumulates at the periphery of viral replication centers (79). However, if SUMO1 conjugation is prevented, E1B-55K with a mutated NES is still present in the cytoplasm. It has been concluded that SUMO1 conjugation is a process which determines the commitment of the Ad5 E1B-55K protein to a CRM1-independent export pathway (79). The consensus sumoylation site (ψKXE, where X is any hydrophobic residue) is present in E1B-55K proteins from some Ad serotypes (2, 5, 7, and 9) but not others (12, 40, and 41) (Fig. 1). Significantly, when the Ad2/Ad5 sequence was mutated to resemble Ad12 (VKRE→DKRE), the E1B-55K protein failed to transform and to inhibit p53 transactivation (35). It is possible that SUMO1 binding is required only for those E1B-55K proteins which do not localize to the nucleus, such as those for Ad2 and Ad5, but this will have to await confirmation. Despite considerable insights provided into the properties of Ad5 E1B-55K, it is not clear to what extent these observations apply to E1B-55K proteins from other viral serotypes, especially as the Ad12 homologue is probably not phosphorylated or sumoylated.
ROLE OF THE E1B-55K PROTEIN DURING INFECTION
During viral infection, the Ad E1B gene products are required to promote efficient viral replication via a number of different mechanisms. Ad5 E1B encodes at least five gene products from a group of mRNAs by alternative splicing; one of these gene products is the E1B-55K protein (135). The role of this protein during viral infection has been characterized by using mostly the Ad5 serotype, but at least some of its functions appear to be conserved in the large E1B proteins of other serotypes, including Ad12 (61, 139).
(i) Interaction with E4orf6.
In infected cells, the E1B-55K protein performs early and late functions that are essential for viral replication. Early functions include inactivation of p53 and prevention of cellular DNA damage responses. Late functions include the simultaneous inhibition of cellular mRNA export and promotion of viral mRNA export and translation. Most early and late functions of the E1B-55K protein appear to require an interaction with the E4orf6 protein; in support of this, mutant E1B-55K and E4orf6 viruses display similar phenotypes (2, 3, 5, 28, 65, 117, 119, 136, 138).
The Ad5 E1B-55K protein displays a complex localization pattern in infected human cells and requires E4orf6 protein for its nuclear localization and recruitment to viral replication centers (32, 52, 114). However, E4orf6 alone is not sufficient to relocalize the E1B-55K protein to the nucleus; another cellular primate-specific factor, RUNX1, is also required, although the functional consequences of this requirement have yet to be determined (101).
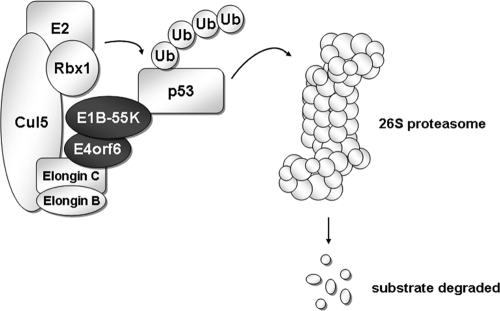
In Ad5-infected cells, the E1B-55K and E4orf6 proteins together form an E3 ubiquitin ligase complex, consisting of the cellular proteins cullin 5 (Cul5), RING-box 1 (Rbx1), and elongins B and C (Fig. 3) (68, 120). E4orf6 is required to form this complex by interacting with elongin C, while the E1B-55K protein has been proposed to be its substrate recognition unit (17). Known cellular early targets of this viral ubiquitin ligase are p53, the MRE11-Rad50-NBS1 (MRN) DNA damage recognition/repair complex, and DNA ligase IV, a protein essential for DNA repair by nonhomologous end joining (5, 120, 138).
FIG. 3.
Ad5 E1B-55K and E4orf6 proteins form an E3 ubiquitin ligase complex that targets substrates such as p53 for proteasomal degradation. E2, ubiquitin-conjugating enzyme; Ub, ubiquitin.
The E1B-55K/E4orf6 ubiquitin ligase also appears to be required for at least some of the late functions of E1B-55K, which involve the simultaneous inhibition of host cell mRNA export and promotion of late viral mRNA transport from the nucleus (9, 18, 154). In infected cells, some E1B-55K and E4orf6 proteins accumulate in and around viral DNA replication and RNA transcription sites (114). The complex has also been shown to actively shuttle between the nucleus and cytoplasm (32, 33).
It has previously been suggested that during Ad infection, E1B-55K and E4orf6 proteins relocalize a cellular protein required for mRNA export from host transcription sites to the periphery of viral replication/transcription centers (114). This would explain how E1B-55K and E4orf6 proteins promote viral mRNA export while simultaneously inhibiting cellular mRNA transport. This model has since been modified to take into consideration the fact that the majority of E1B-55K and E4orf6 proteins exist as an E3 ubiquitin ligase complex. In this updated model, E1B-55K and E4orf6 proteins ubiquitylate a cellular protein, possibly a messenger RNP or a related molecule involved in mRNA transport, which results in its degradation by the proteasome and leads to inhibition of most cellular mRNA export and promotion of viral mRNA export (10). However, a cellular target for E1B-55K protein-mediated ubiquitylation that might promote these functions has not yet been identified.
The precise mechanisms that E1B-55K and E4orf6 proteins use to promote their different functions are still not entirely clear. It has been suggested that all E1B-55K/E4orf6 early and late functions are due to viral ubiquitin-protein ligase activity (18, 154). This idea is supported by the observation that most of the cellular pool of E1B-55K protein is found in a complex with E4orf6 (68, 130). In addition, further work has shown that E4orf6 requires active proteasomes to promote late gene expression (26) and that an E4orf6 mutant unable to form the ubiquitin-protein ligase complex essentially behaves the same as an E4orf6-null virus in terms of its late functions (18). Finally, Ad5 infection of cells expressing a dominant-negative Cul5 mutant fails to induce p53 or MRN degradation, but it also produces a defect in viral nuclear mRNA export and late protein synthesis, which is similar to the phenotype displayed by E1B-55K mutant viruses (154). These results strongly suggest that the majority of E1B-55K and E4orf6 protein functions are linked to viral ubiquitin-protein ligase activity.
(ii) Inactivation of cellular DNA damage responses.
The Ad genome is linear and therefore has double-stranded DNA termini that may represent DNA double-strand breaks (DSBs) to an infected host cell (150). In addition, viral replication intermediates may present the cell with unusual DNA structures which could conceivably activate cellular DNA damage response pathways (150). The first indication that Ad DNA might be recognized by the cell's DNA damage response machinery came from the study of Ad5 E4 mutants. Viruses lacking the E4 region (ΔE4 viruses) are defective in viral replication (65). This defect may be due in part to the fact that ΔE4 viruses produce large concatemers of viral DNA, which correspond to covalently linked viral genomes (149). Without E4 gene products, infected cells recognize viral genomes as DSBs and actively attempt to “repair” them (151).
Ad5 genome concatenation is prevented specifically by expression of either the E4orf3 or E4orf6 proteins, indicating that these proteins interfere with DNA repair processes (149). The major mechanism through which E4 proteins interfere with the DNA damage response and prevent genome concatenation is by inactivation of the MRN complex (22, 138). MRN levels in Ad-infected cells decrease rapidly in an E1B-55K and E4orf6 protein-dependent manner (138). Therefore, at least one subunit of the MRN complex is likely to be a substrate for the E1B-55K/E4orf6 ubiquitin ligase. However, although E1B-55K antibodies have been shown to coimmunoprecipitate MRN subunits (22), a direct interaction between E1B-55K and MRN subunits has not yet been demonstrated, nor have MRN subunits been shown to be ubiquitylated by the E1B-55K/E4orf6 complex.
Individuals with hypomorphic mutations in MRE11 suffer from ataxia-telangiectasia-like disorder (ATLD), with patients' cells that express no detectable MRE11 protein showing considerably reduced levels of Rad50 and NBS1 (137). Conversely, Nijmegen breakage syndrome patients, or individuals whose cells contain hypomorphic mutations in RAD50 that result in low levels of translated protein, still express wt or slightly reduced levels of MRE11 (21, 82), suggesting that MRE11 plays a unique role in maintaining MRN complex stability. ATLD patient cells expressing virtually no detectable MRE11 protein and low levels of Rad50 and NBS1 show no further reduction in the expression levels of these proteins during Ad infection, making it likely that MRE11 is the E1B-55K/E4orf6 protein degradation target within the MRN complex (132). It remains to be seen whether MRE11 is a direct ubiquitylation target or whether the complex is degraded through an indirect mechanism such as inactivation of a ubiquitin-specific protease by E1B-55K/E4orf6 protein.
End joining of viral genomes suggests that infection with a ΔE4 virus activates a cellular DNA damage response. The DNA damage response is controlled by two principal kinases, ataxia-telangiectasia mutated kinase (ATM) and ATM- and Rad3-related kinase (ATR). Analysis of phosphorylation events characteristic of an activated DNA damage response demonstrates that ATM and ATR are activated during infection with Ad5 ΔE4 mutants (22). Activated ATM and the MRN complex accumulate at viral replication centers in cells infected with ΔE4 mutants, but these proteins do not accumulate at viral replication centers in cells infected with wt Ad5 (22), consistent with a requirement for NBS1 in recruiting ATM to sites of DSBs (39, 88).
Viral genome concatemers are not observed during ΔE4 infection of cells expressing mutant DNA ligase IV (138). Recently, it has been demonstrated that DNA ligase IV is degraded in an E1B-55K/E4orf6-dependent manner during Ad5 infection (5). As with the MRN complex, it remains to be seen whether the protein is a direct substrate of the Ad ubiquitin ligase; nonetheless, these data highlight the importance of inactivating DSB repair pathways at multiple levels to promote viral replication.
(iii) Interaction with E4orf3 and localization in cytoplasmic aggresomes.
Normal human cells each have around 5 to 30 promyelocytic leukemia (PML) bodies (also called nuclear domain 10 or PML oncogenic domains), but their cellular functions are still not clear (30). It has been proposed that PML bodies are highly sensitive DNA damage sensors, among other things (31). Certainly, a number of critical DNA damage response proteins are constituents of PML bodies, including components of the MRN complex (94). During Ad infection, PML bodies are disrupted and PML is relocalized into nuclear track-like structures of discrete lengths in a manner which depends on the Ad E4orf3 protein (23, 34). Although E1B-55K interacts with E4orf6 at viral replication centers in infected cells, the protein also transiently localizes to the same nuclear tracks containing the E4orf3 protein and PML (34, 114). Subsequently, it has been shown that the E1B-55K protein interacts with E4orf3 and that this interaction is required for E1B-55K to localize to PML-containing nuclear tracks during Ad5 infection (89).
Cells infected with a mutant Ad5 virus lacking E1B-55K and E4orf6 proteins express normal levels of MRE11, Rad50, and NBS1 but still avoid genome concatenation (138). It has been shown that, like PML, E4orf3 relocalizes the MRN complex during Ad5 infection and in isolation (38, 138). This ability can be separated from PML reorganization and appears to be specific to the Ad5 E4orf3 protein, as Ad4 and Ad12 E4orf3 proteins cannot relocalize MRN components (139). Thus, Ad5 E4orf3 is able (in the absence of the E1B-55K protein) to prevent DNA repair by nonhomologous end joining and, therefore, viral genome concatemer formation by promoting the mislocalization of the MRN complex.
In both Ad5-infected and -transformed cells, a proportion of the E1B-55K protein is localized in large juxtanuclear bodies in the cytoplasm, which also contain p53 (93, 114, 158). Recently, it has been suggested that these bodies are aggresomes, formed at the microtubule-organizing centers as a response to misfolded, aggregated proteins (1, 93). E4orf3, E4orf6, Cul5, and MRN components are also found with the E1B-55K protein in aggresomes, and MRN complex sequestration has been shown to greatly increase its proteasomal degradation by the E1B-55K/E4orf6 ubiquitin ligase complex (1, 93). Thus, during Ad5 infection, the MRN complex is initially relocalized into PML-containing nuclear tracks by E4orf3 (38), where it subsequently binds to the E1B-55K protein. Complexes containing MRN, E1B-55K, E4orf3, and E4orf6 proteins are then transported out of the nucleus into cytoplasmic aggresomes to promote rapid MRN degradation (93).
It is interesting that Ad4 and Ad12 E1B mutant viruses replicate less well than Ad5 mutants (86, 143). It has been proposed that this growth advantage may be due to the differences between the Ad5 E4orf3 protein and the proteins of other serotypes (86), as Ad5 E4orf3 can relocalize MRN components, whereas Ad4 and Ad12 proteins cannot (139).
(iv) Effects on cell cycle regulation.
It is now widely accepted that a major function of Ad E1A during viral infection is to ensure progression of the cell into S phase to facilitate replication. However, it appears that the E1B-55K protein can also contribute to deregulation of the cell cycle during lytic infection (53, 55). For example, infection of HeLa cells in G1 phase with an E1B mutant virus produced only low levels of progeny, whereas infection during S phase gave rise to progeny levels comparable to those seen following wt infection (53). The observation that an E4orf6 mutant virus has a similar phenotype indicates that cell cycle restriction on viral replication is probably linked to functions of the E1B-55K/E4orf6 complex (55). Whether this indicates the existence of another cellular target for viral ubiquitin ligase activity is unknown. The E1B-55K protein may also interfere with normal cell cycle control through the upregulation of cyclin E to promote viral replication (161).
ROLE OF THE E1B-55K PROTEIN IN CELL TRANSFORMATION AND TUMORIGENESIS
In this section, we concentrate primarily on properties of the E1B-55K protein in the absence of E4orf6. It was originally shown that transformation of mammalian cells can be achieved with DNA fragments encompassing only the Ad E1 region, contained within the 5′ end of the Ad2/Ad5 genomes (45, 56). Although rodent cells can be transformed, to a limited extent, with DNA encoding just E1A or E1A together with the E1B-19K protein, it is much more efficient if the E1B-55K protein is also expressed (19, 46, 97, 144). Transformation of baby rat kidney (BRK) cells with E1A and either one of two E1B proteins (19K or 55K) is also appreciably less efficient than if the entire Ad E1 region is used, suggesting perhaps that the E1B-55K protein is required for more than just an antiapoptotic function (13, 107, 152). Significantly, transformation of human cells is only possible with the full Ad E1 region (19, 47, 153). In tumorigenicity assays, when transformed BRK cells are introduced into the syngeneic host, Ad2/Ad5 E1-expressing cells do not produce tumors. However, the equivalent Ad12 transformed cells do (42, 100). This is essentially due to the ability of the Ad12 E1A to downregulate major histocompatibility complex class I expression on the cell surface (12). When introduced into immunocompromised animals (e.g., nude mice), however, both sets of Ad E1-transformed cells induce tumors. In this case, the source of the E1B-55K protein is significant, such that substitution of the Ad12 E1B-55K protein for the Ad5 homologue increases the frequency of tumor production from 25% or less of the animals to 100%, as well as reducing the tumor latency period by about one-half (11). Furthermore, rat cells expressing only E1A and E1B-19K have limited or no tumorigenicity in nude mice, again suggesting a major role for the E1B-55K protein (46, 76).
Although rodent cells can be efficiently transformed with the Ad5 E1 region, the transformation frequency can be increased by the addition of the E4 region encoding E4orf3 and/or E4orf6 (108, 111, 112). In addition, the Ad5 E1A/E1B/E4orf6-transformed cells are appreciably more tumorigenic in nude mice (108, 111). Whether this property of the cells is determined by the increased ability of E1B-55K and E4orf6 proteins to target p53 and/or the MRN complex for degradation is unknown.
The role of the E1B-55K protein in transformation and tumorigenesis is not clear. Certainly, the Ad5 protein binds p53 strongly, translocates much of it to cytoplasmic bodies (aggresomes), and inhibits its transcriptional activation properties, as discussed later (156). Similarly, the Ad12 E1B-55K protein also inhibits p53 transcriptional activation, probably through a relatively weak association; p53 is retained in the nucleus in this case. In addition to its inhibition of p53 activity, the Ad12 E1B-55K protein also increases p53 levels, irrespective of E1A, probably through a direct interaction which inhibits binding by MDM2, and it is likely that the Ad5 protein has the same effect (60).
It is not known whether the other interactions attributable to the E1B-55K protein contribute to transformation and/or the transformed phenotype. It has been shown that the expression of E1B-55K-associated protein 5 (E1B-AP5) or PML can reduce Ad5 E1-mediated transformation of rat cells (44, 112), suggesting that these cellular proteins might be targets, but as yet, no mechanism is apparent. However, based on the mutational analysis of Ad5 E1B-55K with respect to transformation, it appears that there is no absolute correlation between any of its interactions with cellular proteins and the ability of mutant viruses to transform BRK cells (see Table 1). p53 binding and/or inhibition of its transcriptional activation properties have been considered necessary for transformation, but recent evidence suggests that this may not necessarily be the case (69). In these latter experiments, the transforming ability of the Ad5 E1B-55K protein was linked to MRN binding or to “nontranscriptional regulatory” properties, such as localization to aggresomes. As mentioned earlier, it has, however, been possible to link certain specific mutations to the loss or gain of transforming ability, such that phosphorylation of residues at the C terminus of the Ad5 protein and sumoylation of K104 are required, whereas loss of the NES increases transformation frequency (35, 141).
E1B-55K AND CELLULAR BINDING PROTEINS
Although the E1B-55K protein has evolved to optimize its activity in a complex with E4orf6, it has long been known to have distinct properties in isolation. As noted above, it can act as a cooperating oncogene together with E1A. This biological property presumably depends on the interactions of E1B-55K with cellular proteins.
(i) Interaction with p53.
The relationship of Ads to the tumor suppressor p53 is complex. Ad E1A increases p53 expression following DNA transfection and infection with ΔE1B-55K and ΔE4 viruses (29, 57, 95). The interaction of the Ad5 E1B-55K protein with p53 is well established (129), and it is apparent that a major role of the E1B-55K protein is to neutralize detrimental p53 activity. In the context of viral infection, this can be through p53 degradation by the E1B-55K/E4orf6 E3 ubiquitin ligase, but in transformed cells, the E1B-55K protein inhibits p53 transcriptional activity without degrading it due to the absence of E4orf6.
It has been widely assumed that the E1B-55K protein is necessary in infected cells to counter the proapoptotic effects of p53, although there is little direct evidence for this assumption. This is not least because many studies of cells infected with E1B mutant viruses have been carried out with HeLa cells that contain human papilloma virus E6, which degrades p53 via the ubiquitin ligase activity of E6-AP (75). Furthermore, some studies have shown that apoptosis is not induced even when p53 accumulates in ΔE1B-55K mutant-infected cells (20, 115); this is probably due to the presence of the E1B-19K protein. There is also some evidence that p53 might actually be required for efficient viral replication, possibly because wt p53 is necessary for lysis of infected cells (66, 123, 125).
There is some disagreement over whether p53 is transcriptionally active in Ad-infected cells in the absence of the E1B-55K protein (58, 60, 69, 72, 81, 115). It appears that p53 overexpressed as a result of infection with a ΔE1B virus is able to stimulate transcription as determined by using a reporter assay (58, 60) but is not able to promote the expression of downstream genes, such as p21 (72, 115). This may be due to the effects of E1A expression (72). Interestingly, it has been noted that even if the p53 in infected cells is not transcriptionally active, it can promote apoptosis in the absence of the E1B-19K protein, although this seems to have only a limited effect on viral replication (72). Clearly, the question of why the E1B-55K protein targets p53 during viral infection requires further investigation.
In Ad E1-transformed cells, the E1B-55K protein can inhibit transcriptional activation by p53 through direct interaction (59, 156). Binding of the E1B-55K protein to p53 blocks interaction with transcriptional coactivators and results in the recruitment of a strong repressor to p53 (102, 103, 157). This is largely because the complex has a much higher affinity for p53-binding sites than p53 alone (102). Inhibition of the protein's transcriptional activity results in attenuation of both p53-dependent cell cycle arrest and apoptosis (74).
In Ad E1-transformed cells, p53 levels are markedly elevated due to the action of both E1A and E1B-55K proteins (95, 147, 158). In the absence of E1A, high concentrations of p53 can be distinguished due to the action of the E1B-55K protein in increasing the protein half-life (60). The Ad5 E1B-55K protein binds close to the N terminus of p53 in a region similar to that used by MDM2, which stabilizes the protein and inhibits its transcriptional activity (90). The binding site for p53 on the E1B-55K protein is more difficult to define. A number of mutations in the central region of the E1B-55K protein negate binding, but it must be borne in mind that these are mainly insertion mutants which may destabilize the protein, as discussed below. However, when chimeric Ad2/Ad12 E1B-55K proteins were constructed and expressed, it was observed that the inclusion of Ad2 E1B-55K aa 216 to 235 in the Ad12 protein converted it to a strong p53 binder from a weak one (61). This is generally consistent with the results of mutational analysis and glutathione S-transferase pulldown experiments, which suggest a binding site between aa 155 and 261 for p53 in the Ad2/Ad5 protein (118, 134).
(ii) Interaction with E1B-AP5.
E1B-55K-AP5, also called hnRNP U-like protein 1 (HNRPUL1), is a cellular RNA-binding protein first identified in a screening for E1B-55K-binding proteins and has been shown to bind to the Ad5 E1B-55K protein in both infected and transformed cells (44). Subsequently, it was found to associate with tyrosine kinase-interacting protein-associated protein (TAP), also called nuclear export factor 1 (NXF1), a protein that interacts with the nuclear pore complex and is involved in mRNA export (4). E1B-AP5 also interacts with two TAP homologues, NXF2 and NXF3 (71). Furthermore, a number of groups have isolated E1B-AP5 as a component of several large RNA-processing complexes (25, 63, 70, 121). These results made E1B-AP5 a likely candidate target for E1B-55K protein-mediated proteasomal degradation; however, it is not degraded but is in fact upregulated at later stages of Ad5 or Ad12 infection (15). In addition, although E1B-AP5 is relocalized to viral replication centers during Ad infection, this relocalization is E1B independent (15). E1B-AP5 depletion by RNA interference in Ad-infected cells does not affect p53 or MRE11 degradation, viral replication, or late viral protein production (A.B., unpublished observations), making it unlikely that E1B-AP5 functionally associates with the E1B-55K/E4orf6 E3 ubiquitin ligase complex. The significance of the interaction between E1B-55K protein and E1B-AP5 during Ad infection therefore remains unknown at present. It may be that E1B-AP5 relocalization is part of a cellular DNA damage response activated by viral infection, especially as ATRIP, RPA, and other ATR-activating proteins are also redistributed to viral replication centers, and E1B-AP5 itself promotes ATR-dependent phosphorylation of RPA during infection (15).
E1B-AP5 is a multifunctional protein; in addition to its role in mRNA metabolism, it may play a part in transcriptional regulation via interactions with transcription factors such as bromodomain-containing protein 7 (BRD7), p53, and small and mothers against decaplentaplegic-related protein 2 (SMAD2) (7, 85, 96). By using transient reporter assays, it was shown that E1B-AP5 is a repressor of basal transcription driven by several cellular and viral promoters (85). The E1B-55K protein has also been shown to be a strong repressor of basal transcription (157), but interestingly, this activity requires a cellular corepressor which has not yet been identified (103). The E1B-55K protein interacts with a Sin3A/histone deacetylase 1 corepressor complex, but this is apparently not required for the transcriptional repression activity of the E1B-55K protein (118, 160). It is therefore tempting to speculate that the identity of this elusive corepressor is E1B-AP5, and it will be interesting to determine if this is the case.
(iii) Interaction with other cellular proteins.
Several proteins in addition to p53 and E1B-AP5 have been isolated as Ad5 E1B-55K protein-binding partners, although the biological significance of these interactions is still not clear. p53 is acetylated by transcriptional coactivators p300/CBP and the p300/CBP-associated factor (PCAF), which enhances the sequence-specific DNA-binding properties of p53 (64, 128). The E1B-55K protein interacts with p53 but has additionally been shown to bind to PCAF (92). This interaction has been suggested to inhibit p53 acetylation by PCAF and to reduce the DNA-binding ability of p53 in transformed cells through disruption of p53-PCAF binding (92).
The Ad5 E1B-55K protein also binds to the Wilms' tumor protein WT1, through its two N-terminal zinc fingers. p53, E1B-55K, and WT1 proteins form a trimeric complex localized in the aggresomes of Ad5 E1-transformed BRK and human embryonic kidney 293 cells (99). As a result of the interaction, the Ad protein is able to overcome growth inhibition by WT1. Similarly, it appears that the Ad5 E1B-55K protein localizes sequence-specific single-stranded DNA-binding protein 2 (SSBP2) to aggresomes in 293 cells (40). Interaction of the Ad5 E1B-55K protein with Daxx in 293 cell nuclei has also been reported (159). It appears that both the Ad12 and Ad5 proteins compete with PML for Daxx, localizing it away from PML bodies (148, 159). As the E1B-55K protein is a powerful transcriptional repressor, it has been suggested that this localization could be achieved through recruitment of additional repressor molecules. Indeed, the Ad2 E1B-55K protein interacts with an enzymatically active HDAC1/mSin3A complex (118), as mentioned earlier. Further studies have shown that the Ad12 E1B-55K protein interacts directly with mSin3A, but the Ad2 protein does not. Interaction occurs through a conserved leucine-rich motif close to the N terminus of E1B-55K proteins from Ad12, -7, -9, -25, and -40 (LXψLA/LXA/ψA; aa 30 to 37 in Ad12). However, mSin3A binding is not a prerequisite of E1B-55K transcriptional repression, although it may contribute to it (160).
It has recently been shown that in Ad-transformed cell lines, the E1B-55K protein induces sumoylation of p53 and that this requires both direct interaction of E1B-55K with p53 and the sumoylation of the E1B-55K protein itself (109). It would be interesting to determine whether the E1B-55K protein also stimulates sumoylation of some of its other cellular binding partners in Ad-infected or -transformed cells and whether this affects their functions.
MUTATIONAL ANALYSIS OF THE E1B-55K PROTEIN
(i) E1B-55K mutant viruses as oncolytic therapy.
It has been suggested that Ad E1B mutants could be used as oncolytic therapeutic agents (14). This suggestion was initially based on the idea that the E1B-55K-null mutant Ad5 virus dl1520 (also called ONYX-015) would replicate efficiently in tumor cells with deregulated p53 activity, but not in normal cells expressing wt p53 (14). However, it was subsequently shown that tumor selectivity of dl1520 is not linked to p53 status (54, 66, 67, 124, 143) but instead may rely in part on cancer cells to provide the mRNA export functions of the E1B-55K protein (115). It has also been reported that viral DNA replication is reduced >10-fold in primary human fibroblasts infected with a ΔE1B-55K mutant virus (51). This phenotype probably also contributes to the tumor cell selectivity of dl1520. Finally, some data suggest that p53 might actually be required for efficient viral replication, as mentioned above (66, 123, 125). Despite these findings, a number of phase II clinical trials using dl1520 have been carried out, with some success, for patients with head and neck cancer (78, 110, 122).
(ii) Effects of mutations on E1B-55K protein function.
Understanding the tumor selectivity of E1B-55K mutants is key to developing an effective oncolytic virus. It should be noted that mutations in dl1520 which block E1B-55K protein production also prevent transcription of three smaller, related E1B polypeptides, E1B-156R, -93R, and -83R proteins (6). It is therefore not possible to assign functions directly to the E1B-55K protein on the basis of phenotypes exhibited by dl1520. However, a number of E1B-55K protein insertion and point mutants have been developed to date, and their properties during infection and cell transformation have been studied. A list of these mutants, together with their known attributes and associated references, is shown in Table 1. At first glance, a number of mutations appear to give rise to proteins that have effectively lost all properties of the wt protein, with viruses carrying these mutations behaving like E1B-55K protein-null viruses. Such viruses include mutant Ad2/Ad5 hybrid proteins with insertions at residues H180, H215, A262, R309, and H474. As discussed previously, the E1B-55K protein is believed to be highly structured, and it is likely that amino acid insertions at these locations in its sequence disrupt its secondary and tertiary structure, giving rise to unstable or misfolded proteins that are dysfunctional and in some cases are more rapidly degraded (50, 93). It has thus not been easy to show definitively which residues or regions within E1B-55K are specifically required for particular functions.
However, a number of mutations introduced into the Ad2/Ad5 E1B-55K protein amino acid sequence clearly give rise to separation-of-function mutants. These include the R240A point mutant, which does not bind p53, and the H354 insertion mutant, which binds p53 and DNA ligase IV but not the MRN complex (22, 86, 132, 134). Consequently, the R240A mutant virus induces the MRN complex and DNA ligase IV but not p53 degradation, whereas the H354 mutant induces p53 and DNA ligase IV but not MRN complex degradation. Evidently, downregulation of individual substrates targeted by the E1B-55K protein to the viral ubiquitin ligase complex can occur separately, and substrate affinity is a major (but not the sole) determinant of E1B-55K protein-mediated degradation (132).
Until recently, it was unclear whether DNA damage response signaling and Ad genome concatemer formation, rather than the MRN complex directly, were limiting to viral DNA replication. However, by use of the R240A and H354 E1B-55K separation-of-function mutants, it has become clear that activation of DNA damage signaling and concatemer formation do not inhibit viral DNA replication (86). Both R240A and H354 mutants induce DNA ligase IV degradation and so prevent concatemer formation, but only R240A (which degrades MRN) can promote DNA replication (Table 1). It appears, therefore, that the MRN complex plays a direct role in limiting viral DNA replication (38, 86, 104). It has been shown that the binding of MRE11 to viral DNA during Ad5 E4 mutant DNA replication requires NBS1 (105), and that the NBS1 C terminus is required for the inhibition of Ad mutant DNA replication (86). These observations are consistent with data showing that MRN relocalization by Ad5 E4orf3 promotes viral replication (38).
It has been reported that the E1B-55K mutant H260A (also called ONYX-053) cannot stably bind to E4orf6 or p53 and, therefore, cannot induce p53 degradation (134). The protein is also defective in its nuclear localization, consistent with reports showing that E4orf6 is required to transport the Ad5 E1B-55K protein to the nucleus (32, 52, 114, 115). However, the H260A mutant still appears to show only minor defects in viral replication and late functions, such as mRNA export (115). It would be interesting to determine whether this protein still promotes MRN or DNA ligase IV degradation, as it is conceivable that there is enough residual E1B-55K/E4orf6 protein binding in the nucleus to carry out this function and thereby promote viral replication.
It is clear from Table 1 that there remain certain issues to be addressed regarding the phenotypes of some E1B-55K mutants. Matters are complicated by reports suggesting that cell type is important when considering E1B-55K mutant function, with differences being reported between proteins expressed in primary cells and tumor cell lines (115). Also, there appear to be differences in the absolute requirements for substrate degradation, depending on whether proteins are expressed in transfected cells or in the context of viral infection (132). It would be interesting to characterize fully all available mutant E1B-55K proteins in the context of viral infection in order to determine the effect of specific mutations on both early and late functions of E1B-55K, although this would be a considerable undertaking.
CONCLUSIONS
The most striking properties of the E1B-55K protein have long been known. Interaction with p53 was reported in 1982 and interaction with Ad E4orf6 in 1984 (129, 130); localization of the E1B-55K protein to aggresomes in Ad5 transformed cells was reported soon after (158). Similarly, the involvement of the E1B-55K protein in the transport of late viral mRNAs and shut-off of host protein synthesis has been known for over 20 years (2, 8, 9).
So, how far have we come in the intervening decades? We would suggest that the overriding role of the E1B-55K protein during viral infection has been established and that it is to form an E3 ubiquitin ligase complex with E4orf6 and target potentially detrimental cellular proteins for rapid degradation (5, 68, 120, 138). It has more recently become apparent that this activity plays an important role in the regulation of mRNA export during infection, although the target for degradation remains unknown (18, 154). During Ad E1-mediated transformation, the interaction with p53 is presumably of considerable significance. However, it seems likely that other properties of the protein can have an effect, particularly in the light of the recent report that transformation can occur in the absence of p53 binding (69). The determination of whether this may be linked to the strong transcriptional repression properties of the E1B-55K protein or primarily to MRN binding will require further study.
Research into the properties of the E1B-55K protein has been hampered by the difficulty of easily characterizing mutant proteins, as discussed earlier. In particular, it seems likely that the protein is significantly structured, with predictions showing appreciable areas of β sheets but relatively few α helices. The consequence of the apparently structured nature of the E1B-55K protein is twofold: binding sites for partner proteins can comprise amino acids from disparate regions, making it difficult to pinpoint binding sites; additionally, mutation in the p53-binding site, for example, could disrupt the structure in other parts of the E1B-55K protein (where other proteins bind), causing additional loss of function which is not connected to p53 interaction. It also seems that certain small mutations disrupt the E1B-55K protein structure sufficiently to render the protein unstable; this has to be borne in mind in consideration of all the reported mutational analyses.
It must also be remembered that almost all of the studies of the E1B-55K protein have been carried out using Ad2/Ad5 proteins, and there has been a tendency to assume that the results will apply to all serotypes. However, it is clear that there are distinct differences in the properties of the E1B-55K protein among Ad5, Ad12, and other viral serotypes (A.B. and R.J.A.G., unpublished observations). The Ad12 protein in particular may have unique properties, such as induction of chromosomal damage at specific and random loci (131) and the ability to overcome cellular senescence checkpoints when expressed alone in primary human fibroblasts (48).
The significance of many observations regarding the E1B-55K protein remains unknown. Certain interactions with cellular proteins have been reported, but no functional consequences have been definitively assigned to them (e.g., the interactions of the E1B-55K protein with E1B-AP5, Daxx, SSBP2, and WT1 proteins); they therefore require further investigation.
Finally, there may be surprising properties of the E1B-55K protein which have yet to be discovered. In particular, it seems likely that there remain one or more substrates for the E1B-55K/E4orf6 ubiquitin ligase complex. The identification of these putative substrates will no doubt help answer outstanding questions as to the mechanisms of E1B-55K protein functions during both viral infection and cell transformation.
Acknowledgments
We are most grateful to Cancer Research UK and the School of Cancer Sciences at the University of Birmingham for financial support.
We thank Sally Roberts for critical readings of the manuscript. We apologize to authors whose work could not be cited due to space limitations.
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Araujo, F. D., T. H. Stracker, C. T. Carson, D. V. Lee, and M. D. Weitzman. 2005. Adenovirus type 5 E4orf3 protein targets the Mre11 complex to cytoplasmic aggresomes. J. Virol. 7911382-11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiss, L. E., and H. S. Ginsberg. 1984. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J. Virol. 50202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babiss, L. E., H. S. Ginsberg, and J. E. Darnell. 1985. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol. Cell. Biol. 52552-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachi, A., I. C. Braun, J. P. Rodrigues, N. Panté, K. Ribbeck, C. von Kobbe, U. Kutay, M. Wilm, D. Görlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 6136-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker, A., K. J. Rohleder, L. A. Hanakahi, and G. Ketner. 2007. Adenovirus E4 34k and E1b 55k oncoproteins target host DNA ligase IV for proteasomal degradation. J. Virol. 817034-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology 156107-121. [DOI] [PubMed] [Google Scholar]
- 7.Barral, P. M., A. Rusch, A. S. Turnell, P. H. Gallimore, P. J. Byrd, T. Dobner, and R. J. Grand. 2005. The interaction of the hnRNP family member E1B-AP5 with p53. FEBS Lett. 5792752-2758. [DOI] [PubMed] [Google Scholar]
- 8.Bello, L. J., and H. S. Ginberg. 1967. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J. Virol. 1843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltz, G. A., and S. J. Flint. 1979. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J. Mol. Biol. 131353-373. [DOI] [PubMed] [Google Scholar]
- 10.Berk, A. J. 2005. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 247673-7685. [DOI] [PubMed] [Google Scholar]
- 11.Bernards, R., A. Houweling, P. I. Schrier, J. L. Bos, and A. J. van der Eb. 1982. Characterization of cells transformed by Ad5/Ad12 hybrid early region 1 plasmids. Virology 120422-432. [DOI] [PubMed] [Google Scholar]
- 12.Bernards, R., P. I. Schrier, J. L. Bos, and A. J. van der Eb. 1983. Role of adenovirus types 5 and 12 early region 1b tumor antigens in oncogenic transformation. Virology 12745-53. [DOI] [PubMed] [Google Scholar]
- 13.Bernards, R., M. G. de Leeuw, A. Houweling, and A. J. van der Eb. 1986. Role of adenovirus early region IB tumour antigens in transformation and lytic infection. Virology 150126-139. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274373-376. [DOI] [PubMed] [Google Scholar]
- 15.Blackford, A. N., R. K. Bruton, O. Dirlik, G. S. Stewart, A. M. Taylor, T. Dobner, R. J. Grand, and A. S. Turnell. 2008. A role for E1B-AP5 in ATR signaling pathways during adenovirus infection. J. Virol. 827640-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair Zajdel, M. E., and G. E. Blair. 1988. The intracellular distribution of the transformation-associated p53 in adenovirus transformed rodent cells. Oncogene 2579-584. [PubMed] [Google Scholar]
- 17.Blanchette, P., C. Y. Cheng, Q. Yan, G. Ketner, D. A. Ornelles, T. Dobner, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2004. Both BC-box motifs of adenovirus protein E4orf6 are required to efficiently assemble an E3 ligase complex that degrades p53. Mol. Cell. Biol. 249619-9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanchette, P., K. Kindsmüller, P. Groitl, F. Dallaire, T. Speiseder, P. E. Branton, and T. Dobner. 2008. Control of mRNA export by adenovirus E4orf6 and E1B55K proteins during productive infection requires E4orf6 ubiquitin ligase activity. J. Virol. 822642-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd, P. J., K. B. Brown, and P. H. Gallimore. 1982. Malignant transformation of human embryo retinoblasts by cloned adenovirus 12 DNA. Nature 29869-71. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso, F. M., S. E. Kato, W. Huang, S. J. Flint, and R. A. Gonzalez. 2008. An early function of the adenoviral E1B 55 kDa protein is required for the nuclear relocalization of the cellular p53 protein in adenovirus-infected normal human cells. Virology 378339-346. [DOI] [PubMed] [Google Scholar]
- 21.Carney, J. P., R. S. Maser, H. Olivares, E. M. Davis, M. Le Beau, J. R. Yates, L. Hays, W. F. Morgan, and J. H. Petrini. 1998. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93477-486. [DOI] [PubMed] [Google Scholar]
- 22.Carson, C. T., R. A. Schwartz, T. H. Stracker, C. E. Lilley, D. V. Lee, and M. D. Weitzman. 2003. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J. 226610-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho, T., J. S. Seeler, K. Ohman, P. Jordan, U. Pettersson, G. Akusjärvi, M. Carmo-Fonseca, and A. Dejean. 1995. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J. Cell Biol. 13145-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, Y., and G. Varani. 2005. Protein families and RNA recognition. FEBS J. 2722088-2097. [DOI] [PubMed] [Google Scholar]
- 25.Chen, Y. I., R. E. Moore, H. Y. Ge, M. K. Young, T. D. Lee, and S. W. Stevens. 2007. Proteomic analysis of in vivo-assembled pre-mRNA splicing complexes expands the catalog of participating factors. Nucleic Acids Res. 353928-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbin-Lickfett, K. A., and E. Bridge. 2003. Adenovirus E4-34kDa requires active proteasomes to promote late gene expression. Virology 315234-244. [DOI] [PubMed] [Google Scholar]
- 27.Culp, J. S., L. C. Webster, D. J. Friedman, C. L. Smith, W. J. Huang, F. Y.-H. Wu, M. Rosenberg, and R. P. Ricciardi. 1988. The 289-amino acid E1A protein of adenovirus binds zinc in a region that is important for trans-activation. Proc. Natl. Acad. Sci. USA 856450-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cutt, J. R., T. Shenk, and P. Hearing. 1987. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J. Virol. 61543-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debbas, M., and E. White. 1993. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 7546-554. [DOI] [PubMed] [Google Scholar]
- 30.Dellaire, G., and D. P. Bazett-Jones. 2004. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays 26963-977. [DOI] [PubMed] [Google Scholar]
- 31.Dellaire, G., R. W. Ching, K. Ahmed, F. Jalali, K. C. Tse, R. G. Bristow, and D. P. Bazett-Jones. 2006. Promyelocytic leukemia nuclear bodies behave as DNA damage sensors whose response to DNA double-strand breaks is regulated by NBS1 and the kinases ATM, Chk2, and ATR. J. Cell Biol. 17555-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobbelstein, M., J. Roth, W. T. Kimberly, A. J. Levine, and T. Shenk. 1997. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 164276-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dosch, T., F. Horn, G. Schneider, F. Krätzer, T. Dobner, J. Hauber, and R. H. Stauber. 2001. The adenovirus type 5 E1B-55K oncoprotein actively shuttles in virus-infected cells, whereas transport of E4orf6 is mediated by a CRM1-independent mechanism. J. Virol. 755677-5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doucas, V., A. M. Ishov, A. Romo, H. Juguilon, M. D. Weitzman, R. M. Evans, and G. G. Maul. 1996. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 10196-207. [DOI] [PubMed] [Google Scholar]
- 35.Endter, C., J. Kzhyshkowska, R. Stauber, and T. Dobner. 2001. SUMO-1 modification required for transformation by adenovirus type 5 early region 1B 55-kDa oncoprotein. Proc. Natl. Acad. Sci. USA 9811312-11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endter, C., and T. Dobner. 2004. Cell transformation by human adenoviruses. Curr. Top. Microbiol. Immunol. 273163-214. [DOI] [PubMed] [Google Scholar]
- 37.Endter, C., B. Härtl, T. Spruss, J. Hauber, and T. Dobner. 2005. Blockage of CRM1-dependent nuclear export of the adenovirus type 5 early region 1B 55-kDa protein augments oncogenic transformation of primary rat cells. Oncogene 2455-64. [DOI] [PubMed] [Google Scholar]
- 38.Evans, J. D., and P. Hearing. 2005. Relocalization of the Mre11-Rad50-Nbs1 complex by the adenovirus E4 ORF3 protein is required for viral replication. J. Virol. 796207-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falck, J., J. Coates, and S. P. Jackson. 2005. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434605-611. [DOI] [PubMed] [Google Scholar]
- 40.Fleisig, H. B., N. I. Orazio, H. Liang, A. F. Tyler, H. P. Adams, M. D. Weitzman, and L. Nagarajan. 2007. Adenoviral E1B55K oncoprotein sequesters candidate leukemia suppressor sequence-specific single-stranded DNA-binding protein 2 into aggresomes. Oncogene 264797-4805. [DOI] [PubMed] [Google Scholar]
- 41.Flint, S. J., and R. A. Gonzalez. 2003. Regulation of mRNA production by the adenoviral E1B 55-kDa and E4 Orf6 proteins. Curr. Top. Microbiol. Immunol. 272287-330. [DOI] [PubMed] [Google Scholar]
- 42.Freeman, A. E., P. H. Black, E. A. van der Pool, P. H. Henry, J. B. Austin, and R. J. Huebner. 1967. Transformation of primary rat embryo cells by adenovirus type 2. Proc. Natl. Acad. Sci. USA 581205-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisch, S. M., and J. S. Mymryk. 2002. Adenovirus E1A: paradox and paradigm. Nat. Rev. Mol. Cell Biol. 3441-452. [DOI] [PubMed] [Google Scholar]
- 44.Gabler, S., H. Schütt, P. Groitl, H. Wolf, T. Shenk, and T. Dobner. 1998. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J. Virol. 727960-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallimore, P. H., P. A. Sharp, and J. Sambrook. 1974. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA in nine lines of transformed rat cells using specific fragments of the viral genome. J. Mol. Biol. 8949-72. [DOI] [PubMed] [Google Scholar]
- 46.Gallimore, P. H., P. J. Byrd, J. L. Whittaker, and R. J. Grand. 1985. Properties of rat cells transformed by DNA plasmids containing adenovirus type 12 E1 DNA or specific fragments of the E1 region: comparison of transforming frequencies. Cancer Res. 452670-2680. [PubMed] [Google Scholar]
- 47.Gallimore, P. H., R. J. Grand, and P. J. Byrd. 1986. Transformation of human embryo retinoblasts with simian virus 40, adenovirus and ras oncogenes. Anticancer Res. 6499-508. [PubMed] [Google Scholar]
- 48.Gallimore, P. H., P. S. Lecane, S. Roberts, S. M. Rookes, R. J. Grand, and J. Parkhill. 1997. Adenovirus type 12 early region 1B 54K protein significantly extends the life span of normal mammalian cells in culture. J. Virol. 716629-6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gamsjaeger, R., C. K. Liew, F. E. Loughlin, M. Crossley, and J. P. MacKay. 2007. Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 3263-70. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez, R., and S. J. Flint. 2002. Effects of mutations in the adenoviral E1B 55-kilodalton protein coding sequence on viral late mRNA metabolism. J. Virol. 764507-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez, R., W. Huang, R. Finnen, C. Bragg, and S. J. Flint. 2006. Adenovirus E1B 55-kilodalton protein is required for both regulation of mRNA export and efficient entry into the late phase of infection in normal human fibroblasts. J. Virol. 80964-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodrum, F. D., T. Shenk, and D. A. Ornelles. 1996. Adenovirus early region 4 34-kilodalton protein directs the nuclear localization of the early region 1B 55-kilodalton protein in primate cells. J. Virol. 706323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodrum, F. D., and D. A. Ornelles. 1997. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J. Virol. 71548-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodrum, F. D., and D. A. Ornelles. 1998. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J. Virol. 729479-9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodrum, F. D., and D. A. Ornelles. 1999. Roles of the E4orf6, orf3, and E1B 55-kilodalton proteins in cell cycle-independent adenovirus replication. J. Virol. 737474-7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham, F. L., A. J. van der Eb, and H. L. Heijneker. 1974. Size and location of the transforming region in human adenovirus type 5 DNA. Nature 251687-691. [DOI] [PubMed] [Google Scholar]
- 57.Grand, R. J., M. L. Grant, and P. H. Gallimore. 1994. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology 203229-240. [DOI] [PubMed] [Google Scholar]
- 58.Grand, R. J., P. S. Lecane, D. Owen, M. L. Grant, S. Roberts, A. J. Levine, and P. H. Gallimore. 1995. The high levels of p53 present in adenovirus early region 1-transformed human cells do not cause up-regulation of MDM2 expression. Virology 210323-334. [DOI] [PubMed] [Google Scholar]
- 59.Grand, R. J., T. Mustoe, S. Roberts, and P. H. Gallimore. 1995. The quaternary structure of the adenovirus 12 early region 1B 54K protein. Virology 207255-259. [DOI] [PubMed] [Google Scholar]
- 60.Grand, R. J., D. Owen, S. M. Rookes, and P. H. Gallimore. 1996. Control of p53 expression by adenovirus 12 early region 1A and early region 1B 54K proteins. Virology 21823-34. [DOI] [PubMed] [Google Scholar]
- 61.Grand, R. J., J. Parkhill, T. Szestak, S. M. Rookes, S. Roberts, and P. H. Gallimore. 1999. Definition of a major p53 binding site on Ad2E1B58K protein and a possible nuclear localization signal on the Ad12E1B54K protein. Oncogene 18955-965. [DOI] [PubMed] [Google Scholar]
- 62.Grand, R., and D. P. Molloy. The relationship of structure to function of the adenovirus early region 1A oncoprotein. In K. Yoshida (ed.), Molecular biology of tumor virus gene products, in press. Research Signpost, Karala, India.
- 63.Gregory, R. I., K. P. Yan, G. Amuthan, T. Chendrimada, B. Doratotaj, N. Cooch, and R. Shiekhattar. 2004. The Microprocessor complex mediates the genesis of microRNAs. Nature 432235-240. [DOI] [PubMed] [Google Scholar]
- 64.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90595-606. [DOI] [PubMed] [Google Scholar]
- 65.Halbert, D. N., J. R. Cutt, and T. Shenk. 1985. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J. Virol. 56250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall, A. R., B. R. Dix, S. J. O'Carroll, and A. W. Braithwaite. 1998. p53-dependent cell death/apoptosis is required for a productive adenovirus infection. Nat. Med. 41068-1072. [DOI] [PubMed] [Google Scholar]
- 67.Harada, J. N., and A. J. Berk. 1999. p53-independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J. Virol. 735333-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harada, J. N., A. Shevchenko, A. Shevchenko, D. C. Pallas, and A. J. Berk. 2002. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J. Virol. 769194-9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Härtl, B., T. Zeller, P. Blanchette, E. Kremmer, and T. Dobner. 2008. Adenovirus type 5 early region 1B 55-kDa oncoprotein can promote cell transformation by a mechanism independent from blocking p53-activated transcription. Oncogene 273673-3684. [DOI] [PubMed] [Google Scholar]
- 70.Hartmuth, K., H. Urlaub, H. P. Vornlocher, C. L. Will, M. Gentzel, M. Wilm, and R. Lührmann. 2002. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl. Acad. Sci. USA 9916719-16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herold, A., M. Suyama, J. P. Rodrigues, I. C. Braun, U. Kutay, M. Carmo-Fonseca, P. Bork, and E. Izaurralde. 2000. TAP (NXF1) belongs to a multigene family of putative RNA export factors with a conserved modular architecture. Mol. Cell. Biol. 208996-9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hobom, U., and M. Dobbelstein. 2004. E1B-55-kilodalton protein is not required to block p53-induced transcription during adenovirus infection. J. Virol. 787685-8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horridge, J. J., and K. N. Leppard. 1998. RNA-binding activity of the E1B 55-kilodalton protein from human adenovirus type 5. J. Virol. 729374-9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Houweling, A., P. J. van den Elsen, and A. J. van der Eb. 1980. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology 105537-550. [DOI] [PubMed] [Google Scholar]
- 75.Huibregtse, J. M., and S. L. Beaudenon. 1996. Mechanism of HPV E6 proteins in cellular transformation. Semin. Cancer Biol. 7317-326. [DOI] [PubMed] [Google Scholar]
- 76.Jochemsen, H., G. S. G. Daniels, J. J. L. Hertoghs, P. I. Schrier, P. J. van den Elsen, and A. J. van der Eb. 1982. Identification of adenovirus-type 12 gene products involved in transformation and oncogenesis. Virology 12215-28. [DOI] [PubMed] [Google Scholar]
- 77.Kao, C. C., P. R. Yew, and A. J. Berk. 1990. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55K proteins. Virology 179806-814. [DOI] [PubMed] [Google Scholar]
- 78.Khuri, F. R., J. Nemunaitis, I. Ganly, J. Arseneau, I. F. Tannock, L. Romel, M. Gore, J. Ironside, R. H. MacDougall, C. Heise, B. Randlev, A. M. Gillenwater, P. Bruso, S. B. Kaye, W. K. Hong, and D. H. Kirn. 2000. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 6879-885. [DOI] [PubMed] [Google Scholar]
- 79.Kindsmüller, K., P. Groitl, B. Härtl, P. Blanchette, J. Hauber, and T. Dobner. 2007. Intranuclear targeting and nuclear export of the adenovirus E1B-55K protein are regulated by SUMO1 conjugation. Proc. Natl. Acad. Sci. USA 1046684-6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitchingman, G. R., S. P. Lai, and H. Westphal. 1977. Loop structures in hybrids of early RNA and the separated strands of adenovirus DNA. Proc. Natl. Acad. Sci. USA 744392-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koch, P., J. Gatfield, C. Lober, U. Hobom, C. Lenz-Stoppler, J. Roth, and M. Dobbelstein. 2001. Efficient replication of adenovirus despite the over-expression of active and non-degradable p53. Cancer Res. 615941-5947. [PubMed] [Google Scholar]
- 82.Kozlov, S. V., M. E. Graham, C. Peng, P. Chen, P. J. Robinson, and M. F. Lavin. 2006. Involvement of novel autophosphorylation sites in ATM activation. EMBO J. 253504-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kratzer, F., O. Rosorius, P. Heger, N. Hirschmann, T. Dobner, J. Hauber, and R. H. Stauber. 2000. The adenovirus type 5 E1B-55K oncoprotein is a highly active shuttle protein and shuttling is independent of E4orf6, p53 and Mdm2. Oncogene 19850-857. [DOI] [PubMed] [Google Scholar]
- 84.Krishna, S. S., I. Majumdar, and N. V. Grishin. 2003. Structural classification of zinc fingers. Nucleic Acids Res. 31532-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kzhyshkowska, J., A. Rusch, H. Wolf, and T. Dobner. 2003. Regulation of transcription by the heterogeneous nuclear ribonucleoprotein E1B-AP5 is mediated by complex formation with the novel bromodomain-containing protein BRD7. Biochem. J. 371385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lakdawala, S. S., R. A. Schwartz, K. Ferenchak, C. T. Carson, B. P. McSharry, G. W. Wilkinson, and M. D. Weitzman. 2008. Differential requirements of the C terminus of Nbs1 in suppressing adenovirus DNA replication and promoting concatemer formation. J. Virol. 828362-8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lane, D. P., and L. V. Crawford. 1979. T antigen is bound to a host protein in SV40-transformed cells. Nature 278261-263. [DOI] [PubMed] [Google Scholar]
- 88.Lee, J. H., and T. T. Paull. 2005. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 308551-554. [DOI] [PubMed] [Google Scholar]
- 89.Leppard, K. N., and R. D. Everett. 1999. The adenovirus type 5 E1b 55K and E4 Orf3 proteins associate in infected cells and affect ND10 components. J. Gen. Virol. 80997-1008. [DOI] [PubMed] [Google Scholar]
- 90.Lin, J., J. Chen, B. Elenbaas, and A. J. Levine. 1994. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 81235-1246. [DOI] [PubMed] [Google Scholar]
- 91.Linzer, D. I., and A. J. Levine. 1979. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1743-52. [DOI] [PubMed] [Google Scholar]
- 92.Liu, Y., A. L. Colosimo, X. J. Yang, and D. Liao. 2000. Adenovirus E1B 55-kilodalton oncoprotein inhibits p53 acetylation by PCAF. Mol. Cell. Biol. 205540-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu, Y., A. Shevchenko, A. Shevchenko, and A. J. Berk. 2005. Adenovirus exploits the cellular aggresome response to accelerate inactivation of the MRN complex. J. Virol. 7914004-14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lombard, D. B., and L. Guarente. 2000. Nijmegen breakage syndrome disease protein and MRE11 at PML nuclear bodies and meiotic telomeres. Cancer Res. 602331-2334. [PubMed] [Google Scholar]
- 95.Lowe, S. W., and H. E. Ruley. 1993. Stabilization of the p53 tumour suppressor is induced by adenovirus 5 E1a and accompanies apoptosis. Genes Dev. 7535-545. [DOI] [PubMed] [Google Scholar]
- 96.Luo, Q., E. Nieves, J. Kzhyshkowska, and R. H. Angeletti. 2006. Endogenous transforming growth factor-beta receptor-mediated Smad signaling complexes analyzed by mass spectrometry. Mol. Cell. Proteomics 51245-1260. [DOI] [PubMed] [Google Scholar]
- 97.Lupker, J. H., A. Davis, H. Jochemsen, and A. J. van der Eb. 1981. In vitro synthesis of adenovirus type 5 T antigens. I. Translation of early region 1-specific RNA from lytically infected cells. J. Virol. 37524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mackay, J. P., and M. Crossley. 1998. Zinc fingers are sticky together. Trends Biochem. Sci. 231-4. [DOI] [PubMed] [Google Scholar]
- 99.Maheswaran, S., C. Englert, S. B. Lee, R. M. Ezzel, J. Settleman, and D. A. Haber. 1998. E1B-55K sequesters WT1 along with p53 within a cytoplasmic body in adenovirus-transformed kidney cells. Oncogene 162041-2050. [DOI] [PubMed] [Google Scholar]
- 100.Mak, S., I. Mak, J. R. Smiley, and F. L. Graham. 1979. Tumourigenicity and viral gene expression in rat cells transformed by Ad 12 virions or by the EcoRI c fragment of Ad 12 DNA. Virology 98456-460. [DOI] [PubMed] [Google Scholar]
- 101.Marshall, L. J., A. C. Moore, M. Ohki, I. Kitabayashi, D. Patterson, and D. A. Ornelles. 2008. RUNX1 permits E4orf6-directed nuclear localization of the adenovirus E1B-55K protein and associates with centers of viral DNA and RNA synthesis. J. Virol. 826395-6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin, M. E., and A. J. Berk. 1998. Adenovirus E1B 55K represses p53 activation. in vitro. J. Virol. 723146-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin, M. E., and A. J. Berk. 1999. Corepressor required for adenovirus E1B 55,000-molecular-weight protein repression of basal transcription. Mol. Cell. Biol. 193403-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mathew, S. S., and E. Bridge. 2007. The cellular Mre11 protein interferes with adenovirus E4 mutant DNA replication. Virology 365346-355. [DOI] [PubMed] [Google Scholar]
- 105.Mathew, S. S., and E. Bridge. 2008. Nbs1-dependent binding of Mre11 to adenovirus E4 mutant viral DNA is important for inhibiting DNA replication. Virology 37411-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McBride, W. D., and A. Wiener. 1964. In vitro transformation of hamster kidney cells by human adenovirus type 12. Proc. Soc. Exp. Biol. Med. 115870-874. [DOI] [PubMed] [Google Scholar]
- 107.McLorie, W., C. J. McGlade, D. Takayesu, and P. E. Branton. 1991. Individual adenovirus E1B proteins induce transformation independently but by additive pathways. J. Gen. Virol. 721467-1471. [DOI] [PubMed] [Google Scholar]
- 108.Moore, M., N. Horikoshi, and T. Shenk. 1996. Oncogenic potential of the adenovirus E4orf6 protein. Proc. Natl. Acad. Sci. USA 9311295-11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muller, S., and T. Dobner. 2008. The adenovirus E1B-55K oncoprotein induces SUMO modification of p53. Cell Cycle 7754-758. [DOI] [PubMed] [Google Scholar]
- 110.Nemunaitis, J., I. Ganly, F. Khuri, J. Arseneau, J. Kuhn, T. McCarty, S. Landers, P. Maples, L. Romel, B. Randlev, T. Reid, S. Kaye, and D. Kirn. 2000. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 606359-6366. [PubMed] [Google Scholar]
- 111.Nevels, M., S. Rubenwolf, T. Spruss, H. Wolf, and T. Dobner. 1997. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc. Natl. Acad. Sci. USA 941206-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nevels, M., B. Tauber, E. Kremmer, T. Sprus, H. Wolf, and T. Dobner. 1999. Transforming potential of the adenovirus type 5 E4orf3 protein. J. Virol. 731591-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nevins, J. R., H. S. Ginsberg, J. M. Blanchard, M. C. Wilson, and J. E. Darnell. 1979. Regulation of the primary expression of the early adenovirus transcription units. J. Virol. 32727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ornelles, D. A., and T. Shenk. 1991. Localization of the adenovirus early region 1B 55-kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J. Virol. 65424-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O'Shea, C. C., L. Johnson, B. Bagus, S. Choi, C. Nicholas, A. Shen, L. Boyle, K. Pandey, C. Soria, J. Kunich, Y. Shen, G. Habets, D. Ginzinger, and F. McCormick. 2004. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 6611-623. [DOI] [PubMed] [Google Scholar]
- 116.Pelka, P., J. N. Ablack, G. J. Fonseca, A. F. Yousef, and J. S. Mymryk. 2008. Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes. J. Virol. 827252-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pilder, S., M. Moore, J. Logan, and T. Shenk. 1986. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol. Cell. Biol. 6470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Punga, T., and G. Akusjärvi. 2000. The adenovirus-2 E1B-55K protein interacts with a mSin3A/histone deacetylase 1 complex. FEBS Lett. 476248-252. [DOI] [PubMed] [Google Scholar]
- 119.Querido, E., R. C. Marcellus, A. Lai, R. Charbonneau, J. G. Teodoro, G. Ketner, and P. E. Branton. 1997. Regulation of p53 levels by the E1B 55-kilodalton protein and E4orf6 in adenovirus-infected cells. J. Virol. 713788-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Querido, E., P. Blanchette, Q. Yan, T. Kamura, M. Morrison, D. Boivin, W. G. Kaelin, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2001. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a Cullin-containing complex. Genes Dev. 153104-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rappsilber, J., U. Ryder, A. I. Lamond, and M. Mann. 2002. Large-scale proteomic analysis of the human spliceosome. Genome Res. 121231-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reid, T., E. Galanis, J. Abbruzzese, D. Sze, L. M. Wein, J. Andrews, B. Randlev, C. Heise, M. Uprichard, M. Hatfield, L. Rome, J. Rubin, and D. Kirn. 2002. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 626070-6079. [PubMed] [Google Scholar]
- 123.Ridgway, P. J., A. R. Hall, C. Myers, and A. W. Braithwaite. 1997. p53/E1b58kDa complex regulates adenovirus replication. Virology 237404-413. [DOI] [PubMed] [Google Scholar]
- 124.Rothmann, T., A. Hengstermann, N. J. Whitaker, M. Scheffner, and H. zur Hausen. 1998. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J. Virol. 729470-9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Royds, J. A., M. Hibma, B. R. Dix, L. Hananeia, I. A. Russell, A. Wiles, D. Wynford-Thomas, and A. W. Braithwaite. 2006. p53 promotes adenoviral replication and increases late viral gene expression. Oncogene 251509-1520. [DOI] [PubMed] [Google Scholar]
- 126.Rubenwolf, S., H. Schütt, M. Nevels, H. Wolf, and T. Dobner. 1997. Structural analysis of the adenovirus type 5 E1B 55-kilodalton-E4orf6 protein complex. J. Virol. 711115-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruley, H. E. 1983. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 304602-606. [DOI] [PubMed] [Google Scholar]
- 128.Sakaguchi, K., J. E. Herrera, S. Saito, T. Miki, M. Bustin, A. Vassilev, C. W. Anderson, and E. Appella. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 122831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarnow, P., Y. S. Ho, J. Williams, and A. J. Levine. 1982. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell 28387-394. [DOI] [PubMed] [Google Scholar]
- 130.Sarnow, P., P. Hearing, C. W. Anderson, D. N. Halbert, T. Shenk, and A. J. Levine. 1984. Adenovirus early region 1B 58,000-dalton tumor antigen is physically associated with an early region 4 25,000-dalton protein in productively infected cells. J. Virol. 49692-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schramayr, S., D. Caporossi, I. Mak, T. Jelinek, and S. Bacchetti. 1990. Chromosomal damage induced by human adenovirus type 12 requires expression of the E1B 55-kilodalton viral protein. J. Virol. 642090-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwartz, R. A., S. S. Lakdawala, H. D. Eshleman, M. R. Russell, C. T. Carson, and M. D. Weitzman. 2008. Distinct requirements of adenovirus E1b55K protein for degradation of cellular substrates. J. Virol. 829043-9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shaw, A. R., and E. B. Ziff. 1980. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3′ coterminal mRNAs and five late families. Cell 22905-916. [DOI] [PubMed] [Google Scholar]
- 134.Shen, Y., G. Kitzes, J. A. Nye, A. Fattaey, and T. Hermiston. 2001. Analyses of single-amino-acid substitution mutants of adenovirus type 5 E1B-55K protein. J. Virol. 754297-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sieber, T., and T. Dobner. 2007. Adenovirus type 5 early region 1B 156R protein promotes cell transformation independently of repression of p53-stimulated transcription. J. Virol. 8195-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steegenga, W. T., N. Riteco, A. G. Jochemsen, F. J. Fallaux, and J. L. Bos. 1998. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene 16349-357. [DOI] [PubMed] [Google Scholar]
- 137.Stewart, G. S., R. S. Maser, T. Stankovic, D. A. Bressan, M. I. Kaplan, N. G. Jaspers, A. Raams, P. J. Byrd, J. H. Petrini, and A. M. Taylor. 1999. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell 99577-587. [DOI] [PubMed] [Google Scholar]
- 138.Stracker, T. H., C. T. Carson, and M. D. Weitzman. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418348-352. [DOI] [PubMed] [Google Scholar]
- 139.Stracker, T. H., D. V. Lee, C. T. Carson, F. D. Araujo, D. A. Ornelles, and M. D. Weitzman. 2005. Serotype-specific reorganization of the Mre11 complex by adenoviral E4orf3 proteins. J. Virol. 796664-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Teodoro, J. G., T. Halliday, S. G. Whalen, D. Takayesu, F. L. Graham, and P. E. Branton. 1994. Phosphorylation at the carboxy terminus of the 55-kilodalton adenovirus type 5 E1B protein regulates transforming activity. J. Virol. 68776-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Teodoro, J. G., and P. E. Branton. 1997. Regulation of p53-dependent apoptosis, transcriptional repression, and cell transformation by phosphorylation of the 55-kilodalton E1B protein of human adenovirus type 5. J. Virol. 713620-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Trentin, J. J., Y. Yabe, and G. Taylor. 1962. The quest for human cancer viruses. Science 137835-841. [DOI] [PubMed] [Google Scholar]
- 143.Turnell, A. S., R. J. Grand, and P. H. Gallimore. 1999. The replicative capacities of large E1B-null group A and group C adenoviruses are independent of host cell p53 status. J. Virol. 732074-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van der Eb, A. J., H. van Ormondt, P. I. Schrier, J. A. Lupker, H. Jochemsen, P. J. van der Elsen, R. J. DeLeys, J. Maat, C. P. van Beveren, R. Dykema, and A. de Waard. 1980. Structure and function of the transforming genes of human adenovirus and SV40. Cold Spring Harb. Symp. Quant. Biol. 44383-399. [DOI] [PubMed] [Google Scholar]
- 145.van den Elsen, P. J., A. Houweling, and A. van der Eb. 1983. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology 128377-390. [DOI] [PubMed] [Google Scholar]
- 146.van den Elsen, P. J., A. Houweling, and A. J. van der Eb. 1983. Morphological transformation of human adenoviruses is determined to a large extent by gene products of region E1a. Virology 131242-246. [DOI] [PubMed] [Google Scholar]
- 147.van den Heuvel, S. J. L., T. van Laar, W. M. Kast, C. J. M. Melief, A. Zantema, and A. J. van der Eb. 1990. Association between the cellular p53 and the adenovirus 5 E1B-55kd proteins reduces the oncogenicity of Ad-transformed cells. EMBO J. 92621-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wan, Y. P., Y. M. Wu, C. M. Zhu, W. G. Yin, H. L. Cai, and M. J. Yu. 2004. Transcriptional repression of hDaxx enhanced by adenovirus 12 E1B55-kDa oncoprotein interacting with hDaxx. Chin. Med. J. 117753-757. [PubMed] [Google Scholar]
- 149.Weiden, M. D., and H. S. Ginsberg. 1994. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc. Natl. Acad. Sci. USA 91153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weitzman, M. D., C. T. Carson, R. A. Schwartz, and C. E. Lilley. 2004. Interactions of viruses with the cellular DNA repair machinery. DNA Repair 31165-1173. [DOI] [PubMed] [Google Scholar]
- 151.Weitzman, M. D., and D. A. Ornelles. 2005. Inactivating intracellular antiviral responses during adenovirus infection. Oncogene 247686-7696. [DOI] [PubMed] [Google Scholar]
- 152.White, E., and R. Cipriani. 1990. Role of adenovirus E1B proteins in transformation altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol. Cell. Biol. 10120-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whittaker, J. L., P. J. Byrd, R. J. Grand, and P. H. Gallimore. 1984. Isolation and characterization of four adenovirus type 12-transformed human embryo kidney cell lines. Mol. Cell. Biol. 4110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Woo, J. L., and A. J. Berk. 2007. Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export. J. Virol. 81575-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yew, P. R., C. C. Kao, and A. J. Berk. 1990. Dissection of functional domains in the adenovirus 2 early 1B 55K polypeptide by suppressor-linker insertional mutagenesis. Virology 179795-805. [DOI] [PubMed] [Google Scholar]
- 156.Yew, P. R., and A. J. Berk. 1992. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature 35782-85. [DOI] [PubMed] [Google Scholar]
- 157.Yew, P. R., X. Liu, and A. J. Berk. 1994. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 8190-202. [DOI] [PubMed] [Google Scholar]
- 158.Zantema, A., P. I. Schrier, A. Davis-Olivier, T. van Laar, R. T. Vaessen, and A. J. van der Eb. 1985. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol. Cell. Biol. 53084-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao, L. Y., A. L. Colosimo, Y. Liu, Y. Wan, and D. Liao. 2003. Adenovirus E1B 55-kilodalton oncoprotein binds to Daxx and eliminates enhancement of p53-dependent transcription by Daxx. J. Virol. 7711809-11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao, L. Y., A. Santiago, J. Liu, and D. Liao. 2007. Repression of p53-mediated transcription by adenovirus E1B 55-kDa does not require corepressor mSin3A and histone deacetylases. J. Biol. Chem. 2827001-7010. [DOI] [PubMed] [Google Scholar]
- 161.Zheng, X., X. M. Rao, J. G. Gomez-Gutierrez, H. Hao, K. M. McMasters, and H. S. Zhou. 2008. Adenovirus E1B55K region is required to enhance cyclin E expression for efficient viral DNA replication. J. Virol. 823415-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]